Abstract
Background
Acquired resistance to therapeutic drugs has become an important issue in treating ovarian cancer. Studies have shown that the prevalent chemotherapy resistance (cisplatin, paclitaxel etc.) for ovarian cancer occurs partly because of decreased production of reactive oxygen species within the mitochondria of ovarian cancer cells.
Main Body
Nuclear erythroid-related factor-2 (Nrf2) mainly controls the regulation of transcription of genes through the Keap1-Nrf2-ARE signaling pathway and protects cells by fighting oxidative stress and defending against harmful substances. This protective effect is reflected in the promotion of tumor cell growth and their resistance to chemotherapy drugs. Therefore, inhibition of the Nrf2 pathway may reverse drug resistance. In this review, we describe the functions of Nrf2 in drug resistance based on Nrf2-associated signaling pathways determined in previous studies.
Conclusions
Further studies on the relevant mechanisms of Nrf2 may help improve the outcomes of ovarian cancer therapy.
Keywords: Nrf2, Drug resistance, Reactive oxidative stress, Ovarian cancer
Background
Malignant ovarian tumors are one of the most common malignant tumors of the female reproductive organ. Among them, ovarian epithelial cancer has the highest mortality rate, posing a serious threat to women’s life. Early stage ovarian tumors are usually located deep inside the pelvis, exhibit no typical symptoms and are thus discovered only at the advanced stage. The treatment options for advanced ovarian cancer are usually cytoreductive surgery and chemotherapy. However, the current chemoresistance in ovarian cancer(OC)has become a key cause of treatment failure and OC-related deaths [1]. Although extensive research has been carried out on complex factors, including increased drug efflux, drug inactivation, alteration in drug target, and increased DNA repair, the existing mechanisms fail to completely account for the drug resistance in OC [2, 3]. In recent years, the level of oxygen species (ROS) has also been reported to play a vital role in the development of drug resistance in OC, and thus targeting ROS levels may be a promising strategy to conquer cancer chemoresistance.
Oxidative stress refers to the process of oxidative damage caused by an imbalance between the production and scavenging of oxygen free radicals in the body or cells, resulting in the accumulation of ROS and RNS in the body or cells. Increased ROS levels activate relevant signaling pathways, inhibit the function of tumor suppressor genes, and induce oncogenic mutations, ultimately leading to tumorigenesis [4, 5]. Moreover, the significance of elevated ROS lies in facilitating genomic instability and DNA damage in tumors with drug resistance and recurrence [6, 7]. Consequently, more researches on ROS regulation would assist us to overcome drug resistance in OC.
Nrf2 exerts a modifying influence on cellular oxidative stress response. At the same time, by modulating the expression of antioxidant genes, Nrf2 can help prevent cell damage from ROS and electrophiles and keep the balance of intracellular redox homeostasis [8, 9]. Conversely, findings from previous studies suggest that continuous activation of antioxidant Nrf2 may be beneficial to the growth of cancer cells, and may become a way for cancer cells to escape the attack of chemotherapy drugs, providing conditions for cancer cells to develop drug resistance [7, 10–12]. Accordingly, the purpose of this review is to review recent research on Nrf2-related drug resistance and mechanisms in OC to provide reference for clinical treatment.
Nrf2 and ROS
ROS regulation in OC
Recently, several studies showed that the generation of ROS is associated with drug resistance [13–16]. On the one hand that ROS mediate cytotoxicity induced by drugs in tumor cells. On the other hand ,cancer cells are surrounded by antioxidant molecules to keep ROS in the tumor microenvironment (TME), which contributes to the maintenance of drug resistance in OC [17]. This phenomenon may be caused by the different concentrations of ROS in cancer cells [18]. Usually, at low levels, ROS stimulate cell proliferation and survival in the form of mitogens [19, 20]. At medium levels, ROS may hinder the cell cycle process at varying degrees and induce cell differentiation [21]. At high levels, ROS might impair fundamental cellular substances such as proteins, DNAs, RNAs, and cause gene mutations—inhibition of tumor suppressor genes(P53,PTEN)and activation of oncogenes(K-ras, ERK,AKT), resulting in tumorigenesis in normal cells or multidrug resistance in cancers [18]. Consistently, Meng et al. and Dharmaraja et al. have identified that platinum-resistant OC cells can maintain steady high levels of ROS,which results in DNA damage [13, 22, 23]. In addition, several studies have indicated that in the TME, hypoxia-induced ROS cause cisplatin resistance by downregulating p-Drp1 (Ser637) and Mfn1 in OC cells [15, 16]. Common radio- and chemotherapeutic agents affect tumor outcome by modulating ROS; therefore, the impact of ROS modulation is essential for cancer treatment.
Nrf2 regulation in OC
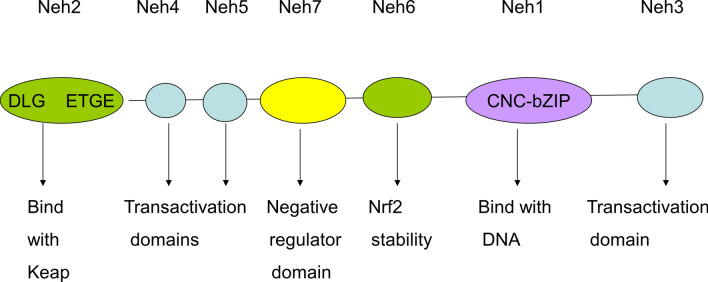
Nrf2 is a member of the Cap-n-Collar (CNC) regulatory protein family and is a transcription factor with a highly conserved basic leucine zipper structure. Nrf2 is a regulatory protein containing seven domains, Neh1–7, and has diverse features (Fig. 1). The Neh1 domain consists of the CNC-bZIP region, in which DNA binds to sMaf proteins as Nrf2 dimerization partners [24, 25]. The Neh2 domain contains two sites, namely DLG and ETGE, which combine with the cytoplasmic protein Keap1, a negative regulator of Nrf2 transcriptional activity [26]. Neh3–5 can bind to coactivating factors and are transactivating structural domains of Nrf2 [27, 28]. Neh6 is a serine-rich region associated with the negative regulation of Nrf2 stability, which relies on Keap1 [29]. Neh7 containing the retinoid X receptor inhibits the transcriptional activity of Nrf2 [30].
Fig. 1.
Structure of the human Nrf2 protein. The Neh1 domain consists of the CNC-bZIP region, in which DNA binds the sMaf proteins as Nrf2 dimerization partners. The Neh2 domain contains two sites, named as DLG and ETGE, which combine with the cytoplasmic protein Keap1, a negative regulator of Nrf2 transcriptional activity. Neh3–5 can bind with coactivating factors and are transactivating structural domains of Nrf2.Neh6 is a serine-rich region associated with the negative regulation of Nrf2 stability, which relies on Keap1. Neh7 containing the retinoid X receptor inhibits the transcriptional activity of Nrf2
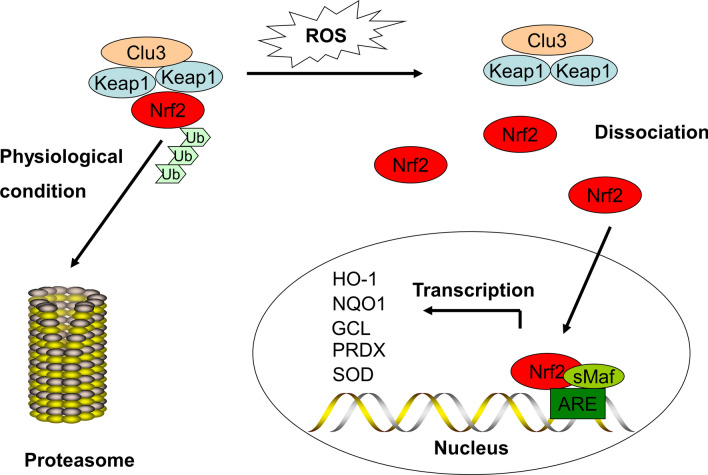
Under physiological conditions, Nrf2 is anchored in the cytoplasm by Keap1 as a substrate for the cullin 3-dependent E3 ubiquitin ligase complex and can induce ubiquitination and promptly degrade Nrf2 via the proteasome. However, when ROS or electrophiles attack cells, Nrf2 detaches from Keap1 and is rapidly translocated into the nucleus, forming a heterodimer with the sMaf protein and then integrating with the ARE, thereby transcriptionally activating Nrf2-regulated antioxidant gene expression including HO-1,NQO1,GCL,PRDX and SOD to exert anti-oxidative effects (Fig. 2). Nrf2 has a short half-life of around 10–30 min, and thus the high turnover of Nrf2 induced by Keap1 maintains ultra-low levels of Nrf2 [31, 32]. The protein products of these genes mediate detoxification through glutathione coupling and participate in ATP-dependent drug efflux, which may be involved with cisplatin resistance in OC [33]. High levels of Nrf2 provide a protective environment in both normal and cancer cells.
Fig. 2.
The classical view of Nrf2 activation and response. Under physiological conditions, Nrf2 is anchored in the cytoplasm by Keap1 as a substrate for Cullin 3-dependent E3 ubiquitin ligase complex and can induce ubiquitination and promptly degrade Nrf2 via the proteasome. However, when ROS or electrophiles attack cells, Nrf2 detaches from Keap1 and is rapidly transferred into the nucleus, forming a heterodimer with the sMaf protein and then integrating with the ARE, thereby transcriptionally activating Nrf2-regulated antioxidant gene expression including HO-1,NQO1,GCL,PRDX and SOD to exert anti-oxidative effects
Excessive activation of Nrf2 is considered as an intermediate link in cell proliferation and causes drug resistance in cancer therapy as well [34–36]. To be specific, Nrf2 activation and Keap1 inactivation mutations are the precursors of permanent constitutive activation of the Nrf2-dependent AR pathway, which is frequently observed in cancer. Besides, anti-cancer radiation and chemotherapies, rely heavily on the production of ROS to induce cytotoxicity [37–39]. Hence, excessive activation of the Nrf2-dependent AR pathway will reduce the effectiveness of such treatments [40, 41].
A clinical study has indicated that high cytoplasmic Nrf2 expression (the inactivated form of Nrf2) in serous carcinoma subtypes is associated with longer survival (p < 0.05), which appears to correlate with high ERα expression (p < 0.05) [42]. The same team found that Nrf2 expression in the cytoplasm was positively correlated with PR expression (p < 0.05) [43]. Furthermore, a retrospective study of the relationship between Nrf2 expression and clinical prognosis in 108 patients with different subtypes of OC showed that a high expression of Nrf2 in OC indicates short DFS (HR: 2.084; 95% CI: 1.229–3.536) and OS (HR: 2.487; 95% CI: 1.443–4.286) [44]. Konstantinopoulos et al. found that among 64 advanced EOC patients, the upregulation of Nrf2 promoted cisplatin resistance in OC patients and was associated with a short OS (P < 0.05) [45]. However, another study showed that chemoresistance is not significantly correlated with Nrf2 expression, although patients with low Nrf2 expression have higher recurrence rates and death rates than patients with high Nrf2 expression. [46] Hence, further studies on the relationships between clinical prognosis and Nrf2 expression, as well as relevant drug resistance mechanisms related to Nrf2, are needed.
Effect of Nrf2 on treatments for OC
Oncogenic mutations in OC may promote drug resistance by activating Nrf2
Disorder of Nrf2/Keap1 caused by mutation and activation of up-stream oncogenes is associated with nuclear transportation and constitutive activation of Nrf2. Gina et al. have confirmed that oncogenic mutations in primary murine cells, such as Kras, Braf and Myc, separately increased the constitutive transcription of Nrf2 to stabilize the basal Nrf2 level and hence reduce intracellular ROS, ultimately causing cells to escape from apoptosis and promoting tumorigenesis, metastasis and chemoresistance [9, 47]. In view of the relationship of ROS and Nrf2 with tumorigenesis, Nrf2 appears to be a significant target for cancer treatment.
Role of Nrf2 in ROS-mediated therapy resistance in OC
Role of Nrf2 in ROS-mediated cisplatin resistance in OC
As mentioned earlier, ROS play an indispensable role in the development of drug resistance. As the main antioxidant regulator, Nrf2, which is involved in ROS detoxification, tightly regulates drug resistance of tumors. It has been reported that during oxidative stress, as the transcription target of Nrf2, p62/SQSTM1 competes with Nrf2 for binding to Keap1 and forms a positive feedback loop between p62 and Nrf2 [48]. Xia et al. showed that overexpressed p62 may protect cells from vitamin K3-induced ROS generation by up-regulating antioxidant genes downstream of Nrf2, including HO-1 and NQO1, in OC [49]. Additionally, recent cases reported by Wu et al. also support the hypothesis that overexpression of CD99, a significant downstream gene of Nrf2, facilitates Nrf2-mediated cisplatin resistance in OC [50, 51]. Bao et al. suggested that low levels of Nrf2 suppressed the expression of ABCF2 and enhanced cisplatin sensitivity in OC cells by mediating the drug efflux pump mechanism [52]. Chen et al. argued that knockdown of Nrf2 in the SKVO3 cell line increased the production of ROS induced by cisplatin by increasing the phosphorylation level of p38-JNK.This subsequently led to elevation of ATF2 levels, followed by decreased expression of AKR1C1,which is involved in apoptosis, ultimately promoting the sensitivity of OC to cisplatin [53]. It was recently reported that activation of Nrf2 promotes activation of its downstream gene AKR1C1, which converts progesterone to an inactive form and promotes platinum resistance in ovarian cancer, while metformin reverses this process by increasing PR expression [54]. Mechanistically, Sun et al. found that SIRT5 contributes to the cisplatin resistance of OC by inhibiting cisplatin-mediated DNA damage via ROS through Nrf2 pathway modulation [55]. SLC40A1, as a novel iron metabolism-associated gene, serves as the only iron exporter gene with several putative Nrf2 binding sites. Wu et al. found that Nrf2 is highly expressed in cisplatin-resistant OC cells. Significantly increased gene expression of SLC401, a transferrin that inhibits Nrf2 translocation into the nucleus, reverses iron overload—induced cisplatin resistance in OC cells [56].
Molecular factors involved in Nrf2 regulation contribute to paclitaxel resistance
Paclitaxel is a first-line adjuvant drug for the treatment of OC, but only about half of OC patients respond to it [57, 58]. It is a new anti-microtubule drug that promotes tubulin polymerization to inhibit depolymerization, keeps tubulin stable, and inhibits cell mitosis. These different mechanisms cause a cascade of toxic effects in OC, such as the reduction of Δψm or elevation of ROS, which will eventually lead to cell death [59]. Enhancing the sensitivity of OC patients to paclitaxel is of great significance to improve prognosis. Stimulation of NADPH oxidase to accumulate ROS is an important part of paclitaxel cytotoxicity in cancer cells [60, 61]. Woo et al. held the view that inhibition of Nrf2 can enhance the chemosensitivity of cancer cells to paclitaxel [62]. We have reason to believe that targeting Nrf2 levels in OC cells may play an important role in overcoming paclitaxel resistance.
Role of Nrf2 in ROS-mediated PARP inhibitor sensitivity in OC
At present, under the condition of platinum resistance in OC, PARPi have shown encouraging effects in the first [63–65] and second-line [66, 67] maintenance therapy for patients with BRCA1/2 mutation and HRD [68]. Cells with HRD must depend on the replaceable mechanisms of NHEJ and BER, both of which require PARP enzymes [69]. BRCA1/2 mutant cancer cells may develop PARPi resistance by restoring HR repair and/or protecting replication forks [70].
Mitochondrial metabolism and ROS production cause DNA oxidative damage and genomic instability in cancers [71]. HRD OCcells require high levels of NAD + and ATP to power PARP-dependent DNA repair [72]. Besides, some scholars have found that PARPi enhanced the effect of Nrf2 inhibitors in BRCA1-mutant OC cells without fear of side effects from the combination of Nrf2 inhibitors with chemotherapeutics [73]. From the above findings, we can speculate that Nrf2 may play an irreplaceable role in PARPi repair of ROS-DNA oxidative damage.
Role of Nrf2 in ROS-mediated pertuzumab and trastuzumab resistance in OC
Several studies have proved that HER2/HER3, Nrf2,and ROS play a key role in promoting growth and drug resistance in cancer cells [74–79]. Specifically, as a key regulator of the Nrf2 pathway, ROS can regulate the HER2/HER3 complex and activate its function. When pertuzumab and trastuzumab, which target HER2/HER3 receptors, are used to treat with OC cell lines, Nrf2 inhibition suppress the Nrf2-dependent antioxidant response pathway, thereby allowing OC cells to overcome resistance to monoclonal antibodies. Khalil et al. proved that Nrf2 is a key factor driving the drug resistance in OC; this provides a new treatment idea in the sensitization of OC to immune targeted therapy [80].
Nrf2 inhibition increases the sensitivity of OC cells to adriamycin, one of the chemicals used in the treatment of OC [81]. Besides, Nrf2 modulates the sensitivity of OC cells to lapatinib and erlotinib by regulating the HER1 receptor [82].
Role of Nrf2 in ROS-mediated Mppa-PDT resistance in OC
PDT is a new type of tumor treatment method that has emerged in response to the development of medicine. It uses a photosensitizer that specifically accumulates in tumor tissues—currently, Mppa has a wide range of clinical application prospects due to its good absorption, high energy density, and strong permeability [83, 84]. It is activated under a specific wavelength of light, and a complex photochemical reaction occurs to generate ROS, which lead to irreversible tumor damage [85–87]. According to a previous research, Nrf2 silencing enhanced PDT sensitivity in breast, colon, renal, and glioblastoma cancer cells based on Mppa, which can increase the accumulation of photosensitizers by down-regulating ABCG2, thereby promoting the production of ROS [88]. Coincidentally, Tian et al. found that the inhibition of Nrf2- ABCG2 /HO-1 signaling increased ROS levels by attenuating antioxidants or pumping Mppa out of OC cells—suggesting that Nrf2-ABCG2 signaling might be involved in the intrinsic resistance to Mppa-PDT [89].
Role of Nrf2 in ROS-mediated ferroptosis resistance in OC
Ferroptosis is a novel mode of cell death first discovered by Dixon et al. in 2012 that is,—associated with unique morphological structure, biochemical, and genetic manifestations; it is essentially oxidative damage caused by excessive accumulation of iron ion-dependent lipid peroxidation products, mainly mitochondrial alterations [90]. Under normal conditions, Nrf2 remains inactive; when induced by ROS stimulation or electrophile substances, Nrf2 changes its molecular conformation and activates downstream antioxidant enzymes to play the role of an antioxidant and inhibit cellular ferroptosis [91]. There are two pathways to synthesize glutathione, which plays an essential role in combating oxidative stress, reducing lipid peroxidation, and protecting tissue cells, —in tumor cells: (a) The classical XC-system: the key factor is SLC7A11; and (b) Reverse transsulfuration pathway, and the key enzyme in this pathway is CBS; The above pathways can be activated by the ability of GPx4 to specifically convert highly toxic lipid hydrogen peroxide to non-toxic lipid alcohols, breaking down hydrogen peroxide to water, and its inactivation can induce excessive production of lipid ROS, which can contribute to ferroptosis. It has been reported that GPx4 is an Nrf2 downstream gene and that Nrf2 upregulation or GPX4 overexpression may be significantly associated with ferroptosis resistance in head and neck cancer, but this has not been confirmed in OC [92, 93]. In addition, Liu et al. showed that in OC, Nrf2 also causes erastin-induced ferroptosis resistance by activating CBS [94].
Natural inhibitors of Nrf2
Given that Nrf2 has a protective effect on tumors and can cause chemotherapy resistance, in recent years, many chemical substances and plant extracts have been reported to inhibit Nrf2 to confront the problem of drug resistance [95–100].
Brusatol, a quassinoid compound derived from Brucea javanica, is considered as a general translation inhibitor that results in decreased levels of short-lived proteins including Nrf2 [95]. For this reason, brusatol’s ability to overcome chemoresistance is compromised. Recently, Chen et al. isolated a plCSA-binding peptide from the malaria protein VAR2CSA, which effectively promotes the binding of brustol to OC, thus overcoming the drawback mentioned above [96]. In addition, Cucci et al. showed that ailanthone from Ailanthus altissima could significantly inhibit the expression of Nrf2 and YAP protein and subsequently inhibit the growth and colony formation of cisplatin-sensitive and cisplatin-resistant OC cells, and exert greater inhibitory effects on the migration of targeted cisplatin-resistant cells [97].
There are also some compounds that have not been proven in OC. Ascorbic acid, an inhibitor of Nrf2, partially restored cell sensitivity to imatinib by down-regulating Nrf2 and reducing the expression of γ-GCSL and the level of glutathione [98], and increased the sensitivity of HeLa cells to cisplatin and adriamycin [99]. Apigenin, a flavonoid extracted from various vegetables and fruits, inhibits the Nrf2 pathway, thereby making doxorubicin-resistant liver cancer cells sensitive to doxorubicin and increasing intracellular doxorubicin [100].
Conclusions
The Keap1-Nrf2-ARE system is a critical defense mechanism to protect cells from oxidative stress and electrophilic stress. While temporary Nrf2 activation during stress is advantageous for cell proliferation [101], sustained Nrf2 activation in cancer cells confers chemoresistance and aggressive tumorigenic activity, which has deleterious effects on the cancer patients [102–105]. Since Nrf2 increases the antioxidant and detoxification capacity of cancer cells, sustained high levels of Nrf2 activity can enhance therapeutic resistance of cancer cells. Nrf2 also drives metabolic reprogramming and cooperates with other oncogenic pathways to establish cellular metabolic processes that favor cell proliferation.
Most patients with OC treated by chemotherapy, immunotherapy, and molecular targeted therapy eventually develop resistance and show poor outcomes. In fact, there are many proteins that regulate the process of drug resistance in OC;—for example, downregulation of 14-3-3ζ, a key protein involved in ovarian development and gamete function [106–108], by RNA interference in OC cells results in enhanced sensitivity to cisplatin-induced cell death [109]. Meanwhile, multiple isoforms of 14-3-3 protein strongly interact with the cell cycle protein CDC25B, which is inactivated in Nrf2−/− cells, to regulate cell cycle in oocyte [110, 111]. Why did we choose to review Nrf2 as a key pivot in the regulation of drug resistance in OC? As described above, Nrf2, as the main regulator of the antioxidant response pathway, has received increasing attention for its significant effect in drug-resistant OC and thus, may be targeted for treating advanced OC. So far, several Nrf2 inhibitors have been used for overcoming drug resistance in OC. In addition to Nrf2 inhibitors, new potential therapeutic targets related to Nrf2 for overcoming drug resistance in OC are being identified (Fig. 3; Table 1). However, the mechanisms of Nrf2-associated drug resistance in OC cells remain unclear and should therefore be further investigated. There is also a need to develop appropriate animal models to evaluate the therapeutic efficacy of Nrf2-related therapeutic targets in drug-resistant OC.
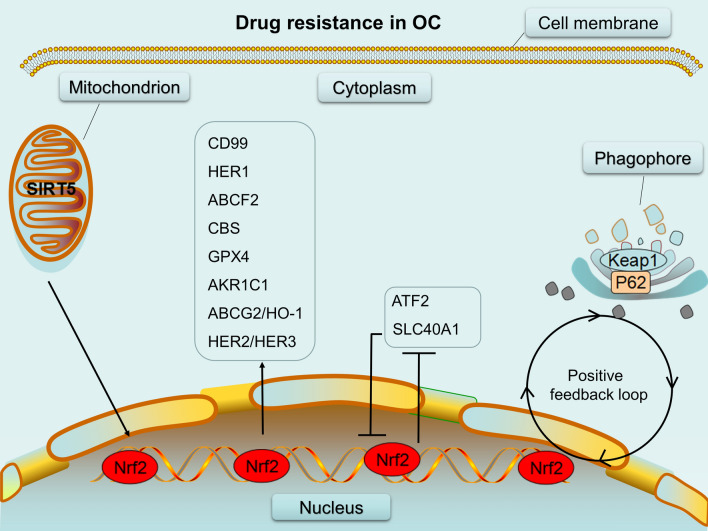
Fig. 3.
Various drug resistance mechanisms associated with Nrf2. SIRT5,CD99,ABCG2/HO-2,HER1,HER2/HER3,ABCF2,GPX4,AKR1C1 and CBS have a positive relationship with Nrf2 as molecules regulated by Nrf2 or regulating Nrf2;while ATF and SLC40A1 have a negative relationship with Nrf2; As the transcription target of Nrf2, p62/SQSTM1 competes with Nrf2 for binding with Keap1 and form a positive feedback loop between p62 and Nrf2.(“→” represents “activation” “— ” means “inhibition”.)
Table 1.
Overview of Nrf2-interacting factors in cancer cell lines
| Gene | Effects on Nrf2 | Effects on cell | Tumor model | Resistance to | References |
|---|---|---|---|---|---|
| P62 | Activator | Protective | SKOV3/DDP | Cisplatin | [48] |
| CD99 | Activator | Protective | A2780,COC1/DDP | Cisplatin | [50, 51] |
| ABCF2 | Activator | Protective | A2780 | Cisplatin | [52] |
| ATF2 | Inhibitor | Cytotoxic | SKOV3 | Cisplatin | [53] |
| AKR1C1 | Activator | Protective | – | Platinum | [54] |
| SIRT5 | Activator | Protective | A2780,SKOV3,CAOV3 | Cisplatin | [55] |
| SLC40A1 | Inhibitor | Cytotoxic | A2780CP,PEO4,COC1/DDP | Cisplatin | [56] |
| HER1 | Activator | Protective | PEO1, SKOV3, and OVCAR3 | lapatinib and erlotinib | [82] |
| HER2/HER3 | Activator | Protective | PEO4,OVCAR4,SKOV3 | Pertuzumab/Trastuzumab/Docetaxel | [80] |
| ABCG2 | Activator | Protective | SKOV3 | Mppa-PDT | [89] |
| GPX4 | Activator | Protective | AMC-HN2–11/SNU | Ferroptosis | [92, 93] |
| CBS | Activator | Protective | SKOV3 and OVCA429 | Ferroptosis | [94] |
| Compounds | |||||
| Brusatol | Inhibitor | Cytotoxic | SKOV3/HEC-1-A/A549 | – | [96] |
| Ailanthone | Inhibitor | Cytotoxic | A2780/CP70 | Cisplatin | [97] |
| Ascorbic acid | Inhibitor | Cytotoxic | KCL22/SR; Hela | Imatinib;cisplatin / adriamycin | [98, 99] |
| Apigenin | Inhibitor | Cytotoxic | BEL-7402/ADM | Doxorubicin | [100] |
Besides active exploration and mechanistic research on therapeutic targets associated with Nrf2, studies for discovering diagnostic biomarkers and surrogate markers for refractory OC are also needed. For progress in diagnosis and treatment, further researches and technical improvements are required. Consequently, a thorough elucidation of the function of Nrf2 will help to improve the clinical diagnosis and prognosis of OC.
Acknowledgements
We thank Amanda, Editage (www.editage.cn), for English editing a draft of this manuscript.
Abbreviations
- OC
Ovarian cancer
- Nrf2
Nuclear erythroid-related factor-2
- Keap-1
Kelch-like ECH-associated protein-1
- sMaf
Small musculoaponeurotic fibrosarcoma
- ROS
Reactive oxygen species
- bZIP
Basic leucine zipper
- HO-1
Heme oxygenase 1
- NQO1
NAD(P)H dehydrogenase (quinone) 1
- ABCG2
ATP-binding cassette, subfamily G, member 2
- AKR1C1
Aldo-keto reductase family 1 member C1
- SIRT5
Sirtuin 5
- SLC40A1
Solute carrier family 40 member 1
- TME
Tumor microenvironment
- Mppa-PDT
Methyl pyropheophorbide-amediated photodynamic therapy
- ABC
ATP-binding cassette
- P-gp
P-glycoprotein
- PARPi
Poly-ADP Ribose Polymerase inhibitors
- HRD
Homologous recombination deficiency
- CBS
Cystathionine β-synthase
Authors’ contributions
SZ and XC: conceptualization; DL: writing—original draft preparation; DL,XH and FZ: writing—review and editing; SZ and XC: permission to submit. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All the authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xinxin Ci, Email: cixinxin@jlu.edu.cn.
Songling Zhang, Email: slzhang@jlu.edu.cn.
References
- 1.Capriglione S, Luvero D, Plotti F, et al. Ovarian cancer recurrence and early detection:may HE4 play a key role in this open challenge? A systematic review of literature. Med Oncol. 2017;34:164. doi: 10.1007/s12032-017-1026-y. [DOI] [PubMed] [Google Scholar]
- 2.Zhong YY, Chen HP, Tan BZ, et al. Triptolide avoids cisplatin resistance and induces apoptosis via the reactive oxygen species/nuclear factor-κB pathway in SKOV3PT platinum-resistant human ovarian cancer cells. Oncol Lett. 2013;6(4):1084–1092. doi: 10.3892/ol.2013.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren F, Shen J, Shi H, et al. Novel mechanisms and approaches to overcome multidrug resistance in the treatment of ovarian cancer. Biochim Biophys Acta. 2016;1866(2):266–75. doi: 10.1016/j.bbcan.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14(11):709–21. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moloney JN, Stanicka J. Cotter,Subcellular localization of the FLT3-ITD oncogene plays a significant role in the production of NOX-and p22phox-derived reactive oxygen species in acute myeloid leukemia. Leuk Res. 2017;52:34–42. doi: 10.1016/j.leukres.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Dharmaraja AT. Role of Reactive Oxygen Species (ROS) in therapeutics and drug resistance in cancer and bacteria. J Med Chem. 2017;60(8):3221–3240. doi: 10.1021/acs.jmedchem.6b01243. [DOI] [PubMed] [Google Scholar]
- 7.Cui Q, Wang J-Q, Assaraf YG, et al. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist Updates. 2018;41:1–25. doi: 10.1016/j.drup.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Latella G. Redox imbalance in intestinal fibrosis: beware of the TGFβ-1, ROS, and Nrf2 connection. Dig Dis Sci. 2018;63(2):312–320. doi: 10.1007/s10620-017-4887-1. [DOI] [PubMed] [Google Scholar]
- 9.Sajadimajd S, Khazaei M. Oxidative stress and cancer: the role of Nrf2. Curr Cancer Drug Targets. 2018;18(6):538–557. doi: 10.2174/1568009617666171002144228. [DOI] [PubMed] [Google Scholar]
- 10.Alvin JL, Chia CE, Goldring NR, Kitteringham S, Wong Q, Morgan P, Park BK. Differential effect of covalent protein modification and glutathione depletion on the transcriptional response of Nrf2 and NF-kappaB. Biochem Pharmacol. 2010;80(3):410–21. doi: 10.1016/j.bcp.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27(20):2179–91. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roh J-L, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng Y, Chen C-W, Mingo MH, Yung al.DUOXA1-mediated ROS production promotes cisplatin resistance by activating ATR-Chk1 pathway in ovarian cancer. Cancer Lett. 2018;1:104–16. doi: 10.1016/j.canlet.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonelli C, Chio IIC, Tuveson DA. Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Y, Kim B, Cho U, et al. Mitochondrial fission causes cisplatin resistance under hypoxicconditions via ROS in ovarian cancer cells. Oncogene. 2019;38(45):7089–105. doi: 10.1038/s41388-019-0949-5. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Zhang D, Shen L, et al. Redox homeostasis protects mitochondria through accelerating ROS conversion to enhance hypoxia resistance in cancer cells. Sci Rep. 2016;6:22831. doi: 10.1038/srep22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salatino A, Aversa I, Battaglia AM. H-Ferritin affects cisplatin-induced cytotoxicity in ovarian cancer cells through the modulation of ROS. Oxid Med Cell Longev. 2019;2019:3461251. doi: 10.1155/2019/3461251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui Q, Wang JQ, Assaraf YG, et al. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist Updates. 2018;41:1–25. doi: 10.1016/j.drup.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;19(10):R453–62. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill JG, Piskounova E, Morrison SJ. Cancer, oxidative stress, and metastasis. Cold Spring Harb Symp Quant Biol. 2016;81:163–75. doi: 10.1101/sqb.2016.81.030791. [DOI] [PubMed] [Google Scholar]
- 21.Verbon EH, Post JA, Boonstra J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene. 2012;511(1):1–6. doi: 10.1016/j.gene.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 22.Dharmaraja AT. Role of Reactive Oxygen Species (ROS) in therapeutics and drug resistance in cancer and bacteria. J Med Chem. 2017;60(8):3221–3240. doi: 10.1021/acs.jmedchem.6b01243. [DOI] [PubMed] [Google Scholar]
- 23.Maiti AK. Gene network analysis of oxidative stress-mediated drug sensitivity in resistant ovarian carcinoma cells. Pharmacogenom J. 2010;10(2):94–104. doi: 10.1038/tpj.2009.49. [DOI] [PubMed] [Google Scholar]
- 24.Tian B, Lu ZN, Guo XL. Regulation and role of nuclear factor-E2-related factor 2 (Nrf2) in multidrug resistance of hepatocellular carcinoma. Chem Biol Interact. 2018;280:70–6. doi: 10.1016/j.cbi.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Motohashi H, Katsuoka F, Engel JD, Yamamoto MSmall. Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc Natl Acad Sci USA. 2004;101:6379–84. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katoh Y, Itoh K, Yoshida E, Miyagishi M, Fukamizu A, Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–68. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 28.Nioi P, Nguyen T, Sherratt PJ, Pickett CB. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol Cell Biol. 2005;25:10895–906. doi: 10.1128/MCB.25.24.10895-10906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain,one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–81. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Liu K, Geng M, Gao P, Wu X, Hai Y, et al. RXRalpha inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013;73:3097–108. doi: 10.1158/0008-5472.CAN-12-3386. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278:4536–41. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- 32.Motohashi H, O’Connor T, Katsuoka F, Engel JD, Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/S0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- 33.Konstantinopoulos PA, Spentzos D, Fountzilas E, Francoeur N, Sanisetty S, Grammatikos AP, et al. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011;71(15):5081–9. doi: 10.1158/0008-5472.CAN-10-4668. [DOI] [PubMed] [Google Scholar]
- 34.Gauron C, Rampon C, Bouzaffour M, Ipendey E, Teillon J, Volovitch M, Vris S. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci Rep. 2013;3:2084. doi: 10.1038/srep02084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deeni Y, Khalil HS, Goltsov A, Langdon S, Harrison D, Bown J. Quantitative analysis of proliferation behaviour of ovarian cancer cells with the dynamics of reactive oxygen species production and sequestration. J Biotech. 2014;185:1–126. doi: 10.1016/j.jbiotec.2014.05.025. [DOI] [Google Scholar]
- 36.Khalil HS, Goltsov A, Langdon SP, Harrison DJ, Bown J, Deeni Y. Quantitative analysis of NRF2 pathway reveals key elements of the regulatory circuits underlying antioxidant response and proliferation of ovarian cancer cells. J Biotechnol. 2015;202:12–30. doi: 10.1016/j.jbiotec.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 37.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci USA. 2008;105:13568–73. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, Shibata T, Yamamoto M, Hirohashi S. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–9. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 39.Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N, Itoh K. Yamamoto Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res. 2009;15:3423–32. doi: 10.1158/1078-0432.CCR-08-2822. [DOI] [PubMed] [Google Scholar]
- 40.Pan H, Wang H, Zhu L, Wang X, Cong Z, Sun K, Fan Y. The involvement of Nrf2-ARE pathway in regulation of apoptosis in human glioblastoma cell U251. Neurol Res. 2013;35:71–8. doi: 10.1179/1743132812Y.0000000094. [DOI] [PubMed] [Google Scholar]
- 41.Niture SK, Jaiswal AK. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic Biol Med. 2013;57:119–31. doi: 10.1016/j.freeradbiomed.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Czogalla B, Kahaly M, Mayr D, Schmoeckel E, Niesler B, Kolben T, Burges A, Mahner S, Jeschke U, Trillsch F. Interaction of ERα and NRF2 impacts survival in ovarian cancer patients. Int J Mol Sci. 2018;20(1):112. doi: 10.3390/ijms20010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Czogalla B, Kahaly M, Mayr D, Schmoeckel E, Niesler B, Hester A, Zeder-Göß C, Kolben T, Burges A, Mahner S, Jeschke U, Trillsch F. Correlation of NRF2 and progesterone receptor and its effects on ovarian cancer biology. Cancer Manag Res. 2019;11:7673–7684. doi: 10.2147/CMAR.S210004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liew PL, Hsu CS, Liu WM. Prognostic and predictive values of Nrf2, Keap1, p16 and E-cadherin expression in ovarian epithelial carcinoma. Int J Clin Exp Pathol. 2015;8:5642–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Konstantinopoulos PA, Spentzos D, Fountzilas E, et al. Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res. 2011;71(15):5081–9. doi: 10.1158/0008-5472.CAN-10-4668. [DOI] [PubMed] [Google Scholar]
- 46.Cho HY, Kim K, Kim YB, Kim H, No JH. Expression patterns of Nrf2 and Keap1 in ovarian cancer cells and their prognostic role in disease recurrence and patient survival. Int J Gynecol Cancer. 2017;27(3):412–9. doi: 10.1097/IGC.0000000000000908. [DOI] [PubMed] [Google Scholar]
- 47.DeNicola GM, Karreth FA, Humpton TJ, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–9. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.XIA M, YU H, GU S, et al. p62/SQSTM1 is involved in cisplatin resistance in human ovarian cancer cells via the Keap1-Nrf2-ARE system. Int J Oncol. 2014;45(6):2341–8. doi: 10.3892/ijo.2014.2669. [DOI] [PubMed] [Google Scholar]
- 49.Xia M, Yan X, Zhou L, Xu Y, Su J, Li H. p62 suppressed VK3-induced oxidative damage through Keap1/Nrf2 pathway in human ovarian cancer cells. J Cancer. 2020;11(6):1299–307. doi: 10.7150/jca.34423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jarboe EA, Hirschowitz SL, Geiersbach KB, Wallander ML, Tripp SR, Layfield LJ, et al. Juvenile granulosa cell tumors: immunoreactivity for CD99 and Fli-1 and EWSR1 translocation status: a study of 11 cases. Int J Gynecol Pathol. 2014;33(1):11–5. doi: 10.1097/PGP.0b013e31828309e6. [DOI] [PubMed] [Google Scholar]
- 51.Wu J, Zhang Z, Li H, Wu S, Liu Z. Nrf2 Induced Cisplatin Resistance in Ovarian Cancer by Promoting CD99 Expression. Biochem Biophys Res Commun. 2019;518(4):698–705. doi: 10.1016/j.bbrc.2019.08.113. [DOI] [PubMed] [Google Scholar]
- 52.Bao LJ, Wu JF, Dodson M, Montserrat E, Ning Y, Zhang ZB .al.ABCF2, an Nrf2 Target Gene, Contributes to Cisplatin Resistance in Ovarian Cancer. CellsMol Carcinog. 2017;56(6):1543–53. doi: 10.1002/mc.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J, Fiona Simpkins SC, Simpkins H. The role of Nrf2 and ATF2 in resistance to platinum-based chemotherapy. Cancer Chemother Pharmacol. 2017;79(2):369–380. doi: 10.1007/s00280-016-3225-1. [DOI] [PubMed] [Google Scholar]
- 54.Czogalla B, Kahaly M, Mayr D, Schmoeckel E, Niesler B, Hester A, Zeder-Göß C, Kolben T, Burges A, Mahner S, Jeschke U, Trillsch F. Correlation of NRF2 and progesterone receptor and its effects on ovarian cancer biology. Cancer Manag Res. 2019;11:7673–7684. doi: 10.2147/CMAR.S210004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun X, Wang S, Gai J, Guan J, Li J, Li Y, et al. SIRT5 promotes cisplatin resistance in ovarian cancer by suppressing DNA damage in a ROS-dependent manner via regulation of the Nrf2/HO-1 pathway. Front Oncol. 2019;9:754. doi: 10.3389/fonc.2019.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu JF, Bao LJ, Zhang ZB, Yi XF. Nrf2 induces cisplatin resistance via suppressing the iron export related gene SLC40A1 in ovarian cancer cells. Oncotarget. 2017;8(55):93502–15. doi: 10.18632/oncotarget.19548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ganz PA, Goodwin PJ. Breast cancer survivorship: where are we today? Adv Exp Med Biol. 2015;862:1–8. doi: 10.1007/978-3-319-16366-6_1. [DOI] [PubMed] [Google Scholar]
- 58.Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: an overview. BMC Med. 2015;13:195. doi: 10.1186/s12916-015-0439-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J, Neuwelt Y, Leslie AJ, Muldoon L, Neuwelt EA. Acetaminophen enhances cisplatin- and paclitaxel-mediated cytotoxicity to SKOV3 human ovarian carcinoma. Anticancer Res. 2013;33(6):2391–2400. [PMC free article] [PubMed] [Google Scholar]
- 60.Jérôme A, Batteux F, Nicco C, et al. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 2006;119(1):41–8. doi: 10.1002/ijc.21685. [DOI] [PubMed] [Google Scholar]
- 61.Jérôme A, Lu Y, Hu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67(8):3512–7. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 62.Woo Y, Oh J, Kim J-S. Suppression of Nrf2 activity by chestnut leaf extract increases chemosensitivity of breast cancer stem cells to paclitaxel. Nutrients. 2017;9(7):760. doi: 10.3390/nu9070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Martin A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 65.Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–28. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 66.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Eng J Med. 2012;366:1382–92. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 67.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–84. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 68.Tomao F, Bardhi E, Di Pinto A, et al. Parp inhibitors as maintenance treatment in platinum sensitive recurrent ovarian cancer: An updated meta-analysis of randomized clinical trials according to BRCA mutational status. Cancer Treat Rev. 2019;80:101909. doi: 10.1016/j.ctrv.2019.101909. [DOI] [PubMed] [Google Scholar]
- 69.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.D’Andrea AD. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair. 2018;71:172–176. doi: 10.1016/j.dnarep.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 71.Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, Lleonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12(1):376–90. doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Lahiguera A, Hyroššová P, Figueras A, et al. Tumors defective in homologous recombination rely on oxidative metabolism: relevance to treatments with PARP inhibitors. EMBO Mol Med. 2020;12(6):e11217. doi: 10.15252/emmm.201911217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Wijst MGP, Huisman C, Mposhi A, Roelfes G, Rots MG. Targeting Nrf2 in healthy and malignant ovarian epithelial cells: Protection versus promotion. Mol Oncol. 2015;9(7):1259–73. doi: 10.1016/j.molonc.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang HJ, Yi YW, Hong YB, et al. HER2 confers drug resistance of human breast cancer cells through activation of NRF2 by direct interaction. Sci Rep. 2014;4:7201. doi: 10.1038/srep07201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manandhar S, Choi BH, Jung KA, et al. NRF2 inhibition represses ErbB2 signaling in ovarian carcinoma cells: implications for tumor growth retardation and docetaxel sensitivity. Free Radic Biol Med. 2012;52:1773–85. doi: 10.1016/j.freeradbiomed.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 76.Shimokawa H. Reactive oxygen species promote vascular smooth muscle cell proliferation. Circ Res. 2013;113:1040–2. doi: 10.1161/CIRCRESAHA.113.302049. [DOI] [PubMed] [Google Scholar]
- 77.Gauron C, Rampon C, Bouzaffour M, Ipendey E, Teillon J, Volovitch M, Vriz S. Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci Rep. 2013;3:2084. doi: 10.1038/srep02084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Y, Tian Z, Guo R, Ren F. Nrf2 inhibitor, brusatol in combination with trastuzumab exerts synergistic antitumor activity in HER2-positive cancers by inhibiting Nrf2/HO-1 and HER2-AKT/ERK1/2 pathways. Oxid Med Cell Longev. 2020 doi: 10.1155/2020/9867595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y, Guo R, Tian X, et al. Synergistic anti-tumor activity of Nimotuzumab in combination with Trastuzumab in HER2-positive breast cancer. Biochem Biophys Res Commun. 2017;489(4):523–527. doi: 10.1016/j.bbrc.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 80.Hilal S, Khalil SP, Goltsov LA, et al. A novel mechanism of action of HER2 targeted immunotherapy is explained by inhibition of NRF2 function in ovarian cancer cells. Oncotarget. 2016;7(46):75874–75901. doi: 10.18632/oncotarget.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manandhar S, Lee S, Kwak M-K. Effect of stable inhibition of NRF2 on doxorubicin sensitivity in human ovarian carcinoma OV90 cells. Arch Pharm Res. 2010;33(5):717–26. doi: 10.1007/s12272-010-0511-z. [DOI] [PubMed] [Google Scholar]
- 82.Kankia IH, Khalil HS, Simon P. NRF2 Regulates HER1 Signaling Pathway to Modulate the sensitivity of Ovarian Cancer Cells to Lapatinib and Erlotini. Oxid Med Cell Longev. 2017;2017:1864578. doi: 10.1155/2017/1864578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo T, Wilson BC, Lu Q-B. Evaluation of one- and two-photon activated photodynamic therapy with pyropheophorbide-a methyl ester in human cervical, lung and ovarian cancer cells. J Photochem Photobiol B. 2014;5:102–10. doi: 10.1016/j.jphotobiol.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 84.Guelluy P-H, Fontaine-Aupart M-P, Grammenos A, Lécart S, Maryse Hoebeke PJ. Optimizing photodynamic therapy by liposomal formulation of the photosensitizer pyropheophorbide-a methyl ester: in vitro and ex vivo comparative biophysical investigations in a colon carcinoma cell line. Photochem Photobiol Sci. 2010;9(9):1252-60. [DOI] [PubMed]
- 85.Stanisław Kwiatkowski BK, Przystupski D, et al. Photodynamic therapy—mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 86.Ali M, Rkein DM, Ozog Photodynamic therapy. Dermatol Clin. 2014;32(3):415–25. doi: 10.1016/j.det.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 87.David M, Ozog AM, Rkein G, Fabi S, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42(7):804–27. doi: 10.1097/DSS.0000000000000800. [DOI] [PubMed] [Google Scholar]
- 88.Choi BH, Ryoo IG, Kang HC, Kwak MK. The sensitivity of cancer cells to pheophorbide a-based photodynamic therapy is enhanced by Nrf2 silencing. PLoS One. 2014;9:e107158. doi: 10.1371/journal.pone.0107158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tian S, Yong M, Zhu J, et al. Enhancement of the effect of methyl pyropheophorbide-a-mediated photodynamic therapy was achieved by increasing ROS through inhibition of Nrf2-HO-1 or Nrf2-ABCG2 signaling. Anticancer Agents Med Chem. 2017;17:1824–36. doi: 10.2174/1871520617666170327145857. [DOI] [PubMed] [Google Scholar]
- 90.Dixon SJ, Lemberg KM, Lamprecht M, et al. Ferroptosis: an iron-dependent form of non-apoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bellezza I, Giambanco I, Minelli A. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta. 2018;1865(5):721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 92.Doll S, Proneth B, Tyurina YY, et al. ACSL4 ferroptosis sensitivity by shaping cellular lipid. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shin D, Kim EH, Lee J, Roh J-L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018;129:454–62. doi: 10.1016/j.freeradbiomed.2018.10.426. [DOI] [PubMed] [Google Scholar]
- 94.Nan Liu X, Lin, Huang C. Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin-induced ferroptosis resistance. Br J Cancer. 2020;122(2):279–92. doi: 10.1038/s41416-019-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harder B, Tian W, Clair J, Tan AC, Ooi A, Chapman E, et al. Brusatol overcomes chemoresistance through inhibition of protein translation. Mol Carcinog. 2017;56(5):1493–500. doi: 10.1002/mc.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen X, Yin T, Zhang B, et al. Inhibitory effects of brusatol delivered using glycosaminoglycan-placental chondroitin sulfate A-modified nanoparticles on the proliferation, migration and invasion of cancer cells. Int J Mol Med. 2020;46(2):817–27. doi: 10.3892/ijmm.2020.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cucci MA, Grattarola M, Dianzani C, Damia G, Ricci F, Roetto A, et al. Ailanthone increases oxidative stress in CDDP-resistant ovarian and bladder cancer cells by inhibiting of Nrf2 and YAP expression through a post-translational mechanism. Free Radic Biol Med. 2020;150:125–35. doi: 10.1016/j.freeradbiomed.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 98.Tarumoto T, Nagai T, Ohmine K, et al. Ascorbic acid restores sensitivity to imatinib via suppression of Nrf2-dependent gene expression in the imatinib-resistant cell line. Exp Hematol. 2004;32(4):375–81. doi: 10.1016/j.exphem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 99.Wu T-M, Liu S-T, Chen S-Y, Chen G-S, Wu C-C, Huang S-M. Mechanisms and applications of the anti-cancer effect of pharmacological ascorbic acid in cervical cancer cells. Front Oncol. 2020;10:1483. doi: 10.3389/fonc.2020.01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao AM, Ke ZP, Wang JN, Yang JY, Chen SY, Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34(8):1806–14. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- 101.Motohashi H, Yamamoto M. NRF2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549‐557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 102.Romero R, Sayin VI, Davidson SM, et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med. 2017;23:1362‐1368. doi: 10.1038/nm.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shibata T, Ohta T, Tong KI, et al. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci. 2008;105:13568. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shibata T, Kokubu A, Saito S, et al. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia. 2011;13:864–73. doi: 10.1593/neo.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinez VD, Vucic EA, Thu KL, Pikor LA, Lam S, Lam WL. Disruption of KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex components by multiple genetic mechanisms: association with poor prognosis in head and neck cancer. Head Neck. 2015;37:727‐734. doi: 10.1002/hed.23663. [DOI] [PubMed] [Google Scholar]
- 106.De S, et al. The 14-3-3 (YWHA) proteins in mammalian reproduction. Int Ann Sci. 2020;10(1):52–59. doi: 10.21467/ias.10.1.52-59. [DOI] [Google Scholar]
- 107.Kumrah N, De S. Expression and localization of the 14-3-3 (YWHA) protein family within mammals. NSU Undergraduate Student J. 2020;2020(2).
- 108.De S, Marcinkiewicz JL, Vijayaraghavan S, et al. Expression of 14-3-3 protein isoforms in mouse oocytes, eggs and ovarian follicular development. BMC Res Notes. 2012;5:57. doi: 10.1186/1756-0500-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim HJ, Sung SH, Kim CY, Bae MK, Cho MS, Kim YH, Kim SC, Ju W. 14-3-3ζ overexpression is associated with poor prognosis in ovarian cancer. Yonsei Med J. 2018;59(1):51–6. doi: 10.3349/ymj.2018.59.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eisa AA, De S, Detwiler A, et al. YWHA (14-3-3) protein isoforms and their interactions with CDC25B phosphatase in mouse oogenesis and oocyte maturation. BMC Dev Biol. 2019;19(1):20. doi: 10.1186/s12861-019-0200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reddy NM, Kleeberger SR, Bream JH. Genetic disruption of the Nrf2 compromises cell-cycle progression by impairing GSH-induced redox signaling. Oncogene. 2008;27(44):5821–32. doi: 10.1038/onc.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.