Abstract
Background
The virus-induced genome editing (VIGE) system can be used to quickly identify gene functions and generate knock-out libraries as an alternative to the virus-induced gene silencing (VIGS). Although plant virus-mediated VIGE has been shown to have great application prospects, edited genes cannot be transferred to the next generations using this system, as viruses cannot enter into shoot apical meristem (SAM) in plants.
Results
We developed a novel cotton leaf crumple virus (CLCrV)-mediated VIGE system designed to target BRI1, GL2, PDS genes, and GUS transgene in A. thaliana by transforming Cas9 overexpression (Cas9-OE) A. thaliana. Given the deficiency of the VIGE system, ProYao::Cas9 and Pro35S::Cas9 A. thaliana were transformed by fusing 102 bp FT mRNAs with sgRNAs so as to explore the function of Flowering Locus T (FT) gene in delivering sgRNAs into SAM, thus avoiding tissue culture and stably acquiring heritable mutant offspring. Our results showed that sgRNAs fused with FT mRNA at the 5′ end (FT strategy) effectively enabled gene editing in infected plants and allowed the acquisition of mutations heritable by the next generation, with an efficiency of 4.35–8.79%. In addition, gene-edited offspring by FT-sgRNAs did not contain any components of the CLCrV genome.
Conclusions
FT strategy can be used to acquire heritable mutant offspring avoiding tissue culture and stable transformation based on the CLCrV-mediated VIGE system in A. thaliana.
Keywords: CLCrV, CRISPR/Cas9, FT-sgRNA, VIGE
Background
The acquisition of heritable mutations is essential for identifying gene functions and breeding new crop varieties. Genome editing is a powerful tool that induces DNA double-strand breaks (DSBs) at specific genomic loci, leading to targeted mutations by triggering DNA damage repair mechanisms [1]. Unlike first-generation genome editing tools, Type II CRISPR/Cas9 genome editing, which has been widely applied for crop improvement, involves simple designing and cloning methods [2–6]. During this process, single guide RNAs (sgRNAs) is used to guide the endonuclease Cas9 to cut a double-stranded DNA (dsDNA) at precise target loci. This process leads to DSBs repair through nonhomologous end-joining (NHEJ) or homologous recombination (HR). In the absence of homologous DNA templates, the repair of DSBs through NHEJ may cause deletions, insertions, or substitutions of bases at target loci, resulting in heritable variations at specific loci [7]. However, the application of CRISPR/Cas9 in plants may be challenging because the acquisition of heritable mutations via CRISPR/Cas9 usually requires a pathway of stable transformation, which is both time-consuming and laborious.
Recent studies have found that sgRNAs, a key component in the CRISPR system, are inefficient or invalid at many genomic loci. In addition, their efficiency directly impacts the application of CRISPR/Cas9 in plants, and their activity may not be predicted by bioinformatics methods [8, 9]. Therefore, before acquiring mutations using the CRISPR system, it is necessary to minimize the risk of using invalid sgRNAs, which requires prompt screening of efficient sgRNAs. Protoplast transformation with intact gene editing vectors [10] and Agrobacterium-mediated transient transformation of plant leaves with intact gene editing vectors [11] are the two commonly used methods to identify the activity of sgRNAs. Nevertheless, these methods have a high failure frequency.
Recent studies have shown that sgRNAs can be delivered in plants by using plant viruses, also known as the virus-induced genome editing (VIGE) [12, 13]. This method offers a few advantages compared to other conventional ways of transforming intact gene editing vectors. Firstly, sgRNAs can be produced, reproduced, spread, and accumulated along with virus in the whole plant, thus inducing target gene editing mutations in more cells and significantly improving the detection rate of gene editing. Secondly, VIGE can assemble multiple sgRNAs on one viral genome to enable multiplexed gene editing. Finally, VIGE can identify gene functions without stable transformation. Some RNA viruses, including tobacco rattle virus (TRV) [14], pea early-browning virus (PEBV) [15], tobacco mosaic virus (TMV) [16], and beet necrotic yellow vein virus (BNYVV) [17], have been used for targeted editing in model plants, such as A. thaliana and N. benthamiana. Also, the barley stripe mosaic virus (BSMV)-mediated VIGE system was established in hexaploid wheat [12]. Plant DNA virus-mediated VIGE was also developed and used afterward [13]. Although plant virus-mediated VIGE has shown great application prospects, a CLCrV-mediated VIGE system has not yet been reported.
Viruses can efficiently deliver sgRNAs to enable the editing of target genes. Nevertheless, such editing events can only occur in the somatic cells of infected plants. Edited genes cannot be transferred to the next generations since a special mechanism makes it difficult for viruses to enter into their SAM, necessary to edit germ cells in plants [13, 18, 19]. Gene editing in SAM is required for the acquisition of heritable mutant offspring in the VIGE system. A previous study has demonstrated that Flowering Locus T (FT) mRNA enabled the entry of heterologous RNA fused with it into SAM [20]. Therefore, in this study, we proposed a new FT strategy that enables the fusion of sgRNAs with FT mRNA, allowing the sgRNAs to enter SAM. This process eventually breaks the deficiency of the stable transformation pathway limit and acquire gene-edited offspring.
Cotton leaf crumple virus (CLCrV) is a two-component DNA virus composed of the CLCrV-A genome and CLCrV-B genome. Existing studies have shown that CLCrV as a virus-induced gene silencing (VIGS) vector, can be used to silence the expression of endogenous genes in plants [21]. To this end, we developed a CLCrV-mediated VIGE system and successfully acquired gene-edited offspring in A. thaliana using the FT strategy.
Methods
Plant materials and growth conditions
A. thaliana Col-0 ecotype plants were transformed by the floral dipping method as previously described [22]. For the selection of transformants, seeds were sterilized with 5% NaClO for 5 min and plated on 1/2 MS medium [23] with 30 μg/mL kanamycin. After 2 weeks, resistant seedlings were transplanted to the soil under long-day conditions (16 h light/8 h dark) at 22 °C. Homozygous transgenic seedlings grown for about 15 days were used for inoculation transformation experiments.
Vectors construction
To construct the 35S::Cas9-Ter-P2301 vector, the 35S::Cas9-Ter fragments (5476 bp) were recovered from 35S::Cas9-Ter-SK vector [24] digested with HindIII and XbaI, and recombined into pCAMBIA2301 (with a GUS gene in the vector) to obtain 35S::Cas9-Ter-P2301 vector. Then, 10 μL 35S::Cas9-Ter-P2301 plasmid was introduced into A. tumefaciens strain GV3101. Transformed Agrobacterium cultures were grown at 28 °C for 2 days, and positive clones were selected and cultured in LB medium (containing 50 μg/mL kanamycin and 50 μg/mL rifampicin) to the logarithmic growth stage for A. thaliana genetic transformation.
To construct the sgRNA vector, a truncated AtU6-26 (330 bp) promoter [24] was used to drive sgRNA expression. The 20 bp guide RNA (Additional file 1) was inserted into AtU6-26::sgRNA vector digested with BbsI. Sequencing was used to verify whether the sgRNA vector containing the target sequence was correct. The CLCrV-A coat protein gene was replaced by a multi-cloning site to facilitate the assembly of sgRNA. Different sgRNA vectors were digested with SpeI and PacI, and ligated into CLCrV-A vector. The recombinant plasmids were verified by the restriction enzyme digestion method.
To construct the FT-sgRNA vector, A. thaliana Col-0 ecotype cDNA and PCR primers (Additional file 1) were used to clone FT (528 bp) gene. Then, 102 bp FT was fused to the 5′ end of AtBRI1-sgRNA by transfer PCR [25]. Sequencing was used to verify whether the FT- AtBRI1-sgRNA vector was correct.
RT-PCR analysis of Cas9 expression
Total RNA was isolated from Cas9-OE A. thaliana and reversely transcribed into cDNA. A. thaliana Atactin2 gene was used as the reference gene. RT-PCR was performed at 94 °C for 3 min followed by 27 cycles of amplification (94 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s) and a final extension of 10 min at 72 °C. According to the template amount of the reference gene, the target Cas9 fragment was amplified. Primers used for PCR amplification are listed in Additional file 1.
Transient transformation in Cas9-OE A. thaliana with CLCrV-sgRNA
For transient expression and virus inoculation, all CLCrV-AtU6-26::sgRNA vectors and CLCrV-B were introduced into A. tumefaciens strain GV3101. The Agrobacterium cultures were inoculated in a 5 mL LB medium (containing 50 μg/mL kanamycin and 50 μg/mL rifampicin) and grown overnight in a 28 °C shaker. Agrobacterium cultures were harvested and resuspended in infiltration buffer (10 mM MgCl2, 10 mM MES and 200 μM acetosyringone), adjusted to an optical density at 600 nm of 1.0, and incubated at room temperature for 3 to 4 h. For virus inoculation, Agrobacterium cultures containing CLCrV-B and CLCrV-A or their derivatives were mixed at a 1:1 ratio. The mixed Agrobacterium solutions were infiltrated into A. thaliana leaves with a 1 ml syringe.
GUS staining and quantitative analysis
The upper systemic leaves without injection were GUS stained after infiltration according to a previously described protocol [26]. Briefly, to analyze GUS gene expression, RNA was isolated from the upper systemic leaves of plant inoculated CLCrV-A and CLCrV-B empty vector, GUS-sgRNA and Cas9-OE A. thaliana, and reversely transcribed into cDNA. qRT-PCR was performed amplifying the 168 bp GUS gene. After the reaction was completed, relative gene expression levels were calculated using the 2−∆∆Ct method [27] with A. thaliana Actin2 gene as the reference gene. Primers used for PCR amplification are listed in Additional file 1.
Mutation detection
For target gene editing detection, genomic DNA was isolated from A. thaliana leaves inoculated with CLCrV-B and CLCrV-A derivatives. PCR/RE assay was performed according to previously described methods [11]. Briefly, PCR amplified a genomic fragment containing the target site, and appropriate restriction enzymes were used to digest PCR products in order to confirm mutations at the target site. The undigested PCR amplicons were cloned into a Blunt Zero cloning vector and sequenced. All primers used for PCR amplification are listed in Additional file 1.
Statistical analyses
Statistical analysis of the numerical data was performed using Excel 2013 and SPSS 18.0. For multiple pairwise comparisons, the data were compared using a one-way ANOVA test. * p < 0.05 was considered statistically significant, and ** p < 0.01 was considered extremely significant.
Results
Design of CLCrV-mediated sgRNA delivery system
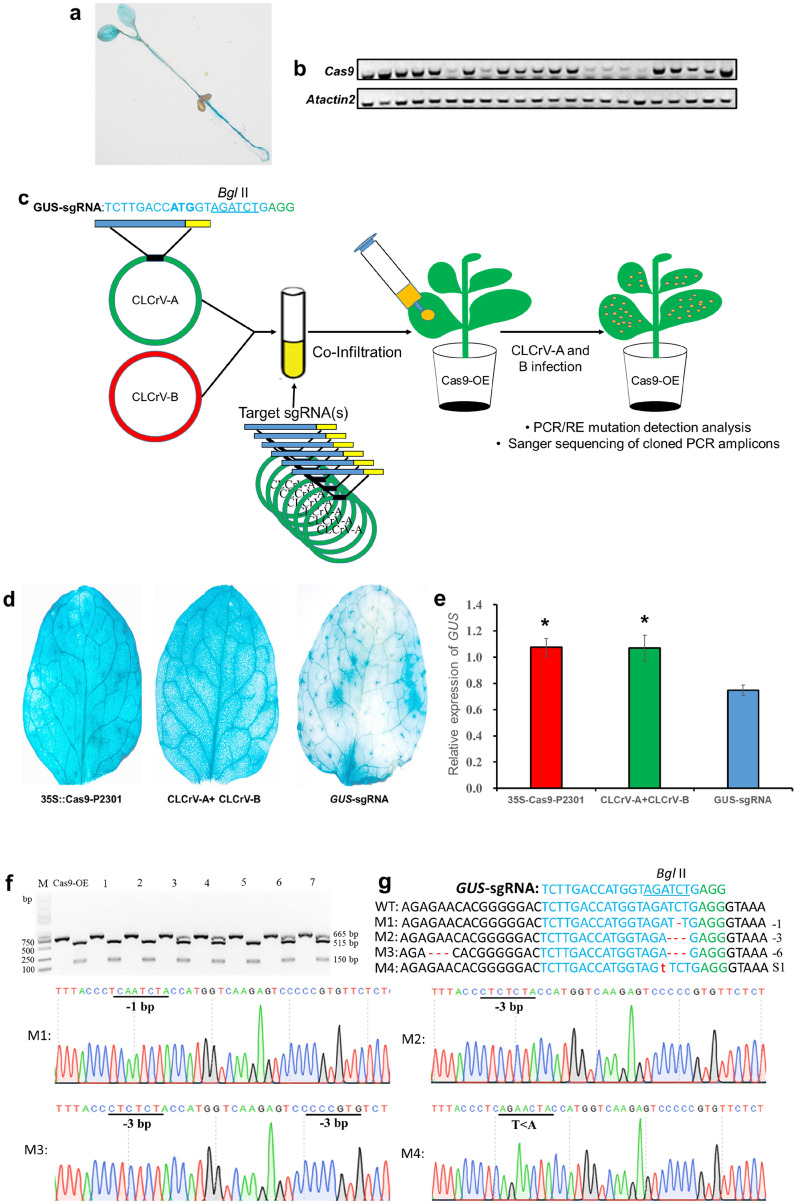
The feasibility of CLCrV-mediated VIGE was analyzed in A. thaliana to determine whether the geminivirus CLCrV could be used to deliver sgRNAs for targeted genes in plants. The high expression of Cas9 and sgRNAs is the key step in the acquisition of a high mutation rate [28, 29]. Considering the size of Cas9, transforming cells with an intact CRISPR genome editing vector may be challenging and usually not enough to enable valid targeted editing. Thus, in this study, we established a transgenic Cas9-OE A. thaliana in which GUS is overexpressed before delivering sgRNAs using CLCrV. Transgene-positive plants were blue when they were GUS stained (Fig. 1a). RT-PCR was further used to verify the valid expression of Cas9 in transgenic plants (Fig. 1b). Transgenic plants with higher Cas9 expressions were used in later studies.
Fig. 1.
GUS reporter gene system targeted by CLCrV-mediated VIGE. a GUS staining of Cas9-OE A. thaliana seedling. b Cas9 expression levels in transgenic plants determined by RT-PCR. c Experimental scheme of the CLCrV-mediated genome editing. d GUS staining in Cas9-OE A. thaliana leaves infiltrated with Agrobacterium carrying different constructs. GUS staining of leaves inoculated with CLCrV-A and CLCrV-B empty vectors that served as a control, the blue area of leaves inoculated with GUS-sgRNA decreased. e GUS expression levels determined by qRT-PCR. Asterisks indicate significant differences (* p < 0.05). f and g Detection of GUS-sgRNA targeted mutations. f Cas9-OE plant served as a control, 1–7 were plant numbers. The gel image shows PCR products of the GUS gene, and digested PCR products with BglII. g The undigested PCR products lacking the BglII site (due to the presence of a mutation) that were subsequently purified, cloned, and analyzed by sequencing. The green color indicated the PAM sequence. The BglII restriction site on the target sequence is underlined in blue. M indicates the mutation sequence. Deletions are shown in red dashes. Substitutions are denoted with red lowercase letters
The GUS reporter system was used to detect the efficiency of the CLCrV-mediated VIGE system. A guide RNA was designed to target BglII site downstream start codon on the GUS gene. The CLCrV-AtU6-26::GUS-sgRNA was co-transformed into the leaves of Cas9-OE A. thaliana with CLCrV-B (Fig. 1c). The blue zones in leaves were significantly narrowed, while the corresponding leaves were infiltrated with the CLCrV vector without sgRNA remained blue, which was similar to that observed in transgenic GUS plants (Fig. 1d). Furthermore, the qRT-PCR proved the decrease of GUS gene expression in plants infiltrated with GUS-sgRNA (Fig. 1e).
The PCR/RE analysis confirmed the mutations at BglII site in the GUS gene in A. thaliana infiltrated with GUS-sgRNA, which was not observed in the control group (Fig. 1f). Sequencing results showed mutations, including − 1 bp, − 3 bp, and − 6 bp deletions and base substitutions in the target sequence region (Fig. 1g). Among them, − 1 bp deletion caused the region's codon to mutate to the stop codon TGA, resulting in early GUS gene translation termination. The above results demonstrated that delivery of CLCrV-mediated sgRNA into the Cas9-OE plants could effectively induce targeted gene editing in plants.
CLCrV-mediated targeted mutagenesis in endogenous genes in A. thaliana
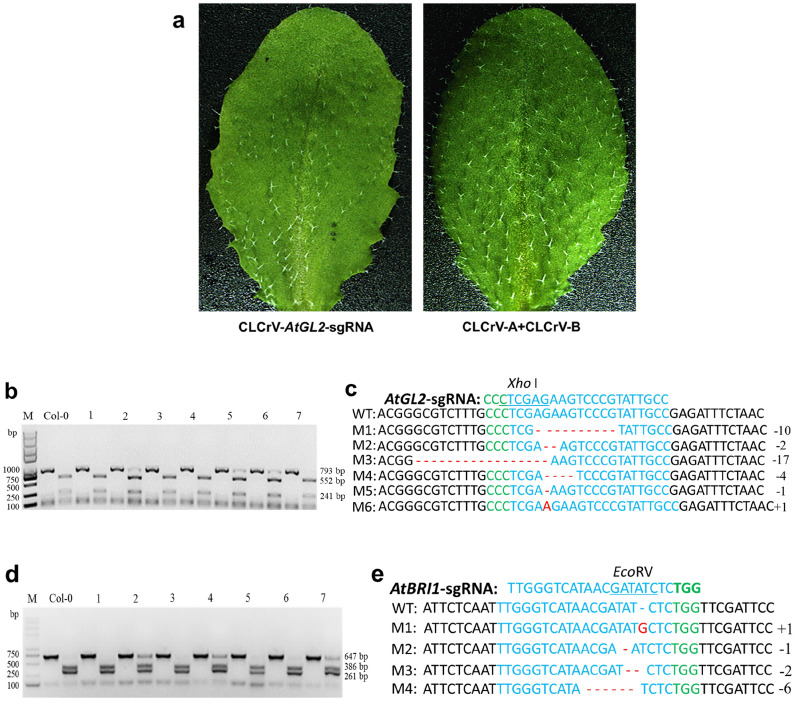
In order to determine whether CLCrV-mediated VIGE was capable of targeted editing of endogenous genes in A. thaliana and causing mutation phenotypes, BRI1-gene modulating development [30] and GL2 gene-regulating trichome development in A. thaliana [31] were used as targets. CLCrV-AtU6-26::AtBRI1-sgRNA and CLCrV-AtU6-26::AtGL2-sgRNA were injected into the leaves of Cas9-OE A. thaliana, respectively. The leaves transformed with CLCrV-AtU6-26::AtGL2-sgRNA exhibited fewer trichomes compared with empty CLCrV vectors (Fig. 2a), while the leaves transformed with CLCrV-AtU6-26::AtBRI1-sgRNA showed no mutation phenotypes. Mutations in AtGL2 and AtBRI1 genes were further detected by PCR/RE and sequencing. Results showed that the gene mutations were base deletions or insertions in the target sequence region (Fig. 2b-e and Additional files 2, 3). We observed no BRI1 mutation phenotype, probably because the edited cell's phenotype was covered by the normal cell. The system also enabled targeted editing of the PDS gene in A. thaliana (Additional file 4). As indicated by our experimental results, CLCrV-mediated VIGE could be used to enable targeted editing of endogenous genes in A. thaliana, which was useful for studying gene functions.
Fig. 2.
CLCrV-mediated targeted mutagenesis of AtGL2 and AtBRI1 in A. thaliana. a Mutant phenotype of systemically infected Cas9-OE A. thaliana leaves at 15–25 days post-infiltration with CLCrV-AtGL2-sgRNA. A. thaliana plants inoculated with CLCrV-A and CLCrV-B empty vectors served as a control. b and c Detection of AtGL2-sgRNA targeted mutations. b Col-0 served as a control, 1–7 were plant numbers. The gel image shows PCR products of the AtGL2 gene and digested PCR products with XhoI. c The undigested PCR products lacking the XhoI site (due to the presence of a mutation) that were subsequently purified, cloned, and analyzed by sequencing. d and e Detection of AtBRI1-sgRNA targeted mutations. d Col-0 served as a control, 1–7 were plant numbers. The gel image shows PCR products of the AtBRI1 gene and digested PCR products with EcoRV. e The undigested PCR products lacking the EcoRV site (due to the presence of a mutation) that were subsequently purified, cloned, and analyzed by sequencing. The green color indicated the PAM sequence. The restriction site on the target sequence is underlined in blue. M indicates the mutation sequence. Deletions are shown in red dashes. Insertions are denoted with red capital letters
Tissue-specific Cas9 system improving gene editing efficiency
Cas9 gene driven by tissue-specific promoters could effectively improve editing efficiency [32]. To improve the mutation efficiency of CLCrV-mediated VIGE, we used ProYao::Cas9 transgenic A. thaliana in which Cas9 is preferentially expressed in meristem as the transformation receptor [32]. AtBRI1 and AtGL2 genes were used as targets, PCR/RE and sequencing results showed that the editing efficiency of ProYao::Cas9 in AtBRI1 and AtGL2 genes were significantly higher (50.00% and 62.50%, respectively) than that of Pro35S::Cas9 (18.75% and 18.75%, respectively) (Table 1 and Additional file 5). Results indicated that ProYao::Cas9 could be used as an ideal receptor for CLCrV-mediated VIGE.
Table 1.
Comparison of mutation frequency between Pro35S::Cas9 and ProYao::Cas9 in CLCrV-mediated VIGE system in A. thaliana
| Transgenic receptor | Target | Mutation frequency (%) |
|---|---|---|
| ProYao::Cas9 | AtBRI1-sgRNA | 50.0% (8/16) |
| AtGL2-sgRNA | 62.5% (10/16) | |
| Pro35S::Cas9 | AtBRI1-sgRNA | 18.75% (3/16) |
| AtGL2-sgRNA | 18.75% (3/16) |
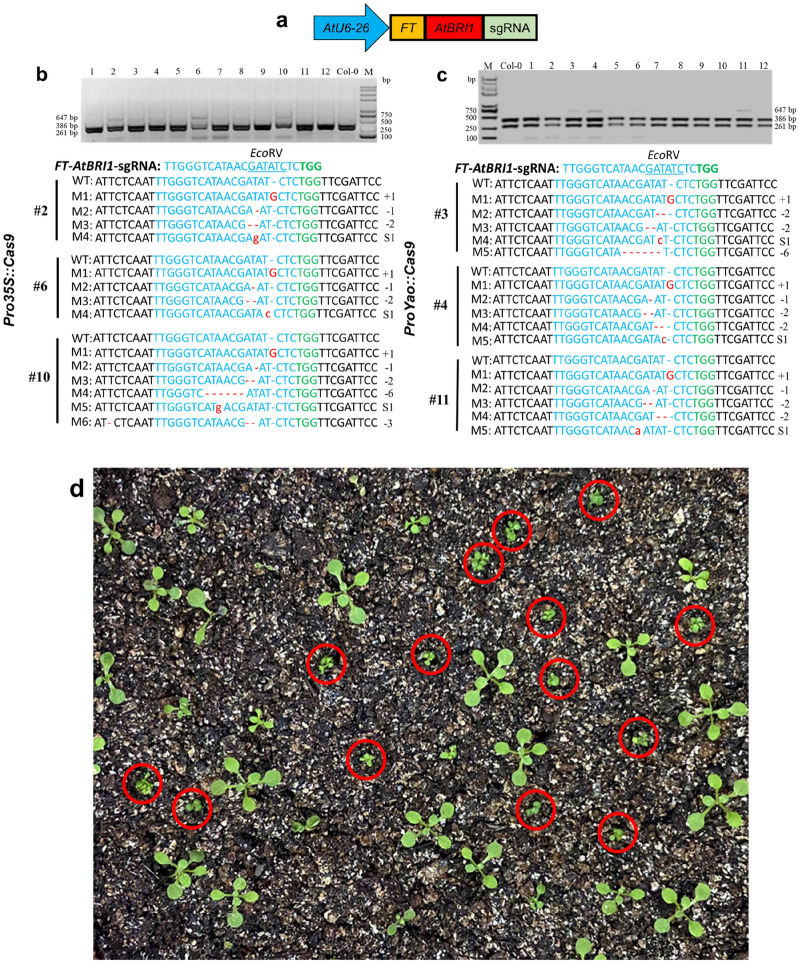
Heritable gene editing using FT mobile guide RNAs
Flowering Locus T (FT) gene has an important role in inducing flowering in A. thaliana [33–36]. FT mRNA could systematically diffuse in plants and migrate to the SAM of plants independent of FT proteins. FT encoding sequences from the initiation codon to the 102nd nucleotides were responsible for FT mobility [37, 38], which can take the heterologous RNA fused with it into SAM [20]. Therefore, we investigated whether FT mRNA could deliver sgRNAs to SAM, causing editing events in meristem and finally acquiring stably heritable mutations without stable transformation. The feasibility of this approach was verified in A. thaliana. AtBRI1 gene of A. thaliana was used as the target. FT (102 bp) gene was fused to the 5′ end of AtBRI1 sgRNA since the first 102 bp FT segments were responsible for FT mRNA mobility (Fig. 3a). The leaves of Pro35S::Cas9 and ProYao::Cas9 A. thaliana were transformed with FT-sgRNA expression vector-mediated with A. tumefaciens. Results showed that AtU6-26::FT-AtBRI1-sgRNA caused editing event in infiltrated leaves of Pro35S::Cas9 plants (#2, #6 and #10) and ProYao::Cas9 (#3, #4 and #11) (Fig. 3b, c and Additional file 6), which in turn indicated that the fusion of 102 bp FT mRNA to the 5′ end of sgRNA could also effectively enable gene editing.
Fig. 3.
FT-sgRNA targeted mutagenesis of AtBRI1 in A. thaliana. a Truncated FT RNA was fused to the 5′ end of AtBRI1-sgRNA. b and c Detection of FT-AtBRI1-sgRNA targeted mutations in Pro35S::Cas9 and ProYao::Cas9 A. thaliana. Col-0 served as a control, 1–12 were plant numbers. The gel image shows digested PCR products of the AtBRI1 gene with EcoRV, the undigested PCR products lacking the EcoRV site (due to the presence of a mutation) that were subsequently purified, cloned, and analyzed by sequencing. The green color indicated the PAM sequence. The restriction site on the target sequence is underlined in blue. M indicates the mutation sequence. Deletions are shown with red dashes. Insertions are denoted with red capital letters. Substitutions are denoted with red lowercase letters. d Phenotype of bri1 mutations in M2 generation. The red circle shows the bri1 mutant
To test whether FT mRNA takes sgRNA into SAM and leads to the mutation in a germ cell, seeds of the progenies of Cas9-OE plants with mutation made by FT-sgRNA and unmodified sgRNA were sown. No phenotype was observed in the offspring (M1) of transformed FT-AtBRI1-sgRNA and unmodified sgRNA plants. However, PCR/RE and sequencing analysis was performed on M1 plants, revealing AtBRI1 gene mutations in some offspring of transformed FT-AtBRI1-sgRNA. The mutation efficiency of AtBRI1 in the M1 generation ranged from 4.35 to 8.79%, no significant difference was observed between Pro35S::Cas9 plants and ProYao::Cas9 plants. Sequencing results showed that most of the mutant plants contained only two genotypes (deletions of -AT and WT, and insertions of + G and WT) and were presumed to be heterozygotes. In comparison, only a few of them contained two or more genotypes and were presumed to be chimeras. No AtBRI1 mutations were detected in the offspring of transformed unmodified sgRNA plants (Table 2). The seeds (M2 generation) from the mutant plants (M1) were sown and transplanted into the soil, and the BRI1 mutation phenotype was obviously present in some plants within two weeks (Fig. 3d). It was inferred that mutations were inherited in the form of heterozygotes according to statistical results of the phenotypic segregation ratio of the M2 generation of BRI1 (Table 3) and the Chi-squared Test result of 3:1. The results showed that the FT-sgRNA strategy in A. thaliana could cause heritable mutations in the form of heterozygotes, while homozygotes could only be acquired in the M2 generation.
Table 2.
Mutation efficiency of AtBRI1 gene in system leaf and transgenic M1 generation
| Transgenic receptor | Modification | Target | System leaf mutation frequency of M0 | Plant number | No. of M1 edited plants showing excepted phenotype | No. of M1 edited plants | Mutation frequency of M1 |
|---|---|---|---|---|---|---|---|
| Pro35S::Cas9 | Unmodified sgRNA | BRI1-sgRNA | (3/12) 25% | #2 | N/A | 0 | 0.00%(0/68) |
| #4 | N/A | 0 | 0.00%(0/48) | ||||
| #7 | N/A | 0 | 0.00%(0/72) | ||||
| FT-sgRNA | FT-BRI1-sgRNA | (3/12) 25% | #2 | N/A | 3 | 4.35%(3/69) | |
| #6 | N/A | 4 | 8.33%(4/48) | ||||
| #10 | N/A | 8 | 8.79%(8/91) | ||||
| ProYao::Cas9 | Unmodified sgRNA | BRI1-sgRNA | (8/16)50% | #12 | N/A | 0 | 0.00%(0/44) |
| #13 | N/A | 0 | 0.00%(0/60) | ||||
| #14 | N/A | 0 | 0.00%(0/64) | ||||
| FT-sgRNA | FT-BRI1-sgRNA | (3/12) 25% | #3 | N/A | 3 | 6.52%(3/46) | |
| #4 | N/A | 5 | 8.06%(5/62) | ||||
| #11 | N/A | 3 | 5.56%(3/54) |
Table 3.
Genetic analysis of BRI1 mutations in M2 generation
| Transgenic receptor | Plant number | Total number of plants | No. of M2 edited plants | No. of M2 no edited plants | χ2 |
|---|---|---|---|---|---|
| Pro35S::Cas9 | #2–6 | 52 | 12 | 40 | 0.0256 |
| #2–10 | 68 | 14 | 54 | 0.4902 | |
| #2–12 | 49 | 11 | 38 | 0.0612 | |
| ProYao::Cas9 | #4–5 | 79 | 18 | 61 | 0.1055 |
| #4–10 | 71 | 14 | 57 | 0.7934 | |
| #4–36 | 47 | 10 | 37 | 0.1773 |
The table above shows the statistical analysis results of effects of unmodified sgRNAs and FT-sgRNA vectors on mutation efficiency of system leaf and M1 generation at AtBRI1 target loci. N/A indicates the absence of an obvious phenotype.
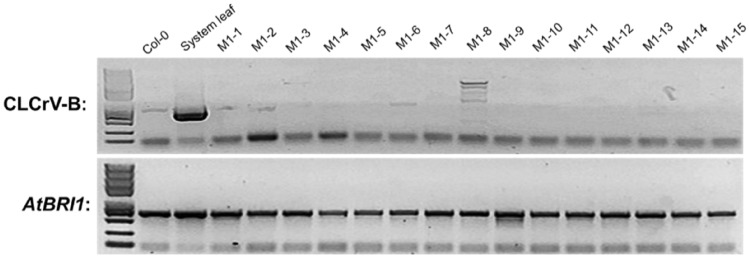
Virus detection in mutant offspring (M1)
In order to detect whether CLCrV is transmitted to the next generation, PCR amplification of CLCrV-B genome and AtBRI1 gene were performed using DNA samples isolated from FT-AtBRI1-sgRNA (#2) system leaf and part of its M1 generation. Results showed that except for the detection of virus accumulation in system leaves, no virus accumulation was detected in the M1 generation (Fig. 4).
Fig. 4.
Detection of virus accumulation in M1 generation
Discussion
The broad application prospects of gene editing systems, such as identifying gene functions and molecular design breeding, have been extensively applied in plants. Yet, this approach requires a complete process of stable transformation, i.e., tissue culture in most plants, which is time-consuming, thus making it impossible to identify gene functions on a large scale. A recently developed virus-mediated gene editing system (VIGE) [12, 13] does not require tissue culture because sgRNA expression is accompanied by the replication, accumulation, and spread of viruses. This new method can induce editing events in almost the whole plant, causing an obvious phenotype due to defective genes.
Cotton leaf crumple virus (CLCrV) is a DNA virus whose infection is not affected by coat protein deficiency [39]. The virus can carry 800 bp of exogenous DNA segments for gene silencing [21]. SgRNA and Cas9 are two essential components of the CRISPR/Cas9 system, Cas9 does not change regardless of which locus of a genome is edited. The carrying capacity of CLCrV is enough for sgRNA expression, although not suitable for Cas9 protein expression. Therefore, in this study, we designed a new system in which the Cas9 overexpression line was used as a transformation receptor, coat protein genes of CLCrV were replaced with sgRNAs, and transcription of sgRNAs was driven by truncated AtU6-26 (330 bp) promoters [24]. This approach only required CLCrV to deliver sgRNAs, thus avoiding transforming intact editing vectors. By using the CLCrV-mediated delivery system, the GUS gene in Cas9 transgenic A. thaliana was successfully targeted, which proves that CLCrV could be useful in delivering sgRNAs (Fig. 1) and enabling targeted editing of endogenous genes in A. thaliana (Fig. 2).
Previous studies have reported that heritable editing events are recovered in the TRV-mediated VIGE system with a very low genome-editing efficiency [14]. Still, the CLCrV-mediated sgRNA delivery system fails to cause heritable mutations in A. thaliana compared with the TRV-mediated VIGE system in N. benthamiana. CLCrV-mediated VIGE cannot occur in SAM and can only exist in somatic cells due to the virus's limited replication [40]. Therefore, we further explored the function of the Flowering Locus T (FT) gene in A. thaliana to deliver sgRNAs to the SAM of plants. We fused FT mRNA to the 5′ end of sgRNA and transiently transforming Pro35S::Cas9 and ProYao::Cas9 transgenic A. thaliana. Our data showed that FT-sgRNA has the same editing efficiency as unmodified sgRNA in the infected plant and can pass on the mutation to offspring. In addition, gene-edited offspring by FT-sgRNAs contains no components of the CLCrV genome (Fig. 4). Compared to Pro35S::Cas9, the CLCrV-mediated VIGE system had no apparent advantage in heritable gene editing frequencies in ProYao::Cas9 transgenic A. thaliana (Table 2), although it showed higher editing efficiency in the infected plant (Table 1). It was recently reported that PDS mutations were acquired in the offspring of N. benthamiana without tissue culture by fusing FT mRNAs to the 3′ end of PDS sgRNAs and assembling the construction in RNA virus TRV vectors, which were transformed in Cas9-OE N. benthamiana. The highly-efficient mutant acquisition proved that FT mRNAs could deliver the RNAs fused with it to the SAM of plants over long distances, thus significantly improving the application efficiency of gene-editing technology in the plant [41]. Our results also showed that FT mRNA was capable of delivering sgRNAs to the SAM of plants, which could acquire heritable mutant offspring when fused FT mRNAs to the 5′ end of sgRNAs. Our results also proved that the FT-sgRNA strategy could work, although the efficiency of FT-sgRNA gene editing mediated by CLCrV (4.35% to 8.79%) was much lower than that mediated by TRV (65% to 100%). Based on the fact that the movement efficiency of FT-RNAs to SAM did significantly differ between different plant species [42, 43], we assumed that the mutant progeny is lower, possibly because of the inefficient mobility of FT mRNAs to SAM in A. thaliana [42].
Conclusions
CLCrV-mediated VIGE enables efficient gene editing in A. thaliana. The FT-sgRNA strategy was successfully applied in A. thaliana, thus suggesting its broad application prospects in functional genomics and molecular design breeding in crops.
Supplementary Information
Additional file 1. Primer sequences involved in this study.
Additional file 2. The DNA sequence of targeted editing of AtGL2 by AtGL2-sgRNA.
Additional file 3. The DNA sequence of targeted editing of AtBRI1 by AtBRI1-sgRNA.
Additional file 4. CLCrV-mediated targeted knockout of AtPDS.
Additional file 5. Tissue-specific Cas9 system for mutation detection of AtBRI1 and AtGL2.
Additional file 6. The DNA sequence of targeted editing of AtBRI1 by FT-AtBRI1-sgRNA.
Acknowledgements
We thank Professor Xueping Zhou from Zhejiang University for providing the CLCrV-A and CLCrV-B vectors. We also thank Professor Jiankang Zhu from Shanghai Research Center for Plant Adversity Biology, Chinese Academy of Sciences, for providing the ProYao::Cas9 A. thaliana seeds.
Authors’ contributions
JF Lei and XD Liu designed the experiments; JF Lei performed most of the experiments and analyzed the data, other authors assisted in experiments; JF Lei and XD Liu wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2016YFD0101006); the Fundamental Research Funds for the Central Universities (KYYJ201701); Xinjiang Uygur Autonomous Region postgraduate research and innovation project (XJ2019G131); Xinjiang Agricultural University cotton team development fund (XNMH2019003).
Availability of data and materials
The authors are pleased to share analyzed/raw data and plant materials upon reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13007-021-00719-4.
References
- 1.Gaj T, Gersbach CA, Barbas CF. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding D, Chen K, Chen Y, Li H, Xie K. Engineering introns to express RNA guides for Cas9- and Cpf1-mediated multiplex genome editing. Mol Plant. 2018;11(4):542–552. doi: 10.1016/j.molp.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Veillet F, Chauvin L, Kermarrec MP, Sevestre F, Merrer M, Terret Z, Szydlowski N, Devaux P, Gallois JL, Chauvin JE. The Solanum tuberosum GBSSI gene: a target for assessing gene and base editing in tetraploid potato. Plant Cell Rep. 2019;38(9):1065–1080. doi: 10.1007/s00299-019-02426-w. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Zhu J, Wu H, Liu C, Huang C, Lan J, Zhao Y, Xie C. Precise base editing of non-allelic acetolactate synthase genes confers sulfonylurea herbicide resistance in maize. Crop J. 2020;8(3):449–456. doi: 10.1016/j.cj.2019.10.001. [DOI] [Google Scholar]
- 5.Okada A, Arndell T, Borisjuk N, Sharma N, Watson-Haigh NS, Tucker EJ, Baumann U, Langridge P, Whitford R. CRISPR/Cas9-mediated knockout of Ms1 enables the rapid generation of male-sterile hexaploid wheat lines for use in hybrid seed production. Plant Biotechnol J. 2019;17(10):1905–1913. doi: 10.1111/pbi.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amin NA, Ahmad N, Wu N, Pu X, Ma T, Du Y, Bo X, Wang N, Sharif R, Wang P. CRISPR-Cas9 mediated targeted disruption of FAD2–2 microsomal omega-6 desaturase in soybean (Glycine max.L) BMC Biotechnol. 2019;19(1):1–10. doi: 10.1186/s12896-019-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waterworth WM, Drury GE, Bray CM, West C. Repairing breaks in the plant genome: the importance of keeping it together. New Phytol. 2011;192(4):805–822. doi: 10.1111/j.1469-8137.2011.03926.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhao C, Wang Y, Nie X, Han X, Liu H, Li G, Yang G, Ruan J, Ma Y, Li X, Cheng H, Zhao S, Fang Y, Xie S. Evaluation of the effects of sequence length and microsatellite instability on single-guide RNA activity and specificity. Int J Biol Sci. 2019;15(12):2641–2653. doi: 10.7150/ijbs.37152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie S, Shen B, Zhang C, Huang X, Zhang Y. sgRNAcas9: a software package for designing CRISPR sgRNA and evaluating potential off-target cleavage sites. PLoS ONE. 2014;9(6):1–9. doi: 10.1371/journal.pone.0100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Lu X, Shu N, Wang S, Wang J, Wang D, Guo L, Ye W. Targeted mutagenesis in cotton (Gossypium hirsutum L.) using the CRISPR/Cas9 system. Sci Rep. 2017;7:44304. doi: 10.1038/srep44304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao W, Long L, Tian X, Xu F, Liu J, Singh PK, Botella JR, Song C. Genome editing in cotton with the CRISPR/Cas9 system. Front Plant Sci. 2017;8:1364. doi: 10.3389/fpls.2017.01364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J, Li S, Li Z, Li H, Song W, Zhao H, Lai J, Xia L, Li D, Zhang Y. A barley stripe mosaic virus-based guide RNA delivery system for targeted mutagenesis in wheat and maize. Mol Plant Pathol. 2019;20(10):1463–1474. doi: 10.1111/mpp.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin K, Han T, Liu G, Chen T, Wang Y, Alice YY, Liu Y. A geminivirus-based guide RNA delivery system for CRISPR/Cas9 mediated plant genome editing. Sci Rep. 2015;5:1–10. doi: 10.1038/srep14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali Z, Abul-faraj A, Li L, Ghosh N, Piatek M, Mahjoub A, Aouida M, Piatek A, Baltes NJ, Voytas DF, Dinesh-Kumar S, Mahfouz MM. Efficient virus-mediated genome editing in plants using the CRISPR/Cas9 system. Mol Plant. 2015;8(8):1288–1291. doi: 10.1016/j.molp.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Ali Z, Eid A, Ali S, Mahfouz MM. Pea early-browning virus-mediated genome editing via the CRISPR/Cas9 system in Nicotiana benthamiana and Arabidopsis. Virus Res. 2018;244:333–337. doi: 10.1016/j.virusres.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Cody WB, Scholthof HB, Mirkov TE. Multiplexed gene editing and protein overexpression using a tobacco mosaic virus viral vector. Plant Physiol. 2017;175(1):23–35. doi: 10.1104/pp.17.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang N, Zhang C, Liu J, Guo Z, Zhang Z, Han C, Wang Y. Development of Beet necrotic yellow vein virus-based vectors for multiple-gene expression and guide RNA delivery in plant genome editing. Plant Biotechnol J. 2019;17:1302–1315. doi: 10.1111/pbi.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qu F, Ye X, Hou G, Sato S, Clemente TE, Morris TJ. RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in Nicotiana benthamiana. J Virol. 2005;79:15209–15217. doi: 10.1128/JVI.79.24.15209-15217.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwach F, Vaistij FE, Jones L, Baulcombe DC. An RNA-dependent RNA polymerase prevents meristem invasion by potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol. 2005;138(4):1842–1852. doi: 10.1104/pp.105.063537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo K, Huang N, Yu T. Selective targeting of mobile mRNAs to plasmodesmata for cell-to-cell movement. Plant Physiol. 2018;177(2):604–614. doi: 10.1104/pp.18.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Z, Huang C, Li F, Zhou X. A versatile system for functional analysis of genes and microRNAs in cotton. Plant Biotechnol J. 2014;12(5):638–649. doi: 10.1111/pbi.12169. [DOI] [PubMed] [Google Scholar]
- 22.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 23.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum. 1962;15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 24.Feng Z, Zhang B, Ding W, Liu X, Yang D, Wei P, Cao F, Zhu S, Zhang F, Mao Y, Zhu J. Efficient genome editing in plant using CRISPR/Cas system. Cell Res. 2013;23(10):1229–1232. doi: 10.1038/cr.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erijman A, Shifman JM, Peleg Y. A single-tube assembly of DNA using the transfer-PCR (TPCR) platform. Methods Mol Biol. 2014;1116:89–101. doi: 10.1007/978-1-62703-764-8_7. [DOI] [PubMed] [Google Scholar]
- 26.Long L, Guo D, Gao W, Yang W, Hou L, Ma X, Miao Y, Botella JR, Song C. Optimization of CRISPR/Cas9 genome editing in cotton by improved sgRNA expression. Plant Methods. 2018;14:85. doi: 10.1186/s13007-018-0353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using Real-Time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Cho S, Shin J, Cho BK. Applications of CRISPR/Cas system to bacterial metabolic engineering. Int J Mol Sci. 2018;19(4):1089. doi: 10.3390/ijms19041089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Wu S, Xu J, Sui C, Wei J. Application of CRISPR/Cas9 in plant biology. Acta Pharm Sin B. 2017;7(3):292–302. doi: 10.1016/j.apsb.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90(5):929–938. doi: 10.1016/S0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 31.Rerie WG, Feldmann KA, Marks MD. The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Gene Dev. 1994;8(12):1388–1399. doi: 10.1101/gad.8.12.1388. [DOI] [PubMed] [Google Scholar]
- 32.Yan L, Wu Y, Li H, Yang W, Wei S, Hu R, Xie Q. High efficiency genome editing in Arabidopsis using Yao promoter-driven CRISPR/Cas9 system. Mol Plant. 2015;8(12):1820–1823. doi: 10.1016/j.molp.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Takada S, Goto K. Terminal flower 2, an Arabidopsis homology of heterochromatin protein 1, counteracts the activation of FLOWERING LOCUS T by constans in the vascular tissues of leaves to regulate flowering time. Plant Cell. 2003;15(12):2856–2865. doi: 10.1105/tpc.016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309(5737):1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- 35.An HL, Roussot C, Suarez-Lopez P, Corbesier L, Vincent C, Pineiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, Coupland G. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development. 2004;131(15):3615–3626. doi: 10.1242/dev.01231. [DOI] [PubMed] [Google Scholar]
- 36.Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309(5737):1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- 37.Li C, Zhang K, Zeng X, Jackson S, Zhou Y, Hong Y. A cis element within flowering locus T mRNA determines its mobility and facilitates trafficking of heterologous viral RNA. J Virol. 2009;83(8):3540–3548. doi: 10.1128/JVI.02346-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Gu M, Shi N, Zhang H, Yang X, Osman T, Liu Y, Wang H, Vatish M, Jackson S, Hong Y. Mobile FT mRNA contributes to the systemic florigen signalling in floral induction. Sci Rep. 2011;1:73. doi: 10.1038/srep00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuttle JR, Haigler CH, Robertson D. Method: low-cost delivery of the cotton leaf crumple virus-induced gene silencing system. Plant Methods. 2012;8(1):27. doi: 10.1186/1746-4811-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H, Qu X, Dong Z, Luo L, Shao C, Forner J, Lohmann JU, Su M, Xu M, Liu X, Zhu L, Zeng J, Liu S, Tian Z, Zhao Z. WUSCHEL triggers innate antiviral immunity in plant stem cells. Science. 2020;370(6513):227–231. doi: 10.1126/science.abb7360. [DOI] [PubMed] [Google Scholar]
- 41.Evan EE, Ugrappa N, Maria EG, Pin-jui H, Savithramma DK, Daniel FV. Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat Plant. 2020;6(6):620–624. doi: 10.1038/s41477-020-0670-y. [DOI] [PubMed] [Google Scholar]
- 42.Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316(5827):1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 43.Tamaki S, Matsuo S, Wong H, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316(5827):1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Primer sequences involved in this study.
Additional file 2. The DNA sequence of targeted editing of AtGL2 by AtGL2-sgRNA.
Additional file 3. The DNA sequence of targeted editing of AtBRI1 by AtBRI1-sgRNA.
Additional file 4. CLCrV-mediated targeted knockout of AtPDS.
Additional file 5. Tissue-specific Cas9 system for mutation detection of AtBRI1 and AtGL2.
Additional file 6. The DNA sequence of targeted editing of AtBRI1 by FT-AtBRI1-sgRNA.
Data Availability Statement
The authors are pleased to share analyzed/raw data and plant materials upon reasonable request.