Abstract
OBJECTIVES
Options for paediatric aortic valve replacement (AVR) are limited if valve repair is not feasible. Results of paediatric Ross procedures are inferior to adult Ross results, and mechanical AVR imposes constant anticoagulation with the inherent risks.
METHODS
The study design was a prospective, multicentre follow-up of all paediatric patients receiving decellularized aortic homografts (DAHs) for AVR in 8 European centres.
RESULTS
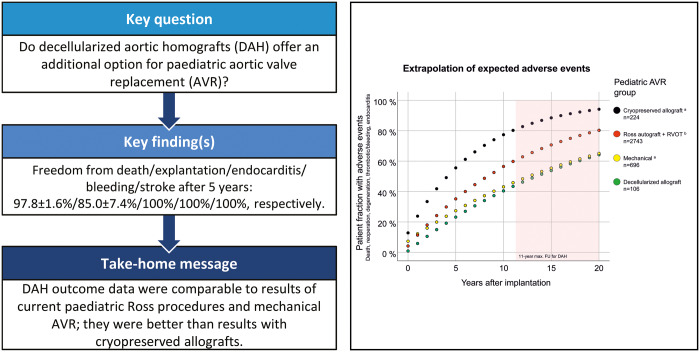
A total of 106 children (77 boys) were operated (mean age 10.1 ± 4.8 years, DAH diameter 20.5 ± 3.8 mm). A total of 60 (57%) had undergone previous surgical interventions: 34 with 1, 15 with 2 and 11 with ≥3. There was one early death in a 12-year-old girl, who underwent her fourth aortic valve operation, due to intracerebral haemorrhage on extracorporeal membrane oxygenation after coronary reimplantation problems following 3-sinus reconstruction 1 year earlier. One 2-year-old patient died due to sepsis 2 months postoperatively with no evidence for endocarditis. In addition, a single pacemaker implantation was necessary and a 2.5-year-old girl underwent successful HTx due to chronic myocardial failure despite an intact DAH. After a mean follow-up of 3.30 ± 2.45 years, primary efficacy end points mean peak gradient (18.1 ± 20.9 mmHg) and regurgitation (mean 0.61 ± 0.63, grade 0–3) were very good. Freedom from death/explantation/endocarditis/bleeding/stroke at 5 years was 97.8 ± 1.6/85.0 ± 7.4/100/100/100% respectively. Calculated expected adverse events were lower for DAH compared to cryopreserved homograft patients (mean age 8.9 years), lower than in Ross patients (9.4 years) and in the same range as mechanical AVR (12.8 years).
CONCLUSIONS
Even though the overall number of paediatric DAH patients and the follow-up time span are still limited, our data suggest that DAHs may present a promising additional option for paediatric AVR.
Keywords: Children, Aortic valve disease, Decellularization, Allografts
INTRODUCTION
Options for paediatric aortic valve replacement (AVR) are limited where aortic valve repair is not feasible. The durability of the autograft and the biological conduit used for pulmonary valve replacement are inferior in paediatric Ross patients compared with adult Ross patients [1–4]. In addition, the Ross procedure is unsuitable for some children due to anatomical reasons or previous surgical procedures [5, 6].
Conventional xenogenic biological aortic valve prostheses in children frequently undergo early degeneration, sometimes with rapid increase in valvular gradients, and sudden cardiac death has been reported in such situations [7, 8]. Mechanical aortic valves have shown steady improvements over the past decades, and simple mechanical failure of these prostheses has become a rare occurrence. Thromboembolic events, however, constitute still a major limitation for mechanical valves. According to recent meta-analyses, the annual risk for major thromboembolic events should be calculated with 0.5–1.0% for children and 1.2–2.3% for young adults, leading to a substantial life-time risk [9, 10]. In addition, industry currently does not produce sufficient numbers of small-sized mechanical valves and new technological developments are lacking. Although new anticoagulants are in the pipeline, their potential clinical value for paediatric mechanical AVR is currently unclear [11].
Decellularized aortic allografts were introduced clinically over a decade ago for both adult and paediatric patients with encouraging early clinical results [12, 13]. Decellularized pulmonary homografts showed superior 10-year results in a matched comparison with conventional cryopreserved homografts and bovine jugular vein conduits [14]. Recently, we demonstrated the extent of spontaneous in vivo recellularization in decellularized allografts, which forms the basis for regeneration and a robust long-term function [15].
The reduced immunogenicity of decellularized aortic homografts (DAHs) may in theory lead to better durability than other biological valve alternatives for paediatric AVR. However, there also have been reports about significant calcification occurring in DAH using other proprietary decellularization protocols, which make prospective long-term follow-up of all DAHs mandatory [16, 17].
The aim of this study is to present an update on the current results of paediatric DAH implantation and to compare these results with contemporary data on the Ross procedure and other AVR options for children.
MATERIALS AND METHODS
Study setting
The study design was a prospective, multicentre follow-up of all paediatric patients receiving DAHs for AVR in 8 European centres (Hannover, Leuven, Leiden, Zürich, Chisinau, Stuttgart, Erlangen, Vienna).
The primary end points were periprocedural complications, heart valve dysfunction and repeat procedure for valve-related dysfunction (surgical or interventional therapy).
Indication for AVR according to the current clinical guidelines of the European Association for Paediatric Cardiology was the key inclusion criterion. Children with active endocarditis were not included.
Approval for this non-interventional follow-up study was granted by Hannover Medical School ethics committee (no. 1503-2012) at the study outset, and informed consent was obtained appropriately from all parents.
Surgical procedures were performed according to the locally established standard procedures under cardiopulmonary bypass (CPB). Postoperatively, patients were recommended aspirin/acetylsalicylic acid at 2–3 mg/kg per day for 3–6 months and, in some adolescent patients, warfarin therapy was recommended for 2 months followed by continued acetylsalicylic acid medication. All DAH implantations were performed as a root replacement with coronary reimplantation and without significant reinforcement procedures.
Homograft procurement and processing
Homografts were procured in line with the current European Directive 2004/23, as amended, via 3 different tissue banks (European Homograft Bank, Brussels, Dr R. Jashari; German Society for Tissue Transplantation—DGFG, Hannover, M. Börgel; EuroTissue Bank, Rotterdam, A. van den Bogaerdt) and shipped to Hannover for processing at Corlife oHG (www.corlife.eu).
The DAH was authorized by the German competent authority as a medicinal product: ‘Cell-free aortic heart valve, Arise AV’ (# PEI.G.11766.01.1). The processing of each homograft comprises ∼30 different steps using a detergent-based, non-cryopreservation approach as described previously [14]. Microbiological assessment was performed as a part of the incoming inspection, both during and after processing with a final 14-day quarantine. Each individual homograft was assessed histologically following processing, and the residual dsDNA content was measured before and after processing prior to final release. Reference samples of all homografts were stored in accordance with German law for at least 1 year.
Statistical analysis
Summaries of the numeric data are given as means and standard deviation or median and interquartile range as appropriate. Normal distribution of factors was assessed using the Kolmogorov–Smirnov test. Categorical variables are given as counts and percentages. The proportion of explanted and dysfunctional grafts over time was calculated, and a peak echocardiographic gradient of ≥50 mmHg and regurgitation ≥moderate was defined as dysfunctional.
Time-related events, such as freedom from explantation and degeneration, were evaluated according to the Kaplan–Meier method.
Status observations after DAH implantation were grouped per postoperative year, and the frequency of each functional status was calculated group-wise for all respective DAHs. Since the status frequencies applied only for non-explanted conduits, these frequencies were multiplied by the fraction of conduits that were not explanted by the middle of the relevant year. Hence, the functional status frequencies presented here refer to all initially implanted DAH in the respective period.
In the ARISE Registry, we calculated perioperative mortality and annually reported rates of adverse events such as late death, reoperation or reintervention, valve degeneration, thrombotic and bleeding events and endocarditis for all paediatric DAH implanted to date. DAH data were compared with the results of recent large-scale meta-analyses for paediatric AVR, which included studies of 2743 paediatric Ross patients, 696 children with mechanical AVR and 224 patients undergoing AVR using a standard cryopreserved homograft [10, 18]. This long-term extrapolation was performed by simply adding the observed and reported early adverse events and annual rates of adverse events. Annual event rates were calculated to the respective event-free patient fraction. In addition, actual observed adverse paediatric DAH events are shown in Kaplan–Meier function equivalent ± 95% confidence interval.
We refrained from testing for statistical significance here, as we compared prospectively collected multicentre data with meta-analyses summarizing retrospective and single-centre studies.
SPSS 25 (IBM Corporation, Somers, NY, USA) was used for the analyses.
RESULTS
Perioperative outcome of paediatric decellularized aortic homografts implantation
A total of 106 paediatric patients (77 males) were operated between February 2008 and September 2019 with a mean age of 10.1 ± 4.8 years; the follow-up was complete. Forty (38%) patients underwent previous catheter interventions, and 60 (57%) had undergone previous surgical interventions (34 with 1, 15 with 2, 11 with ≥3 previous operations). The mean implanted DAH diameter was 20.5 ± 3.8 mm, and the mean operation duration was 399 ± 129 min with a mean CPB time of 246 ± 101 min and a mean cross-clamp time of 154 ± 51 min.
There was 1 early death in a 12-year-old girl, who had undergone her fourth aortic valve operation. Death was caused by intracerebral haemorrhage while on extracorporeal membrane oxygenation (ECMO) support due to coronary reimplantation problems following a 3-sinus reconstruction 1 year earlier. One 2-year-old patient died due to sepsis 2 months postoperatively with no echocardiographic evidence for endocarditis; an autopsy was declined by the parents.
In addition, 1 pacemaker implantation was necessary and a 2.5-year-old girl underwent successful HTx due to chronic myocardial failure despite an intact DAH.
In 4 children, there was simultaneous implantation of a decellularized pulmonary homograft during the AVR procedure.
Table 1 provides the study cohort characteristics.
Table 1:
Patient characteristics for the paediatric DAH cohort and the ARISE Registry cohort including all DAHs implanted to date
| Paediatric AVR (N = 106) | All DAH (N = 259) | |
|---|---|---|
| Implantation period (years) | 2008–2019 | 2008–2019 |
| Age at implantation, median (IQR) | 10.1 (4.8) | 21.3 (11.6–43.8) |
| Follow-up, median (IQR) | 3.40 (1.8–4.9) | 2.5 (1.5–4.4) |
| Total follow-up | 350 | 713 |
| Sex (male), n (%) | 77 (73) | 176 (68) |
| Number of previous operations | ||
| 0 | 46 | 145 |
| 1 | 34 | 69 |
| 2 | 15 | 29 |
| >2 | 11 | 16 |
| Type of previous procedures | ||
| 1 × AVR | 11 | 32 |
| 2 × AVR | 2 | 7 |
| 3 × AVR | 1 | 1 |
| Catheter-based intervention | 40 | 48 |
| Aortic valve repair | 15 | 23 |
| Mean allograft diameter (mm), median (IQR) | 21 (19–23) | 23 (21–25) |
| 10–18 | 19 | 19 |
| 19–22 | 56 | 98 |
| 23–29 | 31 | 142 |
| Implantation time (min), median (IQR) | ||
| Total operation | 370 (311–466) | 324 (245–423) |
| Cardiopulmonary bypass | 228 (191–289) | 174 (130–240) |
| Cross-clamp | 145 (124–181) | 127 (99–158) |
| Latest echocardiography | ||
| Aortic annulus (mm), mean (SD) | 20.5 (3.8) | 22.0 (4.0) |
| Aortic annulus, z-score, mean (SD) | −0.01 (1.49) | 0.18 (1.49) |
| Effective orifice area (cm2), mean (SD) | 2.4 (0.8) | 2.9 (0.8) |
| Peak gradient (mmHg), median (IQR) | 12 (8–21) | 11 (7–17) |
| Regurgitation (grade 0–3), median (IQR) | 0.5 (0–1) | 0.5 (0–1) |
| LV ejection fraction (%), mean (SD) | 62.3 (9.1) | 62.7 (8.4) |
Mean and SD are for normally distributed factors and median and IQR are for factors with no normal distribution.
AVR: aortic valve replacement; DAH: decellularized aortic homografts; IQR: interquartile range; LV: left ventricular; SD: standard deviation.
Coronary reimplantation complications
In 4/106 paediatric patients (3.8%) with a mean age of 8.5 ± 4.6 years, problems occurred during coronary reimplantation. In 3 patients, stent implantations were performed in the proximal right coronary artery or left coronary artery to remedy persisting postoperative ECG alteration. One of these 3 patients died while on ECMO support as described above.
Posterior left ventricular (LV) impairment after CPB led to an A. mammaria free-graft to the left circumflex artery (LCx) in a 7-year-old boy, which immediately resolved LV hypo-motility. Interestingly, a coronary angiography performed 6 months later showed a normal LV function and normal left coronary artery and LCx. The arterial bypass to the LCx was almost totally occluded, rendering a temporary LCx perfusion problem by a thrombus or an intima flap the most likely explanation for the impairment.
In the 3 surviving patients, normal LV function was observed at the most recent follow-up.
Midterm outcome of paediatric decellularized aortic homografts
After a mean follow-up of 3.30 ± 2.45 years, the primary efficacy end points of peak gradient (18.1 ± 20.9 mmHg) and regurgitation (mean 0.61 ± 0.63, grade 0–3) were very good. The mean LV ejection fraction was 62 ± 9%, and the mean aortic valve diameter was 20.5 ± 3.8 mm, with a mean z-score of −0.01 ± 1.49 and a mean effective orifice area of 2.36 ± 0.84 cm2.
Freedom from death/explantation/endocarditis/bleeding/stroke at 5 years was 97.8 ± 1.6/85.0 ± 7.4/100/100/100%, respectively. Figure 1 provides freedom from explantation according to the Kaplan–Meier method and the functional status of all implanted DAH in children with up to 11 years of follow-up, as well as the results for the entire DAH cohort, including adults.
Figure 1:
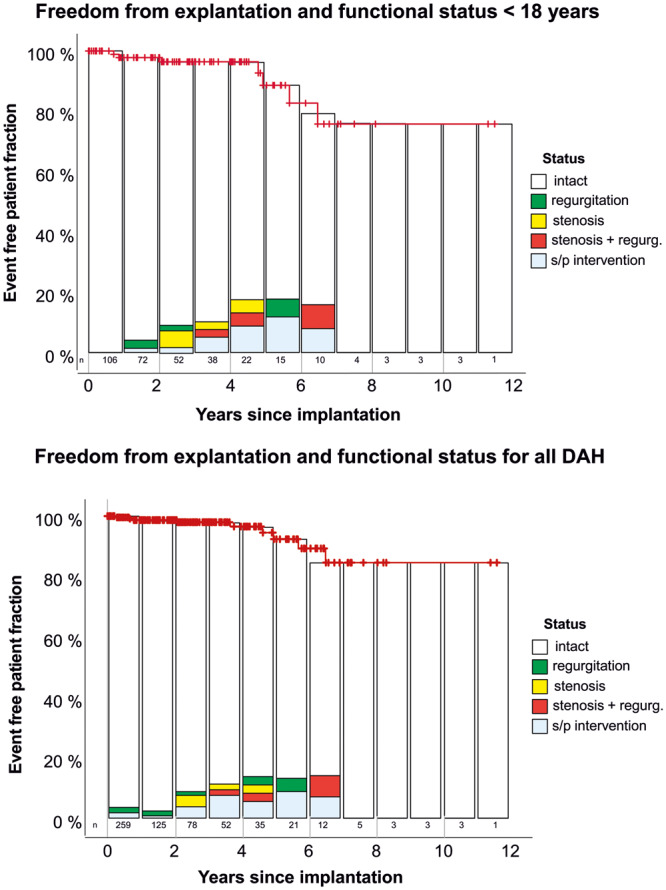
Freedom from explantation according to the Kaplan–Meier method and functional status of all implanted DAH in children and for all DAHs, including those in adults. Displayed functional status frequencies refer to all DAHs implanted in the respective period. DAH: decellularized aortic homograft.
Decellularized aortic homografts failure in paediatric patients
DAH required explantation in 7/106 patients with a mean age of 5.9 ± 5.7 years (vs 10.1 ± 4.8 years, P = 0.03). All of these patients underwent previous procedures prior to DAH implantation, including 2 patients with 3 previous surgical procedures.
In a 2.5-year-old patient, LV function, already severely impaired preoperatively, did not recover despite excellent graft function and normal coronary blood flow, resulting ultimately in heart transplantation. The histological results, including the degree of recellularization after 8 months, have been published recently [15].
One case of DAH explantation after 4.5 years due to the development of subvalvular stenosis has also been published [12].
In a 7-year-old patient, a rapid calcification occurred within months after implantation and endocarditis was suspected, but no specific bacteria were identified despite a thorough microbiological work-up [15].
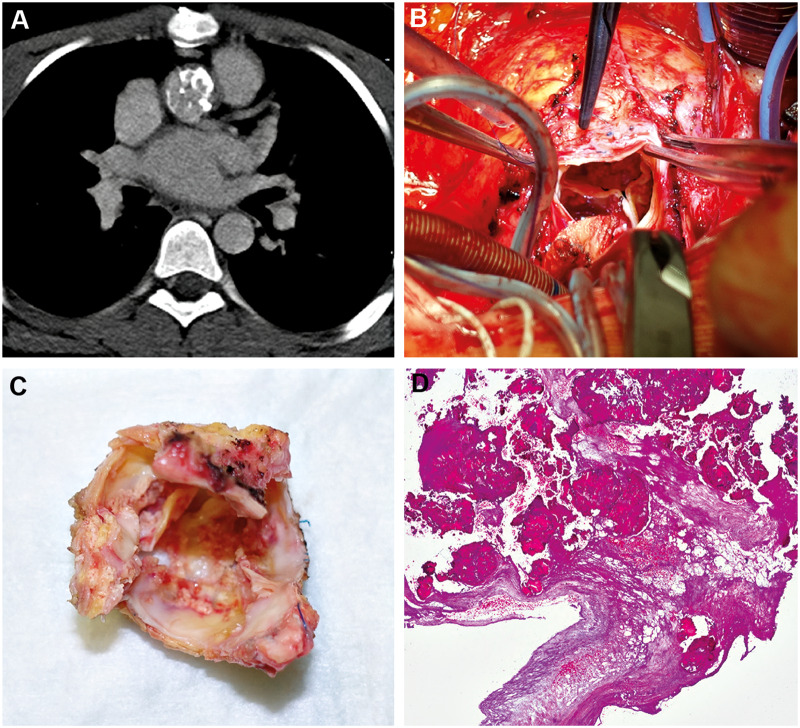
In the remaining 4 patients, valvular degeneration was observed leading to DAH explantation. Figure 2 shows one of these patients, a 14-year-old boy with a small, unrepaired ventricular septal defect (VSD). In 2010, he suffered from pancarditis during a Streptococcus pneumonia infection, which led to multiple heart valve complications. He subsequently underwent aortic, pulmonary and tricuspid valve repair and VSD closure in 2010, mechanical AVR with a 16-mm ATS in 2011, and received a DAH following stenosis of the ATS valve due to a subvalvular pannus formation in November 2015. Unfortunately, the DAH developed early degeneration and calcification, leading to explantation in November 2017 and replacement by a mechanical valve (On-X 21 mm). Histological analysis confirmed extensive calcification and matrix destruction (Fig. 2).
Figure 2:
A fourteen-year-old boy 2 years after decellularized aortic homograft implantation following multiple previous procedures due to endocarditis. The computed tomography scan showed a decellularized aortic homograft cusp and wall calcification, which were confirmed at explantation and histologically.
In the other 3 patients who also underwent multiple surgical procedures prior to DAH implementation, lesser DAH degeneration was observed but extended to the homograft wall and cusps. In one of these patients, a mechanical AVR was performed during a procedure for mechanical mitral valve replacement due to progressive mitral stenosis to avoid an additional sternotomy, although the DAH was only mildly stenotic (29 mm peak).
Four of the 7 patients underwent redo AVR procedures with DAH and showed normal homograft function up to 4 years postoperatively. Two patients received a mechanical valve at reoperation. There were no mortalities in any of the 6 AVR redo procedures, and 1 successful HTx was performed.
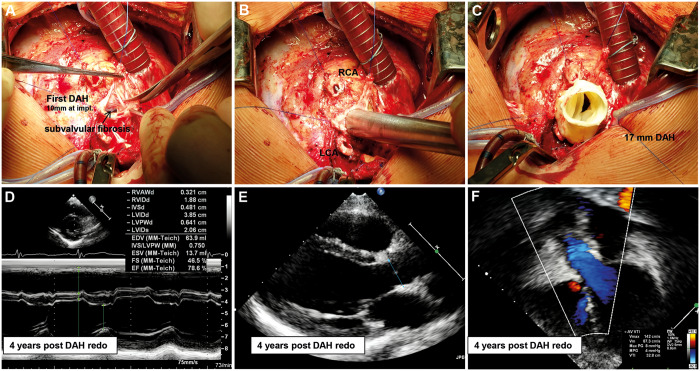
Figure 3 shows the patient with the longest follow-up after redo DAH implantation.
Figure 3:
A 0.2-year-old boy, S/P 2 × aortic valve balloon valvuloplasty, aortic valve replacement with DAH 10 mm in 2010. Intraoperative images at redo aortic valve replacement in 2015 with a 17-mm DAH due to subvalvular stenosis leading to aortic regurgitation by jet-lesion destruction of 1 cusp. The left ventricle and homograft were functioning normally 4 years after redo. DAH: decellularized aortic homograft; RCA: right coronary artery.
A 0.2-year-old boy, following 2 balloon dilatations, underwent AVR with a 10-mm DAH in 2010 and redo AVR in 2015 with a 17-mm DAH due to subvalvular stenosis leading to aortic regurgitation by jet-lesion destruction of 1 cusp. The LV and homograft were functioning normally 4 years after the redo procedure.
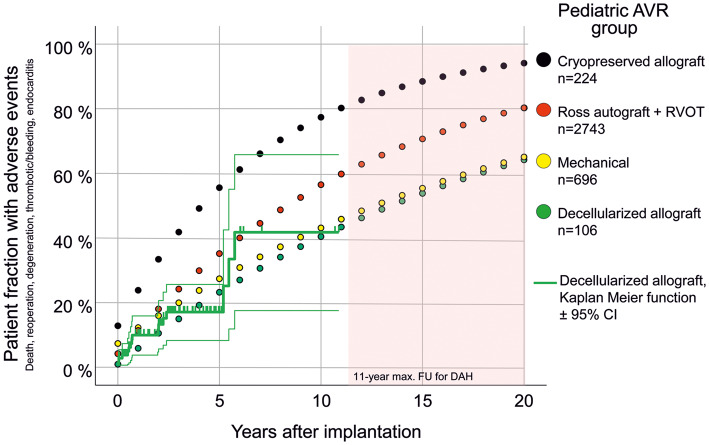
Expected adverse events for contemporary aortic valve replacement options in children
Perioperative and annually reported adverse events, such as death, reoperation or reintervention, valve degeneration, thrombotic and bleeding events and endocarditis, were calculated for conventional cryopreserved allografts (patients mean age 8.9 years), mechanical valves (patients mean age 12.8 years), the Ross procedure (patients mean age 9.4 years) and DAH (patients mean age 10.1 ± 4.8 years) to provide an overview of expected adverse events per patient (Table 2).
Table 2:
Observed perioperative mortality and annual adverse events for DAH in children in comparison to reported results of paediatric Ross procedures, mechanical AVR and standard allograft implantation in children
| DAH | Ross [18] | Mechanical [10] | Allograft [10] | |
|---|---|---|---|---|
| Early death (%) | 0.94 | 4.19 | 7.34 | 12.82 |
| Late mortality (%/years) | 0.38 | 0.54 | 1.23 | 1.59 |
| Reoperation and intervention (%/years) | 2 | 3.04 | 1.07 | 5.44 |
| Structural valve degeneration (%/years) | 2.29 | 3.25 | 0.86 | 2.93 |
| Thrombosis/bleeding (%/years) | 0 | 0.35 | 1.15 | 1.99 |
| Endocarditis (%/years) | 0.29 | 0.27 | 0.45 | 0.66 |
AVR: aortic valve replacement; DAH: decellularized aortic homografts.
Figure 4 shows that according to the limited follow-up data available so far, DAHs in children perform comparably to the conventional alternatives of mechanical valves and the Ross procedure, and better than conventional cryopreserved homografts.
Figure 4:
All paediatric DAHs implanted to date in comparison to recently published meta-analysis data for several AVR options in children. Perioperative and annual adverse events such as death, reoperation or reintervention, valve degeneration, thrombotic and bleeding events and endocarditis were summarized to provide an extrapolation of expected adverse events per patient in the long term. In addition, actual observed adverse paediatric DAH events are shown in Kaplan–Meier function equivalent ± 95% CI. Data taken from Etnel et al. [10, 18]. AVR: aortic valve replacement; CI: confidence interval; DAH: decellularized aortic homografts; FU: follow-up; RVOT: right ventricular outflow tract.
DISCUSSION
The current study adds important information on options for children undergoing AVR, who are not suitable candidates for the Ross procedure. Midterm outcome for DAH in children showed comparable results to those for the Ross procedure and mechanical AVR, and better results than conventional cryopreserved homografts in cohorts balanced for age and extent of previous procedures.
No specific handling issues were experienced during DAH implantation in a multicentre setting. Early mortality rates were lower with DAH than in paediatric Ross procedures (2.2% vs 4.2%, P = 0.3). Coronary reimplantation complications occurred in 3.8% of the DAH patients, which is comparable to the rates of 6.5% in children and 5% in young adults for intraoperative coronary artery bypass grafting associated with the Ross procedure [10, 18].
We observed progressive degeneration in a subset of ∼10% of paediatric patients receiving DAH. In general, children of younger age were more likely to experience allograft degeneration. The mean age of the reoperated children was significantly lower with 5.9 vs 10.1 years (P = 0.03) in the whole paediatric DAH cohort. Younger children requiring AVR often have poorer anatomical preconditions and have undergone several previous surgical procedures. As a consequence, residual LV outflow tract obstruction may occur after AVR and non-laminar flow is likely to influence the durability of the delicate structures of any biological valve. Non-laminar flow may also have a negative impact on spontaneous recellularization [15].
The potential of DAH for recellularization in children on the other hand can be illustrated by the case of a 2.4-year-old girl, in whom DAH was explanted during heart transplantation due to pre-existing myocardial failure despite normal graft function. Significant recellularization of the macroscopically normal homograft by non-immunogenic cells was observed 8 months after implantation, resembling 75% of a normal aortic valve [15].
Furthermore, we hypothesize that immunological aspects play an important role in DAH failure in young children as the morphological and histological examinations of explanted DAH resembled degeneration observed in conventional cryopreserved homografts. Although each DAH allograft undergoes extensive quality control measurements, rendering incomplete decellularization highly improbable, there appears to be an individual sensitization mechanism at work. This sensitization may be directed towards the collagen matrix structure of the DAH or towards remaining antigens within extracellular matrix substances, which are not removed during the decellularization process [19]. We have initial unpublished data showing different immune responses towards different decellularized xenografts by the same individual. Human immune responses towards different decellularized allografts are currently under analysis in healthy controls at our institution, and early data indicate considerable variance in the individual immune responses towards decellularized pulmonary and aortic homografts. This work has prompted the consideration of preoperative allograft matching tests, which may help to avoid this type of sensitization and hence reduce reoperation rates in young children.
In 4 of 6 redo AVR within the paediatric DAH cohort, we performed a second DAH implantation following open and thorough consultation with the respective parents.
Allograft wall degeneration and calcification was manageable intraoperatively including uneventful coronary transfer. All reoperations were successful with no mortalities, matching the successful results of redo aortic root replacement procedures in adults [20]. We observed no early degeneration of the second DAH during the follow-up of up to a maximum of 4 years so far.
While the current results for paediatric DAH are not yet ideal and despite the limitations of the restricted availability of allografts, we nonetheless consider that DAH constitutes an additional therapeutic option in particular for children who have undergone multiple previous aortic procedures. DAH allows better reconstruction of the LVOT than a pulmonary autograft. The associated dilatation of the ascending aorta can be simultaneously treated using a long DAH, thereby avoiding composite grafts, which are often more prone to endocarditis. There are also a growing number of paediatric patients requiring double semilunar valve replacement, for whom double valve replacement with decellularized allografts represents the only viable biological option [21].
Limitations
The short follow-up currently available for DAH constitutes the biggest limitation, and long-term tracking of DAH patients will be mandatory especially in view of the long life expectancy for paediatric patients.
Moreover, there are a number of limitations to the present study, including the one-armed study design and the restrictions inherent in the comparison of prospectively collected multicentre data with retrospectively conducted single-centre analyses and meta-analyses based on such reports. A relative strength of the analysis is the uniquely large size of the comparative groups for AVR and the age matching of these groups to the DAH cohort, which allowed direct comparison.
The applicability of the conclusions from this study to other proprietary decellularization protocols is limited due to potential differences in immunogenicity and, consequently, recellularization capability.
CONCLUSION
Even though the overall number of paediatric DAH patients and the follow-up time span are still limited, our data suggest that DAH may offer a promising additional option for paediatric AVR. The reported preliminary DAH outcome data in children were comparable with the results of current paediatric Ross procedures and mechanical AVR, and better than standard cryopreserved allografts.
ACKNOWLEDGEMENTS
The authors specifically thank the procuring tissue banks and their respective executive teams for their support in implementing this study. The authors also thank Nina McGuinness for editorial assistance.
Funding
This study was supported by a grant from the European Union’s HORIZON 2020 Programme under Grant Agreement No. 643597 (www.arise-clinicaltrial.eu).
Conflict of interest: Axel Haverich holds shares in Corlife oHG, the company providing the patented service of processing decellularized allografts used in this study. Igor Tudorache is a medical consultant for Corlife oHG involved in the approval of homografts.
Ramadan Jashari is the director of the European Homograft Bank. All other authors declare that there are no conflicts of interest.
Author contributions
Alexander Horke: Conceptualization; Data curation; Formal analysis; Investigation; Writing—review & editing. Dmitry Bobylev: Conceptualization; Investigation; Writing—review & editing. Murat Avsar: Data curation; Formal analysis; Investigation; Writing—review & editing. Bart Meyns: Conceptualization; Data curation; Formal analysis; Investigation; Writing—review & editing. Filip Rega: Data curation; Formal analysis; Investigation; Writing—review & editing. Mark Hazekamp: Data curation; Formal analysis; Investigation; Writing—review & editing. Michael Huebler: Data curation; Formal analysis; Investigation; Writing—review & editing. Martin Schmiady: Data curation; Formal analysis; Investigation; Writing—review & editing. Ioannis Tzanavaros: Data curation; Formal analysis; Investigation; Writing—review & editing. Robert Cesnjevar: Data curation; Formal analysis; Investigation; Writing—review & editing. Anatol Ciubotaru: Conceptualization; Data curation; Formal analysis; Investigation; Writing—review & editing. Günther Laufer: Data curation; Formal analysis; Investigation; Project administration; Writing—review & editing. Daniel Zimpfer: Data curation; Formal analysis; Funding acquisition; Writing—review & editing. Ramadan Jashari: Data curation; Formal analysis; Investigation; Resources; Writing—review & editing. Dietmar Boethig: Conceptualization; Data curation; Formal analysis; Methodology; Resources; Software; Visualization; Writing—review & editing. Serghei Cebotari: Conceptualization; Data curation; Formal analysis; Investigation; Supervision; Writing—review & editing. Philipp Beerbaum: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing—review & editing. Igor Tudorache: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—review & editing. Axel Haverich: Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Writing—review & editing. Samir Sarikouch: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing—original draft.
Presented at the 33rd Annual Meeting of the European Association for Cardio-Thoracic Surgery, Lisbon, Portugal, 3–5 October 2019.
ABBREVIATIONS
- AVR
Aortic valve replacement
- CPB
Cardiopulmonary bypass
- DAH
Decellularized aortic homograft
- ECMO
Extracorporeal membrane oxygenation
- LCx
Left circumflex artery
- LV
Left ventricular
- VSD
Ventricular septal defect
REFERENCES
- 1. Mookhoek A, Charitos EI, Hazekamp MG, Bogers AJ, Horer J, Lange R. et al. Ross procedure in neonates and infants: a European multicenter experience. Ann Thorac Surg 2015;100:2278–84. [DOI] [PubMed] [Google Scholar]
- 2. Brown JW, Patel PM, Ivy Lin JH, Habib AS, Rodefeld MD, Turrentine MW.. Ross versus non-ross aortic valve replacement in children: a 22-year single institution comparison of outcomes. Ann Thorac Surg 2016;101:1804–10. [DOI] [PubMed] [Google Scholar]
- 3. David TE, Ouzounian M, David CM, Lafreniere-Roula M, Manlhiot C.. Late results of the Ross procedure. J Thorac Cardiovasc Surg 2019;157:201–8. [DOI] [PubMed] [Google Scholar]
- 4. Sharabiani MT, Dorobantu DM, Mahani AS, Turner M, Peter Tometzki AJ, Angelini GD. et al. Aortic valve replacement and the Ross operation in children and young adults. J Am Coll Cardiol 2016;67:2858–70. [DOI] [PubMed] [Google Scholar]
- 5. David TE. Aortic valve replacement in children and young adults. J Am Coll Cardiol 2016;67:2871–3. [DOI] [PubMed] [Google Scholar]
- 6. Nelson JS, Maul TM, Wearden PD, Pasquali SK, Romano JC.. National practice patterns and early outcomes of aortic valve replacement in children and teens. Ann Thorac Surg 2019;108:544–51. [DOI] [PubMed] [Google Scholar]
- 7. Saleeb SF, Newburger JW, Geva T, Baird CW, Gauvreau K, Padera RF. et al. Accelerated degeneration of a bovine pericardial bioprosthetic aortic valve in children and young adults. Circulation 2014;130:51–60. [DOI] [PubMed] [Google Scholar]
- 8. Saleeb SF, Gauvreau K, Mayer JE, Newburger JW.. Aortic valve replacement with bovine pericardial tissue valve in children and young adults. Circulation 2019;139:983–5. [DOI] [PubMed] [Google Scholar]
- 9. Korteland NM, Etnel JRG, Arabkhani B, Mokhles MM, Mohamad A, Roos-Hesselink JW. et al. Mechanical aortic valve replacement in non-elderly adults: meta-analysis and microsimulation. Eur Heart J 2017;38:3370–7. [DOI] [PubMed] [Google Scholar]
- 10. Etnel JR, Elmont LC, Ertekin E, Mokhles MM, Heuvelman HJ, Roos-Hesselink JW. et al. Outcome after aortic valve replacement in children: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2016;151:143–52.e1–3. [DOI] [PubMed] [Google Scholar]
- 11. von Zur Muhlen C, Bothe W, Bode C.. The optimal anti-thrombotic regimen after surgical bioprosthetic aortic valve replacement-does it really matter? Thromb Haemost 2019;119:189–90. [DOI] [PubMed] [Google Scholar]
- 12. Tudorache I, Horke A, Cebotari S, Sarikouch S, Boethig D, Breymann T. et al. Decellularized aortic homografts for aortic valve and aorta ascendens replacement. Eur J Cardiothorac Surg 2016;50:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. da Costa FD, Costa AC, Prestes R, Domanski AC, Balbi EM, Ferreira AD. et al. The early and midterm function of decellularized aortic valve allografts. Ann Thorac Surg 2010;90:1854–60. [DOI] [PubMed] [Google Scholar]
- 14. Boethig D, Horke A, Hazekamp M, Meyns B, Rega F, Van Puyvelde J. et al. A European study on decellularized homografts for pulmonary valve replacement: initial results from the prospective ESPOIR trial and ESPOIR Registry data. Eur J Cardiothorac Surg 2019;56:503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sarikouch S, Theodoridis K, Hilfiker A, Boethig D, Laufer G, Andreas M. et al. Early insight into in vivo recellularization of cell-free allogenic heart valves. Ann Thorac Surg 2019;108:581–9. [DOI] [PubMed] [Google Scholar]
- 16. Helder MR, Kouchoukos NT, Zehr K, Dearani JA, Maleszewski JJ, Leduc C. et al. Late durability of decellularized allografts for aortic valve replacement: a word of caution. J Thorac Cardiovasc Surg 2016;152:1197–9. [DOI] [PubMed] [Google Scholar]
- 17. Bando K. A proposal for prospective late outcome analysis of decellularized aortic valves. J Thorac Cardiovasc Surg 2016;152:1202–3. [DOI] [PubMed] [Google Scholar]
- 18. Etnel JRG, Grashuis P, Huygens SA, Pekbay B, Papageorgiou G, Helbing WA. et al. The Ross procedure: a systematic review, meta-analysis, and microsimulation. Circ Cardiovasc Qual Outcomes 2018;11:e004748. [DOI] [PubMed] [Google Scholar]
- 19. Dziki JL, Wang DS, Pineda C, Sicari BM, Rausch T, Badylak SF.. Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. J Biomed Mater Res A 2017;105:138–47. [DOI] [PubMed] [Google Scholar]
- 20. Jassar AS, Desai ND, Kobrin D, Pochettino A, Vallabhajosyula P, Milewski RK. et al. Outcomes of aortic root replacement after previous aortic root replacement: the “true” redo root. Ann Thorac Surg 2015;99:1601–8; discussion 1608–9. [DOI] [PubMed] [Google Scholar]
- 21. Bobylev D, Sarikouch S, Tudorache I, Cvitkovic T, Soylen B, Boethig D. et al. Double semilunar valve replacement in complex congenital heart disease using decellularized homografts. Interact CardioVasc Thorac Surg 2019;28:151–7. [DOI] [PubMed] [Google Scholar]