Abstract
In chronic spontaneous urticaria (CSU), itchy wheals, angioedema, or both occur regularly, often daily, and for years. An effective therapy for CSU aims at achieving complete symptom control. The current guideline for the management of CSU patients recommends non-sedative anthistamines in standard or up to 4-fold higher dosages as 1 and 2 line treatment. For most CSU patients this treatment is not sufficient; for them, the anti-IgE antibody omalizumab is the therapy of choice. Although good to very good symptom control can be achieved in most cases, there are many patients with insufficient response. For these patients, but also as an alternative to therapy with omalizumab, numerous other biologicals are currently under development. In this review, we provide an overview of possible future biologic therapies for chronic urticaria.
Keywords: urticaria, angioedema, biologicals, monoclonal antibodies
Introduction
Chronic spontaneous urticaria (CSU) is characterized by recurrent wheals, angioedema, or both over a period of more than 6 weeks without a specific trigger [49]. The average duration of CSU is mostly reported to be 5 years [12, 43, 44], but in some cases the disease may last much longer. Recent data suggest that CSU patients with angioedema, patients with coexisting chronic inducible urticaria (CINDU), or with a positive autologous skin serum test (ASST), in particular, are more likely to have a longer course of the disease [4, 19, 24, 40, 44]. CSU is one of the most common skin diseases, with an estimated point prevalence of 0.5 – 1% [33], and it causes significant impairment of quality of life in many affected individuals, comparable to that of patients with severe ischemic heart disease [36].
Although our understanding of the pathophysiological processes of CSU has improved significantly in recent years, some aspects of its pathogenesis remain largely unexplained. For example, the central role of mast cells (MC) in the disease is well established [50]. However, the influence of other cells such as B cells, T cells, or eosinophils is unclear, as are the details of the exact mechanisms of MC activation. Numerous findings in recent years indicate that two different autoimmune mechanisms are essentially responsible for MC activation in CSU: In the majority of patients, type I autoimmunity (“autoallergy”) is present, i.e., an auto-IgE-mediated immediate reaction against an autoantigen, an endogenous allergen. In a smaller proportion of CSU patients, type IIb autoimmunity is present, in which IgG and IgM antibodies are directed against cellular structures on MCs, for example the IgE receptor FcεRI, leading to MC activation [18, 26, 30]. The presence of such autoantibodies can be detected, for example, in a basophil histamine release assay (BHRA) [8]. Patients who have a positive BHRA (~ 20% of all CSU patients) are not only more likely to have an overall more severe and prolonged CSU, but also respond significantly worse to therapy with omalizumab, an anti-IgE monoclonal antibody, which is otherwise very successful in CSU [14].
As of to date, there is no curative treatment for CSU, and all currently recommended treatment options are symptomatic therapies to control and prevent urticarial symptoms. The current guideline for the treatment of CSU recommends second-generation non-sedating antihistamines in standard doses as the first step. If control is not adequate (after 2 – 4 weeks or earlier, if symptoms are intolerable), the antihistamine dose should be increased up to 4-fold. If this does not result in sufficient control, omalizumab is additionally administered [49]. For patients who do not achieve symptom relief, the current guideline recommendation is the administration of cyclosporine. Most, but by no means all, patients with CSU achieve improvement in their CSU with this therapeutic algorithm. For patients who respond only partially, not at all, or slowly to available therapies, or who cannot be optimally treated due to side effects or concomitant diseases, other safe and effective alternative or additional treatment options are needed. Currently, several such therapies are in clinical development and testing [26]. In this review, we present potential future treatments with monoclonal antibodies for patients with CSU.
Biologics in CSU
MCs are the essential effector cells in urticaria; therefore, the blockade of MC activation is a promising approach in the treatment of urticaria [17]. In light of this, the current guideline for the treatment of chronic urticaria recommends omalizumab and cyclosporine as the third and fourth lines of treatment, respectively. Omalizumab prevents MC activation through IgE-mediated mechanisms, and cyclosporine can inhibit MC activation by inhibiting signal transduction. Novel biologic-based approaches in the therapy of MC-mediated diseases can be broadly divided into three groups: 1) inhibition of signals leading to MC activation, 2) activation of inhibitory receptors on MC, and 3) depletion of MC (Figure 1). In the following, we present the targets and associated monoclonal antibodies that are currently being developed or discussed for future therapy of CSU.
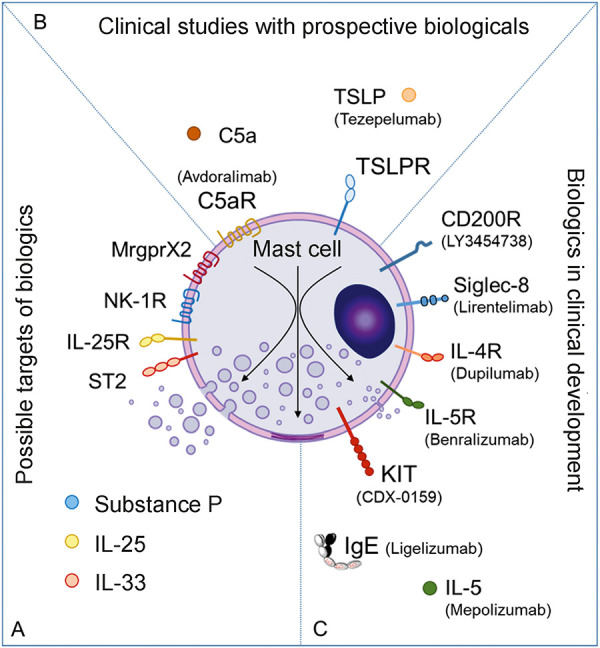
Figure 1. Biologics other than omalizumab for chronic spontaneous urticaria (CSU). Target structures for which biologics should be developed or existing biologics which should be clinically tested (A), drugs that are likely to be clinically tested for the indication urticaria (B), receptors and mediators for which biologics are already in clinical development for treatment of CSU, i.e., for which studies on the effectiveness and safety in patients with CSU have already been carried out or are currently being carried out (C).
Antibodies leading to inhibition of MC-activating signals
Anti-IgE antibodies
In recent years, activation of skin MC via IgE and the IgE receptor FcεRI has been shown to be a major contributor to the pathogenesis of chronic urticaria. Treatment with omalizumab, an anti-IgE antibody, is effective in CSU and is now an established therapy [1, 28, 29, 31, 32, 35, 41, 48]. With the novel anti-IgE antibody ligelizumab, there is now another monoclonal antibody that has ~ 50 times higher affinity for IgE than omalizumab. The results of a recently published multicenter, randomized, controlled phase II study show that ligelizumab is not only a highly effective therapy for CSU compared to placebo, but also has a higher rate of complete responders, i.e., patients who no longer show any symptoms of urticaria, than those treated with omalizumab [27]. Ligelizumab showed not only a very rapid and effective response, but also a longer lasting effect. At the dose of 240 mg, a recurrence of symptoms after the last administration of ligelizumab occurred on average after more than 10 weeks, whereas with omalizumab urticarial symptoms recurred after 4 weeks. Whether the better clinical efficacy of ligelizumab is due to its higher affinity for IgE or whether additional effects, for example on IgE production by B cells, are responsible for this better outcome has not yet been conclusively clarified [13]. Phase 3 trials in adults and adolescents with CSU are currently ongoing and have yet to confirm these results in a larger number of patients (NCT03580369, NCT03580356). In addition to ligelizumab, other anti-IgE biologics in development include GI-301 (GI-Innovation), a novel long-acting IgE trap-Fc fusion protein that, like omalizumab and ligelizumab, binds circulating IgE. GI-301, like ligelizumab, exhibits higher and more durable binding to IgE than omalizumab.
Antibodies against alarmins
The cytokines interleukin (IL)-33, IL-25, and thymic stromal lymphopoietin (TSLP), also known as alarmins, have previously been shown to activate MCs and are thought to contribute to the pathogenesis of CSU [15, 21]. For example, it has been shown that there are significantly more cells expressing IL-33, IL-25, and TSLP in the lesional skin of CSU patients compared with that of healthy controls [15]. However, whether blockade of these cytokines actually leads to an improvement in CSU symptoms is still unknown but could represent an interesting new treatment strategy for CSU. With, for example, tezepelumab (anti-TSLP), etokimab, itepekimab, and MEDI3506 (all anti-IL-33), as well as astegolimab and GSK3772847 (both anti-ST2 (subunit of the IL-33 receptor, also called IL1RL1)), biologics are ready to test this clinically.
Antibodies against Th2 cytokines
MCs express a variety of receptors, including those for the Th2 cytokines IL-4 and IL-5. It has been known for some time that both cytokines contribute to the survival of MCs and they can enhance FcεRI-mediated degranulation [37, 46]. The concentrations of IL-4 in the serum of patients with CSU are increased, and IL-4-expressing cells are found more frequently in the skin of CSU patients [7, 47], suggesting a contribution of IL-4 to the pathogenesis of CSU. Recently, a small case series has now demonstrated that dupilumab, an anti-IL-4Rα antibody, can be effective in patients with CSU [20]. The efficacy of dupilumab in urticaria is currently being investigated in several clinical trials, both in CSU and cholinergic urticaria (NCT03749135, NCT03749148, NCT04180488).
IL-5 is another cytokine that may contribute to the pathogenesis of CSU not only through its effects on MCs but also by acting on eosinophils and basophils, which are known to be increased in the lesional skin of CSU patients. Benralizumab, an anti-IL-5 receptor antibody, as well as the anti-IL-5 antibodies mepolizumab and reslizumab have been successfully used in the treatment of individual patients with CSU and CINDU [5, 6, 22]. In addition, positive results of a smaller controlled trial with benralizumab were recently published [23]. Benralizumab and mepolizumab are currently in clinical trials to test their efficacy in CSU (NCT04612725, NCT03494881).
Antibodies against G protein-coupled receptors (GPCRs)
MCs express numerous GPCRs that lead to activation and degranulation of MCs. These receptors include, for example, the complement C5a receptor (C5aR, CD88), to which the anaphylatoxin C5a binds, and the Mas-related G protein-coupled receptor X2 (MRGPRX2). Signals leading to activation of these receptors are thought to be involved in the pathogenesis of CSU. Interestingly, C5aR is selectively expressed by MCs of the skin but not by those in the lung or intestine. Since the symptoms of CSU are primarily or even exclusively manifested in the skin, this may argue for a role of C5aR. In addition, it has been shown that degranulation of MCs by autoantibodies from patients with autoimmune type IIb CSU is mediated, at least in part, by activation of C5aR [10, 16]. The efficacy of the anti-C5aR antibody avdoralimab is currently being evaluated in bullous pemphigoid, a disease in which a major role of MCs is also suspected, and other indications. The use of drugs that prevent activation of C5aR also appears to be an interesting approach for the therapy of CSU.
Another interesting MC receptor is MRGPRX2, which, like C5aR, is preferentially expressed by skin MCs and whose expression is upregulated in the skin of patients with severe CSU [11]. MRGPRX2 is a receptor that is activated by various endogenous and exogenous substances. Endogenous agonists of the receptor include substance P, major basic protein, and eosinophil peroxidase, all of which can be found at higher concentrations in the serum of patients with chronic inflammatory skin diseases such as CSU. For substance P, a neuropeptide and agonist of both MRGPRX2 and the neurokinin 1 receptor, elevated levels are detected in the serum of CSU patients, and serum levels of substance P correlate with CSU disease activity [34, 45]. Therefore, targeted blockade of MRGPRX2 and/or its agonists, for example substance P, represents a promising mechanism to decrease MC activation in patients with CSU.
Antibodies that bind to inhibitory receptors on MCs
The vast majority of receptors expressed by MCs are activating receptors, i.e., binding of corresponding ligands leads to degranulation, migration, differentiation, or proliferation of MCs. In contrast, a small group of MC receptors mediate inhibitory signals, i.e., binding of agonists to these receptors results in inhibition of MC activation, including degranulation. Two of these inhibitory MC receptors are Siglec-8 and CD200R. Antibodies directed against these receptors are currently being developed for the therapy of CSU. For example, lirentelimab, an anti-Siglec-8 monoclonal antibody, was recently shown to inhibit MC activation and lead to extensive depletion of eosinophils. Lirentelimab has been successfully tested in an open-label phase IIa pilot study in patients with omalizumab-naïve and omalizumab-refractory CSU, as well as in patients with symptomatic dermographism or cholinergic urticaria [2]. However, larger controlled studies to confirm the safety and efficacy of the drug in the treatment of CSU are still pending. Initial recently published findings on the safety of this antibody were demonstrated in a study of the therapy of eosinophilic gastritis and duodenitis [9].
Binding of activating antibodies to the receptor CD200R on MCs also results in inhibition of activation and degranulation of MCs [3]. The monoclonal antibody LY3454738 directed against CD200R is currently in a randomized, controlled phase 2 trial to test the efficacy of therapy in patients with CSU (NCT04159701).
Antibodies that deplete MCs
MCs are among the few cells that express Kit, the receptor for stem cell factor (SCF), in the mature state. SCF is the essential factor responsible for differentiation, activation, migration, proliferation, and survival of MCs [38]. Slightly increased numbers of MCs are found in the skin of patients with CSU, which may be due to the action of SCF; moreover, SCF is a potent activator of MCs [39, 42]. Neutralization of SCF, for example by anti-SCF or anti-Kit antibodies, could reduce the number of MCs and inhibit their activation. Since any MC-mediated disease would benefit from this, it is also likely that this could be an effective therapeutic approach in CSU. Preliminary results from a phase 1 study in healthy volunteers with the anti-Kit antibody CDX-0159 indicate that treatment leads to a substantial reduction of MCs. In this study with a total of 32 healthy volunteers, a single intravenous dose-dependent administration led to an almost complete reduction of basal tryptase in the blood after only a few days. Tryptase is an MC-specific protease (only basophils still contain small amounts), and basal tryptase levels in blood (based on constitutive release by MC) correlate with the number of MC. The decrease of basal tryptase in blood after administration of CDX-0159 to levels below the detection limit therefore suggests an effective reduction of the number of MCs. In the two higher doses, there was a sustained suppression of tryptase until the end of the observation period of 71 days [25]. CDX-0159 is currently in ongoing clinical trials for CSU and CINDU.
Conclusion
Until the approval of the anti-IgE antibody omalizumab for the treatment of CSU in 2014, the management of CSU patients was challenging. Because a large proportion of patients do not respond at all or inadequately to antihistamines, the advent of biologics therapy with anti-IgE represented a breakthrough in patient care. Over the years, however, it became apparent that despite the great successes with omalizumab, numerous CSU patients still could not be treated effectively. Not only to have additional therapeutic options besides omalizumab, but also to be able to treat those patients who do not respond or do not respond sufficiently to omalizumab therapy, new, safe, and effective therapeutic options are needed for CSU, but also for CINDU, for which there is still no therapy at all beyond antihistamines.
The biologics presented here all have the potential to be equally or even more effective than omalizumab in the therapy of CSU. For some, this has already been shown in clinical trials (ligelizumab). While for some targets only the idea of a potential therapy exists so far (for example MRGPRX2), other molecules are currently already in phase 3 trials (for example ligelizumab, dupilumab). So we can be excited and optimistic about what the next years will bring for us and our patients with CSU in terms of new therapeutic options.
Funding
None.
Conflict of interest
M. Metz has received honoraria as a speaker and/or consultant for Amgen, Aralez, argenx, Moxie, Novartis, Roche, Sanofi, and Uriach.
M. Maurer is or has recently been a speaker and/or consultant for and/or has received research funding from Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Celldex, Centogene, CSL Behring, FAES, Genentech, GIInnovation, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novar-tis, Roche, Sanofi/Regeneron, Third HarmonicBio, UCB, and Uriach.
References
- 1. Altrichter S Chuamanochan M Knoth H Asady A Ohanyan T Metz M Maurer M Real-life treatment of cholinergic urticaria with omalizumab. J Allergy Clin Immunol. 2019; 143: 788–791.e8. [DOI] [PubMed] [Google Scholar]
- 2. Altrichter S Staubach P Pasha M Rasmussen HS Singh B Chang AT Bernstein JA Siebenhaar F Maurer M Efficacy and safety data of AK002, an anti-siglec-8 monoclonal antibody, in patients with multiple forms of uncontrolled chronic urticar-ia (CU): Results from an open-label phase 2a study. Allergy. 2020; 74: 120. [Google Scholar]
- 3. Bachelet I Munitz A Levi-Schaffer F Abrogation of allergic reactions by a bispecific antibody fragment linking IgE to CD300a. J Allergy Clin Immunol. 2006; 117: 1314–1320. [DOI] [PubMed] [Google Scholar]
- 4. Beltrani VS An overview of chronic urticaria. Clin Rev Allergy Immunol. 2002; 23: 147–169. [DOI] [PubMed] [Google Scholar]
- 5. Bergmann KC Altrichter S Maurer M Benefit of benralizumab treatment in a patient with chronic symptomatic dermographism. J Eur Acad Dermatol Venereol. 2019; 33: e413–e415. [DOI] [PubMed] [Google Scholar]
- 6. Bernstein JA Singh U Rao MB Berendts K Zhang X Mutasim D Benralizumab for Chronic Spontaneous Urticaria. N Engl J Med. 2020; 383: 1389–1391. [DOI] [PubMed] [Google Scholar]
- 7. Caproni M Cardinali C Giomi B Antiga E D’Agata A Walter S Fabbri P Serological detection of eotaxin, IL-4, IL-13, IFN-gamma, MIP-1alpha, TARC and IP-10 in chronic autoimmune urticaria and chronic idiopathic urticaria. J Dermatol Sci. 2004; 36: 57–59. [DOI] [PubMed] [Google Scholar]
- 8. Chang TW Chen C Lin CJ Metz M Church MK Maurer M The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2015; 135: 337–342. [DOI] [PubMed] [Google Scholar]
- 9. Dellon ES Peterson KA Murray JA Falk GW Gonsalves N Chehade M Genta RM Leung J Khoury P Klion AD Hazan S Vaezi M Bledsoe AC Durrani SR Wang C Shaw C Chang AT Singh B Kamboj AP Rasmussen HS Anti-Siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med. 2020; 383: 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrer M Nakazawa K Kaplan AP Complement dependence of histamine release in chronic urticaria. J Allergy Clin Immunol. 1999; 104: 169–172. [DOI] [PubMed] [Google Scholar]
- 11. Fujisawa D Kashiwakura J Kita H Kikukawa Y Fujitani Y Sasaki-Sakamoto T Kuroda K Nunomura S Hayama K Terui T Ra C Okayama Y Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 2014; 134: 622–633.e9. [DOI] [PubMed] [Google Scholar]
- 12. Gaig P Olona M Muñoz Lejarazu D Caballero MT Domínguez FJ Echechipia S García Abujeta JL Gonzalo MA Lleonart R Martínez Cócera C Rodríguez A Ferrer M Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004; 14: 214–220. [PubMed] [Google Scholar]
- 13. Gasser P Tarchevskaya SS Guntern P Brigger D Ruppli R Zbären N Kleinboelting S Heusser C Jardetzky TS Eggel A The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat Commun. 2020; 11: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gericke J Metz M Ohanyan T Weller K Altrichter S Skov PS Falkencrone S Brand J Kromminga A Hawro T Church MK Maurer M Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J Allergy Clin Immunol. 2017; 139: 1059–1061.e1. [DOI] [PubMed] [Google Scholar]
- 15. Kay AB Clark P Maurer M Ying S Elevations in T-helper-2-initiating cytokines (interleukin-33, interleukin-25 and thymic stromal lymphopoietin) in lesional skin from chronic spontaneous (‘idiopathic’) urticaria. Br J Dermatol. 2015; 172: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 16. Kikuchi Y Kaplan AP A role for C5a in augmenting IgG-dependent histamine release from basophils in chronic urticaria. J Allergy Clin Immunol. 2002; 109: 114–118. [DOI] [PubMed] [Google Scholar]
- 17. Kolkhir P Altrichter S Munoz M Hawro T Maurer M New treatments for chronic urticaria. Ann Allergy Asthma Immunol. 2020; 124: 2–12. [DOI] [PubMed] [Google Scholar]
- 18. Kolkhir P Church MK Weller K Metz M Schmetzer O Maurer M Autoimmune chronic spontaneous urticaria: What we know and what we do not know. J Allergy Clin Immunol. 2017; 139: 1772–1781.e1. [DOI] [PubMed] [Google Scholar]
- 19. Kulthanan K Jiamton S Thumpimukvatana N Pinkaew S Chronic idiopathic urticaria: prevalence and clinical course. J Dermatol. 2007; 34: 294–301. [DOI] [PubMed] [Google Scholar]
- 20. Lee JK Simpson RS Dupilumab as a novel therapy for difficult to treat chronic spontaneous urticaria. J Allergy Clin Immunol Pract. 2019; 7: 1659–1661.e1. [DOI] [PubMed] [Google Scholar]
- 21. Lin W Zhou Q Liu C Ying M Xu S Increased plasma IL-17, IL-31, and IL-33 levels in chronic spontaneous urticaria. Sci Rep. 2017; 7: 17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magerl M Terhorst D Metz M Altrichter S Zuberbier T Maurer M Bergmann KC Benefit of mepolizumab treatment in a patient with chronic spontaneous urticaria. J Dtsch Dermatol Ges. 2018; 16: 477–478. [DOI] [PubMed] [Google Scholar]
- 23. Maurer M Altrichter S Metz M Zuberbier T Church MK Bergmann KC Benefit from reslizumab treatment in a patient with chronic spontaneous urticaria and cold urticaria. J Eur Acad Dermatol Venereol. 2018; 32: e112–e113. [DOI] [PubMed] [Google Scholar]
- 24. Maurer M Bindslev-Jensen C Gimenez-Arnau A Godse K Grattan CE Hide M Kaplan AP Makris M Simons FE Zhao Z Zuberbier T Church MK Chronic idiopathic urticaria (CIU) is no longer idiopathic: time for an update. Br J Dermatol. 2013; 168: 455–456. [DOI] [PubMed] [Google Scholar]
- 25. Maurer M Crew L Murphy M Hawthorne T Alvarado D Forsberg E Morani PA Paradise E Aneiro L Thomas LJ Keler T Crowley E Heath-Chiozzi M Young D Kankam M CDX-0159, an anti-KIT monoclonal antibody, demonstrates dose-dependent reductions in serum tryptase and a favorable safety profile in a phase 1a healthy volunteer study. Allergy. 2020; 75:280. [Google Scholar]
- 26. Maurer M Eyerich K Eyerich S Ferrer M Gutermuth J Hartmann K Jakob T Kapp A Kolkhir P Larenas-Linnemann D Park HS Pejler G Sánchez-Borges M Schäkel K Simon D Simon HU Weller K Zuberbier T Metz M Urticaria: Collegium Internationale Allergologicum (CIA) Update 2020. Int Arch Allergy Immunol. 2020; 181: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maurer M Giménez-Arnau AM Sussman G Metz M Baker DR Bauer A Bernstein JA Brehler R Chu CY Chung WH Danilycheva I Grattan C Hébert J Katelaris C Makris M Meshkova R Savic S Sinclair R Sitz K Staubach P Ligelizumab for Chronic Spontaneous Urticaria. N Engl J Med. 2019; 381: 1321–1332. [DOI] [PubMed] [Google Scholar]
- 28. Maurer M Kaplan A Rosén K Holden M Iqbal A Trzaskoma BL Yang M Casale TB The XTEND-CIU study: Long-term use of omalizumab in chronic idiopathic urticaria. J Allergy Clin Immunol. 2018; 141: 1138–1139.e7. [DOI] [PubMed] [Google Scholar]
- 29. Maurer M Metz M Brehler R Hillen U Jakob T Mahler V Pföhler C Staubach P Treudler R Wedi B Magerl M Omalizumab treatment in patients with chronic inducible urticaria: A systematic review of published evidence. J Allergy Clin Immunol. 2018; 141: 638–649. [DOI] [PubMed] [Google Scholar]
- 30. Maurer M Metz M Magerl M Neues zur Pathogenese der chronischen spontanen Urtikaria. Ärztliches. J Dermatol. 2018; 2: 20–22. [Google Scholar]
- 31. Maurer M Rosén K Hsieh HJ Saini S Grattan C Gimenéz-Arnau A Agarwal S Doyle R Canvin J Kaplan A Casale T Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013; 368: 924–935. [DOI] [PubMed] [Google Scholar]
- 32. Maurer M Schütz A Weller K Schoepke N Peveling-Oberhag A Staubach P Müller S Jakob T Metz M Omalizumab is effective in symptomatic dermographism-results of a randomized placebo-controlled trial. J Allergy Clin Immunol. 2017; 140: 870–873.e5. [DOI] [PubMed] [Google Scholar]
- 33. Maurer M Weller K Bindslev-Jensen C Giménez-Arnau A Bousquet PJ Bousquet J Canonica GW Church MK Godse KV Grattan CE Greaves MW Hide M Kalogeromitros D Kaplan AP Saini SS Zhu XJ Zuberbier T Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011; 66: 317–330. [DOI] [PubMed] [Google Scholar]
- 34. Metz M Krull C Hawro T Saluja R Groffik A Stanger C Staubach P Maurer M Substance P is upregulated in the serum of patients with chronic spontaneous urticaria. J Invest Dermatol. 2014; 134: 2833–2836. [DOI] [PubMed] [Google Scholar]
- 35. Metz M Schütz A Weller K Gorczyza M Zimmer S Staubach P Merk HF Maurer M Omalizumab is effective in cold urticaria-results of a randomized placebo-controlled trial. J Allergy Clin Immunol. 2017; 140: 864–867.e865. [DOI] [PubMed] [Google Scholar]
- 36. O’Donnell BF Urticaria: impact on quality of life and economic cost. Immunol Allergy Clin North Am. 2014; 34: 89–104. [DOI] [PubMed] [Google Scholar]
- 37. Ochi H De Jesus NH Hsieh FH Austen KF Boyce JA IL-4 and -5 prime human mast cells for different profiles of IgE-dependent cytokine production. Proc Natl Acad Sci USA. 2000; 97: 10509–10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okayama Y Kawakami T Development, migration, and survival of mast cells. Immunol Res. 2006; 34: 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petersen LJ Brasso K Pryds M Skov PS Histamine release in intact human skin by monocyte chemoattractant factor-1, RANTES, macrophage inflammatory protein-1 alpha, stem cell factor, anti-IgE, and codeine as determined by an ex vivo skin microdialysis technique. J Allergy Clin Immunol. 1996; 98: 790–796. [DOI] [PubMed] [Google Scholar]
- 40. Sánchez-Borges M Caballero-Fonseca F Capriles-Hulett A González-Aveledo L Maurer M Factors linked to disease severity and time to remission in patients with chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2017; 31: 964–971. [DOI] [PubMed] [Google Scholar]
- 41. Staubach P Metz M Chapman-Rothe N Sieder C Bräutigam M Maurer M Weller K Omalizumab rapidly improves angioedema-related quality of life in adult patients with chronic spontaneous urticaria: X-ACT study data. Allergy. 2018; 73: 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Terhorst D Koti I Krause K Metz M Maurer M In chronic spontaneous urticaria, high numbers of dermal endothelial cells, but not mast cells, are linked to recurrent angio-oedema. Clin Exp Dermatol. 2018; 43: 131–136. [DOI] [PubMed] [Google Scholar]
- 43. Toubi E Kessel A Avshovich N Bamberger E Sabo E Nusem D Panasoff J Clinical and laboratory parameters in predicting chronic urticaria duration: a prospective study of 139 patients. Allergy. 2004; 59: 869–873. [DOI] [PubMed] [Google Scholar]
- 44. van der Valk PG Moret G Kiemeney LA The natural history of chronic urticaria and angioedema in patients visiting a tertiary referral centre. Br J Dermatol. 2002; 146: 110–113. [DOI] [PubMed] [Google Scholar]
- 45. Vena GA Cassano N Di Leo E Calogiuri GF Nettis E Focus on the role of substance P in chronic urticaria. Clin Mol Allergy. 2018; 16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yanagida M Fukamachi H Ohgami K Kuwaki T Ishii H Uzumaki H Amano K Tokiwa T Mitsui H Saito H Iikura Y Ishizaka T Nakahata T Effects of T-helper 2-type cytokines, interleukin-3 (IL-3), IL-4, IL-5, and IL-6 on the survival of cultured human mast cells. Blood. 1995; 86: 3705–3714. [PubMed] [Google Scholar]
- 47. Ying S Kikuchi Y Meng Q Kay AB Kaplan AP TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol. 2002; 109: 694–700. [DOI] [PubMed] [Google Scholar]
- 48. Zhao ZT Ji CM Yu WJ Meng L Hawro T Wei JF Maurer M Omalizumab for the treatment of chronic spontaneous urticaria: A meta-analysis of randomized clinical trials. J Allergy Clin Immunol. 2016; 137: 1742–1750.e4. [DOI] [PubMed] [Google Scholar]
- 49. Zuberbier T Aberer W Asero R Abdul Latiff AH Baker D Ballmer-Weber B Bernstein JA Bindslev-Jensen C Brzoza Z Buense Bedrikow R Canonica GW Church MK Craig T Danilycheva IV Dressler C Ensina LF Giménez-Arnau A Godse K Gonçalo M Grattan C The EAACI/GA²LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018; 73: 1393–1414. [DOI] [PubMed] [Google Scholar]
- 50. Church MK Kolkhir P Metz M Maurer M The role and relevance of mast cells in urticaria. Immunol Rev. 2018; 282: 232–247. [DOI] [PubMed] [Google Scholar]


