Abstract
We implemented universal face shield use for all healthcare personnel upon entry to facility in order to counter an increase in SARS-COV2 cases among healthcare personnel and hospitalized patients. There was a marked reduction of infections in both healthcare personnel and hospitalized patients between pre and post intervention. Our results support universal face shield use as part of a multifaceted approach in areas of high SARS-COV2 community transmission.
Keywords: Face shields, PPE, COVID-19, Occupational health
Introduction
SARS-COV2 transmission to healthcare personnel (HCP) and hospitalized patients is a significant challenge (Kluytmans-van den Bergh et al., 2020, Lai et al., 2020, Rickman et al., 2020). Our hospital is a quaternary healthcare system with more than 500 beds and 8,000 HCP. Between April 1 and April 17, 2020, we instituted several infection prevention strategies to limit transmission of SARS-COV2 including: limiting entry to facility, screening for symptoms and temperature, universal masking for HCP, universal masking for patients, social distancing (avoid having lunch with other people) and limiting meeting size to less than 10.
As a part of our pandemic response, we started a surveillance program for HCP and patients (started April 17). This program included: biweekly testing for HCP in high risk units (ED, transplant units, and COVID-19 units), weekly testing for HCP in cluster areas (> = 3 cases of HCP with COVID-19 diagnosis or any case of hospital-acquired infection [HAI]) and testing all asymptomatic patients on admission and every 7 days. Testing was voluntary for HCP and patients. HCP in other areas were allowed to be tested if desired or if there was exposure history. HCP or patients with previous positive COVID-19 diagnosis were excluded.
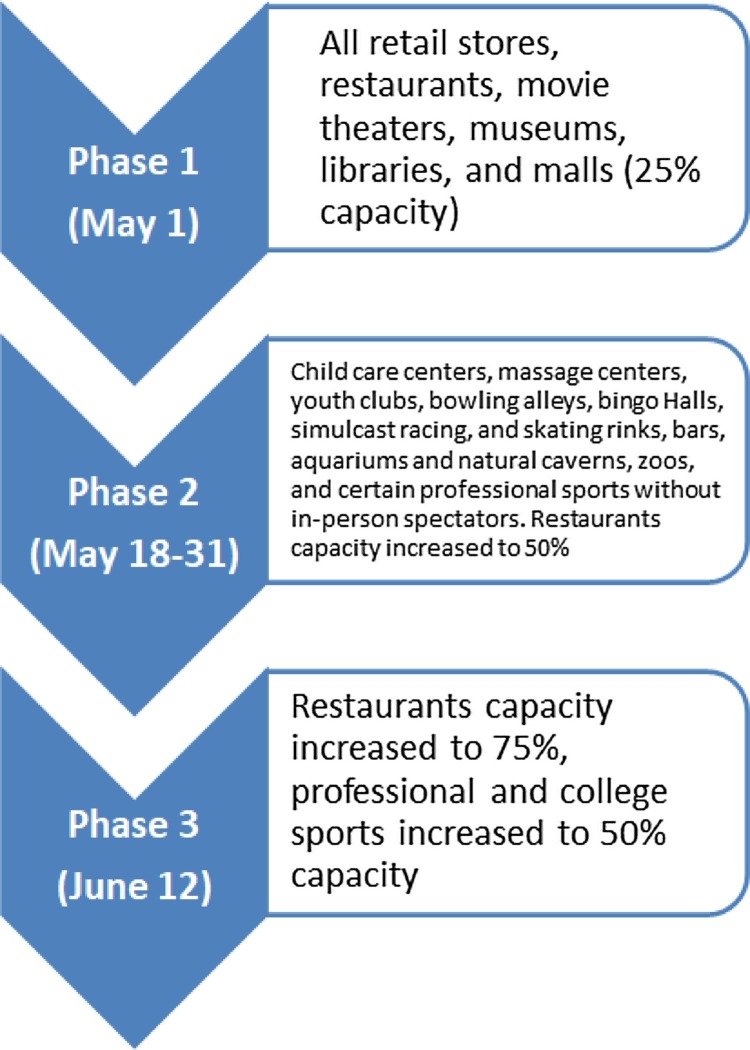
As the state of Texas started reopening (Figure 1 ), we have seen an increase in HCP testing positive for SARS-COV2 and patients acquiring HAI. In one week (June 29, 2020 to July 5, 2020), 112 employees (15.9%) tested positive for SARS-COV2 with clusters in 2 ICUs, operation room, environmental services, and cardiac catheterization laboratory. Most clusters started in the community and then spread staff to staff (data not shown). In addition, we saw 7 cases of HAI (3.4 cases per 1000 patient-days) during that week (Figure 2 ).
Figure 1.
Texas reopening phases.
This figure represents the three phases of Texas reopening.
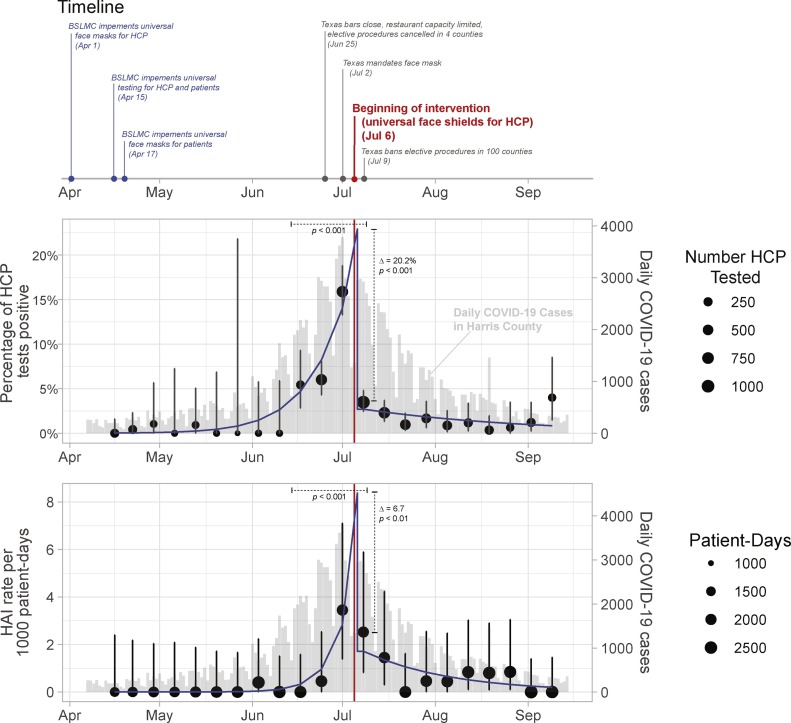
Figure 2.
Interrupted time series analysis.
This figure represents the timeline of key state and local interventions (top), interrupted time series for percent of healthcare worker personnel with positive SARS-COV2 (middle), and possible or confirmed hospital acquired infections (bottom). Note: the intervention was implemented on July 6, 2020. HCP: healthcare personnel; HAI: hospital acquired infections; BSLMC; Baylor St. Luke’s Medical Center, Houston, TX.
On July 6, 2020, we implemented universal face shield requirements for all healthcare personnel upon entry to facility. We decided to add face shields in order to reduce the potential for autoinoculation by preventing the HCP from touching their face, and to protect from viral entry to the eyes, nose and mouth. In addition, they may be used as a barrier of transmission from HCP to other HCP and patients, and they can be reused for a long time, easily cleaned and available at low cost (USD $2 when 3D printed locally) (Centers for Disease Control and Prevention, 2021, Perencevich et al., 2020). In one study, face shields were able to reduce influenza transmission by 96% within 18 inches of a cough when used by a simulated HCP. They also blocked the majority of small particle aerosols (68%) after 30 min of exposure (Lindsley et al., 2014).
The aim of our quality improvement study was to assess the impact of face shield policy on SARS-COV2 infection among HCP and hospitalized patients.
Methods
The pre-intervention period (April 17, 2020-July 5, 2020) included implementation of universal face masks and surveillance testing of HCP and patients. The intervention period (July 6, 2020-September 7, 2020) included the addition of face shields (Lazarus 3D, Corvallis, OR, USA) to all HCP (for patient encounters and staff-to-staff encounters). Goggles were allowed as an alternative for those unable to tolerate face shields. Face shields or goggles were not provided to patients.
We obtained data for the number of HCP who were tested and the rate of positivity per week from the surveillance clinic. HCP were excluded from testing if they had a previous positive SARS-COV2 test. Using electronic medical records, we obtained the number of patients with a positive SARS-COV2 after 5 days of admission. Possible hospital-acquired infection (HAI) was defined as a positive SARS-COV2 test between 5-13 days from admission with no previous positive test. Confirmed HAI was defined as a positive SARS-COV2 test after 14 days from admission with no previous positive test.
We calculated the proportion of positive HCW tests with 95% CI and the weekly rate of HAI per 1000 patient-days with 95% CI using the epitools package (##version 0.5-10.1) (Aragon et al., 2020). We used interrupted time series analysis with segmented regression to examine the effect of our intervention on the difference in proportion of HCP positive for SARS-COV2 (using logistic regression) and possible or confirmed HAI (using Poisson regression), comparing pre-intervention and post-intervention rates. We defined significance as P values < 0.05. We used R 4.0.2 and RStudio 1.3.1056 to perform the statistical analysis. This study was deemed exempt from IRB review.
Results
Of 6527 HCP tested, 246 tested positive for SARS-COV2 (3.8%). In the preintervention period, the weekly positivity rate among HCP increased from 0% to 12.9% (Figure 2, Table 1 ). During the intervention period, the weekly positivity rate among HCP decreased to 2.3%, with segmented regression showing a change in predicted proportion positive in week 13 (22.9% to 2.7%, p < 0.001) and change in the post-intervention slope on the log odds scale (p < 0.001). Full statistical analysis is presented in the appendix.
Table 1.
Number of health care providers (HCP) tested (total and positive), total patient-days, and number of hospital acquired infections (HAI).
| Testing Dates (Mon-Fri) | Total HCP Tested | Number HCP COVID+ | Total Patient-Days | Number of HAI |
|---|---|---|---|---|
| 4/15-4/17 | 229 | 0 | 1540 | 0 |
| 4/20-4/24 | 239 | 1 | 1699 | 0 |
| 4/27-5/1 | 96 | 1 | 1814 | 0 |
| 5/4-5/8 | 49 | 0 | 1772 | 0 |
| 5/11-5/15 | 108 | 1 | 1965 | 0 |
| 5/18-5/22 | 52 | 0 | 2151 | 0 |
| 5/25-5/29 | 15 | 0 | 2215 | 0 |
| 6/1-6/5 | 62 | 0 | 2494 | 1 |
| 6/8-6/12 | 61 | 0 | 2447 | 0 |
| 6/15-6/19 | 221 | 12 | 2335 | 0 |
| 6/22-6/26 | 649 | 39 | 2205 | 1 |
| 6/29-7/3 | 705 | 112 | 2030 | 7 |
| Beginning of Intervention (July 6): Universal face shields for HCP | ||||
| 7/6-7/10 | 1034 | 36 | 1980 | 5 |
| 7/13-7/17 | 696 | 16 | 2071 | 3 |
| 7/20-7/24 | 517 | 5 | 2280 | 0 |
| 7/27-7/31 | 360 | 6 | 2181 | 1 |
| 8/3-8/7 | 342 | 3 | 2254 | 1 |
| 8/10-8/14 | 256 | 3 | 2387 | 2 |
| 8/17-8/21 | 281 | 1 | 2497 | 2 |
| 8/24-8/28 | 157 | 1 | 2374 | 2 |
| 8/31-9/4 | 248 | 3 | 2650 | 0 |
| 9/7-9/11 | 150 | 6 | 2543 | 0 |
A total of 25 HAI cases were identified (16 possible and 9 confirmed). In the preintervention period, HAI cases increased from 0 to 7 (Figure 2, Table 1). During the intervention period, HAI cases decreased to 0. The change in predicted HAI rate due to the intervention was significantly different in week 13 with and without the intervention (8.7 vs 1.7 per 1000 patient-days, p < 0.001), and change between pre-intervention and post-intervention slope on the log scale was also significant (p < 0.001). County community data accessed from the Harris County Public Health COVID Data Dashboard “Epi Curve” are included in Figure 2 (Harris County Public Health, 2019a).
Discussion
Our study showed that the universal use of face shields was associated with significant reduction in SARS-COV2 infection among HCP and hospitalized patients. This could be explained by a reduction in viral transmission after universal face shield introduction. Similar results were described in community health workers in India after implementation of face shields (Bhaskar and Arun, 2020).
In general, face shields were well-tolerated by the majority of our staff (quantitative data not available). This is similar to another study reporting the use of face shields in interventional radiology (Sapoval et al., 2020). This study indicated that 3D-printed face shields were tolerable and not associated with visual discomfort.
There were several state measures that were implemented during the time of the intervention that led to reduction in community cases and might have confounded the results (Harris County Public Health, 2019b). However, the number of SARS-COV2 infections declined more rapidly among HAI and HCP compared to the decline in the community (Figure 2). We did not assess for compliance of other infection prevention measures, which could be a confounder in the study. We did not have access to individual-level data for healthcare workers, and so cannot differentiate differences in effect based on position (physician, nurse, respiratory therapist, etc.) or location (ER, ICU, floor). Finally, compliance with testing increased during the surge which could be another confounder.
In conclusion, our results suggest the universal face shield use as a part of a multifaceted approach in areas of high SARS-COV2 community transmission.
Conflict of interest
All authors report no conflict of interest or financial disclosure
Funding source
None.
Ethical approval
This study was deemed exempt from IRB review.
Acknowledgement
None.
References
- Aragon T.J., Fay M.P., Wollschlaegeret D., Omidpanah A. 2020. Epitools: Epidemiology Tools.https://CRAN.R-project.org/package=epitools [Google Scholar]
- Bhaskar M.E., Arun S. SARS-CoV-2 infection among community health workers in India before and after use of face shields. JAMA. 2020;324(13):1348–1349. doi: 10.1001/jama.2020.15586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2021. Operational considerations for personal protective equipment in the context of global supply shortages for coronavirus disease 2019 (COVID-19) pandemic: non-US healthcare settings. (Accessed 10 February 2021) [Google Scholar]
- Harris County Public Health . 2019. Novel coronavirus.https://publichealth.harriscountytx.gov/Resources/2019-Novel-Coronavirus [Google Scholar]
- Harris County Public Health . 2019. Harris County/ Houston COVID-19 Cases.https://harriscounty.maps.arcgis.com/apps/opsdashboard/index.html#/c0de71f8ea484b85bb5efcb7c07c6914 [Google Scholar]
- Kluytmans-van den Bergh M., Buiting A., Pas S., Bentvelsen R., van den Bijllaardt W., van Oudheusden A. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open. 2020;3(May (5)):e209673. doi: 10.1001/jamanetworkopen.2020.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X., Wang M., Qin C., Tan L., Ran L., Chen D. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3(5):e209666. doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley W.G., Noti J.D., Blachere F.M., Szalajda J., Beezhold D.H. Efficacy of face shields against cough aerosol droplets from a cough simulator. J Occup Environ Hyg. 2014;11(8):509–518. doi: 10.1080/15459624.2013.877591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perencevich E.N., Diekema D.J., Edmond M.B. Moving personal protective equipment into the community: face shields and containment of COVID-19. JAMA. 2020;323(22):2252–2253. doi: 10.1001/jama.2020.7477. [DOI] [PubMed] [Google Scholar]
- Rickman H.M., Rampling T., Shaw K., Martinez-Garcia G., Hail L., Coen P. Nosocomial transmission of COVID-19: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis. 2020;20(June):ciaa816. doi: 10.1093/cid/ciaa816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapoval M., Gaultier A., Giudice C.D., Pellerin O., Kassis-Chikhani N., Lemarteleur B.V. 3D-printed face protective shield in interventional radiology: evaluation of an immediate solution in the era of COVID-19 pandemic. Diagn Interv Imaging. 2020;101(June (6)):413–415. doi: 10.1016/j.diii.2020.04.004. Epub 2020 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]