Abstract
Background
Convalescent plasma (CP) is being used as a treatment option in hospitalized patients with COVID-19. Till date, there is conflicting evidence on efficacy of CP in reducing COVID-19 related mortality.
Objective
To evaluate the effect of CP on 28-day mortality reduction in patients with COVID-19.
Methods
We did a multi-centre, retrospective case control observational study from 1st May 2020 to 31st August 2020. A total of 1079 adult patients with moderate and severe COVID-19 requiring oxygen, were reviewed. Of these, 694 patients were admitted to ICU. Out of these, 333 were given CP along with best supportive care and remaining 361 received best supportive care only.
Results
In the overall group of 1079 patients, mortality in plasma vs no plasma group was statistically not significant (22.4% vs 18.5%; p = 0.125; OR = 1.27, 95% CI: 0.94‐–1.72). However, in patients with COVID-19 admitted to ICU, mortality was significantly lower in plasma group (25.5% vs 33.2%; p = 0.026; OR = 0.69, 95%CI: 0.50–0.96). This benefit of reduced mortality was most seen in age group 60 to 74 years (26.7% vs 43.0%; p = 0.004; OR = 0.48, 95% CI: 0.29–0.80), driven mostly by females of this age group (23.1% vs 53.5%; p = 0.013; OR = 0.26, 95% CI: 0.09–0.78). Significant difference in mortality was observed in patients with one comorbidity (22.3% vs 36.5%; p = 0.004; OR = 0.50, 95% CI: 0.31–0.80). Moreover, patients on ventilator had significantly lower mortality in the plasma arm (37.2% vs 49.3%; p = 0.009; OR = 0.61, 95% CI: 0.42–0.89); particularly so for patients on invasive mechanical ventilation (63.9% vs 82.9%; p = 0.014; OR = 0.37, 95% CI: 0.16–0.83).
Conclusion
The use of CP was associated with reduced mortality in COVID-19 elderly patients admitted in ICU, above 60 years of age, particularly females, those with comorbidities and especially those who required some form of ventilation.
Keywords: Convalescent plasma therapy, COVID-19, ICU cases, Mortality, Subgroup analysis
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), the novel coronavirus causing COVID-19, originated from Wuhan, China and has spread rapidly across the globe [1]. As no definitive treatment options are currently available for COVID-19, researchers the world over have been investigating a variety of drugs like azithromycin, hydroxychloroquine, remdesivir, tociluzimab, anticoagulants and dexamethasone [[2], [3], [4], [5], [6]]. Some of these are repurposed drugs and have been approved by regulators of various countries to be used as “Emergency Use Approval” (EUA) or “off label” medication [7].
Convalescent plasma (CP) has been used as a passive source of antibodies against various bacterial (tetanus and diphtheria), viral diseases (poliomyelitis, measles, mumps) [8] and influenza A H1N1 [9]. CP was also considered in earlier pandemics of Spanish flu, West Nile Virus, Middle East Respiratory Syndrome (MERS), Severe Acute Respiratory Syndrome (SARS), and more recently Ebola virus [[10], [11], [12]]. The convalescent plasma therapy (CPT) for COVID-19 has also been recently approved by US FDA and Indian Central Drugs Standard Control Organization [13]. It has been approved by the Ministry of Health and Family Welfare (MoHFW), Govt. of India, as “off label” use in patients with moderate and severe COVID-19 who are not improving and have increasing oxygen requirement despite use of steroids [7].
Evidence suggests that CP contains receptor binding domain specific antibodies which have potent antiviral activity [14,15]. Use of convalescent plasma, is known to be well-tolerated with only a few easily managed adverse effects [16].
There have been, till date, few larger randomized controlled trials (RCT) [17,18], some retrospective observational studies [16,19,20] and many small case reports [[21], [22], [23]] on the benefits of CPT in COVID-19 patients with conflicting results. There is still not much clarity whether CPT offers mortality benefit and, if yes, in which category of COVID-19 patients. The present study was designed to answer some of these questions.
2. Patients and methods
2.1. Study design
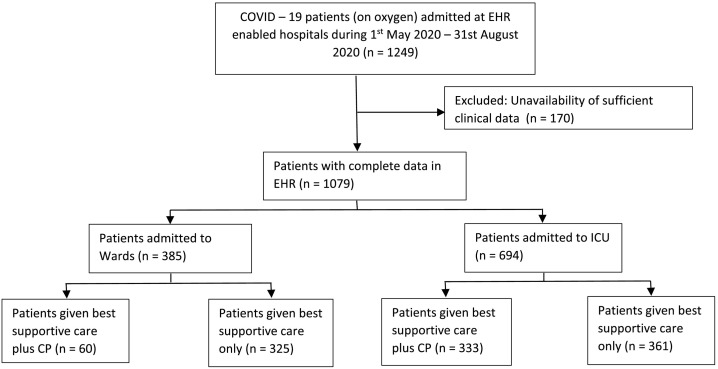
This is a multicentre, retrospective, observational case control study. COVID-19 patients admitted at various tertiary care teaching hospitals in Delhi of the same network, with electronic heath records (EHR), were included. Clinical and laboratory data was retrieved from EHR. Patients admitted from 01 May 2020 to 31 Aug 2020, with moderate and severe and/or life threatening COVID-19, who required oxygen therapy, were included. Of a total of 1249 such patients admitted during this period, 170 patients were excluded due to unavailability of sufficient clinical data. Remaining 1079 patients were included in the final analysis. All patients in either arm received various treatments as per the attending physician's discretion, institutional protocol, and/or national guidelines for the management of COVID-19 issued by the government from time to time. A variety of medicines were used in these patients and included hydroxychloroquine (HCQ), azithromycin, doxycycline, ivermectin, remdesivir, anticoagulants, other broad spectrum antibiotics, steroids (methylprednisolone or dexamethasone), tocilizumab and oxygen support/ventilation as required. Prior to introduction of remdesivir in India, HCQ was routinely being used. After availability of remdesivir in India and its emergency use approval (EUA) on 13th June 2020, it is being used in moderate to severe COVID-19 patients [7]. Routine use of steroids, in moderate to severe COVID-19, was made a part of standard treatment guidelines by Ministry of Health and Family Welfare version 3 (dated 13th June 2020) [7]. Use of these treatment modalities remained same across both the groups (henceforth called “Best Supportive Care Only Group” and “Plasma with Best Supportive Care Group”). Fig. 1 shows the CONSORT diagram. All patients were followed for 28 days after discharge by tele-calling. The detailed analysis was performed on 694 patients admitted to ICU – 333 of these received plasma in addition to the best supportive care and the other 361 were on the best supportive care only.
Fig. 1.
CONSORT chart for the study.
Majority of COVID-19 patients who were given CP came from one major hospital complex, which contributed to 239 out of 333 (71.8%) patients. The physicians in this hospital started using CP from early days of COVID-19 outbreak in India and, because of this, much higher use was observed in this hospital complex. Other hospitals, where either the usage of CP or availability of CP was low, contributed 28.2% of plasma cases and a greater number to the control arm of Best Supportive Care only. Otherwise, the standard of care in all hospitals, across the network remained uniform.
2.2. Inclusion criteria
Eligible patients included adults (>18 years) with evidence of SARS-COV-2 infection confirmed by RT-PCR test of nasopharyngeal and oropharyngeal swab. Criteria for classifying patients into moderate and severe category were as per the clinical management protocols of MoHFW [7].
Moderate disease: pneumonia with no signs of severe disease, and having one or more:
-
-
Dyspnoea, fever, and cough
-
-
SPO2 < 94% on room air
-
-
Respiratory rate > 24/min
Severe disease: patient having one or more of the following conditions:
-
-
Clinical signs of pneumonia plus one of the following: respiratory rate > 30/min or severe respiratory distress requiring ventilation or SPO2 < 90% on room air.
-
-
ARDS (new onset bilateral opacities & SpO2/FiO2 ≤ 315)
-
-
Sepsis
-
-
Septic shock
Patients meeting these criteria and requiring oxygen therapy were included in the study.
2.3. Donor selection criteria [24]
Donors had to meet the following criteria: 1) prior diagnosis of COVID-19 documented by a laboratory test; 2a) complete resolution of symptoms at least 28 days prior to donation or 2b) complete resolution of symptoms at least 14 days prior to donation. In such cases, if the patient was discharged without negative RT-PCR for COVID-19, 2 samples of naso- and oropharyngeal swab collected 24 h apart were sent for RT-PCR test for COVID-19, and negative result ascertained before considering the recovered patients as a potential donor. 3) Only males and nulliparous female donors of weight > 55 kg, 4) Donor with Hb > 12.5 g/dL and platelet count >150,000 per microliter of blood and TLC within normal limits, 5) HIV, HBV and HCV by serology and NAT negative patients, 6) syphilis and malaria negative donors and 7) total serum protein >6 g/dL all donor selection criteria for blood donation were followed as per Drugs and Cosmetics (second Amendment) Rules, 2020 [25].
In the absence of a standard viral neutralization test, and limited possibility of assessment of neutralizing antibody titres of >1:640, we adopted a policy of the use of an assay to assess quantitative antibodies of anti-SARS COV2 antibodies prior to harvest of CP from the eligible donors. Antibody testing was done prospectively in donors from mid-June onwards, prior to which donor samples were stored at −80 °C and were retrospectively tested. In our larger centre, the available platform which we used was an FDA approved (DiaSorin), Quantitative IgG antibody assay against the S1&S2 protein of SARS COVID-2 virus. The cut-off of 15 AU/ml was taken for a positive result which is equivalent to 1:40 Plaque Neutralization antibody & 80 AU/ml is equivalent to 1:160 plaque neutralizing antibody assay. In the other two smaller centres, Ortho Clinical Diagnostic Vitros Anti SARS-COV-2 IgG Chemiluminescent Immuno Assay was used. The Ortho Clinical IgG is a qualitative assay. Result of this assay are based on the sample signal-to-cut-off (S/CO) ratio, with values <1.0 and ≥1.0 corresponding the Negative and Positive results.
For the purpose of the present study, the cut-off for low titre and high titre IgG neutralizing antibodies (NAb) positivity, was 15–80 AU/ml and >80 AU/ml for the DiaSorin kit and 5–13 S/CO and >13 S/CO, respectively, for the Ortho Vitros kit.
The volume of donated plasma was 400 ml. The first 200 ml (one unit) plasma was infused over one to one and half hours and if the patient did not show any improvement after 24 h, based on the decision of the responsible physician, another unit of plasma was administered.
2.4. Exclusion criteria
Patients not requiring oxygen therapy were excluded. Pregnant and lactating mothers were also excluded.
2.5. Approvals
This retrospective observational study was approved by the Institutional Ethics Committee (IEC) vide ref. no. RS/MSSH/GMHR CMS/IEC/20-16, dated 3rd Nov, 2020.
A proper informed consent for various treatments, including convalescent plasma therapy, was taken from every patient.
2.6. Objectives
Primary objective of the study was to study 28-day mortality in patients who received convalescent plasma therapy versus those who did not.
Secondary objectives were to assess if CP reduced the chances of patients going on to invasive ventilation and whether it offered any mortality benefits in critically ill patients on ventilator and vasopressors.
2.7. Statistical analysis
Baseline characteristics such as age, gender, and comorbidities in the plasma and no plasma group were compared by chi-square test. The number of patients on invasive and non-invasive ventilators and mortality in plasma and no plasma group in various subgroup of patients were also compared with chi-square test. p-Value <0.05 was considered statistically significant. In the case of multiple comparisons, the level of significance was 0.05/6 = 0.008 as per Bonferroni correction since there are no more than 6 multiple comparisons. SPSS21 was used for statistical calculations. The sample size in the two groups was adequate to detect a difference of at least 10% in overall mortality between plasma and no plasma group with power of 80% when the mortality in ICU cases in the standard care group is 25%.
3. Results
The mean (SD) age of patients was 60.1 (12.1) years vs 58.9 (13.8) years in plasma vs no plasma group (p = 0.231). However, the age-gender distribution and the comorbidity-gender distribution were different for the following subgroups (Table 1 ).
Table 1.
Baseline comparison of cases in the Best Supportive Care Only Group and Plasma with Best Supportive Care Group.
| Characteristic | Best Supportive Care Only |
Plasma with Best Supportive Care |
p-Value | ||
|---|---|---|---|---|---|
| Count | Percent | Count | Percent | ||
| All in ICU | 361 | 52.6% | 333 | 84.7% | <0.001 |
| <45 | 62 | 17.2% | 40 | 12.0% | 0.055 |
| 45–59 | 115 | 31.9% | 122 | 36.6% | 0.185 |
| 60–74 | 149 | 41.3% | 135 | 40.5% | 0.844 |
| 75+ | 35 | 9.7% | 36 | 10.8% | 0.628 |
| Males | 261 | 72.3% | 267 | 80.2% | 0.015 |
| <45 | 50 | 19.2% | 32 | 12.3% | 0.023 |
| 45–59 | 80 | 30.7% | 96 | 36.8% | 0.196 |
| 60–74 | 106 | 40.6% | 109 | 41.8% | 0.961 |
| 75+ | 25 | 9.6% | 30 | 11.5% | 0.533 |
| Females | 100 | 27.7% | 66 | 19.8% | Same as for males |
| <45 | 12 | 12.0% | 8 | 12.1% | 0.981 |
| 45–59 | 35 | 35.0% | 26 | 39.4% | 0.566 |
| 60–74 | 43 | 43.0% | 26 | 39.4% | 0.645 |
| 75+ | 10 | 10.0% | 6 | 9.1% | 0.846 |
| No: of comorbidities | 361 | 52.6% | 333 | 84.7% | <0.001 |
| None | 92 | 25.5% | 72 | 21.6% | 0.231 |
| 1 | 181 | 50.1% | 166 | 49.8% | 0.939 |
| 2 | 61 | 16.9% | 74 | 22.2% | 0.077 |
| 3+ | 27 | 7.5% | 21 | 6.3% | 0.543 |
| Males | 261 | 72.3% | 267 | 80.2% | 0.015 |
| None | 72 | 27.6% | 63 | 23.6% | 0.293 |
| 1 | 128 | 49.0% | 136 | 50.9% | 0.663 |
| 2 | 45 | 17.2% | 53 | 19.9% | 0.441 |
| 3+ | 16 | 6.1% | 15 | 5.6% | 0.802 |
| Females | 100 | 27.7% | 66 | 19.8% | Same as for males |
| None | 20 | 20.0% | 9 | 13.6% | 0.291 |
| 1 | 53 | 53.0% | 30 | 45.5% | 0.341 |
| 2 | 16 | 16.0% | 21 | 31.8% | 0.017 |
| 3+ | 11 | 11.0% | 6 | 9.1% | 0.691 |
| Specific comorbidity M + F | |||||
| HTN | 157 | 43.5% | 182 | 54.7% | 0.003 |
| DM | 66 | 18.3% | 61 | 18.3% | 0.990 |
| CAD | 51 | 14.1% | 25 | 7.5% | 0.005 |
| Hypothyroidism | 33 | 9.1% | 42 | 12.6% | 0.141 |
HTN: Hypertension; DM: Diabetes mellitus; CAD: Coronary artery disease.
Males were significantly higher in the plasma group (80.2% vs 72.3%) (p = 0.015) and less of younger (<45 years) age (12.3% vs 19.2%), (p = 0.023). There were more females in the plasma group with multiple comorbidities, than in the no plasma group. Specific comorbidities examined were hypertension (HTN), diabetes mellitus (DM), coronary artery disease (CAD), and hypothyroidism. These were the most commonly occurring comorbidities in these cases. All other comorbidities (CKD, cancer, stroke, etc.) were reported in less than 10% cases. Hypertension was more common in plasma group (54.7% vs 43.5%, p = 0.003) and CAD less common (7.5% vs 14.1%, p = 0.005). However, as mentioned later, the difference in mortality in these subgroups was not statistically significant.
The percentage of patients on ventilator, invasive and non-invasive, and on vasopressor were not significantly different (minimum p = 0.166) between the two groups (Table 2 ).
Table 2.
Need for ventilation and/or vasopressors in Best Supportive Care Only Group and Plasma with Best Supportive Care Group.
| Characteristic | Best Supportive Care Only (n = 361) |
Plasma with Best Supportive Care (n = 333) |
p-Value | ||
|---|---|---|---|---|---|
| Count | Percent | Count | Percent | ||
| No ventilator | 132 | 36.6% | 115 | 34.5% | 0.577 |
| Any ventilator | 229 | 63.4% | 218 | 65.5% | |
| Non-invasive (NIV) | 159 | 69.4% | 157 | 72.0% | 0.412 |
| Invasive (IV) | 70 | 30.6% | 61 | 28.0% | 0.548 |
| Vasopressor in IV group | 36 | 51.4% | 24 | 39.3% | 0.166 |
| NIV to IV (out of IV group) | 47 | 20.5% | 54 | 24.8% | 0.283 |
| Vasopressor in NIV to IV group | 23 | 48.9% | 23 | 42.6% | 0.523 |
For the total of 1079 patients on oxygen, overall mortality in patients was 22.4% in the plasma group and 18.5% in the no plasma group, This difference was not statistically significant (p = 0.125; OR = 1.27, 95% CI: 0.94–1.72). A total of 694 (64.3%) patients required transfer to intensive care unit (ICU). Three hundred thirty-three (48.0%) patients received convalescent plasma in addition to the best supportive care and the remaining 361 (52.0%) were on best supportive care only. In the ICU patients, mortality was 25.5% vs 33.2% in plasma vs no plasma group (p = 0.026; OR = 0.69, 95% CI: 0.50–0.96). Reduced mortality in plasma group was particularly seen in patients of age 60–74 years (26.7% vs 43.0%, p = 0.004; OR = 0.48, 95% CI: 0.29–0.80), driven mostly by females of this age group (23.1% vs 53.5%, p = 0.013; OR = 0.26, 95% CI: 0.09–0.78) although not statistically significant at strict level of 0.008.
In one of the main hospitals, where the experience with the use of CP was maximum, out of a total of 342 patients in ICU, 239 (69.8%) received plasma while 103 (30.1%) did not. Overall mortality in the plasma group was 49/239 (20.5%) and significantly lower than 36/103 (34.9%) in the no plasma group (p = 0.0014).
Significant difference in mortality was observed in patients with one comorbidity (22.3% vs 36.5%, p = 0.004; OR = 0.50, 95% CI: 0.31–0.80). This also was primarily due to females in this subgroup (mortality 6.7% vs 49.1%, p < 0.001; OR = 0.07, 95% CI: 0.02–0.34) in the plasma vs no plasma group (Table 3 ). We observed a similar reduction in mortality in patients with 2 comorbidities (35.1% vs 49.2%) but this did not reach statistical significance (p = 0.099; OR = 0.56, 95% CI: 0.28–1.12). The mortality in patients with specific comorbidity such as hypertension, diabetes, and coronary artery disease were not different (least p = 0.099) but was significantly different in patients with hypothyroidism (19.0% vs 42.4%, p = 0.027; OR = 0.32, 95% CI: 0.11–0.90). Of 38 women of age 60–74 years with one comorbidity, 12 were in plasma group and 26 in the no plasma group. Only one of these (8.3%) died in the plasma group whereas 13 (50.0%) died in the no plasma group (p = 0.013).
Table 3.
Mortality in various groups in cases on Best Supportive Care Only and cases on Plasma with Best Supportive Care.
| Characteristic | Best Supportive Care Only (n = 361) |
Plasma with Best Supportive Care (n = 333) |
p-Value |
OR |
95% CI of OR |
|||
|---|---|---|---|---|---|---|---|---|
| Deathsa | Mortality | Deathsa | Mortality | Lower | Upper | |||
| All in ICU | 120 | 33.2% | 85 | 25.5% | 0.026 | 0.69 | 0.50 | 0.96 |
| <45 | 14 | 22.6% | 7 | 17.5% | 0.536 | 0.73 | 0.26 | 2.00 |
| 45–59 | 27 | 23.5% | 28 | 23.0% | 0.923 | 0.97 | 0.53 | 1.77 |
| 60–74 | 64 | 43.0% | 36 | 26.7% | 0.004 | 0.48 | 0.29 | 0.80 |
| 75+ | 15 | 42.9% | 14 | 38.9% | 0.734 | 0.85 | 0.33 | 2.19 |
| Male | 73 | 28.0% | 72 | 27.0% | 0.796 | 0.95 | 0.65 | 1.39 |
| <45 | 9 | 18.0% | 6 | 18.8% | 0.932 | 1.05 | 0.33 | 3.30 |
| 45–59 | 13 | 16.3% | 24 | 25.0% | 0.156 | 1.72 | 0.81 | 3.65 |
| 60–74 | 41 | 38.7% | 30 | 27.5% | 0.082 | 0.60 | 0.34 | 1.07 |
| 75+ | 10 | 40.0% | 12 | 40.0% | 1.000 | 1.00 | 0.34 | 2.95 |
| Female | 47 | 47.0% | 13 | 19.7% | <0.001 | 0.28 | 0.13 | 0.57 |
| <45 | 5 | 41.7% | 1 | 12.5% | 0.325 | 0.20 | 0.02 | 2.18 |
| 45–59 | 14 | 40.0% | 4 | 15.4% | 0.037 | 0.27 | 0.08 | 0.96 |
| 60–74 | 23 | 53.5% | 6 | 23.1% | 0.013 | 0.26 | 0.09 | 0.78 |
| 75+ | 5 | 50.0% | 2 | 33.3% | 0.633 | 0.50 | 0.06 | 4.09 |
| No: of comorbidities | ||||||||
| None | 16 | 17.4% | 14 | 19.4% | 0.736 | 1.15 | 0.52 | 2.54 |
| 1 | 66 | 36.5% | 37 | 22.3% | 0.004 | 0.50 | 0.31 | 0.80 |
| 2 | 30 | 49.2% | 26 | 35.1% | 0.099 | 0.56 | 0.28 | 1.12 |
| 3+ | 8 | 29.6% | 8 | 38.1% | 0.537 | 1.46 | 0.44 | 4.89 |
| Males | 73 | 28.0% | 72 | 27.0% | 0.796 | 0.95 | 0.65 | 1.39 |
| None | 8 | 11.1% | 12 | 19.0% | 0.195 | 1.88 | 0.72 | 4.95 |
| 1 | 40 | 31.3% | 35 | 25.7% | 0.321 | 0.76 | 0.45 | 1.30 |
| 2 | 19 | 42.2% | 20 | 37.7% | 0.651 | 0.83 | 0.37 | 1.87 |
| 3+ | 6 | 37.5% | 5 | 33.3% | 0.809 | 0.83 | 0.19 | 3.64 |
| Females | 47 | 47.0% | 13 | 19.7% | <0.001 | 0.28 | 0.13 | 0.57 |
| None | 8 | 40.0% | 2 | 22.2% | 0.351 | 0.43 | 0.07 | 2.61 |
| 1 | 26 | 49.1% | 2 | 6.7% | <0.001 | 0.07 | 0.02 | 0.34 |
| 2 | 11 | 68.8% | 6 | 28.6% | 0.015 | 0.18 | 0.04 | 0.75 |
| 3+ | 2 | 18.2% | 3 | 50.0% | 0.169 | 4.50 | 0.49 | 41.25 |
| Specific comorbidity M + F | ||||||||
| HTN | 59 | 37.6% | 53 | 29.1% | 0.099 | 0.68 | 0.43 | 1.08 |
| DM | 17 | 25.8% | 18 | 29.5% | 0.636 | 1.21 | 0.55 | 2.63 |
| CAD | 24 | 47.1% | 11 | 44.0% | 0.802 | 0.88 | 0.34 | 2.31 |
| Hypothyroidism | 14 | 42.4% | 8 | 19.0% | 0.027 | 0.32 | 0.11 | 0.90 |
HTN: Hypertension; DM: Diabetes mellitus; CAD: Coronary artery disease.
Deaths out of cases mentioned in Table 1 for the respective subgroups.
Out of a total of 694 patients with severe COVID-19 in ICU, 101 (14.6%) patients worsened on non-invasive ventilation (NIV) and needed to be shifted to invasive ventilator. Fifty-four (53.46%) of these patients were given CP. The other 316 (45.5%) patients remained on NIV only and 157 (49.6%) of these received CP. Overall, CP did not affect the chances of being put on invasive ventilator from NIV (p = 0.508).
Patients on ventilator had significantly lower mortality in plasma arm (37.2% vs 49.3%, p = 0.009; OR = 0.61, 95% CI: 0.42–0.89) (Table 4 ). This was particularly so for patients on invasive ventilator (IV) with mortality 63.9% vs 82.9% (p = 0.014; OR = 0.37, 95% CI: 0.16–0.83), respectively, in plasma vs no plasma group. Patients on an invasive ventilator and on vasopressor had high (almost 90%) mortality in both the groups (Table 4).
Table 4.
Comparative Mortality of patients on ventilation & vasopressor between Best Supportive Care Only Group & Plasma with Best Supportive Care Group.
| Characteristic | Best Supportive Care Only (n = 361) |
Plasma with Best Supportive Care (n = 333) |
p-Value | OR | 95% CI of OR |
|||
|---|---|---|---|---|---|---|---|---|
| Deathsa | Mortality | Deathsa | Mortality | Lower | Upper | |||
| No ventilator | 7 | 5.3% | 4 | 3.5% | 0.488 | 0.64 | 0.18 | 2.26 |
| Any ventilator | 113 | 49.3% | 81 | 37.2% | 0.009 | 0.61 | 0.42 | 0.89 |
| Non-invasive (NIV) | 55 | 34.6% | 42 | 26.8% | 0.131 | 0.69 | 0.43 | 1.12 |
| Invasive (IV) | 58 | 82.9% | 39 | 63.9% | 0.014 | 0.37 | 0.16 | 0.83 |
| Vasopressor with invasive ventilation (IV) | 33 | 91.7% | 21 | 87.5% | 0.675 | 0.64 | 0.12 | 3.45 |
Deaths out of cases mentioned in Table 2 in the respective rows.
Twenty-six out of 333 (7.8%) received second unit of CP, as per the physician's decision. Ten of these patients (38%) died.
3.1. IgG NAb titre of CP
At our larger hospital complex, out of the 239 ICU patients who were given CPT, 176 (73.6%) patients received CP wherein IgG Nab were tested, using DiaSorin kit. As this kit became available only from mid-June 2020, all CP transfusions, from that time onwards, were carried out only after checking for IgG Nab titres. Some of the plasma samples from the CP donations, prior to this period (May till mid-June 2020) were stored at −80 °C and retrospectively analyzed for IgG Nab titres. Out of 80 such patients, it was found that 6 patients had received CP with antibody titre <15 AU/ml. AT the other 2 smaller hospitals, out of 94 ICU patients who got CP, 76 (80.8%) patients received CP wherein IgG Nab were tested using Ortho Vitros kit. Overall, we could not check IgG NAb in 81 out of 333 (24.3%) of our patients in ICU who received CP. After mid-June 2020, CP with only adequate IgG NAb titre (>15 AU/ml by DiaSorin kit or >5 S/CO by Ortho Vitros kit) was accepted for transfusion.
Out of 158 patients who received low-titre NAb CP, 40 (25.3%) patients died. Out of 82 patients who received high-titre NAb CP, 25 (30%) died. This difference was not statistically significant (p = 0.393). In the group of 81 patients, where the data regarding NAb titre was not available, 21 patients (25.9%) died. Out of the 6 patients, who were retrospectively found to have been transfused CP with very low titres of NAb, one (16.7%) patient died.
3.2. Safety of CPT
Convalescent plasma infusion was largely safe and no major adverse effects were observed in any of our patients. Two patients with minor allergic reactions (rashes) were reported to the Blood Bank Officer.
4. Discussion
The current study was undertaken to evaluate the effectiveness of CP in patients with moderate and severe COVID-19 disease. To date, this is the largest retrospective case control study from India, presently reporting world's second largest number of COVID-19 cases.
There are a number of reports comprising small number of patients treated with CP [[21], [22], [23]] from different countries across the world. Duan et al. were first to report benefit of CP in a small cohort of 10 patients with improvement in all 10 patients and undetectable viral load in 7/10 patients [21]. In contrast, Zeng et al. reported mortality in 5/6 patients who received CP [26]. Table 5 summarizes the literature review of relevant studies on CP, with more than 100 patients and/or RCTs.
Table 5.
Review of various studies (larger RCTs and/or >100 matched cases) on effectiveness of Convalescent Plasma in COVID-19.
| Ref | Number of patients | Study | Patient population | Antibody titre used | Results | Adverse effects | Author conclusions | Our comment |
|---|---|---|---|---|---|---|---|---|
| Ling Li et al. [18] | 103 | Open label RCT, multicentre | 23 vs 22 Severe 29 vs 29 Life-threatening COVID-19 |
1:640 | No significant difference in time to clinical improvement or in 28 days mortality between patients who received CP versus control | Chills and rashes −1 Shortness of breath, cyanosis, and severe dyspnea −1 |
CP not effective | Not powered to predict the difference |
| Abolghasemi et al. [28] | 189 115 in plasma group and 74 in control group |
Multicentre nonrandomized clinical study | Moderate severity | Antibody titre cut off >1.1 using semi quantitative ELISA and Rapid strip test | CP reduced all cause mortality in treatment group compared with control group (14.8% vs 24.3%); however this was not statistically significant (p = 0.09). it had a significant impact of length of stay (p = 0.002) and need for intubation (p = 0.006) | CP effective | Control group was much smaller and had milder cases | |
| Salazar E et al. [30] | 136 cases and 251 controls | Prospective propensity matched study | Severe and/or life threatening COVID-19 | Anti-RBD IgG titre of 1:1350 | Significant reduction (p = 0.047) in mortality within 28 days, specifically in patients transfused within 72 h of admission with CP with anti-RBD IgG titre of >1:1350 | Transfusion of high anti-RBD IgG titre CP early in hospitalisation reduces mortality | ||
| Xia et al. [20] | 138 cases and 1568 controls | Retrospective observational study | Severe or critical COVID-19 | Mortality in CP group was 2.2% as compared to 4.1% in non CP group also from CP group, only 2.4% got shifted to ICU whereas in non CP group, 5.1% patients got ICU transfer | 3 patients had minor allergic reactions (pruritus or erythema) during transfusion | Patients with SCSS >5 before therapy showed no improvement after CP. | Authors classified patients who were given CP into responders, partial responders and nonresponders. | |
| Altuntas et al. [29] | Cases 888 Control 888 |
Retrospective case control study | Severe and critically ill COVID-19 | IgG antibody not routinely done | Duration in ICU, rate of mechanical ventilation support and vasopressor support were lower in CP group (p = 0.001, p = 0.02, p = 0.001, respectively). CFR was 24.7 in CP group and 27.7 in control group (p = 0.15) | Not available | Antibody titres of plasma donors were not available | |
| Agarwal A et al. & PLACID trial collaborators [17] | Cases: 235 Controls: 229 |
Open label parallel are phase II Multicentre randomized controlled trial |
Moderately ill COVID-19 patients | Nearly 2/3rd of donor had NAb titre >1:20 with a median titre of 1:40 | Mortality in intervention arm was 14.5% vs 13.5% in control arm (odd ratios was 1.06). There was no difference in the 2 arms with respect to duration of respiratory support, proportion of patient requiring invasive ventilation and vasopressor support | Minor adverse event of pain, chills, nausea in one patient; fever and tachycardia in 3 patients; dyspnoea and IV catheter blockage in 2 patients each. Mortality was assessed as possibly related to CP in 3 patients (1.3%). | CP not effective | Three reported cases of mortality related to CP raised a significant concern regarding safety profile of CP. |
| Our study | 1079 (total cases in ward and ICU) In ICU: 694 Plasma group in ICU:333 No plasma group in ICU: 361 | Retrospective case control observational study | Moderate and Severe COVID-19 | In one large hospital, Quantitative IgG antibody assay with cut off 15 AU/ml using Diaosorin kit. In other smaller hospitals, qualitative assay using Ortho Vitros kit with cut-off of >5 S/CO. | Overall no statistically significant difference in mortality between CP and non CP group (22.4% vs 18.5%; p = 0.125). However, significantly reduced mortality in COVID-19 patients admitted in ICU (25.5% vs 33.2%; p = 0.026) especially in age group 60–74 (26.7% vs 43%; p = 0.004), females (23.1% vs 53.5%; p = 0.013), those with one comorbid medical condition (22.3% vs 36.5%; p = 0.004) and on invasive mechanical ventilation (63.9% vs 82.9%; p = 0.014) | 2 patients had mild allergic reaction (rashes) during plasma transfusion | Highest mortality benefit with CP seen in COVID-19 patients above 60 years of age, admitted in ICU, especially females and those on ventilator and having medical comorbid conditions. |
The results of our study are quite different from that of the only other large-scale study from India - a multicentre prospective RCT PLACID trial, which included 464 patients of moderate severity COVID-19, from 39 different hospitals across India [17]. This study did not find any difference in mortality between plasma vs no plasma group (14.5% vs 13.5%; OR = 1.06, 95% CI: 0.61–1.83). However, this study did not include very severe or life threatening COVID-19 patients. Even in our study, if we see the overall cohort of 1079 patients (moderate and severe), CP did not have any mortality benefit (22.4% vs 18.5%; p = 0.125; OR = 1.27, 95% CI: 0.94–1.72). The benefit emerged only in more critically ill patients of COVID-19 who were in ICU (25.5% vs 33.2%; p = 0.026; OR = 0.69, 95% CI: 0.50–0.96). This happened despite plasma group had more elderly males, where anyway the mortality is expected to be higher [27]. Also, the main hospital, which had maximum experience with the use of CP, showed a highly significant mortality benefit in severe COVID-19 (20.5% vs 34.9%; p = 0.0014; OR = 0.48, 95% CI: 0.29–0.80).
A similar study involving moderately ill COVID-19 patients by Abolghasemi et al., showed that significantly higher number of patients who received CP were discharged from the hospital within 5 days versus control (28.1% vs 8.9%; p = 0.010) [28]. They reported that CP significantly reduced need for mechanical ventilation in treatment versus control group (7% vs 20.3%). All-cause mortality in CP group was 14.8% versus 24.3% in control arm in the study but this was not statistically significant (p = 0.09). RCT by Ling Li et al. also showed no significant difference in time to clinical improvement (28 days vs 18 days; p = 0.26; HR = 1.4, 95% CI: 0.79–2.49) or in 28-day mortality (15.7% vs 24%; p = 0.3; OR = 0.59, 95% CI: 0.22–1.59) between patients who received CP vs control [18].
Study by Xia et al. also showed that there was less likelihood of patient who received CP to be transferred to ICU as compared to those who did not receive CP (2.4% vs 5.1%); however, this was not statistically significant (p = 0.2) [20]. This study did not show any significant difference between need for NIV (20.3% vs 16.7%) or invasive mechanical ventilation (1.4% vs 0.2%) between those who received CP and those who did not. Mortality rate in the group that received CP was 2.2% versus 4.1% in the standard treatment arm. Our study also did not show any benefit with CP in terms of reducing the chances of patients going onto invasive ventilation from NIV. The Indian PLACID trial also showed no benefit of CP in terms of total and post enrolment duration of respiratory support, and proportion of patients who had to be put on invasive ventilation and those needing vasopressor support [17]. In the study by Altuntas et al., the duration of stay in ICU (9 days vs 12 days; p = 0.001), rate of mechanical ventilation (49.3% vs 55%; p = 0.02) and vasopressor requirement (24.7% vs 34.3%; p = 0.001) were lower in the CP group versus control respectively [29]. They however, found no statistically significant difference in the fatality rate between the CP and control groups (24.7% vs 27.7%, p = 0.150). In our study, however patients on ventilator had significantly lower mortality in plasma arm as compared to no plasma arm (37.2% vs 49.3%, p = 0.009; OR = 0.61, 95% CI: 0.42–0.89). This was particularly so for patients on invasive ventilator with mortality 63.9% and 82.9% (p = 0.014; OR = 0.37, 95% CI: 0.16–0.83) in plasma and no plasma arm, respectively. Joyner et al. reported overall 7-day and 30-days mortality of 17.6% and 41% among adult COVID-19 patients on mechanical ventilation, who received CP [30].
Patient outcome in our study was quite similar to the result reported by Salazar et al. where they found significant mortality reduction in severe and life threatening COVID-19 patients (2.7% vs 8.9%; p = 0.04; PE = 3.64, 95% CI: 1.05–12.62) especially when CP was given within 72 h of admission [31].
In our study, we found maximum benefit of CP in ICU patients, in the age group 60–74 years, where the mortality in the plasma group was 26.7% versus 43% in the no plasma group (p = 0.004; OR = 0.50, 95% CI: 0.31–0.80). Similar findings were reported by Rogers et al. wherein a subgroup analysis of patients above 65-years-old or greater who received CP demonstrated a significantly increased hospital discharge rate among these patients (RR = 1.86, 95% CI: 1.03–3.36); this increased rate of hospital discharge was even more pronounced in a subgroup analysis of elderly patients who received 2 units of CP (RR = 2.70, 95% CI: 1.16–6.28) [32]. This study, however, showed no significant difference in overall in-hospital mortality (12.5% vs 15.8%; p = 0.52; HR = 0.93, 95% CI: 0.39–2.20) or time to hospital discharge (Rate Ratio = 1.28, 95% CI: 0.91–1.81) as compared to a control group who did not receive CP. The authors of this study attributed the beneficial effect of CP in elderly to the waning humoral immunity with age, emphasizing the importance played by humoral immunity in combating this infection.
In a study by Joyner et al. on adult COVID-19 patients who received CP, 7-day and 30-day mortality was found the lowest in the younger people (18–59 years) (7-day mortality 3.1% in 18–39 years and 5.4% in 40–59 years and 30-days mortality 7.5% in 18–39 years and 15.1% in 40–59 years) as compared to older people (60–79 years) (7-days mortality 10% in 60–69 years and 15.3% in 70–79 years and 30-days mortality 27.1% in 60–69 years and 35.3% in 70–79 years) [30]. The mortality is expected to be lower in the younger population anyways. This study had no control arm.
Significant difference in mortality between plasma and no plasma group was also observed in patients in our study with one comorbidity, largely driven by females. This finding has not been reported in literature so far. The mortality in patients with specific comorbidity such as HTN, DM and CAD was not different (minimum p = 0.099; OR = 0.68; 95% CI: 0.43–1.08) but was significantly different in patients with hypothyroidism (19% vs 42.4%, p = 0.027; OR = 0.32, 95% CI: 0.11–0.90). This needs further investigation.
The impact of IgG antibody levels in the transfused plasma on the mortality was analyzed by Joyner et al. [30] This was an open label, Expanded Access Program (EAP) for the treatment of COVID-19 patients with human convalescent plasma. 35,322 transfused patients, across 2807 acute care facilities in the USA, were included. It was observed that patients who received high IgG plasma (>18.45 S/Co), 7-day mortality was 8.9% (95% CI: 6.8%–11.7%); for recipients of medium IgG plasma (4.62–18.45 S/Co) the mortality was 11.6% (95% CI: 10.3%–13.1%); and for recipients of low IgG plasma (<4.62 S/Co) the mortality as 13.7% (95% CI: 11.1% 16.8%) (p = 0.048). The unadjusted dose-response relationship with IgG was significant in 30-day mortality (p = 0.021). These findings were in contrast to those found in the Indian PLACID study, where the primary outcome (all-cause mortality at 28 days or progression to severe disease) did not differ between the subgroups of participants in the intervention arm who received convalescent plasma with detectable neutralizing antibody titres (n = 160) or convalescent plasma with neutralizing antibody titres of 1:80 or higher (n = 67) or convalescent plasma with no detectable neutralizing antibodies (n = 64) and the control arm. In the present study, we used FDA approved (DiaSorin), Quantitative IgG antibody assay against the S1&S2 protein of SARS COVID-2 virus in our larger centre, which contributed to almost 72% of the CP recipients. In the other two smaller centres, Ortho Clinical Diagnostic Vitros Anti SARS-COV-2 IgG Chemiluminescent Immuno Assay was used. As per Luchsinger et al. Ortho anti-SARS-Cov-2 total Ig and IgG high-throughput serological assays and Abbott SARS-CoV-2 IgG assay quantify levels of antibodies that strongly correlate with the results of Nab assay and are consistent with gold standard ELISA results [33]. In our study also, we did not find any significant difference in mortality with the level of IgG NAb in CP. The mortality in low-titre IgG NAb CP group was 25.3% versus 30% in the high-titre group (p = 0.393).
Klassen et al. aggregated patient outcome data from various RCTs, matched-control, case series and case report studies and found that CP exhibited a 51% reduction in mortality rate compared to patients receiving standard treatments (19% vs. 29% mortality; OR:0.49, 95% CI: 0.37, 0.64, p < 0.001) [34].
Our study indicates mortality benefit of CP in severely ill COVID-19 patients requiring some form of ventilation and in the age group >60 years, especially women with 1–2 medical comorbidities. We do not know the exact mechanism of action of CP in COVID-19, it seems that plasma therapy may have a role in reduction of mortality in the highly vulnerable patient population of elderly and on ventilator. Perhaps a little push by convalescent plasma in this segment of patients was able to tilt the balance towards recovery.
5. Limitations of the study
It is a retrospective study. During the study duration, patients received several treatments such as HCQs, remdesevir, ivermectin, azithromycin, steroids (dexamethasone or methylprednisolone), tociluzimab depending on prevailing guidelines at those time points and at treating physician's discretion. Majority of patients in plasma arm were recruited at one centre while no plasma arm patients were at other hubs. We also do not have information if some of the patients had already developed adequate antibodies when CPT was administered. These limitations notwithstanding, we were able to identify a subset of patients with COVID-19, who are likely to benefit the most, from CP.
6. Conclusions
The use of convalescent plasma was associated with reduced mortality in severe COVID-19 elderly patients, above 60 years of age, particularly females, those with comorbidities and especially those who require some form of ventilation. This beneficial effect was lost when the entire cohort of patients across varying severity of illness was compared. Plasma did not seem to offer any mortality benefit in patients of moderate severity or those who were terminally ill. Further research into the mechanism of actions of CP in COVID-19 may help predict the good responders.
CRediT authorship contribution statement
SB provided overall guidance, was involved in patient care and management, data analysis and interpretation and in writing the manuscript; AD, RA, OS, DJ, YPS, AG were involved in clinical care including ICU management; SP and RR were the transfusion medicine experts and contributed in selection of plasma donor and in apheresis and maintaining records of donations and adverse events; AI was the chief bio statistician and helped in data analysis and interpretation and drafting the manuscript; VJ provided data collation oversight and drafting manuscript; RN was involved in data interpretation and writing the manuscript. All authors have seen and approved the final draft.
Acknowledgements
We wish to thank Dr. Vibha Jain, Ms. Shruti Singh, Dr. Menka Loomba, Ms. Taruna Sharma, Ms. Ratnam Shukla, Ms. Aditi Saini. Mr. Rahul Arya, Mr. Rajat Kumar Sharma, Mr. Pushpender Sharma and Ms. Kritika Chadha, for their help towards data collection and validation.
Editor: Mohandas Narla
References
- 1.World Health Organization WHO Director-General's Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020
- 2.Beigel J, Tomashek K, Dodd L et al. Remdesivir for the treatment of Covid-19—Final Report. N Eng J Med. 2020 Oct 8; NEJMoa2007764.doi:10.1056NEJMoa2007764. [DOI] [PMC free article] [PubMed]
- 3.Gautret P., Lagier J.C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 Jul;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020 Apr:ciaa478.doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed]
- 5.Villar J., Ferrando C., Domingo M., et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Resp Med. 2020 Mar 1;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 6.Guaraldi G., Meschiari M., Cozzi-Lepri A., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020 Aug;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ministry of Health & family Welfare. Clinical Management Protocol: COVID-19 version 3, dated 13/6/20 www.mohfw.gov.in/pdf/ClinicalManagementProtocolfornormalCOVID19.pdf.
- 8.Xi Yongzhi. Convalescent plasma therapy for COVID – 19: a tried-and-true old strategy? Signal Transduct Target Ther. 2020;5(1):203. doi: 10.1038/s41392-020-00310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung I.F., To KK, Lee C.K., et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 2011 Feb 15;52(4):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Griensven, Johan et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med 2016; 374(1): 33–42. [DOI] [PMC free article] [PubMed]
- 11.Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann. Intern. Med. 2006 Oct 17;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 12.Marson P., Cozza A., De Silvestro G. The true historical origin of convalescent plasma therapy. Transfus. Apher. Sci. 2020;102847 doi: 10.1016/j.transci.2020.102847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanne J.H. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;368:m1256. doi: 10.1136/bmj.m1256. [DOI] [PubMed] [Google Scholar]
- 14.Zeng Q.L., Yu Z.J., Gou J.J., et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J. Infect. Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyner M.J., Bruno K.A., Klassen S.A., et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin. Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P, on behalf of PLACID Trial Collaborators. Convalescent plasma in the management of moderate Covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 2020;371: m3939. 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed]
- 18.Li L., Zhang W., Hu Y., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19. A randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantore I., Valente P. Convalescent plasma from COVID 19 patients enhances intensive care unit survival rate. A preliminary report. Transfus. Apher. Sci. 2020;102848 doi: 10.1016/j.transci.2020.102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia X, Li K, WU L et al. Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood 2020; 136(6):755–759. [DOI] [PMC free article] [PubMed]
- 21.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen C., Wang Z., Zhao F., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartman W.R., Hess A.S., Connor J.P. Hospitalized COVID-19 patients treated with convalescent plasma in a mid-size city in the Midwest. Transl Med Commun. 2020;5(1):17. doi: 10.1186/s41231-020-00068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ICMR PLACID trial A Phase II, open label, randomized controlled trial to assess the safety and efficacy of convalescent plasma to limit COVID-19 associated complications in moderate disease; convalescent plasma in COVID-19 version 1.5 dated 11th May, 2020 (Cited 2020 July 23) https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadPublic_NoticesFiles/ICMR%20Convalescent%20plasma%20_protocol_v1.5.pdf Available from:
- 25.Final G.S.R. 166(E)_Amendment in Part X B & Part XII B pertains to Blood center and Blood components, Drugs and Cosmetics Act 1940 and Rules 1945, amended 11th March 2020 (accessed 2020 March 18, Cited 2020 July 23) https://cdsco.gov.in/opencms/opencms/en/Notifications/Gazette-Notifications available from.
- 26.Zeng F., Chen X., Deng G. Convalescent plasma for patients with COVID-19 (letter) Proc Natl Acad Scie U S A. 2020;117(23):12528. doi: 10.1073/pnas.2006961117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin J.M., Bai P., He W., et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abolghasemi H., Eshghi P., Cheraghali A.M., et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus. Apher. Sci. 2020 Jul;15:102875. doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altuntas F, Ata N, Yigenoglu TN, et al. Convalescent plasma therapy in patients with COVID-19. Transfus. Apher. Sci. doi: 10.1016/j.transci.2020.102955. [DOI] [PMC free article] [PubMed]
- 30.Joyner MJ, Senefeld JW, Klassen SA, et al, the US EAP COVID-19 Plasma Consortium. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. doi: 10.1101/2020.08.12.20169359. [DOI]
- 31.Salazar E., Christensen P.A., Graviss E.A., et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am. J. Pathol. 2020;190(11):2290–2303. doi: 10.1016/j.ajpath.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers R, Shehadeh F, Eng M, et al. Convalescent plasma for patients with severe COVID-19: a matched cohort study. doi: 10.1101/2020.08.18.20177402. [DOI] [PMC free article] [PubMed]
- 33.Luchsinger L.L., Ransegnola B.P., Jin D.K., et al. Serological assays estimate highly variable SARS-CoV-2 neutralizing antibody activity in recovered COVID-19 patients. J. Clin. Microbiol. 2020;58(12):e02005–e02020. doi: 10.1128/JCM.02005-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klassen SA, Senefeld JW, Johnson PW, et al. Evidence favouring the efficacy of convalescent plasma for COVID-19 therapy. doi: 10.1101/2020.07.29.20162917. [DOI]