Abstract
In the 1918 influenza pandemic, more than 95% of mortalities were ascribed to bacterial pneumonia. After the primary influenza infection, the innate immune system is attenuated, and the susceptibility to bacteria is increased. Subsequent bacterial pneumonia exacerbates morbidity and increases the mortality rate. Similarly, COVID-19 infection attenuates innate immunity and results in pneumonia. In addition, the current pneumococcal conjugate vaccine may have limited defense against secondary pneumococcal infection after influenza infection. Therefore, until a fully protective vaccine is available, a method of increasing immunity may be helpful. Ginseng has been shown to increase the defense against influenza in clinical trials and animal experiments, as well as the defense against pneumococcal pneumonia in animal experiments. Based on these findings, ginseng is suspected to be helpful for providing immunity against COVID-19.
Respiratory infections rank first in infectious disease mortality rate according to the WHO in 2016, with 3 million pneumonia deaths each year. In particular, at least 50 million people died of the influenza pandemic in 1918, and about 1 million people died in the worldwide pandemic in 2009 (https://www.cdc.gov/flu/pandemic-resources/). (Fig. 1)
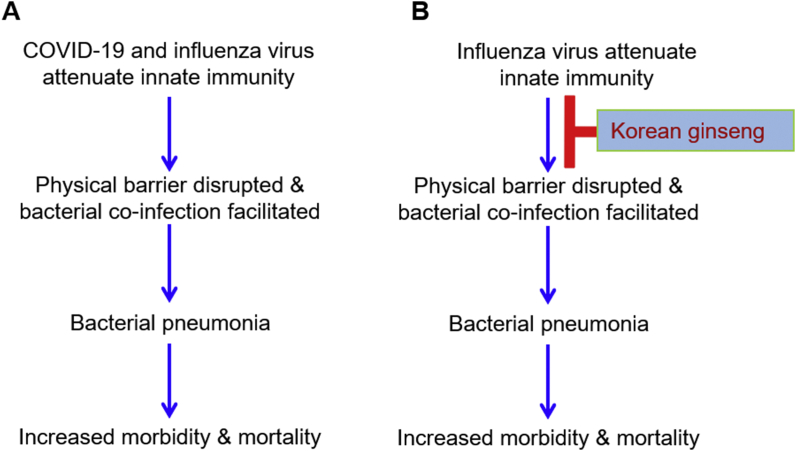
Fig. 1.
Common features of COVID-19 and influenza viruses (A) and preventive effects of ginseng on influenza virus and bacterial pneumonia (B).
Influenza virus attenuates innate immunity and subsequently increases susceptibility to secondary bacterial infection, resulting in increased morbidity and mortality. Consistently, COVID-19 attenuates innate immunity and increases inflammatory cytokine secretion, resulting in pneumonia. Korean ginseng has been shown to be helpful to prevent influenza and pneumococcal pneumonia and may be helpful for alleviating COVID-19 infection.
Annual selection of the influenza virus strains for vaccines is based on epidemiological data. Because of this, the strain may not match circulating strains, resulting in poor vaccine efficacy. Moreover, even if the selected influenza virus strain matches circulating strains, efficacy can be decreased by: 1) the highly plastic nature of the antigenic part of hemagglutinin (HA), which subsequently mutates (antigenic drift); and 2) influenza’s eight-segment genome and ability to acquire a new genome, generating a novel hybrid (antigenic shift). In addition, production of matched pandemic influenza virus vaccines takes 6 months. All of these features have made it difficult to prevent influenza effectively [1,2].
Corona virus (CoV), which comprises Severe Acute Respiratory Syndrome (SARS) and Middle Eastern Respiratory Syndrome (MERS), and influenza virus are RNA viruses. The new virus from China, SARS Corona Virus 2 (SARS-CoV-2; COVID-19), is related to bat-derived SARS-like coronaviruses. These viruses are spread by respiratory droplets from human to human or via direct contact. The most common symptoms of COVID-19 patients with pneumonia are fever and cough (https://www.who.int/health-topics/coronavirus).
Influenza and coronavirus (comprising SARS, MERS, and COVID-19) mutate easily due to selection within the host or low fidelity of RNA replication [3,4]. Moreover, current influenza virus therapeutics such as neuraminidase inhibitors (oseltamivir, peramivir, and zanamivir) increase resistance [5]. Neuraminidase inhibitors for influenza infection can attenuate symptoms and shorten duration of sickness (https://www.cdc.gov/flu). Similarly, remdesivir, which is the only therapeutic allowed for COVID-19 emergency use, can shorten the sickness period from 15 to 11 days but does not increase the survival rate significantly [6].
Mortality of influenza virus infection often is due to bacterial superinfection. Over 95% of the more than 50 million deaths during the 1918 influenza pandemic were ascribed to bacterial pneumonia. Infection with respiratory viruses disrupts lung physiology and the physical barrier, promoting simultaneous bacterial infection [7]. Mortality rate of pneumonia in Spain during 1980 - 2011 ranked highest, but that of influenza virus was lower [8].
When influenza virus infects a host, 1) the innate immune system is weakened, increasing susceptibility to bacteria in the nasopharynx (especially pneumococcus), 2) influenza virus induces lung epithelial damage and potentiates influenza virus and bacterial co-infection, and 3) influenza virus suppresses host tolerance and induces inflammation [9]. Consistently, COVID-19 limits interferon (IFN) induction and induces lower anti-viral innate immunity and imbalanced host responses enabling the virus to override the host [10]. Although COVID-19 infection induces low IFN level and subsequent moderate response of IFN-stimulated genes, permitting sustained viral replication, it induces high level of chemokines and inflammatory cytokines [10]. Thus, co-pathogenesis of virus and bacteria is determined by interactive networks between the host and infecting pathogens, resulting in physical barrier disruption, dysregulation of the immune system, and increased morbidity and mortality [7,9]. Host receptors for bacterial attachment and infection are exposed, and host receptor expression is increased. Viruses and bacteria express factors that overturn, suppress, or eliminate the host immune response. As a result, pathogens can overgrow, increasing the inflammatory response and leading to immune-mediated host damage. Bacteria are generally secondary invaders during influenza infection, but they express virulence factors that promote viral pathogenesis, increasing the amount of virus and decreasing its likelihood to be eliminated. Thus, the disease worsens, and the mortality rate increases [7,9] (Fig. 1A).
The population older than 65 years is increasing globally. Since aged persons are highly susceptible to respiratory diseases due to weakened immune system, the mortality rate of this population could be higher than ever before. The mortality rate in those older than 70 years from pneumonia showed no change from 1990 to 2017, whereas that in those younger than 5 years decreased steadily due to introduction of vaccines (https://ourworldindata.org/pneumonia). The currently used pneumococcal 13-valent conjugate vaccine may have limited defense against secondary pneumococcal infection after primary influenza infection, and this limited protection has been associated with impaired induction of anti-pneumococcal humoral response [11]. In addition, antibodies produced after COVID-19 infection contain a short-acting protective antibody that is effective for only weeks or months [12]. Guidelines of the United States Food & Drug Administration on the approval process for COVID-19 vaccines requires products to show at least 50% prevention or decrease of disease severity (https://www.fda.gov/media/139638).
COVID-19 causes a variety of diseases from respiratory diseases to enteritis. The COVID-19 spike protein binds to the receptor ACE2 (angiotensin-converting enzyme 2) [3] in human epithelial cells in the airway and lung tissue. However, ACE2 in human can be induced by not only influenza infection, but also IFN [13]. ACE2 is present in a wide variety of cells comprising not only lung, but also brain, heart, placenta, pancreas, and enterocytes, suggesting that COVID-19 can affect many organs [13]. The COVID-19 spike protein is identical to eight amino acids of the sodium channel protein in human epithelial cells, suggesting that the virus may interfere with function of this channel. This competition can disrupt or dysregulate sodium channel activation and can explain why COVID-19 patients sometimes experience surplus fluid in the lungs [14]. Therefore, until a vaccine that can fully defend against these infectious respiratory diseases is supplied, a method of increasing immunity may be helpful.
The incidence rate of common cold symptom complex (CCSC) including flu during a 2-year period in Japan demonstrated a 1.38% incidence in patients taking Korean Red Ginseng (KRG) compared to the 4.89% in patients not taking KRG, indicating preventive effects of KRG on the CCSC [15]. Consistently, in mice experiments, KRG increased the defense against influenza virus H1N1 [16] and H5N1 [17,18]. KRG protected tissue after H1N1 influenza virus infection via stimulation of anti-viral cytokine IFN-γ secretion [19]. Moreover, KRG provided protection against bacterial pneumonia-septicemia caused by pneumococcus in animal experiments by decreasing inflammatory cytokine secretion, resulting in a decrease in mortality rate [20]. KRG could enhance vaccine efficacy via increase of phagocytosis, inhibition of reactive oxygen species production, and reduction of apoptosis signaling and inflammation [21].
Taken together, these finding indicate that the pathogenesis of COVID-19 is similar to that of influenza. Lack of efficient pneumococcal vaccine during respiratory viral and bacterial co-infections suggests the need for ginseng products, which have been shown to alleviate respiratory viral and bacterial co-infections, for non-specific immune stimulation and protection as auxiliary agents.
Acknowledgements
This work was supported by a National Research Foundation of Korea grant (NRF-2018R1A2A1A05078102). The funding body played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Krammer F., García-Sastre A., Palese P. Is it possible to develop a "Universal" influenza virus vaccine? Potential target antigens and critical aspects for a universal influenza vaccine. Cold Spring Harb Perspect Biol. 2018 Jul 2;10(7):a028845. doi: 10.1101/cshperspect.a028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krammer F., Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015 Mar;14(3):167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- 3.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue K.S., Moncla L.H., Bedford T., Bloom J.D. Within-host evolution of human influenza virus. Trends Microbiol. 2018 Sep;26(9):781–793. doi: 10.1016/j.tim.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurt A.C. The epidemiology and spread of drug resistant human influenza viruses. Curr Opin Virol. 2014 Oct;8:22–29. doi: 10.1016/j.coviro.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May 16;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. 10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullers J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014 Apr;12(4):252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 8.López-Cuadrado T., Llácer A., Palmera-Suárez R., Gómez-Barroso D., Savulescu C., González-Yuste P., Fernández-Cuenca R. Trends in infectious disease mortality rates, Spain, 1980-2011. Emerg Infect Dis. 2014 May;20(5):782–789. doi: 10.3201/eid2005.131528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia L., Xie J., Zhao J., Cao D., Liang Y., Hou X., Wang L., Li Z. Mechanisms of severe mortality-associated bacterial co-infections following influenza virus infection. Front Cell Infect Microbiol. 2017 Aug 3;7:338. doi: 10.3389/fcimb.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020 May 28;181(5):1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metzger D.W., Furuya Y., Salmon S.L., Roberts S., Sun K. Limited efficacy of antibacterial vaccination against secondary serotype 3 pneumococcal pneumonia following influenza infection. J Infect Dis. 2015 Aug 1;212(3):445–452. doi: 10.1093/infdis/jiv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T. et al. Prevalence of IgG antibodies to SARS-CoV-2 in Wuhan - implications for the ability to produce long-lasting protective antibodies against SARS-CoV-2. medRxiv, doi: https://doi.org/10.1101/2020.06.13.20130252.
- 13.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020 May 28;181(5):1016–1035. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anand P., Puranik A., Aravamudan M., Venkatakrishnan A.J., Soundararajan V. SARS-CoV-2 strategically mimics proteolytic activation of human ENaC. Elife. 2020 May 26;9 doi: 10.7554/eLife.58603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko H., Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004 Jun;95(2):158–162. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- 16.Yoo D.G., Kim M.C., Park M.K., Song J.M., Quan F.S., Park K.M., Cho Y.K., Kang S.M. Protective effect of Korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J Med Food. 2012 Oct;15(10):855–862. doi: 10.1089/jmf.2012.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Jung Y.J., Kim K.H., Kwon Y., Kim Y.J., Zhang Z., Kang H.S., Wang B.Z., Quan F.S., Kang S.M. Antiviral activity of fermented ginseng extracts against a broad range of influenza viruses. Viruses. 2018 Sep 1;10(9):471. doi: 10.3390/v10090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park E.H., Yum J., Ku K.B., Kim H.M., Kang Y.M., Kim J.C., Kim J.A., Kang Y.K., Seo S.H. Red Ginseng-containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic H5N1 influenza virus. J Ginseng Res. 2014 Jan;38(1):40–46. doi: 10.1016/j.jgr.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J.S., Hwang H.S., Ko E.J., Lee Y.N., Kwon Y.M., Kim M.C., Kang S.M. Immunomodulatory activity of red ginseng against influenza A virus infection. Nutrients. 2014 Jan 27;6(2):517–529. doi: 10.3390/nu6020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen C.T., Luong T.T., Lee S.Y., Kim G.L., Kwon H., Lee H.G., Park C.K., Rhee D.K. Panax ginseng aqueous extract prevents pneumococcal sepsis in vivo by potentiating cell survival and diminishing inflammation. Phytomedicine. 2015 Oct 15;22(11):1055–1061. doi: 10.1016/j.phymed.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.O., Lee S., Kim S.J., Rhee D.K. Korean red ginseng enhances pneumococcal Δpep27 vaccine efficacy by inhibiting reactive oxygen species production. J Ginseng Res. 2019 Apr;43(2):218–225. doi: 10.1016/j.jgr.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]