Abstract
Background
Most SARS-CoV-2 infected patients develop IgG antibodies within 2–3 weeks after symptom onset. Antibody levels have been shown to gradually decrease in the first months after infection, but few data are available at six months or later.
Methods
A retrospective multi-center study was performed using 652 samples of 236 PCR-confirmed SARS-CoV-2 infected patients from 2 Belgian University hospitals. Patients were included if at least two samples were available (range 2–7 samples); including at least one sample collected 30 days or later after first positive PCR (range 0–240 days). Of those 236 patients, 19.1 % were classified as mild/asymptomatic (mild) and 80.9 % as moderate to critical (severe). IgG anti-nucleocapsid antibodies (anti-N) were measured using the Abbott Architect immunoassay.
Results
22.2 % of mild and 2.6 % of severe COVID-19 cases never seroconverted (p < 0.001). Of the mild patients who seroconverted 0–59 days after PCR; 18.8 %, 40.0 % and 61.1 % were seronegative in the windows 60–119 days, 120–179 days and 180–240 days after PCR, respectively. In severe patients, these numbers were 1.9 %, 10.8 % and 29.4 % respectively (p < 0.05 each). Antibody levels were significantly higher in severe patients compared to mild patients in each 60 day window (p < 0.001 each).
Conclusions
SARS-CoV-2 anti-N IgG antibody levels steadily decreased after 2 months up to 8 months post PCR. Of severe COVID-19 patients, 70.6 % remained positive up to eight months after infection. Antibody levels were significantly lower in mild SARS-CoV-2 infected patients and 61.1 % became seronegative within 6 months after the first positive PCR.
Abbreviations: CE, Conformité Européenne; CI, 95 % confidence interval; COVID-19, Coronavirus Disease 19; CoV, coronavirus; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; RT-PCR, reverse transcriptase polymerase chain reaction; CMIA, chemiluminescent microparticle immunoassay
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Longitudinal studies, Immunoglobulin G, Antibodies
1. Background
While almost all moderate to critically ill COVID-19 patients develop IgG anti-SARS-CoV-2 antibodies within 2 weeks after the onset of symptoms [1], an estimated 20 % of asymptomatic and mild COVID-19 patients never seroconvert [2]. There are two structural proteins that are commonly used as target antigens for serological assays, the nucleocapsid (N) protein, and the spike (S) protein. IgG anti-N antibodies appear on average 2 days earlier than IgG anti-S antibodies [3].
There are reports that peak antibody levels are lower in patients who had an asymptomatic to mild infection [4]. It has also been suggested that these patients become seronegative faster with some reports of rapid decline within 3 months after infection [5,6]. There are, however, almost no data at 6 months or later. A recent study by Maine et al. reported a sensitivity of more than 97 % 4–12 weeks after onset of symptoms for the Abbott IgG anti-N assay without any significant difference between patients not requiring oxygen and patients requiring oxygen [7]. The authors also reported the detection of IgG anti-N antibodies 3–5 months after onset of symptoms in 92 % of COVID-19 patients, but the number of samples was small (n = 13).
There is significant interest in the long-term kinetics of IgG anti-SARS-CoV-2 antibodies since these might be an indirect measure of protection against reinfection [8]. Reinfections with seasonal coronaviruses have been reported to occur 6–12 months after the first infection [9]. Several cases of reinfections with SARS-CoV-2 have been reported in recent months [10,11], although the risk of reinfection appears to be very low (<0.1 %) up to 4 months after infection based on data from Qatar [12]. It remains unclear whether the risk for reinfection might increase in winter 2020–2021 > 6 months after the first COVID-19 peak in Europe and North-America. Data on antibody levels after 6 months might help to estimate the remaining level of immunity in patients infected during the first peak.
The aim of this study was to characterize the IgG anti-N antibody kinetics up to 8 months after infection and to compare the antibody response in mild and severe COVID-19.
2. Materials & methods
This retrospective study was performed at the University Hospitals Leuven and Ghent University Hospital after approval by the local ethics committees (S63897 and BC07662). We used 652 leftover samples from 236 individuals (139 men and 97 women). All patients tested positive for SARS-CoV-2 with RT-PCR. Asymptomatic and mild COVID-19 cases were considered as “mild patients” since they typically do not require hospitalization, while moderate, severe and critical COVID-19 cases were considered as “severe patients” (see online data supplement for more information on patient classification). Median age was 61 years in mild (range 24–91) and severe (range 23–92) patients.
IgG anti-N antibody levels were measured using the semi-quantitative SARS-CoV-2 IgG chemiluminescent microparticle immunoassay (CMIA) on Architect i2000SR (Abbott, Lake Forest Illinois) using serum or lithium heparin plasma, both appropriate sample types according to the manufacturer’s instructions. A signal/cutoff (S/CO) value ≥ 1.40 was considered positive.
The percentage of patients who seroconverted was compared using Fisher’s exact test and 95 % confidence intervals were calculated using the modified Wald method. Antibody levels were compared using a non-parametric rank sum test (Mann–Whitney–Wilcoxon). A p-value < 0.05 was considered significant. See online data supplement for information about data analysis.
3. Results
3.1. Positivity rate
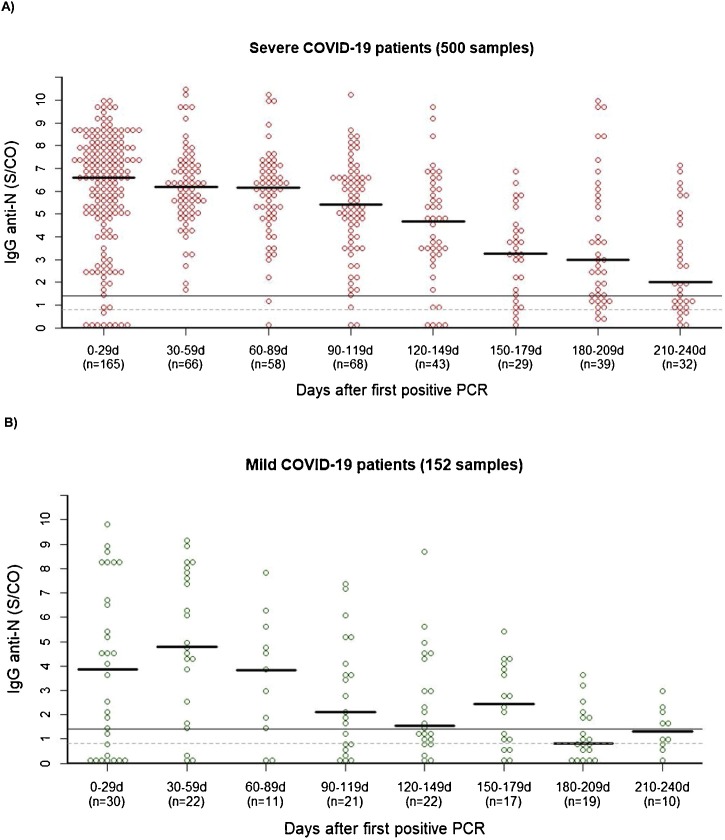
Ten of the 45 mild patients never had a positive sample (22.2 %, [CI: 12.4–36.5]) compared to only 5 of 191 severe patients (2.6 % [CI: 0.1–6.1]) (p < 0.001). The highest proportion of positive samples per time window was noted 30–59 days after PCR, with 100 % [CI: 93.4–100] of samples from severe patients and 86.4 % [CI: 65.8–96.1] of samples from mild patients having a S/CO ≥ 1.40 (Fig. 1 A and B, p < 0.05). In mild patients who had a positive sample 0–59 days after PCR (n = 32); 18.8 % [CI: 5.8–43.8], 40.0 % [CI: 23.3–59.3] and 61.1 % [CI: 38.5–79.8] became seronegative in the 60–119 days, 120–179 days and 180–240 days time windows after PCR, respectively. In severe patients with a positive sample in the first 60-day time window (n = 178), these numbers were 1.9 % [CI: 0.1–7.1], 10.8 % [CI: 5.0–20.9] and 29.4 % [CI: 18.6–43.1] respectively. No patients who became seronegative tested positive again in a later time window.
Fig. 1.
Beeswarm plot of the IgG anti-N results in 191 severe (A) and 45 mild (B) COVID-19 patients. If more than one sample was tested for a patient in the 0-29 or 30-59 day window, only the result from the sample with the highest antibody level was included. If more than one sample was tested for a patient in the other time windows, only the result from the sample that was collected last was included (n = number of patients).
3.2. Long-term antibody kinetics
The median antibody level in severe patients was the highest in the 0–29 day window (S/CO 6.9), followed by a steady decline (Fig. 1A). In mild patients, the highest median antibody level was observed 30–59 days post PCR (S/CO 4.4), with a declining trend in the subsequent time windows. The somewhat less smooth decline in mild patients can be attributed to the smaller number of available specimens.
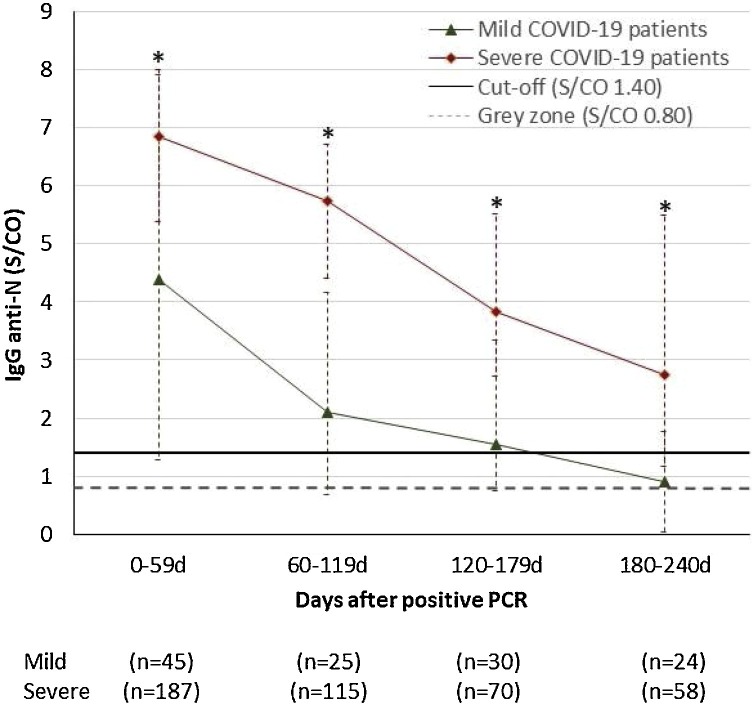
To compare the long-term antibody response in mild and severe patients, we statistically compared the antibody levels per 60 day time window. Antibody levels were significantly higher in severe patients compared to mild patients in each 60 day time window (p < 0.001 each) (Fig. 2 ). While 69.0 % [CI 56.1–79.4] of severe patients were seropositive 180–240 days after positive PCR, only 33.3 % [CI: 17.8–53.4] of mild patients were seropositive after 6 months (p < 0.01). Using a cut-off for a grey zone (0.80 S/CO), 86.2 % [CI: 74.8–93.1] of severe patients were seropositive compared to 58.3 % [CI: 38.8–75.6] of mild patients (p < 0.01) (Supplementary Table 1).
Fig. 2.
Median IgG (P25 and P75 as confidence intervals) anti-N antibody levels after first positive PCR in 191 severe and 45 mild COVID-19 patients. If more than one sample was tested for a patient in the 0-59 day window, only the result from the sample with the highest antibody level was included. If more than one sample was tested for a patient in the other time windows, only the result from the sample that was collected last was included (n = number of patients). * p-value <0.001 vs. mild COVID-19 patients.
4. Discussion
We evaluated the long-term IgG anti-N SARS-CoV-2 antibody response with the Abbott CMIA assay. We found that 97.4 % of severe patients became seropositive compared to only 77.7 % of mild patients and that peak antibody levels in the 60 days post PCR were significantly lower in mild patients. Antibody levels gradually decreased after 2 months in both mild and severe patients but remained significantly higher in severe patients. Of seropositive mild patients, 61.1 % became seronegative within 6 months, whereas 70.6 % of severe patients remained seropositive up to 8 months post PCR.
Our results confirm earlier reports that a significant number of mild and asymptomatic patients never seroconvert and, if they do, have on average lower peak antibody levels than patients with moderate to critical disease [4,13,14]. The fact that severe patients produce more antibodies might be attributable to a stronger stimulation of B-cells and formation of (more) long-lived plasma cells [15]. The apparent lack of a sustained antibody response against SARS-CoV-2 and other human coronaviruses has recently been suggested to be attributable to a dysregulation of the early humoral response [16].
Although the Abbott IgG anti-N assay does not directly measure neutralizing antibodies, the results have been shown to correlate with neutralizing antibody titers and protection against reinfection [[17], [18], [19], [20], [21], [22]]. Neutralizing antibodies are a marker of protective immunity against SARS-CoV-2 in non-human primates [23], but the required neutralizing antibody titer for protective immunity in humans is currently unknown [24]. The fact that 70.6 % of severe patients were still seropositive compared to less than one third of mild patients after 6 months suggests that patients who had a moderate to critical infection (typically requiring hospitalization) during the first wave in spring 2020 might have a lower risk of (symptomatic) reinfection than patients who had an asymptomatic to mild infection.
A strength of our study is that it is one of the first studies to describe the immune response beyond 6 months in mild and severe patients. There are also a number of limitations to our study. First, we did not study anti-S antibody levels, which might have different antibody kinetics and better correlate with protection against reinfection. Second, we did not perform neutralization assays which provide a more direct link with protective humoral immunity. Third, the number of asymptomatic and mild SARS-CoV-2 infections was relatively low compared to the number of moderate to critical patients. Finally, the study design was retrospective and because we used left-over samples, we did not have a sample of every patient in every time window.
5. Conclusions
SARS-CoV-2 anti-N IgG antibody levels steadily decreased after 2 months up to 8 months after positive PCR. Of moderate to critical COVID-19 patients, 70.6 % remained positive up to eight months after infection. Antibody levels were significantly lower in asymptomatic and mild SARS-CoV-2 infected patients in every 60 day time window and 61.1 % of these patients became seronegative within 6 months after the first positive PCR.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
P. Vermeersch is a senior clinical investigator of the FWO-Vlaanderen.
We thank Kat Delaat, Tine De Maeyer, Kelly Gebruers, Kaat D’hooghe and Eveline Neys for their expert technical assistance and Anne Van der Schagt, Caroline Knaepen and Tuur Abst for help in the storage of left-over samples.
The research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2021.104765.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Van Elslande J., Houben E., Depypere M., Brackenier A., Desmet S., André E., Van Ranst M., Lagrou K., Vermeersch P. Diagnostic performance of 7 rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin. Microbiol. Infect. 2020;26:1082–1087. doi: 10.1016/J.CMI.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowitdamrong E., Puthanakit T., Jantarabenjakul W., Prompetchara E., Suchartlikitwong P., Putcharoen O., Hirankarn N. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Elslande J., Decru B., Jonckheere S., Van Wijngaerden E., Houben E., Vandecandelaere P., Indevuyst C., Depypere M., Desmet S., André E., Van Ranst M., Lagrou K., Vermeersch P. Antibody response against SARS-CoV-2 spike protein and nucleoprotein evaluated by 4 automated immunoassays and 3 ELISAs. Clin. Microbiol. Infect. 2020;26(1557):e1–1557. doi: 10.1016/j.cmi.2020.07.038. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A., Hemmings O., O’Byrne A., Kouphou N., Galao R.P., Betancor G., Wilson H.D., Signell A.W., Winstone H., Kerridge C., Huettner I., Jimenez-Guardeño J.M., Lista M.J., Temperton N., Snell L.B., Bisnauthsing K., Moore A., Green A., Martinez L., Stokes B., Honey J., Izquierdo-Barras A., Arbane G., Patel A., Tan M.K.I., O’Connell L., O’Hara G., MacMahon E., Douthwaite S., Nebbia G., Batra R., Martinez-Nunez R., Shankar-Hari M., Edgeworth J.D., Neil S.J.D., Malim M.H., Doores K.J. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., Hu J.L., Xu W., Zhang Y., Lv F.J., Su K., Zhang F., Gong J., Wu B., Liu X.M., Li J.J., Qiu J.F., Chen J., Huang A.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 6.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A., Ferbas K.G., Tobin N.H., Aldrovandi G.M., Yang O.O. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N. Engl. J. Med. 2020;383:1085–1087. doi: 10.1056/nejmc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maine G.N., Lao K.M., Krishnan S.M., Afolayan-Oloye O., Fatemi S., Kumar S., VanHorn L., Hurand A., Sykes E., Sun Q. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using abbott architect. J. Clin. Virol. 2020;133 doi: 10.1016/j.jcv.2020.104663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M., Borgert B.A., Moreno C.A., Solomon B.D., Trimmer-Smith L., Etienne V., Rodriguez-Barraquer I., Lessler J., Salje H., Burke D.S., Wesolowski A., Cummings D.A.T. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat. Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edridge A.W.D., Kaczorowska J., Hoste A.C.R., Bakker M., Klein M., Loens K., Jebbink M.F., Matser A., Kinsella C.M., Rueda P., Ieven M., Goossens H., Prins M., Sastre P., Deijs M., van der Hoek L. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 10.Van Elslande J., Vermeersch P., Vandervoort K., Wawina-Bokalanga T., Vanmechelen B., Wollants E., Laenen L., André E., Van Ranst M., Lagrou K., Maes P. Symptomatic SARS-CoV-2 reinfection by a phylogenetically distinct strain. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim A.Y., Gandhi R.T. Re-infection with SARS-CoV-2: what Goes Around may come back around. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1541. [DOI] [Google Scholar]
- 12.Raddad L.J.A., Chemaitelly H., Ayoub H.H., Al Kanaani Z., Al Khal A., Al Kuwari E., Butt A.A., Coyle P., Jeremijenko A., Kaleeckal A.H., Latif A.N., Rahim H.F.A., Al Kuwari M.G., Eid H., Romaihi A., Al Thani S.M., Bertollini R. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. MedRxiv. 2020 doi: 10.1101/2020.08.24.20179457. 2020.08.24.20179457. [DOI] [Google Scholar]
- 13.Chen Y., Tong X., Li Y., Gu B., Yan J., Liu Y., Shen H., Huang R., Wu C. A comprehensive, longitudinal analysis of humoral responses specific to four recombinant antigens of SARS-CoV-2 in severe and non-severe COVID-19 patients. PLoS Pathog. 2020;16 doi: 10.1371/JOURNAL.PPAT.1008796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amanna I.J., Slifka M.K. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol. Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneko N., Kuo H.H., Boucau J., Farmer J.R., Allard-Chamard H., Mahajan V.S., Piechocka-Trocha A., Lefteri K., Osborn M., Bals J., Bartsch Y.C., Bonheur N., Caradonna T.M., Chevalier J., Chowdhury F., Diefenbach T.J., Einkauf K., Fallon J., Feldman J., Finn K.K., Garcia-Broncano P., Hartana C.A., Hauser B.M., Jiang C., Kaplonek P., Karpell M., Koscher E.C., Lian X., Liu H., Liu J., Ly N.L., Michell A.R., Rassadkina Y., Seiger K., Sessa L., Shin S., Singh N., Sun W., Sun X., Ticheli H.J., Waring M.T., Zhu A.L., Alter G., Li J.Z., Lingwood D., Schmidt A.G., Lichterfeld M., Walker B.D., Yu X.G., Padera R.F., Pillai S. Loss of Bcl-6-expressing t follicular helper cells and germinal centers in COVID-19. Cell. 2020;183:143–157. doi: 10.1016/j.cell.2020.08.025. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel E.U., Bloch E.M., Clarke W., Hsieh Y.-H., Boon D., Eby Y., Fernandez R.E., Baker O.R., Keruly M., Kirby C.S., Klock E., Littlefield K., Miller J., Schmidt H.A., Sullivan P., Piwowar-Manning E., Shrestha R., Redd A.D., Rothman R.E., Sullivan D., Shoham S., Casadevall A., Quinn T.C., Pekosz A., Tobian A.A.R., Laeyendecker O. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padoan A., Bonfante F., Pagliari M., Bortolami A., Negrini D., Zuin S., Bozzato D., Cosma C., Sciacovelli L., Plebani M. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine. 2020;62 doi: 10.1016/j.ebiom.2020.103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jääskeläinen A.J., Kuivanen S., Kekäläinen E., Ahava M.J., Loginov R., Kallio-Kokko H., Vapalahti O., Jarva H., Kurkela S., Lappalainen M. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suhandynata R.T., Hoffman M.A., Huang D., Tran J.T., Kelner M.J., Reed S.L., Mclawhon R.W., Voss J.E., Nemazee D., Fitzgerald R.L. Commercial serology assays predict neutralization activity against SARS-CoV-2. Clin. Chem. 2020 doi: 10.1093/clinchem/hvaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meschi S., Colavita F., Bordi L., Matusali G., Lapa D., Amendola A., Vairo F., Ippolito G., Capobianchi M.R., Castilletti C. Performance evaluation of Abbott ARCHITECT SARS-CoV-2 IgG immunoassay in comparison with indirect immunofluorescence and virus microneutralization test. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumley S.F., O’Donnell D., Stoesser N.E., Matthews P.C., Howarth A., Hatch S.B., Marsden B.D., Cox S., James T., Warren F., Peck L.J., Ritter T.G., de Toledo Z., Warren L., Axten D., Cornall R.J., Jones E.Y., Stuart D.I., Screaton G., Ebner D., Hoosdally S., Chand M., Crook D.W., O’Donnell A.-M., Conlon C.P., Pouwels K.B., Walker A.S., Peto T.E.A., Hopkins S., Walker T.M., Jeffery K., Eyre D.W. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J., Tostanosk L.H., Peter L., Mercad N.B., McMahan K., Mahrokhia S.H., Nkolol J.P., Liu J., Li Z., Chandrashekar A., Martine D.R., Loos C., Atyeo C., Fischinger S., Burk J.S., Slei M.D., Chen Y., Zuiani A., Lelis F.J.N., Travers M., Habibi S., Pessaint L., Van Ry A., Blade K., Brown R., Cook A., Finneyfrock B., Dodson A., Teow E., Velasco J., Zahn R., Wegmann F., Bondzi E.A., Dagotto G., Gebr M.S., He X., Jacob-Dolan C., Kirilova M., Kordana N., Lin Z., Maxfiel L.F., Nampanya F., Nityanandam R., Ventur J.D., Wan H., Cai Y., Chen B., Schmid A.G., Weseman D.R., Bari R.S., Alter G., Andersen H., Lewi M.G., Barou D.H. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrillo J., Izquierdo-Useros N., Ávila-Nieto C., Pradenas E., Clotet B., Blanco J. Humoral immune responses and neutralizing antibodies against SARS-CoV-2; implications in pathogenesis and protective immunity. Biochem. Biophys. Res. Commun. 2020 doi: 10.1016/j.bbrc.2020.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.