Abstract
Background and aims
Metformin has antiviral and anti-inflammatory effects and several cohort studies have shown that metformin lower mortality in the COVID population in a majority white population. There is no data documenting the effect of metformin taken as an outpatient on COVID-19 related hospitalizations. Our aim was to evaluate if metformin decreases hospitalization and severe COVID-19 among minority Medicare patients who acquired the SARS-CoV2 virus.
Methods
We conducted a retrospective cohort study including elderly minority Medicare COVID-19 patients across eight states. We collected data from the inpatient and outpatient electronic health records, demographic data, as well as clinical and echocardiographic data. We classified those using metformin as those patients who had a pharmacy claim for metformin and non-metformin users as those who were diabetics and did not use metformin as well as non-diabetic patients. Our primary outcome was hospitalization. Our secondary outcomes were mortality and acute respiratory distress syndrome (ARDS).
Results
We identified 1139 COVID-19 positive patients of whom 392 were metformin users. Metformin users had a higher comorbidity score than non-metformin users (p < 0.01). The adjusted relative hazard (RH) of those hospitalized for metformin users was 0.71; 95% CI 0.52–0.86. The RH of death for metformin users was 0.34; 95% CI 0.19–0.59. The RH of ARDS for metformin users was 0.32; 95% CI 0.22–0.45. Metformin users on 1000 mg daily had lower mortality, but similar hospitalization and ARDS rates when compared to those on 500–850 mg of metformin daily.
Conclusions
Metformin is associated with lower hospitalization, mortality and ARDS among a minority COVID-19 population. Future randomized trials should confirm this finding and evaluate for a causative effect of the drug preventing disease.
Keywords: COVID-19, Metformin, Diabetes
1. Introduction
There is a critical need for medications to prevent and treat COVID-19 until a vaccine is deemed safe and effective. Only a few medications have modest effects on severe COVID-19 and there are no recommended medications for those with mild COVID-19.[1,2]. The safety profile of a medication for the prevention or treatment of outpatient COVID-19 has to be proven as several medications have recently shown poor safety profiles [3].
Metformin has an excellent safety profile over decades of use, with a small list of contraindications, low cost, and wide availability [4]. Several cohort studies and a meta-analysis have shown that prior use of metformin among diabetics is associated with lower in-hospital mortality during the COVID-19 admission [[5], [6], [7], [8]]. Metformin’s anti-inflammatory effects offer biological plausibility to this finding [4,9]. However, there is still no data on the effect of metformin on COVID-19 related hospitalization, the effect of metformin’s dose or the effect on minority populations most likely to benefit from its use.
Therefore, our aim is two-fold. First, evaluate if metformin is associated with decrease hospitalizations, all-cause mortality and acute respiratory distress syndrome among Medicare patients who tested positive for SARS-CoV2 virus. Second, evaluate if the dose of metformin or the baseline characteristics of the patient population has a differential effect of metformin w these outcomes.
2. Methods
2.1. Study setting
We conducted the study at Chen Senior Medical Centers (CSMC), JenCare Senior Medical Center (JCSMC) and Dedicated Senior Medical Centers (DSMC). These are fully capitated group network practices located across eight states. Patients are insured through Medicare Advantage Plans that serve as fiscal intermediaries for processing claims. As part of a system-wide focus on prevention and wellness, patients are seen virtually or in-person every month by their primary care providers and undergo an initial research screening echocardiogram upon establishing care. The population served is well over 80,000 Medicare advantage patients.
3. Study design and study population
We conducted a retrospective cohort study of all the patients who tested positive for COVID-19 in the clinics between January 1, 2020 and August 14, 2020. Follow-up of the cases concluded on August 17, 2020 and the median number of outpatient visits was 5, interquartile range (IQR) (3–7). We included all consecutive COVID-19 patients and defined COVID-19 positive patients as a positive reverse transcription polymerase chain reaction (RT-PCR) test for SARS-CoV-2. Given the high touch care model with frequent visits [10] and the fact that all of the clinics remained fully operational, we expect that most patients with COVID-19 symptoms would contact their clinic and/or primary care provider for arranging testing and/or care. At the same time we also have real time notification when our patients are in the emergency room and this prompts follow-up by our case management.
4. Metformin use
Our main medication of interest was metformin. We classified metformin use as pharmacy claims or electronic health records and included all those with at least one pharmacy claim before the diagnosis of COVID-19 in 2019 and 2020. We classified non-metformin users as those without pharmacy claims of metformin and this group included those who were diabetics and did not use metformin as well as non-diabetic patients. We also collected the dose of metformin and used the last dose of metformin in 2019 and 2020. To evaluate a metformin dose effect, we compared the use of 500–850 mg per day and of 1000 mg or more per day to not using metformin.
5. Outcome
Our primary outcome was hospitalization while mortality and the diagnosis of acute respiratory distress syndrome (ARDS) were secondary outcomes. We obtained hospitalization status from our electronic health record (EHR) and defined it as any patient who was admitted to the hospital for observation or for more than 24 h. The EHR contains as text files that include selected physician progress notes and procedures of each hospital admission. We reviewed the notes from the hospitalist services, from intensive care units and procedure notes and recorded if patients had ARDS. We defined ARDS if the chest-x ray or chest computed tomography was compatible with ARDS and the physician notes mentioned ARDS in the problem list.
We captured mortality from our EHR as all-cause mortality. All-cause mortality was ascertained and defined as at least one of the following: a) self-report from the patient’s family during monthly calls conducted to all patients by the transitional care team, b) hospitalization reports from the hospitalist team and c) the Medicare claims flag. Our team retrieved data on all-cause mortality from the EHR. Both primary outcome was collected from the medical record by one of the co-authors (B.C) and a second co-author reviewed the collected information regarding the secondary outcomes (E.D).
6. Covariates
We included three types of covariates: socio-economic, clinical and echocardiographic characteristics. Our socio-economic predictors included age, gender, race, census based median household income. Age, gender and race were obtained from the EHR. The clinical predictors from the EHR included the Charlson score as a measure of disease burden [11], diagnoses of diabetes, hypertension and heart failure in the 3 months before the diagnosis of COVID-19. We also collected the values for systolic blood pressure, body mass index, hbA1c, total cholesterol for the last in-person visit before the COVID-19 diagnosis. For this analysis we used the values from the last primary care visit and used the last set of laboratory values available. The echocardiographic predictors included diastolic dysfunction and ejection fraction. These predictors were collected from the EHR as all patients enrolled in the practice undergo a screening echocardiogram as part of an ongoing research project. The screening echocardiogram includes doppler mitral flow and tissue velocities tracings. Diastolic function was classified according to the recommendations of the American Society of Echocardiography (ASE) on diastolic functional evaluation. The grading scheme for diastolic dysfunction was mild or grade I, moderate or grade II, and severe (restrictive pattern) or grade III. We also measured the LV ejection fraction (EF) using the modified biplane Simpson’s method. A mean of three cardiac cycles was used.
We selected three variables for subgroup analysis. Body mass index, race, ejection fraction and hba1c. We classified body mass index as normal, overweight, obese and morbidly obese and both hba1c and ejection fraction as tertiles.
7. Statistical analysis
We reported baseline characteristics by metformin use and compared baseline characteristics using Wilcoxon rank sum test and chi-square.
To evaluate if metformin was associated with the primary or secondary outcome and account for potential confounding we used two approaches. First, a multivariate analysis using cox proportional models to calculate the relative hazard (RH) and corresponding 95% confidence interval (CI) controlling for age, gender, Charlson score, diabetes, hypertension and ejection fraction. Second, we calculated a propensity score using logistic regression. The propensity score calculated the probability of using metformin controlling for the same variables. We then matched by propensity score with a margin of 0.01. We elected to present the unmatched adjusted results as the sample size was higher. We calculated person time as the difference between January 1, 2020 and the time of censoring. The rationale to start counting follow-up time at the same moment in time for all was to assure similar follow-up times between both groups. We censored at the time of hospitalization and at the time of ARDS as well as death, if none of the primary or secondary events occurred we administratively censored on August 17, 2020.
To evaluate differences in the effects of metformin by baseline characteristics or comorbidities we included an interaction term on the multivariate model that included metformin use as a categorical variable and the continuous variable of interest.
The fitness of the data was assessed using the deviance ratio. Analyses were performed using STATA version (College Station, Texas), and all significance tests were two-tailed.
8. Results
8.1. Baseline characteristics
During our study period we identified 1139 SARS-CoV-2 positive patients. Table 1 shows the baseline clinical characteristics of the patients who tested positive for SARS-CoV-2 stratified by metformin use. We identified 392 metformin users. Metformin users had a higher comorbidity score than non-metformin users (p < 0.01), particularly higher rates of heart failure, diabetes and hypertension. Metformin users had higher hba1c and body mass index (p < 0.01). Metformin users also used more sulfonylurea and insulin than non-metformin users (p < 0.01) and the use of newer antidiabetics was uncommon (<5%) and equal between both groups. Both groups had similar ejection fraction but metformin users had more grade 2 and 3 diastolic dysfunction (p < 0.01). Table 1 of the appendix shows the propensity matched baseline characteristics.
Table 1.
Baseline characteristics.
| Characteristic | Metformin users (n = 392) | Non-metformin users (n = 747) | p-value |
|---|---|---|---|
| Age, mean and standard deviation years | 70.9 ± 8.9 | 71.2 ± 8.9 | 0.63 |
| Female gender, % | 61 | 59 | 0.56 |
| Black, % | 71 | 70 | 0.19 |
| Diabetes, % | 99 | 33 | <0.01 |
| Hypertension, % | 60 | 50 | <0.01 |
| Sulfonylurea, % | 21 | 5 | <0.01 |
| Insulin, % | 15 | 6 | <0.01 |
| Charlson score, mean and standard deviation | 3.52 ± 1.22 | 2.62 ± 1.57 | <0.01 |
| Heart failure, % | 49 | 41 | 0.01 |
| Body mass index, mean kg/m2 | 33.2 ± 7.7 | 31.7 ± 9.6 | 0.01 |
| Hba1c, mean % | 7.66 ± 1.54 | 6.37 ± 1.51 | <0.01 |
| Ejection fraction, mean and standard deviation % | 59.1 ± 5.3 | 58.5 ± 7.2 | 0.18 |
| Diastolic dysfunction, % | 43 | 31 | <0.01 |
9. Primary and secondary outcomes
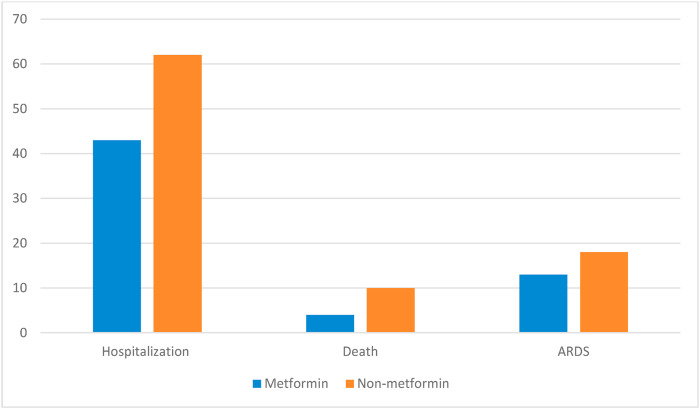
Fig. 1 shows the unadjusted primary and secondary outcome rates by metformin use. Six hundred and thirty two COVID-19 patients were hospitalized. Forty three percent of metformin users were hospitalized compared to 62% of non-metformin users (p < 0.01). The adjusted RH of hospitalized for metformin users was 0.71; 95% CI 0.52–0.86. The propensity matched treatment effect coefficient was −0.04 (p = 0.01) for metformin users compared to non-metformin users. The median length of stay for metformin users was 11 (IQR 5–46) days compared to 14(IQR 6–30) days (p = 0.50).
Fig. 1.
Primary and secondary outcomes by metformin use. p < 0.01 p < 0.01 p < 0.01.
Ninety-one COVID-19 patients died. Four percent of metformin users died compared to 10% of non-metformin users (p < 0.01). The risk adjusted RH of death for metformin users was 0.34; 95% CI 0.19–0.59. The propensity matched treatment effect coefficient was −0.07 (p < 0.01) for metformin users compared to non-metformin users.
One hundred and eighty five COVID-19 patients developed ARDS. Thirteen percent of metformin users developed ARDS compared to 18% of non-metformin users (p < 0.01). The adjusted RH of ARDS for metformin users was 0.72; 95% CI 0.52–0.86. The RH of ARDS for metformin users was 0.32; 95% CI 0.22–0.45. The propensity matched treatment effect coefficient was −0.02 (p = 0.04) for metformin users compared to non-metformin users. Our analysis showed the same results when comparing only diabetic metformin and non-metformin users (see appendix.
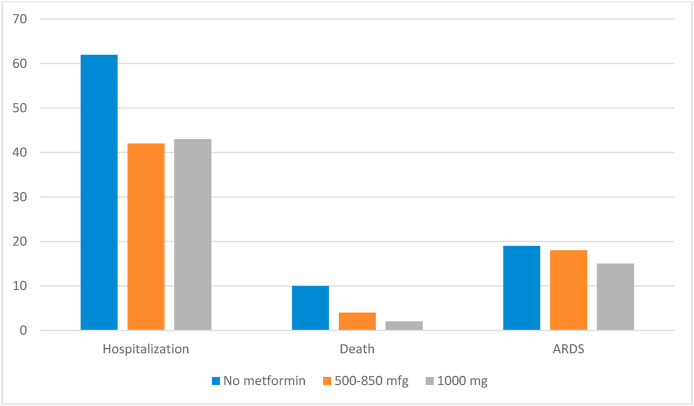
10. Metformin dose effect on outcomes
Patients who used 1000 mg of metformin were younger (68 years) than those on 500–850 mg (72 years) but had similar gender and race distribution. Those on 1000 mg had a higher HbA1c than the lower dose. Those using 1000 mg had lower mortality but similar hospitalization and ARDS rates when compared to those on 500–850 mg (Fig. 2 ). Table 2 shows the adjusted dose effect measures.
Fig. 2.
Unadjusted events by metformin dose.
Table 2.
Relative hazard, 95% confidence interval and p-value by dose of metformin.
| Medication category | Hospitalization | Mortality | ARDS |
|---|---|---|---|
| No metformin use (n = 759) | Reference | ||
| Metformin 500–850 mg (n = 256) | 0.74 (0.51–1.07)0.11 | 0.35 (0.17–0.71)<0.01 | 0.80 (0.66–1.25)0.42 |
| Metformin 1000 mg or more (n = 123) | 0.77 (0.48-1.23)0.27 | 0.23 (0.06–0.78)0.01 | 0.66 (0.33–1.30)0.23 |
11. Subgroup analysis
The interaction term was significant for body mass index (p = 0.03) and HbA1c (p = 0.04). Table 2 of the appendix shows the effect of metformin on each outcome by race, body mass index and hemoglobin A1c. Those with the highest BMI and HbA1c had higher hospitalization and mortality (p < 0.05) all other associations were non-significant.
12. Discussion
Our study found lower COVID-19 related hospitalization, mortality and ARDS among minority Medicare patients who took metformin as an outpatient. The findings were independent from demographic characteristics and comorbidities. In addition, our results suggest a dose effect of metformin on mortality and an interaction between metformin use and baseline characteristics. Increased BMI, Hemoglobin A1C were associated with the benefit from metformin use whereas those with lower values did not have a statistically significant benefit.
Our study has several strengths that lend weight to our findings. Among those are the multicenter, national design, the large sample size, the inclusion of unselected consecutive primary care patients, the availability of relevant clinical, demographic and echocardiographic data to adjust our models and consistency of results using different statistical analysis.
Metformin, besides its effect on glucose, has many other effects that include antiaging and anti-inflammatory properties [4,9,12]. Recently, metformin has shown antiviral properties on ZIKA virus infection by decreasing the production of viral particles. [13]. The mechanism by which metformin affects SARS-CoV2 replication is that metformin phosphorylates the ACE-2 receptor via AMPK and therefore decreasing viral entry to the cell. [14, 15]. This post translational modification of the receptor can also extend the half life of the ACE-2 receptor, which may offer lung protection [14]. Another potential favorable mechanism of metformin is the reduction of inflammatory marker release by affecting the MTOR and NFKB pathways [16]. These mechanisms provide biological plausibility to our findings and support the conduction of rigorous studies evaluating the impact of metformin.
Ten studies and two meta-analysis have already documented the effect of using metformin as an outpatient on COVID-19 related outcomes [[5], [6], [7], [8]].
[17] All studies had a cohort design, included 11,200 and eight studies found a statistically significant reduction in inhospital mortality. Two studies found worsening outcomes. Gao et al. found significant disease progression among 110 hospitalized diabetic patients [18] and Cheng et al. found an increase in acidosis and this increase in lactic acidosis was associated with worse outcomes among 1213 with diabetes [19]. Our study complements this body of literature as we reported on the prevention of hospitalization rather than focusing on inpatient mortality and found a metformin dose effect. However, many of the studies are in pre-print and therefore not peer-reviewed and have significant limitations that include small sample size, residual confounding, inappropriate time series analysis.
We found an interaction between body mass index and hemoglobin A1c on metformin’s effect on outcomes. A potential explanation for this differential effect could be markedly elevated levels of inflammatory markers on obese diabetics out of control and the suppression of inflammatory markers by metformin leading to a lower inflammatory state. Alternatively increased body mass may be less detrimental to the elderly than younger people and therefore may provide a protective effect.
13. Limitations
There are several limitations that deserve mention. First, we relied on a retrospective review of the medical record hence the inclusion of comorbidities using administrative codes could have led to information bias. Second, by using metformin claims we are not sure if patients filled and took their medications. Third, our low incidence could be explained by the fact that at the time of this report widespread COVID-19 testing was not available, we only reported symptomatic patients who had a COVID-19 diagnostic test and we communicate regularly with our patients and remind them the importance of physical distancing, mask wearing and staying at home. Fourth, our dose effect analysis is limited because of small sample size, potential for residual confounding and lack of propensity score analysis. Fifth, we did not include several biomarkers that could contribute to the primary and secondary outcomes.
In conclusion, metformin seems to decrease COVID-19 related hospitalization of diabetic patients. Future randomized studies need to confirm this finding and the potential use of metformin as a treatment for outpatient COVID-19.
Disclosures
No conflicts of interest.
Credit author statement
Conceptualization: Ghany, Dawkins, McCarter, Tamariz, Chung, Palacio, Forbes, Chen.
Analysis: Tamariz, Chung, Dawkins, Palacio.
Data curation: Chung, Dawkins, Forbes, Palacio.
Writing: Tamariz, Palacio.
Review: McCarter, Dawkins, Forbes, Chen, Ghany.
Supervision: Ghany, Chen, McCarter.
Declaration of competing interest
None to declare.
eAppendix.
Table 1.
Baseline characteristics of propensity matched cohort.
| Characteristic | Metformin users (n = 178) | Non-metformin users (n = 124) | p-value |
|---|---|---|---|
| Age, mean and standard deviation years | 75.6 ± 5.4 | 76.5 ± 5.4 | 0.15 |
| Female gender, % | 36 | 36 | 0.49 |
| Black, % | 71 | 70 | 0.19 |
| Diabetes, % | 100 | 100 | 1.0 |
| Hypertension, % | 55 | 55 | 0.31 |
| Charlson score, mean and standard deviation | 3.97 ± 1.18 | 4.19 ± 1.37 | 0.14 |
| Heart failure, % | 49 | 50 | 0.43 |
Table 2.
Subgroup analysis
| Hospitalization |
Mortality |
ARDS |
||||
|---|---|---|---|---|---|---|
| Category | No metformin | Metformin | No metformin | Metformin | No metformin | Metformin |
| Normal BMI (n = 156) | 44 | 33 | 11 | 3 | 13 | 9 |
| Overweight (n = 276) | 38 | 25 | 9 | 4∗ | 13 | 12 |
| Obese (n = 260) | 35 | 33 | 8 | 3∗ | 12 | 10 |
| Morbid obesity (n = 430) | 49 | 40∗ | 12 | 6∗ | 13 | 12 |
| White (n = 147) | 36 | 39 | 13 | 0 | 12 | 9 |
| Black (n = 720) | 46 | 41 | 10 | 5 | 12 | 8 |
| Hispanic (n = 139) | 31 | 30 | 7 | 5 | 15 | 14 |
| HbA1c < 8 (n = 564) | 43 | 39 | 7 | 1∗ | 12 | 18 |
| HbA1c > 8 (n = 575) | 42 | 32∗ | 15 | 7∗ | 14 | 18 |
∗P < 0.05.
Table 3.
Outcome comparison by metformin use in diabetics only.
| Hospitalization | Death | ARDS | |
|---|---|---|---|
| Metformin users (n = 243) | 44% | 4% | 16% |
| Non-metformin users (n = 350) | 52% | 14% | 18% |
| p-value | <0.01 | 0.04 | 0.48 |
| RH and 95% CI | 0.28 (0.15–0.53) | 0.74 (0.53–0.98) | 0.86 (0.45–1.15) |
References
- 1.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 3.Pathak D.S.K., Salunke D.A.A., Thivari D.P. No benefit of hydroxychloroquine in COVID-19: results of systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. 2020;14(6):1673–1680. doi: 10.1016/j.dsx.2020.08.033. S1871-4021(20)30336-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valencia W.M., Palacio A., Tamariz L., Florez H. Metformin and ageing: improving ageing outcomes beyond glycaemic control. Diabetologia. 2017;60(9):1630–1638. doi: 10.1007/s00125-017-4349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramante C., Ingraham N., Murray T. Observational study of metformin and risk of mortality in patients hospitalized with covid-19. Lancet Healthy Longev. 2021;2(1):e34–e41. doi: 10.1016/S2666-7568(20)30033-7. Epub 2020 Dec 3. PMID: 33521772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crouse A., Grimes T., Li P., Might M., Ovalle F., Shalev A. Metformin use is associated with reduced mortality in a diverse population with covid-19 and diabetes. Front Endocrinol (Lausanne) 2021;11 doi: 10.3389/fendo.2020.600439. eCollection 2020. PMID: 33519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hariyanto T.I., Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. 2020;19 doi: 10.1016/j.obmed.2020.100290. 100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo P., Qiu L., Liu Y. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103(1):69–72. doi: 10.4269/ajtmh.20-0375. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barzilai N., Crandall J.P., Kritchevsky S.B., Espeland M.A. Metformin as a tool to target aging. Cell Metabol. 2016;23(6):1060–1065. doi: 10.1016/j.cmet.2016.05.011. S1550-4131(16)30229-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghany R., Tamariz L., Chen G. High-touch care leads to better outcomes and lower costs in a senior population. Am J Manag Care. 2018;24(9):e300–e304. doi: 87709 [pii] [PubMed] [Google Scholar]
- 11.Sharabiani M.T., Aylin P., Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109–1118. doi: 10.1097/MLR.0b013e31825f64d0. 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni A.S., Gubbi S., Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metabol. 2020;32(1):15–30. doi: 10.1016/j.cmet.2020.04.001. S1550-4131(20)30183-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S., Singh P.K., Suhail H. AMP-activated protein kinase restricts zika virus replication in endothelial cells by potentiating innate antiviral responses and inhibiting glycolysis. J Immunol. 2020;204(7):1810–1824. doi: 10.4049/jimmunol.1901310. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Dong J., Martin M. AMP-activated protein kinase phosphorylation of angiotensin-converting enzyme 2 in endothelium mitigates pulmonary hypertension. Am J Respir Crit Care Med. 2018;198(4):509–520. doi: 10.1164/rccm.201712-2570OC. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang Y., Zhang X., Leng W. Assessment of diabetic cardiomyopathy by cardiovascular magnetic resonance T1 mapping: correlation with left-ventricular diastolic dysfunction and diabetic duration. J Diabet Res. 2017;2017 doi: 10.1155/2017/9584278. 9584278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheen A.J. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab. 2020;46(6):423–426. doi: 10.1016/j.diabet.2020.07.006. Epub 2020 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Do J.Y., Kim S.W., Park J.W., Cho K.H., Kang S.H. Is there an association between metformin use and clinical outcomes in diabetes patients with COVID-19? Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.10.006. Online ahead of print. PMID: 33160030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Y., Liu T., Zhong W. Risk of metformin in patients with type 2 diabetes with COVID-19: a preliminary retrospective report. Clin Transl Sci. 2020 doi: 10.1111/cts.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng X., Liu Y.M., Li H. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell Metabol. 2020;32(4):537–547. doi: 10.1016/j.cmet.2020.08.013. S1550-4131(20)30426-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]