To the editor
We recently conducted a study examining whether transcranial electrical stimulation (TES) motor threshold (MT), reverse-calculation transcranial direct current stimulation (tDCS) electric field modeling, or both could potentially be used as methods of individualizing tDCS doses(1). We found that TES MT significantly correlates with a reverse-calculated tDCS dosage in the motor cortex and were intrigued by the possibility of using TES MT as an MRI-free method of individually dosing tDCS(1). A limitation of this previous work was that we did not test the utility of TES MT to estimate reverse-calculation tDCS doses outside of the motor cortex. Here we extend this research by assessing whether TES MT correlates with reverse-calculation electric field models of prefrontal stimulation in a common F3-F4 electrode montage that has been used in depression [2], drug craving [3], working memory [4], and many other conditions.
In this study we used the same dataset as in Ref. [1], in which we acquired transcranial magnetic stimulation (TMS) MT, TES MT, and anatomical T1w MRI scans for 29 healthy adults (15 women, mean age = 26.9, SD = 9.1). We previously described the two-visit study protocol in depth in Ref. [1] but briefly describe it here. In Visit 1, we placed a plastic cap on each participant’s head and used a closed-loop TMS-motor evoked potential (MEP) acquisition setup using single pulses of TMS (Magstim BiStim machine with 70mm figure-of-eight Remote Coil; Whitland, Wales, UK) over the left motor hotspot and electromyography (EMG) electrodes over the contralateral right hand [5]. We defined a positive MEP as having a peak-to-peak amplitude of ≥0.05mV, and used PEST software (https://www.clinicalresearcher.org/software.htm) to determine the next stimulation intensity for MT acquisition [6]. After determining the TMS MT, we cut through the plastic cap to place a 35 × 20mm electrode (Natus Neurology Inc., Pleasanton, CA, USA) on the head at the left motor hotspot and placed a 55 × 42mm electrode (Natus Neurology Inc., Pleasanton, CA, USA) on the left deltoid. We used a Digitimer DS7A (Letchworth Garden City, England, UK) to send single pulses of electrical stimulation through the electrodes, with a pulse width of 200 ms, maximum voltage of 400V, and initial stimulation intensity of 58.0mA. Using this left M1-left deltoid electrode configuration and these stimulation parameters, TES was safe, tolerable, and relatively pain-free for each participant (****See Supplemental Materials S1 in Ref. [1] for tolerability and pain ratings). In addition, a modified PEST algorithm allowed our determination of a TES MT for each participant with just 5 TES pulses [1].
In Visit 2, we acquired anatomical T1w MRI scans for each participant to be used for electric field modeling. To segment each person’s MRI scan we used headreco (https://simnibs.github.io/simnibs/build/html/documentation/command_line/headreco.html), a command that calls SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) and CAT12 (http://www.neuro.uni-jena.de/cat/) and converts NIFTI to MSH files [7]. Using previously published methods, we used visual inspection and a Z-score analysis to evaluate the quality of tissue segmentation of grey matter, white matter, and cerebrospinal fluid (CSF) [1]. We did not identify any improper segmentations in these data.
To perform electric field modeling, we used SimNIBS 3.1.1 (https://simnibs.github.io/simnibs/build/html/index.html) [8] as it can be used to perform region of interest (ROI) analyses and has been validated against ROAST [9]. We placed rectangular 70 × 50mm electrodes over each participant’s F3 and F4, with the longer axis running left/right on the head (Fig. 1A) and 2.0mA of current input into F3 (anode) and −2.0mA for F4 (cathode). We extracted 10mm radius spherical ROIs at MNI coordinates for the cortical projections underneath the electrodes at F3 and F4. This method has previously been used to determine the MNI coordinates of ROIs at the cortical level that underlie TMS coils placed on the scalp(10)(Fig. 1A). We further measured an ROI at the cortical projection midway between the two electrodes underneath Fz [10]. Under each ROI, an average electric field was computed using a grey matter mask. We then reverse-calculated the tDCS dose at the scalp that would be required to produce the group average electric field for each person using the cross-multiplication method detailed in Fig. 1B and regressed the dose against the TES MT for each person in SPSS 25.0 (Armonk, NY, USA: IBM Corp.).
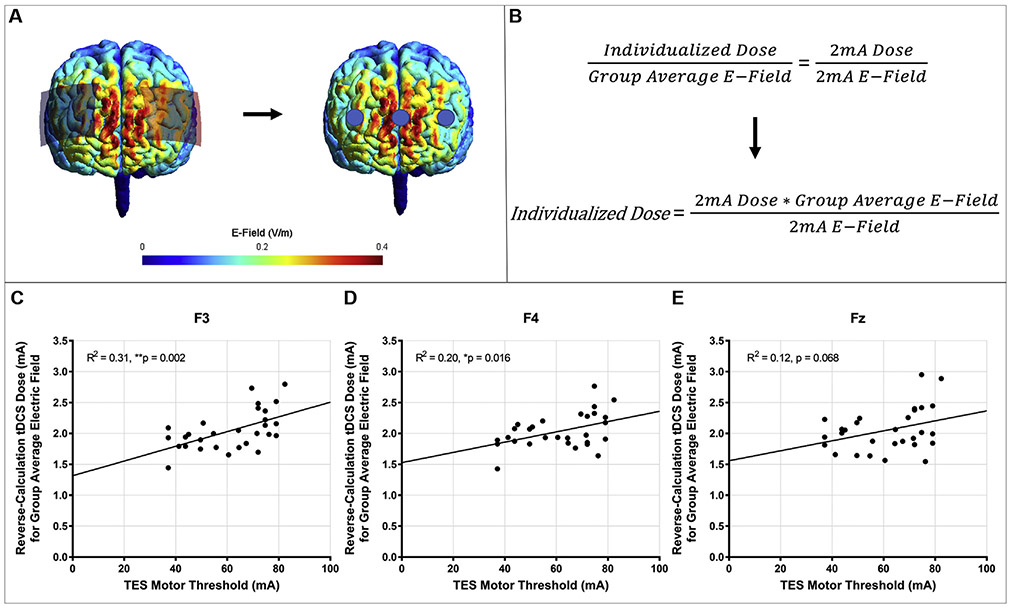
Fig. 1. Reverse-Calculation Modeling ROI Analysis Overview and Results.
1A: We used SimNIBS 3.1.1. modeling to place rectangular 70 × 50mm electrodes at F3 (anode) and F4 (cathode). Blue circles depict the spherical 10mm radius ROIs we extracted at the cortical level using grey matter masks. These ROIs were centered around the cortical locations underneath the anode (F3), cathode (F4), and midway between the two electrodes (Fz). 1B: Reverse-Calculation Formula. The individualized dose was determined using one 2mA model and cross-multiplication to determine the individualized dose that would be required to produce the group average electric field. C-E: Prefrontal F3-F4 Reverse-Calculation Dose x TES MT Regressions. We plotted each individual’s reverse-calculation electric field model underneath F3 (1C), F4 (1D), and Fz (1E) against the measured TES MT at the scalp over the motor hotspot. These TES MT values significantly correlated at the ROIs underneath F3 and F4 and trended toward significance for the ROI underneath Fz. With further evaluation and refinement, it appears that TES MT could be a promising candidate technique for individually dosing tDCS without the use of MRI. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The group average reverse-calculation doses were 2.045mA for the ROI underneath F3 (range = 1.444–2.515mA, SD = 0.320mA), 2.036mA for the ROI underneath F4 (range = 1.426–2.764mA, SD = 0.280mA), and 2.053mA for the ROI underneath Fz (range = 1.545–2.445mA, SD = 0.351mA). TES MT significantly correlated with the reverse-calculation dose based on the ROIs underneath F3, F(1, 27) = 12.03, R2 = 0.31, p = 0.002 and F4, F(1,27) = 6.55, R2 = 0.20, p = 0.016 and trended toward significance at the ROI underneath Fz, F(1, 27) = 3.60, R2 = 0.12, p = 0.068 (Fig. 1C-E). We did not evaluate if TMS MT correlates with prefrontal reverse-calculation doses as we previously found that TMS MT did not correlate with reverse-calculation tDCS doses over the motor hotspot and also that TMS MT only has a trending relationship with TES MT [1].
In sum, we conducted a complementary study to Ref. [1], finding that TES MT acquired over the motor cortex could help to estimate ROI-based reverse-calculation tDCS doses in the prefrontal cortex. With further evaluation in larger sample sizes and in different populations and disease states, TES MT holds promise as an MRI-free technique to individually dose tDCS over not just motor areas [1] but also for prefrontal stimulation. Evaluating MRI-free approaches to individualize tDCS dosage would help to reduce the resources and cost that are required for reverse-calculation tDCS modeling.
It is unclear why the reverse-calculation tDCS electric fields underneath F3 correlated more strongly with TES MT than underneath F4 or Fz. It may be due to the TES MT being acquired over the same left hemisphere as the ROI underneath F3, rather than between hemispheres (Fz) or in the right hemisphere (F4). The Fz location between hemispheres may be particularly prone to variability since it could contain a lower and more variable number of voxels between participants. Reverse-calculation modeling and TES MT acquisition should be further refined and evaluated as methods of individually dosing tDCS. Further research should investigate the use of reverse-calculation tDCS modeling, TES MT, or both to prospectively dose tDCS.
Acknowledgments
Financial Support
This study was funded by the National Center of Neuromodulation for Rehabilitation (NC NM4R). The NC NM4R is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number P2CHD086844. The Brain Stimulation Laboratory also receives funding from the Center for Biomedical Research Excellence (COBRE) in Stroke Recovery. This study was partially funded by grants to MB from NIH (NIH-NINDS 1R01NS101362, NIH-NIMH 1R01MH111896, NIH-NCI U54CA137788/U54CA132378, and NIH-NIMH 1R01MH109289).
Footnotes
Declaration of competing interest
The City University of New York (CUNY) has IP on neurostimulation system and methods with author Marom Bikson as inventor. Marom Bikson has equity in Soterix Medical Inc and is a consultant for GSK, Halo, and X. We confirm that there are no known conflicts of interest associated with this publication and there was no financial support for this work that could have influenced its outcome.
Contributor Information
Kevin A. Caulfield, Brain Stimulation Laboratory, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, USA.
Bashar W. Badran, Brain Stimulation Laboratory, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, USA
Xingbao Li, Brain Stimulation Laboratory, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, USA.
Marom Bikson, Department of Biomedical Engineering, City College of New York, USA.
Mark S. George, Brain Stimulation Laboratory, Department of Psychiatry, Medical University of South Carolina, Charleston, SC, USA; Ralph H. Johnson VA Medical Center, Charleston, SC, USA
References
- [1].Caulfield KA, Badran BW, DeVries WH, Summers PM, Kofmehl E, Li X, et al. Transcranial electrical stimulation motor threshold can estimate individualized tDCS dosage from reverse-calculation electric-field modeling. Brain Stimulation 2020;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brunoni AR, Boggio PS, De Raedt R, Bensenor IM, Lotufo PA, Namur V, et al. Cognitive control therapy and transcranial direct current stimulation for depression: a randomized, double-blinded, controlled trial. J Affect Disord 2014;162:43–9. [DOI] [PubMed] [Google Scholar]
- [3].Alizadehgoradel J, Nejati V, Movahed FS, Imani S, Taherifard M, Mosayebi-Samani M, et al. Repeated stimulation of the dorsolateral-prefrontal cortex improves executive dysfunctions and craving in drug addiction: a randomized, double-blind, parallel-group study. Brain Stimulation 2;13(3):582–93. 10.1016/j.brs.2019.12.028. [DOI] [PubMed] [Google Scholar]
- [4].Nissim NR, O'Shea A, Indahlastari A, Telles R, Richards L, Porges E, et al. Effects of in-scanner bilateral frontal tDCS on functional connectivity of the working memory network in older adults. Front Aging Neurosci 2019;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Badran B, Caulfield K, Lopez J, Cox C, Stomberg-Firestein S, Devries W, et al. Personalized TMS helmets for quick and reliable TMS administration outside of a laboratory setting. Brain Stimulation 2020;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mishory A, Molnar C, Koola J, Li X, Kozel FA, Myrick H, et al. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J ECT 2004;20(3):160–5. [DOI] [PubMed] [Google Scholar]
- [7].Nielsen JD, Madsen KH, Puonti O, Siebner HR, Bauer C, Madsen CG, et al. Automatic skull segmentation from MR images for realistic volume conductor models of the head: assessment of the state-of-the-art. Neuroimage 2018;174:587–98. [DOI] [PubMed] [Google Scholar]
- [8].Saturnino GB, Puonti O, Nielsen JD, Antonenko D, Madsen KH, Thielscher A. SimNIBS 2.1: a comprehensive pipeline for individualized electric field modelling for transcranial brain stimulation Copyright. In: Makarov S, Horner M, Noetscher G, editors. Brain and human body modeling: computational human modeling at EMBC 2018. Cham (CH): Springer; 2019. p. 3–25. The Author(s). 2019. [PubMed] [Google Scholar]
- [9].Huang Y, Datta A, Bikson M, Parra LC. Realistic volumetric-approach to simulate transcranial electric stimulation-ROAST-a fully automated open-source pipeline. J Neural Eng 2019;16(5):056006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 2004;21(1):99–111. [DOI] [PubMed] [Google Scholar]