Abstract
AIM
To evaluate whether portable chest radiography (CXR) scores are associated with coronavirus disease 2019 (COVID-19) status and various clinical outcomes.
MATERIALS AND METHODS
This retrospective study included 500 initial CXR from COVID-19-suspected patients. Each CXR was scored based on geographic extent and degree of opacity as indicators of disease severity. COVID-19 status and clinical outcomes including intensive care unit (ICU) admission, mechanical ventilation, mortality, length of hospitalisation, and duration on ventilator were collected. Multivariable logistic regression analysis was performed to evaluate the relationship between CXR scores and COVID-19 status, CXR scores and clinical outcomes, adjusted for code status, age, gender and co-morbidities.
RESULTS
The interclass correlation coefficients amongst raters were 0.94 and 0.90 for the extent score and opacity score, respectively. CXR scores were significantly (p < 0.01) associated with COVID-19 positivity (odd ratio [OR] = 1.49; 95% confidence interval [CI]: 1.27 - 1.75 for extent score and OR = 1.75; 95% CI: 1.42 - 2.15 for opacity score), ICU admission (OR = 1.19; 95% CI: 1.09 - 1.31 for extent score and OR = 1.26; 95% CI: 1.10 - 1.44 for opacity score), and invasive mechanical ventilation (OR = 1.22; 95% CI: 1.11 - 1.35 for geographic score and OR = 1.21; 95% CI: 1.05 - 1.38 for opacity score). CXR scores were not significantly different between survivors and non-survivors after adjusting for code status (p>0.05). CXR scores were not associated with length of hospitalisation or duration on ventilation (p>0.05).
CONCLUSIONS
Initial CXR scores have prognostic value and are associated with COVID-19 positivity, ICU admission, and mechanical ventilation.
Introduction
Coronavirus disease 2019 (COVID-19), an infectious disease that can cause severe respiratory illness,1, 2, 3 has already infected 13 million people and killed more than 570,000 worldwide.4 COVID-19 continues to strain resources in many hospitals around the world. Lung imaging plays an important role in the management of patients during COVID-19 pandemic. Although reverse transcription polymerase chain reaction (RT-PCR) of a nasopharyngeal or oropharyngeal swab specimen is a reference standard3 , 5 for diagnosing COVID-19 infection, it has a long turnaround time and high false-negative rate.6 Newer tests, such as the lateral flow tests, have rapid turnaround time, but low sensitivity.7 Computed tomography (CT)8 was used in China early on in the pandemic when RT-PCR was less reliable, and CT offers high sensitivity and faster turnaround time; however, CT is prone to cross-contamination and, thus, it is not widely used in the context of COVID-19 in the United States and elsewhere in the world. In addition, CT is not readily accessible in many parts of the world and poses the risk of radiation exposure to patients. By contrast, chest radiography (CXR) is convenient and can be readily disinfected between uses, is more accessible, and has a lower radiation dose than CT, although it has lower sensitivity than CT. Nonetheless, CXRs show characteristic ground-glass opacities and/or consolidation in the lungs associated with COVID-19 infection.9 CXR is often one of the very first clinical assessments that suspected COVID-19 patients receive. Under the appropriate context and along with other clinical assessment, CXR can facilitate diagnosis and triage patients while RT-PCR results are pending. CXR is also well suited to monitor disease progression during hospitalisation, such as sicker patients in the ICU, which is very crucial as those patients cannot be readily moved to the CT machine.
To date, CXR score has not been widely used for staging of disease severity of COVID-19 lung infection in clinical practice. Although a few recent studies have related initial CXR scores of COVID-19 patients to various clinical outcomes, such as ICU admission, need for mechanical ventilation, mortality, length of hospitalisation, and duration on ventilator,10, 11, 12, 13, 14 the results are inconsistent and controversial. The CXR scoring methods varied, and demographics and comorbidities were accounted for in some but not other studies. No studies to date have accounted for whether patients were removed from aggressive therapies due to having a “do not resuscitate” order (i.e., code status), which confound data interpretation and compromise the prognostic value of CXR scores. As such, the potential of CXR to stage disease severity of COVID-19 lung infection is not yet fully realised.
The goal of this study was to further investigate the association of CXR scores of disease severity with COVID-19 status, ICU admission, need for mechanical ventilation, mortality, length of hospitalisation, and duration on ventilator. The types of CXR scores were established carefully for disease severity, one based on geographical extent of involvement and the other based on degree of opacity, with consensus amongst a group of chest radiologists and residents. Analysis accounted for patients' code status, demographics, and co-morbidities.
Materials and methods
Patient selection and inclusion criteria
This is an institutional review board approved retrospective study from University Hospital (for blind review) with waiver of informed consent. There were 4,997 persons under investigation (PUI) for COVID-19, who presented to the emergency department (ED) between 8 March 2020 and 20 April 2020. Only patients with known COVID-19 PCR results and CXRs taken within the first 3 days of ED visit were included. Patients with incomplete information regarding comorbidities were excluded from the study. If there were multiple CXRs for one patient, the one closest to the ED visit was used. This resulted in 500 CXRs with the majority (90%) of the CXRs taken on the day of ED admission from 500 PUIs taken between 8 March 2020 and 13 April 2020 for scoring and analysis.
Clinical data collection
Data were extracted from the electronic medical records both automatically and manually by trained data abstractors via commercial software Cerner's HealtheIntent and established diagnostic codes (such as ICD10 and LOINC codes). These data included patients' demographic information (age and sex), co-morbidities (hypertension [hypertension], diabetes mellitus [DM], asthma, coronary artery disease [CAD], chronic obstructive pulmonary disease [COPD], congestive heart failure [CHF], cancer, immunosuppression, chronic kidney disease [CKD]), clinical outcomes (ICU admission, need for invasive mechanical ventilation, mortality, length of hospital stay, and duration on mechanical ventilator). Data also included patient's duration of symptom onset prior to ED presentation and patient's code status (in which full code indicates all resuscitation interventions to be performed).
Imaging collection and analysis
To establish the disease severity score, a group of four board-certified chest radiologists of 10–20 years of experience and two radiology residents in training worked together to reach consensus by evaluating 12 images of portable CXRs of COVID-19 patients, which was part of the 500 CXRs. The same chest radiologists and residents scored the 500 CXRs for disease severity using the following criteria based on geographical extent and degree of opacity. The extent score of 0–4 was assigned to each of the right and left lungs depending on the extent of involvement with ground glass opacities or consolidation: 0 = no involvement; 1 = <25%; 2 = 25–50%; 3 = 51–75%; 4 = >75% involvement. The opacity score of 0–4 was assigned to each of the right and left lungs as: 0 = no opacity; 1 = ground glass opacity; 2 = mix of consolidation and ground glass opacity (<50% consolidation); 3 = mix of consolidation and ground glass opacity (>50% consolidation); 4 = complete white-out. The right and left lung were scored separately and added together. In short, extent score ranged from 0–8, the opacity score ranged from 0–8. In addition, the sum of these two types of scores (0–16) and the product of the two types of scores (0–64) were computed. To minimise bias, the raters were blinded to the clinical data while scoring the CXRs. Each CXR was scored by two raters independently and the mean of the scores of two raters were calculated.
Statistical methods and performance evaluation
Statistical analysis was performed with IBM SPSS software (Chicago, IL, USA). Intraclass correlation coefficient was calculated to assess inter-reader agreement of the CXR scores. Means and standard deviations of extent score, opacity score, sum of extent and opacity score, product of extent and opacity score were calculated. Multivariable logistic regression analysis were performed to analyse the relationships between CXR scores with ICU admission, mortality, need for ventilation, and gender. Odds ratios were also computed. Linear regression analysis was performed to analyse the correlation between CXR scores and age, length of hospital stay, and duration on ventilation. A p<0.05 is taken to be statistically significant unless otherwise specified.
Results
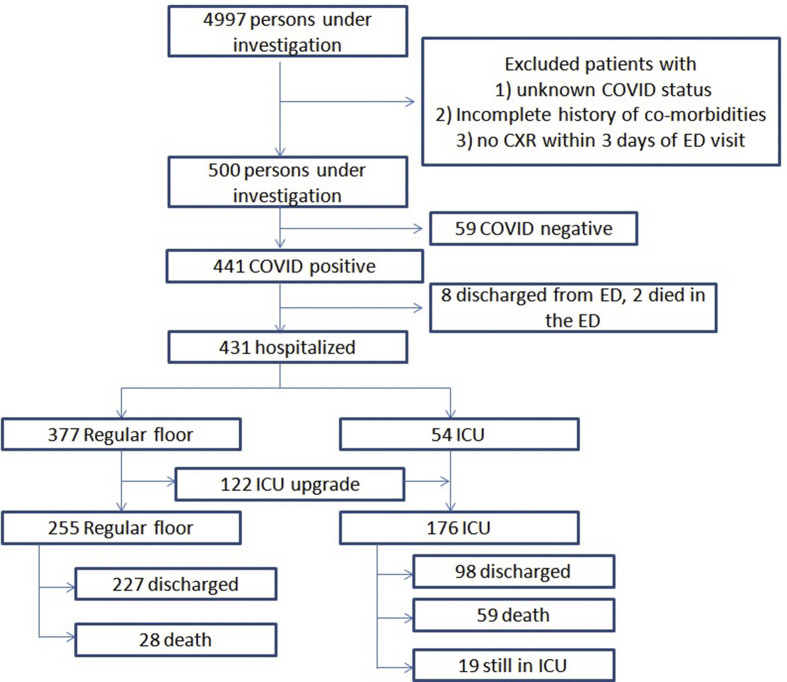
Fig 1 shows the flowchart of patient selection and outcomes of the 500 PUI. Of the 4,997 PUI, after excluding patients with unknown COVID-19 status, incomplete information on co-morbidities, and no CXR within 3 days of ED visit, 500 PUIs were investigated (59 COVID-19 negative, 441 COVID-19 positive). Of the 441 COVID-19 positive patients, eight were discharged from the ED, two died in the ED, 377 were admitted to the regular ward, and 54 were admitted directly to the ICU. Of the 377 patients initially admitted to the regular ward, 255 remained on the regular ward and 122 were subsequently upgraded to the ICU. Of those who remained on the regular ward, 227 were discharged and 28 died. Of the ICU group, 98 were discharged, 59 died while 19 remained on the ICU without a final status. Table 1 shows the demographics and co-morbidities of all the patients in this study.
Figure 1.
Flowchart describing patient selection, triage and outcome of the 500 persons under investigation.
Table 1.
Demographics and co-morbidities of the 500 persons under investigation whose chest radiographs were used in this study.
| Demographics | |
| Age | 60.3±17.8 |
| Gender | 60% male, 40% female |
| Co-morbidities | |
| Hypertension | 44% |
| DM | 26% |
| Asthma | 7% |
| CAD | 15% |
| COPD | 7% |
| CHF | 8% |
| Cancer | 7% |
| Immunosuppression | 7% |
| CKD | 10% |
| None of the above | 35% |
DM: diabetes mellitus, CAD: coronary artery disease, COPD: chronic obstructive pulmonary disease, CHF: congestive heart failure, CKD: chronic kidney disease.
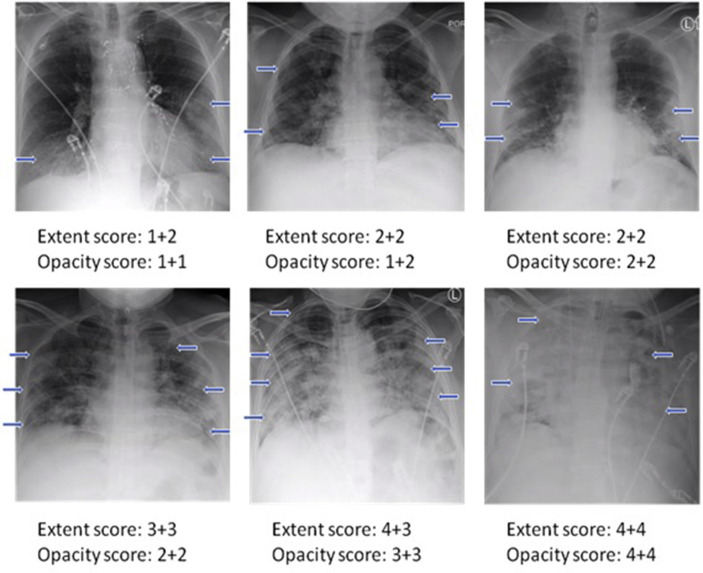
Examples of CXRs with different extent and opacity scores are demonstrated in Fig 2 . CXRs of COVID-19 positive patients showed hazy opacities and/or airspace consolidation, with predominance of bilateral, peripheral and lower lung zone distribution. Each CXR was scored by two raters. The inter-reader agreement assessed by intraclass correlation coefficient, which was 0.94 (95% CI: 0.93–0.95) for the extent score, and 0.90 (95% CI: 0.88–0.92) for the opacity score. The Pearson's correlation coefficient between the extent score and opacity score was 0.82 (p=0.01).
Figure 2.
Examples showing a range of CXR severity scores, which include extent and opacity scores. The two numbers for each are for the right and left lung. Arrows indicate region of ground glass opacities or/and airspace consolidations.
The extent score, opacity score, sum of extent and opacity score, product of extent and opacity score scores were significantly higher in COVID-19 positive patients compared to COVID-19 negative patients, with the odd ratios ranging from 1.08 to 1.75 after adjusting for age, sex and co-morbidities (Table 2 ).
Table 2.
Comparison of CXR scores and distribution of age, gender, and co-morbidities between COVID-19 negative group and COVID-19 positive group.
| COVID-19 negative (n=59) |
COVID-19 positive (n=441) |
Adjusted p-value |
Unadjusted p-value |
Odds ratio (95% CI) |
|
|---|---|---|---|---|---|
| Extent score (0–8) | 2.4±2.2 | 4.1±2.2 | <0.001 | 1.49 (1.27–1.75) | |
| Opacity score (0–8) | 2.0±1.6 | 3.3±1.6 | <0.001 | 1.75 (1.42–2.15) | |
| Sum of extent and opacity score (0–16) | 4.5±3.6 | 7.4±3.6 | <0.001 | 1.29 (1.17–1.41) | |
| Product of extent and opacity score (0–64) | 7.8±9.6 | 16.2±12.5 | <0.001 | 1.08 (1.05–1.13) | |
| Age | 62.1±21.6 | 60.1±17.3 | 0.43 | ||
| Gender (% of Male) | 53% | 61% | 0.20 | ||
| Hypertension | 53% | 43% | 0.18 | ||
| DM | 20% | 27% | 0.26 | ||
| Asthma | 14% | 6% | 0.05 | ||
| CAD | 24% | 14% | 0.05 | ||
| COPD | 12% | 7% | 0.17 | ||
| CHF | 24% | 5% | <0.01 | ||
| Cancer | 19% | 6% | <0.01 | ||
| Immunosuppression | 14% | 6% | 0.05 | ||
| CKD | 17% | 9% | 0.05 |
DM: diabetes mellitus, CAD: coronary artery disease, COPD: chronic obstructive pulmonary disease, CHF: congestive heart failure, CKD: chronic kidney disease.
CXR scores were significantly higher in ICU patients than in regular ward patients, with the odd ratios ranging from 1.04 to 1.26 after adjusting for age, sex and co-morbidities (Table 3 a). CXR scores were also significantly higher in patients on invasive mechanical ventilators than in patients not on ventilators, with the odd ratios ranging from 1.03 to 1.22 after adjusting for age, sex, and co-morbidities (Table 3b). The majority of the ICU patients (85%) were on invasive mechanical ventilators.
Table 3.
(a) Comparison of CXR scores between the regular ward group and the ICU group; (b) distribution of age, gender and co-morbidities between regular ward group and ICU group; and (c) between patients not on ventilation and patients on ventilation.
| (a) | Regular ward patients (n=255) |
ICU patients (n=176) |
Adjusted p-value |
Odds ratio (95% CI) |
|---|---|---|---|---|
| Extent score (0–8) | 3.8±2.2 | 4.6±2.2 | <0.001 | 1.19 (1.09–1.31) |
| Opacity score (0–8) | 3.1±1.5 | 3.7±1.6 | 0.001 | 1.26 (1.10–1.44) |
| Sum of extent and opacity score (0–16) | 6.8±3.5 | 8.3±3.6 | <0.001 | 1.12 (1.06–1.19) |
| Product of extent and opacity score (0–64) |
14.1±11.5 |
19.7±13.3 |
<0.001 |
1.04 (1.02–1.05) |
| (b) |
Regular ward patients (n=255) |
ICU patients (n=176) |
Unadjusted p-value |
|
| Age | 60.9±18.4 | 59.6±15.5 | 0.44 | |
| Gender (% of male) | 57% | 68% | 0.03 | |
| Hypertension | 42% | 47% | 0.34 | |
| DM | 25% | 31% | 0.16 | |
| Asthma | 6% | 7% | 0.53 | |
| CAD | 15% | 12% | 0.38 | |
| COPD | 8% | 5% | 0.22 | |
| CHF | 7% | 4% | 0.24 | |
| Cancer | 7% | 4% | 0.24 | |
| Immunosuppression | 7% | 6% | 0.68 | |
| CKD |
10% |
7% |
0.39 |
|
| (c) |
Non-ventilated (n=281) |
Ventilated (n=150) |
Adjusted p-value |
Odds ratio (95% CI) |
| Extent score (0–8) | 3.8±2.2 | 4.3±2.1 | <0.001 | 1.22 (1.11–1.35) |
| Opacity score (0–8) | 3.2±1.5 | 3.5±1.5 | 0.009 | 1.21 (1.05–1.38) |
| Sum of extent and opacity score (0–16) | 6.9±3.5 | 7.8±3.4 | <0.001 | 1.12 (1.05–1.19) |
| Product of extent and opacity score (0–64) | 14.6±11.7 | 17.5±11.9 | <0.001 | 1.03 (1.02–1.05) |
CXR: chest radiograph, ICU: intensive care unit, DM: diabetes mellitus, CAD: coronary artery disease, COPD: chronic obstructive pulmonary disease, CHF: congestive heart failure, CKD: chronic kidney disease.
For the general floor admission cohort, all CXR scores were significantly higher in non-survivors than in survivors with the odd ratios ranging from 1.09 to 2.18, after adjusting for age, sex, and co-morbidities (Table 4 a). For the ICU cohort, opacity score, sum of extent, and opacity score, and product of extent and opacity score were significantly higher in non-survivors than in survivors with the odds ratios ranging from 1.04 to 1.38, after adjusting for age, sex and co-morbidities, but not the extent score (Table 4b).
Table 4.
Comparison of CXR scores (a) between survivors and non-survivors group on the regular ward, (b) between survivors and non-survivors group in the ICU, and (c) between survivors and non-survivors group in the ICU who were full code only.
| (a) | Regular ward survivors (n=227) |
Regular ward non-survivors (n=28) |
Adjusted p-value |
Odds ratio (95% CI) |
|---|---|---|---|---|
| Extent score (0–8) | 3.6±2.2 | 5.0±1.9 | 0.001 | 1.73 (1.25–2.40) |
| Opacity score (0–8) | 3.0±1.4 | 4.0±1.6 | 0.001 | 2.18 (1.37–3.48) |
| Sum of extent and opacity score (0–16) | 6.6±3.4 | 9.0±3.1 | 0.001 | 1.48 (1.18–1.86) |
| Product of extent and opacity score (0–64) |
13.3±10.9 |
21.3±14.0 |
0.001 |
1.09 (1.04–1.15) |
| (b) |
ICU survivors (n=98) |
ICU non-survivors (n=59) |
Adjusted p-value |
Odds ratio (95% CI) |
| Extent score (0–8) | 4.6±2.2 | 4.7±2.0 | 0.133 | 1.17 (0.95–1.43) |
| Opacity score (0–8) | 3.6±1.6 | 3.8±1.6 | 0.023 | 1.38 (1.05–1.82) |
| Sum of extent and opacity score (0–16) | 8.2±3.7 | 8.5±3.3 | 0.050 | 1.13 (1.00–1.29) |
| Product of extent and opacity score (0–64) |
19.4±12.9 |
20.0±13.5 |
0.034 |
1.04 (1.00–1.07) |
| (c) |
ICU survivors, full code (n=92) |
ICU non-survivors, full code (n=16) |
Adjusted p-value |
Odds ratio (95% CI) |
| Extent score (0–8) | 4.7±2.2 | 4.5±2.1 | 0.983 | 1.01 (0.73–1.41) |
| Opacity score (0–8) | 3.7±1.6 | 3.7±1.9 | 0.714 | 1.09 (0.70–1.68) |
| Sum of extent and opacity score (0–16) | 8.4±3.6 | 8.2±3.7 | 0.827 | 1.02 (0.84–1.25) |
| Product of extent and opacity score (0–64) | 19.9±12.9 | 19.2±14.2 | 0.647 | 1.01 (0.96–1.07) |
CXR: chest radiograph, ICU: intensive care unit.
Mortality with respect to the patient's code status was also analysed. Amongst the regular ward patients, 91% of the survivors and 7% of the non-survivors were full code, which indicates all resuscitation interventions to be performed. Amongst the ICU patients, 93% of the survivors and 27% of the non-survivors were full code. CXR scores between survivors and non-survivors in ICU who were full code were not significantly different (Table 4c). There were only two non-survivors on the general floor who were full code, and they were not further analysed.
CXR scores were not associated with the length of hospital stay both for the regular ward patients (R=0.04 for extent score and R=0.12 for opacity score, p>0.05) and the ICU patients (R=–0.01 for extent score and R=–0.18 for opacity score, p>0.05) when regular patients and ICU patients were analysed separately; however, the extent score correlated weakly with the length of hospital stay when regular ward patients and ICU patients were analysed together (R=0.13, p<0.05) while the opacity score was not (R=0.08, p>0.05). CXR scores were not associated with the duration on ventilator either (R=0.02 for geographic score and R=0.03 for opacity score, p>0.05). The extent score correlated weakly with duration of symptoms prior to ED visit (R=0.11, p<0.05) while the opacity score was not (R=0.09, p>0.05). There were no association of CXR scores with age (p>0.05) or sex (p>0.05).
Discussion
This study investigated whether the initial CXR scores inform COVID-19 status, ICU admission, need for ventilation, mortality, length of hospitalisation, and duration on ventilator. CXR scores were found to be associated with COVID-19 status, ICU admission, need for ventilation, but not with mortality after accounting for code status, length of hospitalisation, or duration on ventilator. A unique CXR scoring system was used in the present study. Different CXR scoring systems have been used to evaluate COVID-19 lung infection, some more sophisticated and some simpler.10, 11, 12, 13, 14, 15 The RALE score, which is more sophisticated, is based on the sum of product of geographical extent and degree of opacity from different lung zones, and has been used to evaluate for severity of lung oedema16 and adopted to score CXR in evaluating COVID-19 lung infection.13 , 14 Several other simpler scoring systems did not take degree of opacity into consideration.10 , 11 , 15 The present scoring system also used both geographic extent and degree of opacity, but to make the system simpler, the lungs were not divided into different zones. The scoring system is relatively easy to use, has a reasonable dynamic range, and has high inter-rater agreement. Interestingly, the extent score and opacity score were highly correlated. The opacity scores generally yielded higher odds ratios than extent scores for clinical outcomes. This suggests that degree of opacity likely reflects disease severity better than the extent of lung infection. This is consistent with CXR and CT findings showing COVID-19 pneumonia progressing from ground-glass opacity to consolidation.9 , 17 The sum and product of the two scores was also investigated. The rationale for using the sum of the score is that the two scores were independently scored, and it is possible that their linear combination could add information. The rationale for using the product of the two scores is that it is possible that their geometric weighted average (extent and magnitude of opacity) could add information when combined linearly together. It is not clear whether individual scores, linear combination, or geometric combination yielded better results. Surprisingly, the sum and product of the two scores yielded similar conclusions, but did not yielded better odds ratios than individual scores alone, suggesting that they might not add value. It would be useful to have a CXR reporting template that can be employed with a practical scoring system embedded in, which could help integration of the scoring system in the clinical workflow.
The initial CXR scores were associated COVID-19 status. This finding suggests that CXR scores can be used in conjunction with clinical observation and laboratory results18 for triage and management of suspected COVID-19 patients while waiting for RT-PCR results. The present results are inconsistent with Wong et al. who reported no differences in CXR scores between COVID-19 positive and negative patients.15 A possible explanation could be their small sample size (58 COVID-19 positive patients and six COVID-19 negative patients). There are differences in the scoring systems. Wong et al. only took into consideration the extent score, which is similar to the present extent score; however, there was a statistically significant difference in the extent score between COVID-19 positive versus negative patients. There is no significant difference between the present study and Wong et al. regarding when the CXRs were acquired. In the present study, CXRs were taken within the first 3 days of the ED visit with the majority (90%) of the CXRs taken on the day of ED visit. In Wong et al., 94% CXRs were taken at the ED presentation and 6% were taken within 48 h.
CXR scores are associated with ICU admission and the need for mechanical ventilation. Cozzi et al. also reported CXR scores were associated with ICU admission but without adjusting for demographics and co-morbidities. Toussie et al. reported CXR severity score ≥3 was an independent predictor of intubation in patients 21–50 years old.11 Kim et al. also found CXR scores were associated with intubation.10
A notable finding is that the initial CXR scores were not associated with mortality after adjusting for code status. Previous studies10, 11, 12, 13, 14, 15 did not account for code status. As a result, it is challenging to determine whether patients who died would have survived if more aggressive measures had been taken among patients assigned to comfort care only. Similarly, no correlations were found between initial CXR scores and length of hospital stay for regular ward patients or ICU patients, and between initial CXR scores and duration on mechanical ventilator. These findings are not surprising because these outcomes are far downstream from the patients' status in the ED, although such an association might exist depending on patient cohorts. The present results are consistent with Toussie et al. who reported CXR scores were not associated with length of hospital stay,11 but inconsistent with Kim et al. who reported CXR scores were associated with length of hospital stay.10 Possible explanations for this discrepancy could be due to differences in patient cohort. Interestingly, there was a weak correlation between the extent score and length of hospital stay when combining regular ward and ICU patients, but did not find a correlation when analysing regular ward and ICU patients separately. One possible explanation is that ICU patients tend to have higher CXR scores and longer length of hospitalisation than regular ward patients.
This study has several limitations. First, although the present CXR score system is easy to use with good inter-rater agreement, there are more sophisticated score schemes that could reflect disease severity better.13 , 14 Second, the present study used only initial CXRs. Longitudinal CXR scoring is needed to better inform clinical trajectory and outcome. Third, although several co-morbidities were included in the study, body mass index (BMI) was not included, which is a known risk factor for COVID-19. Fourth, although the majority of the CXRs (90%) were acquired on the day of ED presentation and presumably before the patients received interventions, it was hard to track whether the patients received interventions prior to CXR acquisition, which could have affected the CXR scores. This is a retrospective study from a single institution. These findings need to be repeated using multiple institutional data to test generalisability. Machine learning approaches to classify COVID-19 lung infection on CXR19 20 and stage COVID-19 disease severity on CXR21 22 have also been recently reported. Future studies could incorporate laboratory variables to better inform clinical disease trajectory and outcome.23, 24, 25
In conclusion, initial CXR scores in the ED are associated with positive COVID-19 status, ICU admission, and need for invasive mechanical ventilation. Initial CXR scores have prognostic value and offer important information that could be used to facilitate identification of high-risk patients and triage and manage patients suspected of or diagnosed with COVID-19, especially when combined with other clinical data, such as vital signs and blood tests.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui D.S., E I.A., Madani T.A. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns Hopkins University . Global Map; 2020. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University.https://coronavirus.jhu.edu/map.html Available at. [Google Scholar]
- 5.Tang Y.W., Schmitz J.E., Persing D.H. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol. 2020;58(6) doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valan A.B., Sture C. Negative nasopharyngeal swabs early in the course of COVID-19. Tidsskr Nor Laegeforen. 2020;140(9) doi: 10.4045/tidsskr.20.0356. [DOI] [PubMed] [Google Scholar]
- 7.Wise J. Covid-19: lateral flow tests miss over half of cases, Liverpool pilot data show. BMJ. 2020;371:m4848. doi: 10.1136/bmj.m4848. [DOI] [PubMed] [Google Scholar]
- 8.Xu X., Yu C., Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobi A., Chung M., Bernheim A. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin Imaging. 2020;64:35–42. doi: 10.1016/j.clinimag.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H.W., Capaccione K.M., Li G. The role of initial chest X-ray in triaging patients with suspected COVID-19 during the pandemic. Emerg Radiol. 2020 Dec;27(6):617–621. doi: 10.1007/s10140-020-01808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toussie D., Voutsinas N., Finkelstein M. Clinical and chest radiography features determine patient outcomes in young and middle age adults with COVID-19. Radiology. 2020 Oct;297(1):E197–E206. doi: 10.1148/radiol.2020201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borghesi A., Zigliani A., Golemi S. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: a study of 302 patients from Italy. Int J Infect Dis. 2020;96:291–293. doi: 10.1016/j.ijid.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cozzi D., Albanesi M., Cavigli E. Chest X-ray in new coronavirus disease 2019 (COVID-19) infection: findings and correlation with clinical outcome. Radiol Med. 2020;125(8):730–737. doi: 10.1007/s11547-020-01232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui T.C.H., Khoo H.W., Young B.E. Clinical utility of chest radiography for severe COVID-19. Quant Imaging Med Surg. 2020;10(7):1540–1550. doi: 10.21037/qims-20-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong H.Y.F., Lam H.Y.S., Fong A.H. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2019:201160. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren M.A., Zhao Z., Koyama T. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73(9):840–846. doi: 10.1136/thoraxjnl-2017-211280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernheim A., Mei X., Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo X., Zhou W., Yan X. Prognostic value of C-reactive protein in patients with COVID-19. Clin Infect Dis. 2020 Nov 19;71(16):2174–2179. doi: 10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain L., Nguyen T., Li H. Machine-learning classification of texture features of portable chest X-ray accurately classifies COVID-19 lung infection. Biomed Eng Online. 2020;19(1):88. doi: 10.1186/s12938-020-00831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kikkisetti S., Zhu J., Shen B. Deep-learning convolutional neural networks with transfer learning accurately classify COVID-19 lung infection on portable chest radiographs. PeerJ. 2020;8 doi: 10.7717/peerj.10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J., Shen B., Abbasi A. Deep transfer learning artificial intelligence accurately stages COVID-19 lung disease severity on portable chest radiographs. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0236621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J.P., Dao L., Roth K. Predicting COVID-19 Pneumonia Severity on Chest X-ray With Deep Learning. Cureus. 2020;12(7):e9448. doi: 10.7759/cureus.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J.S., Ge P., Jiang C. Deep-learning artificial intelligence analysis of clinical variables predicts mortality in COVID-19 patients. J Am Coll Emerg Physicians Open. 2020;1:1364–1373. doi: 10.1002/emp2.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Z., Chen A., Hou W. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0236618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., Ge P., Zhu J., Li H. Deep learning prediction of likelihood of ICU admission and mortality in COVID-19 patients using clinical variables. PeerJ. 2020;8 doi: 10.7717/peerj.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]