Abstract
BACKGROUND
The systematic evaluation of the results of time-series studies of air pollution is challenged by differences in model specification and publication bias.
METHODS
We evaluated the associations of inhalable particulate matter (PM) with an aerodynamic diameter of 10 μm or less (PM10) and fine PM with an aerodynamic diameter of 2.5 μm or less (PM2.5) with daily all-cause, cardiovascular, and respiratory mortality across multiple countries or regions. Daily data on mortality and air pollution were collected from 652 cities in 24 countries or regions. We used overdispersed generalized additive models with random-effects meta-analysis to investigate the associations. Two-pollutant models were fitted to test the robustness of the associations. Concentration–response curves from each city were pooled to allow global estimates to be derived.
RESULTS
On average, an increase of 10 μg per cubic meter in the 2-day moving average of PM10 concentration, which represents the average over the current and previous day, was associated with increases of 0.44% (95% confidence interval [CI], 0.39 to 0.50) in daily all-cause mortality, 0.36% (95% CI, 0.30 to 0.43) in daily cardiovascular mortality, and 0.47% (95% CI, 0.35 to 0.58) in daily respiratory mortality. The corresponding increases in daily mortality for the same change in PM2.5 concentration were 0.68% (95% CI, 0.59 to 0.77), 0.55% (95% CI, 0.45 to 0.66), and 0.74% (95% CI, 0.53 to 0.95). These associations remained significant after adjustment for gaseous pollutants. Associations were stronger in locations with lower annual mean PM concentrations and higher annual mean temperatures. The pooled concentration–response curves showed a consistent increase in daily mortality with increasing PM concentration, with steeper slopes at lower PM concentrations.
CONCLUSIONS
Our data show independent associations between short-term exposure to PM10 and PM2.5 and daily all-cause, cardiovascular, and respiratory mortality in more than 600 cities across the globe. These data reinforce the evidence of a link between mortality and PM concentration established in regional and local studies. (Funded by the National Natural Science Foundation of China and others.)
THE ADVERSE HEALTH EFFECTS OF SHORT-term exposure to ambient air pollution are well documented.1–3 Particulate matter (PM), especially, arouses public health concerns because of its toxicity and the widespread human exposure to this pollutant. PM, which includes inhalable particles with an aerodynamic diameter of 10 μm or less (PM10) and fine particles with an aerodynamic diameter of 2.5 μm or less (PM2.5), is emitted from combustion sources or formed through atmospheric chemical transformation. Given the extensive evidence regarding their effects of health, the daily and annual mean concentrations of PM10 and PM2.5 are regulated according to the World Health Organization (WHO) Air Quality Guidelines4 and standards in major countries.
Numerous time-series studies have examined the associations between short-term PM exposures and daily mortality.5–9 However, most evidence has been obtained from studies in single cities, regions, or countries, and there are challenges in comparing these results and in synthesizing effect estimates because of different modeling approaches and potential publication bias. These limitations can be addressed by performing international, multicenter studies that adopt the same analytic protocol and model specifications to estimate globally representative associations of PM10 and PM2.5 exposures with daily mortality. We established the Multi-City Multi-Country (MCC) Collaborative Research Network to perform a global assessment of the effects of weather or climate on mortality.10,11 This network allowed us to examine and compare the associations of PM concentrations with daily all-cause, cardiovascular, and respiratory mortality at the global, regional, and country level with the use of a standardized analytic framework.
METHODS
DATA COLLECTION
We obtained health and environmental data from the MCC database, which has been described previously.10,12 The current analysis was limited to locations that had available data on air pollution (652 urban areas in 24 countries or regions, with the data covering the period from 1986 through 2015) (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Data on mortality were obtained from local authorities within each country. Causes of death were classified according to codes in the International Classification of Diseases, 9th Revision (ICD-9) or 10th Revision (ICD-10), whichever was available. In each location, mortality was represented by daily counts of either death from nonexternal causes (ICD-9 codes 0 to 799 and ICD-10 codes A0 to R99) or, when such data were unavailable, daily counts of death from any cause. We also collected mortality data for two main causes of death: cardiovascular disease (ICD-10 codes I00 to I99) and respiratory disease (ICD-10 codes J00 to J99).13
We obtained daily data on PM10 in 598 cities and on PM2.5 in 499 cities. Data on both pollutants were available in 445 cities in 16 countries or regions. The geographic distributions of the cities that had data on PM10 and PM2.5, as well as the annual mean PM concentrations over the period studied for each city, are provided in Figure 1 and Figure 2, respectively (also see the interactive map, available at NEJM.org). Daily data on gaseous pollutants (ozone, nitrogen dioxide, sulfur dioxide, and carbon monoxide) were obtained where available. We also collected data on the daily mean temperature and daily mean relative humidity. To avoid potential consequences of including outlying values of exposure data, we used trimmed data, in which the highest 5% and lowest 5% of PM10 and PM2.5 measurements were excluded.14
Figure 1. Distribution of the Cities with Data on PM10.
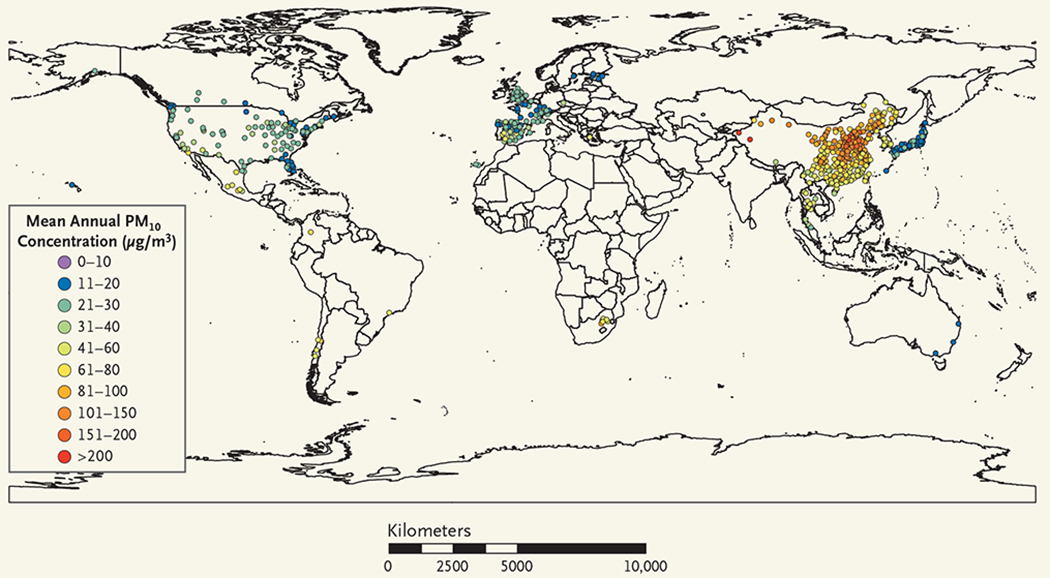
Shown is the geographic distribution of the 598 cities in the 24 countries and regions that had available data on particulate matter with an aerodynamic diameter of 10 μm or less (PM10) and were included in the analysis. Also shown are the annual mean PM10 concentrations. See the interactive map, available at NEJM.org.
Figure 2. Distribution of Cities with Data on PM2.5.
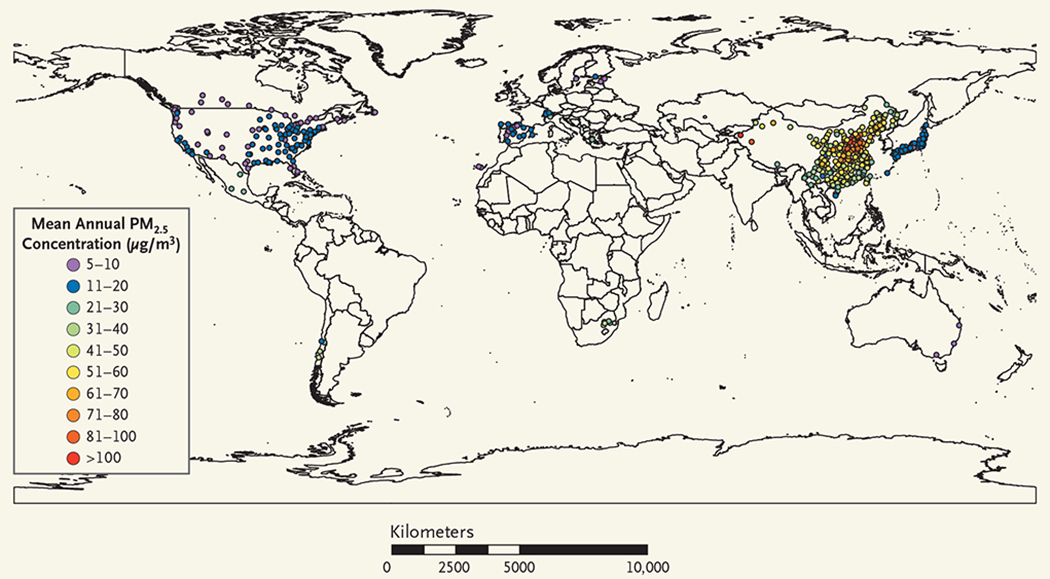
Shown is the geographic distribution of the 499 cities in the 16 countries and regions that had data on particulate matter with an aerodynamic diameter of 2.5 μm or less (PM2.5) and were included in the analysis. Also shown are the annual mean PM2.5 concentrations. See the interactive map, available at NEJM.org.
STATISTICAL ANALYSIS
The associations of PM10 and PM2.5 concentrations with daily all-cause, cardiovascular, and respiratory mortality were assessed in separate analyses with the use of a standard time-series approach. We followed a two-stage analytic protocol, which had been developed and widely applied in previous multicity time-series studies.15,16
In the first stage, we estimated city-specific associations of PM concentration with mortality using quasi-Poisson generalized additive models. In accordance with the approaches used in previous studies,16,17 the following covariates were included in the main model: a natural cubic smooth function with 7 degrees of freedom (df) per year to control for underlying time trends in mortality, an indicator day-of-week variable to account for short-term weekly variations, and natural spline functions with 6 df for temperature and 3 df for relative humidity to control for potentially nonlinear confounding effects of weather conditions in areas where such data were available. To determine an appropriate lag time (i.e., the number of days between exposure and the estimated effect) for PM and temperature to be used in the main analyses, we compared a variety of lag days using generalized cross-validation scores.
In the second stage, we used random-effects models to pool the estimates of the city-specific associations of PM concentrations with mortality.18 We then reported the pooled estimate and related 95% confidence intervals as the percentage change in daily mortality per 10-μg-per-cubic-meter increase in PM concentrations. Between-city heterogeneity was quantified with the use of the I2 statistic.
In addition to the main model described above, we fitted two-pollutant models, each of which included adjustment for one of four gaseous pollutants. The association of PM concentration with mortality was considered robust if the effect estimates in the single-pollutant and two-pollutant models were not significantly different, as determined with a paired z-test.
Using the aforementioned two-stage approach, we also performed regional analyses, with the regions grouped according to WHO region and according to the gross domestic product (GDP) per capita (Table S2 in the Supplementary Appendix), and likelihood-ratio tests were used to determine whether the differences between regions in associations of PM with mortality were significant. To further explore potential effect modifications, we fit meta-regression models with annual mean concentrations of PM and copollutants, annual mean temperature, latitude of locations, WHO region and region classified according to the GDP per capita, rates of missing data on daily mortality and PM10 and PM2.5 concentrations, and GDP per capita.
To estimate the overall shape of the associations between PM10 and PM2.5 concentrations and mortality at the global or country level, we plotted concentration–response curves using the same approach that was used in previous studies.16,19 In brief, we replaced the linear term of PM in the main model with a B-spline function with two knots at the 25th and 75th percentiles of the mean PM concentrations across all cities.
We performed several sensitivity analyses. First, in fitting the concentration–response curves, we placed knots at different PM values. Second, we tested the potential confounding effect of humidity in cities that had available data on this variable by comparing the results of models that adjusted for humidity with the results of models that did not in a paired z-test. Third, we restricted the analyses to data available after the year 2000.
We conducted all statistical analyses with R software, version 3.3.1 (R Foundation for Statistical Computing), using the mgcv package for fitting main models and the rmeta package for performing random-effect models. A P value of less than 0.05 was considered to indicate statistical significance. More details are presented in the Methods section in the Supplementary Appendix.
RESULTS
DESCRIPTIVE ANALYSES
The final analysis included 59.6 million deaths from any cause or nonexternal causes, 20.1 million deaths from cardiovascular diseases, and 5.6 million deaths from respiratory diseases (Table S1 [nontrimmed data] and Table S3 [trimmed data] in the Supplementary Appendix). On average, the annual mean concentration of PM10 in 598 cities was 56.0 μg per cubic meter (median, 44.3 μg per cubic meter [range, 11.0 to 295.0; interquartile range, 37.9 to 70.1]), and the annual mean concentration of PM2.5 in 499 cities was 35.6 μg per cubic meter (median, 31.9 μg per cubic meter [range, 4.1 to 116.9; interquartile range, 21.5 to 43.5]). PM10 was strongly correlated with PM2.5, with a mean Pearson correlation coefficient of 0.78. The mean Pearson correlation coefficients between PM10 and gaseous pollutants were 0.46 with nitrogen dioxide, 0.20 with ozone, 0.38 with sulfur dioxide, and 0.40 with carbon monoxide. The corresponding coefficients between PM2.5 and gaseous pollutants were 0.48, 0.22, 0.40, and 0.45. Other descriptive statistics and the correlations between daily mean PM concentrations and weather variables are summarized in the Results section in the Supplementary Appendix.
REGRESSION ANALYSES
The choice of a 2-day moving average for PM concentration, which represents the average over the same and previous day (lag 0 to 1 day), and a 4-day moving average for temperature, which represents the average of the same and previous 3 days (lag 0 to 3 days), generated the smallest mean generalized cross-validation scores (Tables S4 and S5 in the Supplementary Appendix). These moving averages were then applied in subsequent analyses. For both PM10 and PM2.5, the associations were significant on lag 0 day and then attenuated substantially on lag 1 to 2 days; the estimates of the associations were strongest on lag 0 to 1 day (Table S4 in the Supplementary Appendix).
Overall, we observed positive and significant associations between PM10 and PM2.5 concentrations and all-cause mortality (Table 1). In 598 cities that had data on PM10, an increase of 10 μg per cubic meter in the PM10 concentration was associated with an increase of 0.44% (95% confidence interval [CI], 0.39 to 0.50) in a pooled estimate of daily all-cause mortality. In 499 cities that had data on PM2.5, the same increase in the PM2.5 concentration was associated with an increase of 0.68% (95% CI, 0.59 to 0.77) in a pooled estimate of daily all-cause mortality. The country-specific estimates of the percentage change in daily all-cause mortality showed considerable variations, ranging from 0.03% (for Colombia) to 1.32% (for Australia) in association with a 10-μ-per-cubic-meter increase in PM10 concentration and ranging from 0.03% (for Portugal) to 2.54% (for Greece) in association with the same increase in PM2.5 concentration. Estimates of the effect in France, Estonia, and Switzerland were close to the global median estimate of 0.46% in association with PM10 concentration; estimates of the effect in Switzerland and South Africa were close to the global median estimate of 0.80% in association with PM2.5 concentration.
Table 1.
Percentage Change in All-Cause Mortality per 10-μg-per-Cubic-Meter Increase in 2-Day Moving Average Concentrations of Inhalable Particulate Matter (PM10) and Fine Particulate Matter (PM2.5).*
| Country or Region | PM10 | PM2.5 | ||
|---|---|---|---|---|
| Cities with Available Data | Pooled Estimate | Cities with Available Data | Pooled Estimate | |
| no. | % (95% CI) | no. | % (95% CI) | |
| Australia | 3 | 1.32 (0.22 to 2.44) | 3 | 1.42 (−0.12 to 2.99) |
| Brazil | 1 | 1.22 (0.97 to 1.47) | 0 | NA |
| Canada | 13 | 0.76 (0.25 to 1.27) | 25 | 1.70 (1.17 to 2.23) |
| Chile | 4 | 0.33 (0.14 to 0.53) | 4 | 0.27 (−0.68 to 1.23) |
| China | 272 | 0.28 (0.22 to 0.34) | 272 | 0.41 (0.32 to 0.50) |
| Colombia | 1 | 0.03 (−0.34 to 0.39) | 0 | NA |
| Czech Republic | 1 | 0.40 (−0.02 to 0.82) | 0 | NA |
| Estonia | 4 | 0.46 (−0.69 to 1.63) | 3 | 0.23 (−4.24 to 4.90) |
| Finland | 1 | 0.07 (−0.51 to 0.65) | 1 | 0.14 (−0.55 to 0.83) |
| France | 18 | 0.46 (−0.15 to 1.07) | 0 | NA |
| Greece | 1 | 0.53 (0.17 to 0.90) | 1 | 2.54 (1.28 to 3.83) |
| Italy | 18 | 0.65 (0.26 to 1.04) | 0 | NA |
| Japan | 47 | 1.05 (0.78 to 1.31) | 47 | 1.42 (1.05 to 1.81) |
| Mexico | 8 | 0.67 (0.48 to 0.86) | 3 | 1.29 (0.21 to 2.39) |
| Portugal | 2 | 0.11 (−0.27 to 0.49) | 1 | 0.03 (−1.14 to 1.21) |
| South Africa | 6 | 0.41 (0.14 to 0.68) | 5 | 0.80 (0.16 to 1.44) |
| South Korea | 7 | 0.42 (0.27 to 0.58) | 0 | NA |
| Spain | 45 | 0.87 (0.60 to 1.15) | 19 | 1.96 (1.18 to 2.75) |
| Sweden | 1 | 0.20 (−1.03 to 1.44) | 1 | 0.08 (−1.44 to 1.62) |
| Switzerland | 8 | 0.47 (−0.36 to 1.31) | 4 | 0.79 (−0.96 to 2.58) |
| Taiwan | 3 | 0.25 (−0.03 to 0.53) | 3 | 0.62 (−0.39 to 1.64) |
| Thailand | 19 | 0.61 (0.24 to 0.99) | 0 | NA |
| United Kingdom | 15 | 0.06 (−0.36 to 0.48) | 0 | NA |
| United States | 100 | 0.79 (0.60 to 0.98) | 107 | 1.58 (1.28 to 1.88) |
| Total | 598 | 0.44 (0.39 to 0.50) | 499 | 0.68 (0.59 to 0.77) |
Pooled estimates represent the percentage changes in daily all-cause mortality per 10-μg-per-cubic-meter increase in concentrations of particulate matter (PM) with an aerodynamic diameter of 10 μm or less (PM10) and PM with an aerodynamic diameter of 2.5 μm or less (PM2.5), as determined with the use of trimmed exposure data in which the highest 5% and lowest 5% of PM10 and PM2.5 measurements were excluded. NA denotes not available.
In cause-specific analyses, an increase of 10 μg per cubic meter in PM10 concentration (in 528 cities) was associated with an increase of 0.36% (95% CI, 0.30 to 0.43) in daily cardiovascular mortality and an increase of 0.47% (95% CI, 0.35 to 0.58) in daily respiratory mortality. The corresponding increases in daily cardiovascular and respiratory mortality for the same increase in PM2.5 concentration (in 488 cities) were 0.55% (95% CI, 0.45 to 0.66) and 0.74% (95% CI, 0.53 to 0.95%) (Figs. S1 and S2 in the Supplementary Appendix). In 445 cities that had data on both PM2.5 and PM10, the percentage increases in all-cause mortality per 10-μg-per-cubic-meter increase in PM2.5 concentration were larger than those with the same increase in PM10 concentration, both in the pooled results and in most country-specific estimates (Fig. S3 in the Supplementary Appendix).
Regional analyses indicated differences between areas (Table S6 in the Supplementary Appendix), with higher estimates of the effect in the region of the Americas and smaller estimates in the Western Pacific region. We observed stronger associations between PM10 and PM2.5 concentrations and all-cause mortality in locations with lower annual mean concentrations of PM and higher annual mean temperatures (P<0.001 for all comparisons); there was no significant modification of the effect according to annual mean concentrations of PM and copollutants, latitude of location, WHO region and region classified according to the GDP per capita, rates of missing data on daily mortality and PM10 and PM2.5 concentrations, and GDP per capita (P>0.05 for all comparisons).
In two-pollutant models (Table 2), the magnitude (i.e., the size of the estimated effect) of the associations of PM10 and PM2.5 concentrations on lag 0 to 1 day with all-cause mortality decreased, but all associations between PM and mortality remained significant after adjustment for gaseous pollutants. Notably, the estimates of the percentage change in mortality per 10-μg-per-cubic-meter increase in PM10 concentration decreased significantly after adjustment for nitrogen dioxide (difference of 35%; P<0.001) and sulfur dioxide (difference of 18%; P=0.007). Similarly, the percentage change in mortality with the same increase in PM2.5 concentration decreased by 36% after adjustment for nitrogen dioxide (P<0.001) and by 22% after adjustment for sulfur dioxide (P = 0.007).
Table 2.
Percentage Change in All-Cause Mortality in Association with an Increase of 10 μg per Cubic Meter in the 2-Day Moving Average Concentrations of PM10 and PM2.5, with and without Adjustment for Copollutants.*
| Models | PM10 | PM2.5 | ||||
|---|---|---|---|---|---|---|
| Cities with Available Data | Pooled Estimate | P Value for Difference | Cities with Available Data | Pooled Estimate | P Value for Difference | |
| no. | % (95% CI) | no. | % (95% CI) | |||
| PM and ozone | 559 | 0.90 | 487 | 0.75 | ||
| Single-pollutant model of PM | 0.43 (0.37–0.48) | 0.68 (0.58–0.77) | ||||
| Two-pollutant model of PM with adjustment for ozone | 0.43 (0.37–0.49) | 0.66 (0.56–0.77) | ||||
| PM and nitrogen dioxide | 495 | <0.001 | 466 | <0.001 | ||
| Single-pollutant model of PM | 0.43 (0.37–0.49) | 0.66 (0.57–0.76) | ||||
| Two-pollutant model of PM with adjustment for nitrogen dioxide | 0.28 (0.22–0.35) | 0.42 (0.31–0.53) | ||||
| PM and sulfur dioxide | 495 | 0.007 | 466 | 0.007 | ||
| Single-pollutant model of PM | 0.44 (0.38–0.50) | 0.67 (0.57–0.76) | ||||
| Two-pollutant model of PM with adjustment for sulfur dioxide | 0.36 (0.30–0.42) | 0.52 (0.42–0.62) | ||||
| PM and carbon monoxide | 445 | 0.75 | 416 | 0.50 | ||
| Single-pollutant of PM | 0.40 (0.34–0.46) | 0.61 (0.51–0.71) | ||||
| Two-pollutant model of PM with adjustment for carbon monoxide | 0.39 (0.32–0.46) | 0.57 (0.46–0.68) | ||||
Pooled estimates are of the percentage change in daily all-cause mortality per 10-μg-cubic-meter increase in PM10 or PM2.5 concentration. The P value for difference was calculated by evaluating a binary variable (with and without the adjustment for the copollutant) in a paired z-test with estimates from both single-pollutant and two-pollutant models. A P value of less than 0.05 was considered to be statistically significant for the difference.
The concentration–response associations of daily mean PM10 and PM2.5 concentrations with all-cause mortality were positive, and the curves showed a consistent increase with no discernible thresholds (Fig. 3). The slopes for both curves were steeper at concentrations lower than 40 μg per cubic meter for PM10 and lower than 20 μg per cubic meter for PM2.5. The slopes seemed to flatten at high ranges. In addition, positive associations were still detectable at levels below most global and regional air-quality guidelines or standards. Country-specific concentration–response curves are provided in Figures S4 and S5 in the Supplementary Appendix.
Figure 3. Pooled Concentration–Response Curves.
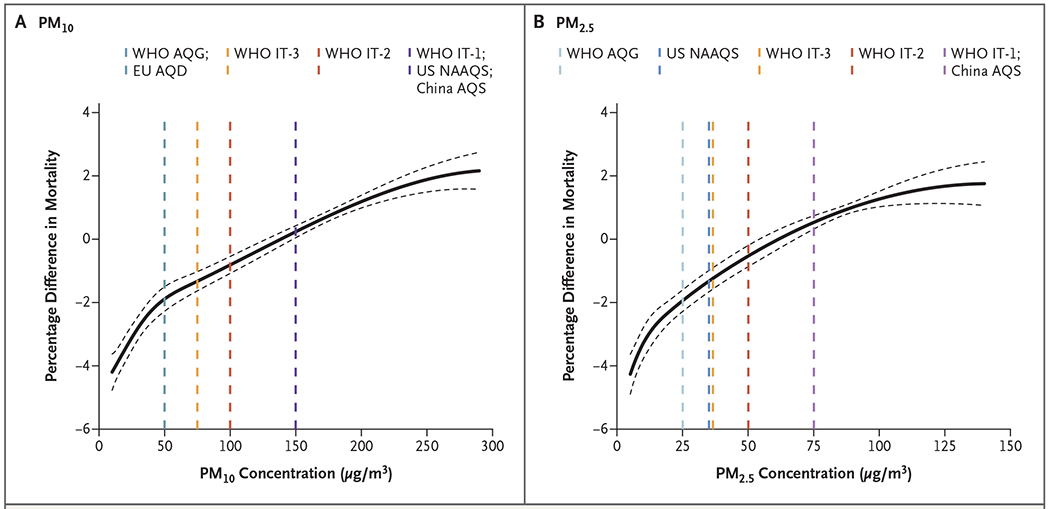
Shown are the pooled concentration–response curves for the associations of 2-day moving average concentrations of PM10 (Panel A) and PM2.5 (Panel B) with daily all-cause mortality. The y axis represents the percentage difference from the pooled mean effect (as derived from the entire range of PM concentrations at each location) on mortality. Zero on the y axis represents the pooled mean effect, and the portion of the curve below zero denotes a smaller estimate than the mean effect. The dashed lines represent the air-quality guidelines or standards for 24-hour average concentrations of PM10 or PM2.5 according to the World Health Organization Air Quality Guidelines (WHO AQG), WHO Interim Target 1 (IT-1), WHO Interim Target 2 (IT-2), WHO Interim Target 3 (IT-3), European Union Air Quality Directive (EU AQD), U.S. National Ambient Air Quality Standard (NAAQS), and China Air Quality Standard (AQS).
Sensitivity analyses confirmed these results. The use of alternative knots did not substantially change the shape of the concentration–response curves, and adjustment for humidity resulted in no significant changes (Figs. S6 and S7 and Table S7 in the Supplementary Appendix). Finally, the analysis in which the subset of data since the year 2000 was used provided similar estimates. Estimates based on nontrimmed PM data are provided in Table S8 in the Supplementary Appendix.
DISCUSSION
Our study analyzed multisite data on air pollution and mortality in 652 cities across different countries and regions, although most countries and cities were in the northern hemisphere. Because the data from each city were analyzed according to the same protocol, the estimate of the percentage change in mortality per 10-μg-per-cubic-meter increase in PM concentration was based on a large data set. This study also provides the statistical power to examine the global concentration–response functions of particulate air pollution at both low and high baseline levels.
In the analysis of PM10, we observed an increase of 0.44% in all-cause mortality per 10-μg-per-cubic-meter increase in PM10 concentration. The magnitude of the association is generally similar to previous findings in other multicity or multicountry studies.7–9,20 For example, the Air Pollution and Health: A European and North American Approach (APHENA) study reported increases of 0.86%, 0.33%, and 0.29% in daily all-cause mortality in Canada, Europe, and the United States, respectively.9 The percentage increase in mortality for the same increase in PM10 concentration was 0.77% in the Multicity Study of Air Pollution and Mortality in Latin America (ESCALA),8 0.55% in the Public Health and Air Pollution in Asia (PAPA) study,7 and 0.19% in the reanalysis of the U.S. National Morbidity Mortality Air Pollution Study (NMMAPS).21
In the analysis of PM2.5, we observed an increase of 0.68% in all-cause mortality per 10-μg-per-cubic-meter increase in PM2.5 concentration. Our estimates were somewhat smaller than those obtained from previous multicity studies and a meta-analysis that used data mainly from developed countries.22,23 This difference may be interpreted as reflecting the nonlinearity of our concentration–response curve, which indicated a steeper slope at lower concentrations. In addition, we found that the associations of mortality with PM concentrations were slightly stronger with PM2.5 than with PM10 in most countries and regions, which added to the evidence that PM2.5 accounted for a larger proportion of the effects of PM10 and PM2.5 combined.6 The stronger effects of PM2.5 may also be supported by the abundant evidence that this particulate fraction contains more small particles that can absorb toxic components from the air and penetrate deep into the lungs.24
The question of whether the observed associations for PM were independent from other pollutants is important for air-quality regulation and health-risk assessment. In our data, although the magnitude of the associations for PM10 and PM2.5 decreased in two-pollutant models, the associations for both remained significant, a finding that provides evidence of the independent health effects of PM. It is notable that the estimates of the percentage change in mortality per 10-μg-per-cubic-meter increase in PM10 and PM2.5 concentrations decreased more after adjustment for nitrogen dioxide and sulfur dioxide than after adjustment for ozone and carbon monoxide, a finding that may be interpreted as reflecting closer correlations of PM with nitrogen dioxide and sulfur dioxide caused by similar sources and seasonal patterns.
In accordance with the findings from the majority of previous studies, the concentration–response curves between PM concentration and daily mortality derived from this global study showed a consistent increase without evidence of a threshold.16,19,22 In both curves, the percentage increase in mortality per unit change in PM concentration seemed to be smaller (i.e., the concentration–response curves seemed to flatten) at high ranges of daily mean PM concentration. This potential saturation effect may be explained by smaller effects of changes in daily mean PM concentration in cities with higher baseline levels of PM, as suggested in our meta-regression analyses. Furthermore, the higher proportion of young people in developing countries may decrease population susceptibility to PM, and less outdoor activity during days with high pollution levels may decrease exposure. Nevertheless, the concentrations of PM below the current air-quality guidelines and standards may still be hazardous to public health. However, associations estimated for extreme PM concentrations are characterized by wider confidence intervals, with greater uncertainty about the actual mortality risk at such values. We should also be cautious about the uncertainty in the concentration–response curves, because they were pooled from cities or countries with diverse PM ranges and varying population susceptibility and data quality and representability.
We found significant evidence of spatial heterogeneity in the associations between PM concentration and daily mortality across countries and regions. A number of factors could contribute to this variability, including different PM components, long-term air pollution levels, population susceptibility, and different lengths of study periods. We also found that higher annual mean concentrations of PM10 and PM2.5 were accompanied by weaker associations with daily mortality, a finding that has been reported in previous studies.16,25 The possible adaptive response to PM in populations living in areas with higher long-term exposure to PM may lead to smaller esti-mate-per-unit changes in exposure. In addition, we identified stronger associations of PM with mortality in regions with higher GDP per capita, which may also be in relation to lower long-term air pollution levels (Pearson coefficient, −0.68 for PM10 and −0.74 for PM2.5) and decreased population susceptibility due to higher socioeconomic status.26 The estimates of the association between PM and mortality in some countries (e.g., France, Finland, Sweden, and the United Kingdom) were smaller and not significant. These countries had fewer cities included and shorter periods evaluated, which may increase the statistical uncertainty in the estimation of the effect. Furthermore, these countries are generally located in areas with a low annual mean temperature, which may decrease the association between PM and mortality, as shown in meta-regression analyses. More interpretations on this issue are provided in the Discussion section in the Supplementary Appendix.
This study has several limitations. First, although the analysis included 24 major countries and regions on six continents, our findings cannot be interpreted as fully globally representative because the 652 cities were mainly located in East Asia, Europe, and North America, with a smaller number of cities in Latin America and Africa. Second, we relied on fixed-site environmental measurements, which could introduce exposure misclassification. Third, diagnostic or coding errors in health data are also inevitable in such a global study that spans multiple decades; the effects of these errors on our results are difficult to evaluate, which presumably makes the estimates of the effects on cause-specific mortality less reliable than those of effects on all-cause mortality. Fourth, there are some missing data, but their influence on our estimates was not substantial (see the Discussion section and Table S9 in the Supplementary Appendix).
Our multicountry time-series analysis provides evidence on positive associations between short-term exposure to PM10 and PM2.5 and daily all-cause, cardiovascular, and respiratory mortality. This study indicated independent associations of PM10 and PM2.5 concentrations with daily mortality after adjustment for gaseous pollutants. Further, concentration–response curves for the effects of PM on mortality showed a consistent increase, with flattening of the slopes at higher concentrations, and the associations were still detectable at concentrations below the current air-quality guidelines and regulatory limits.
Supplementary Material
Acknowledgments
Supported by the National Natural Science Foundation of China (grants 91843302 and 91643205, to Dr. Kan); the China Medical Board Collaborating Program (grant 16-250); the Medical Research Council, United Kingdom (grants MR/R013349/1 and MR/M022625/1, to Drs. Gasparrini, Sera, and Vicedo-Cabrera); the Career Development Fellowship of the Australian National Health and Medical Research Council (grants APP1107107 and APP1163693, to Dr. Guo); the Ministry of Education of Spain (grant PRX17/00705, to Dr. Tobias); the National Plan for I+D+I (grant PI15/00515), cofunded by the Instituto de Salud Carlos III Directorate General for Evaluation and the European Regional Development Fund (FEDER); the Global Research Laboratory (grant K21004000001-10A0500-00710 from the National Research Foundation of Korea, funded by the Ministry of Science, Information and Communication Technologies, to Dr. Kim); the Academy of Finland (grants 310372 and 310373, to Drs. Jaakkola and Ryti); the Estonian Ministry of Education and Research (grant IUT34-17, to Drs. Orru and Indermitte); the Czech Science Foundation (grant 18-22125S, to Drs. Kyselý and Urban); and a Professional Services Agreement with the Health Effects Institute, United States (to Dr. Cohen).
We thank Benjawan Tawatsupa and Kornwipa Punnasiri for providing environmental data for Thailand and Fiorella Acquaotta at the University of Turin for providing the temperature data for South Africa.
Footnotes
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Cong Liu, School of Public Health, Key Laboratory of Public Health Safety of the Ministry of Education and National Health Commission Key Laboratory of Health Technology Assessment, Shanghai, China
Renjie Chen, School of Public Health, Key Laboratory of Public Health Safety of the Ministry of Education and National Health Commission Key Laboratory of Health Technology Assessment, Shanghai, China; Shanghai Key Laboratory of Atmospheric Particle Pollution and Prevention, Shanghai, China
Francesco Sera, Department of Public Health, Environments and Society, London
Ana M. Vicedo-Cabrera, Department of Public Health, Environments and Society, London
Yuming Guo, School of Public Health and Management, Binzhou Medical University, Yantai, China; Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia
Shilu Tong, Fudan University, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China; School of Public Health, Institute of Environment and Population Health, Anhui Medical University, Hefei, China; School of Public Health and Social Work, Queensland University of Technology, Brisbane, Australia
Micheline S.Z.S. Coelho, Institute of Advanced Studies, University of São Paulo, São Paulo
Paulo H.N. Saldiva, Institute of Advanced Studies, University of São Paulo, São Paulo
Eric Lavigne, Air Health Science Division, Health Canada, and the School of Epidemiology and Public Health, University of Ottawa, Ottawa, ON
Patricia Matus, Department of Public Health, Universidad de los Andes, Santiago, Chile
Nicolas Valdes Ortega, School of Nursing and Obstetrics, Universidad de los Andes, Santiago, Chile
Samuel Osorio Garcia, Hospital Vista Hermosa, Bogota, Colombia
Mathilde Pascal, Santé Publique France, French National Public Health Agency, Saint Maurice, France
Massimo Stafoggia, Department of Epidemiology, Lazio Regional Health Service–ASL Roma 1, Rome; Karolinska Institute, Institute of Environmental Medicine, Stockholm, Sweden
Matteo Scortichini, Department of Epidemiology, Lazio Regional Health Service–ASL Roma 1, Rome
Masahiro Hashizume, Department of Pediatric Infectious Diseases, Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan
Yasushi Honda, Faculty of Health and Sport Sciences, University of Tsukuba, Tsukuba, Japan
Magali Hurtado-Díaz, Department of Environmental Health, National Institute of Public Health, Cuernavaca, Mexico
Julio Cruz, Department of Environmental Health, National Institute of Public Health, Cuernavaca, Mexico
Baltazar Nunes, Department of Epidemiology, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon, Portugal
João P. Teixeira, Department of Epidemiology, Instituto Nacional de Saúde Dr. Ricardo Jorge, Lisbon, Portugal; Epidemiology Research Unit–Instituto de Saúde Pública, Universidade do Porto, Porto, Portugal
Ho Kim, Department of Public Health Science, Graduate School of Public Health and Institute of Health and Environment, Seoul National University, Seoul, South Korea
Aurelio Tobias, Institute of Environmental Assessment and Water Research, Spanish Council for Scientific Research, Barcelona, Spain
Carmen Íñiguez, Department of Statistics and Computational Research, University of Valencia Environmental Health Joint Research Unit Fundación para el Fomento de la Investigatión Sanitaria y Biomédica de la Comunitat Valenciana–Universitat de València-Universitat Jaume I de Castellón Biomedical Research Center Network for Epidemiology and Public Health, Valencia, Spain
Bertil Forsberg, Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden
Christofer Åström, Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden
Martina S. Ragettli, Swiss Tropical and Public Health Institute and the University of Basel, Basel, Switzerland
Yue-Leon Guo, Environmental and Occupational Medicine, National Taiwan University, Taipei City; College of Medicine and National Taiwan University Hospital, Taipei City
Bing-Yu Chen, Environmental and Occupational Medicine, National Taiwan University, Taipei City
Michelle L. Bell, School of Forestry and Environmental Studies, Yale University, New Haven, CT
Caradee Y. Wright, Environment and Health Research Unit, South African Medical Research Council, Pretoria, South Africa; Department of Geography, Geo-informatics, and Meteorology, University of Pretoria, Pretoria, South Africa
Noah Scovronick, Department of Environmental Health, Rollins School of Public Health, Emory University, Atlanta
Rebecca M. Garland, Department of Geography, Geo-informatics, and Meteorology, University of Pretoria, Pretoria, South Africa; Natural Resources and the Environment Unit, Council for Scientific and Industrial Research, Pretoria, South Africa; Unit for Environmental Sciences and Management, North-West University, Potchefstroom, South Africa
Ai Milojevic, Department of Public Health, Environments and Society, London
Jan Kyselý, Institute of Atmospheric Physics, Czech Academy of Sciences, Czech University of Life Sciences, Prague, Czech Republic; Faculty of Environmental Sciences, Czech University of Life Sciences, Prague, Czech Republic
Aleš Urban, Institute of Atmospheric Physics, Czech Academy of Sciences, Czech University of Life Sciences, Prague, Czech Republic
Hans Orru, Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden; Institute of Family Medicine and Public Health, University of Tartu, Tartu, Estonia
Ene Indermitte, Institute of Family Medicine and Public Health, University of Tartu, Tartu, Estonia
Jouni J.K. Jaakkola, Center for Environmental and Respiratory Health Research, University of Oulu, Medical Research Center Oulu, and Oulu University Hospital and University of Oulu, Oulu, Finland
Niilo R.I. Ryti, Center for Environmental and Respiratory Health Research, University of Oulu, Medical Research Center Oulu, and Oulu University Hospital and University of Oulu, Oulu, Finland
Klea Katsouyanni, London School of Hygiene and Tropical Medicine, and the School of Population Health and Environmental Sciences, King’s College London, London; Department of Hygiene, Epidemiology and Medical Statistics, School of Medicine, National and Kapodistrian University of Athens, Athens
Antonis Analitis, Department of Hygiene, Epidemiology and Medical Statistics, School of Medicine, National and Kapodistrian University of Athens, Athens
Antonella Zanobetti, Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston
Joel Schwartz, Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston
Jianmin Chen, Department of Environmental Science and Engineering, Shanghai, China
Tangchun Wu, Key Laboratory of Environment and Health, Ministry of Education, and State Key Laboratory of Environmental Health (Incubating), School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Aaron Cohen, Health Effects Institute, Boston; Institute for Health Metrics and Evaluation, University of Washington, Seattle
Antonio Gasparrini, Department of Public Health, Environments and Society, London; Centre for Statistical Methodology, London
Haidong Kan, School of Public Health, Key Laboratory of Public Health Safety of the Ministry of Education and National Health Commission Key Laboratory of Health Technology Assessment, Shanghai, China; Shanghai Key Laboratory of Atmospheric Particle Pollution and Prevention, Shanghai, China; Children’s Hospital of Fudan University, National Center for Children’s Health, China
REFERENCES
- 1.Rückerl R, Schneider A, Breitner S, Cyrys J, Peters A. Health effects of particulate air pollution: a review of epidemiological evidence. Inhal Toxicol 2011;23:555–92. [DOI] [PubMed] [Google Scholar]
- 2.Brunekreef B, Holgate ST. Air pollution and health. Lancet 2002;360:1233–42. [DOI] [PubMed] [Google Scholar]
- 3.Héroux ME, Anderson HR, Atkinson R, et al. Quantifying the health impacts of ambient air pollutants: recommendations of a WHO/Europe project. Int J Public Health 2015;60:619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: global update 2005: summary of risk assessment. Geneva: World Health Organization, 2006. [PubMed] [Google Scholar]
- 5.Dai L, Zanobetti A, Koutrakis P, Schwartz JD. Associations of fine particulate matter species with mortality in the United States: a multicity time-series analysis. Environ Health Perspect 2014;122:837– 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu F, Xu D, Cheng Y, et al. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ Res 2015;136:196–204. [DOI] [PubMed] [Google Scholar]
- 7.Wong CM, Vichit-Vadakan N, Kan H, Qian Z. Public Health and Air Pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ Health Perspect 2008;116:1195– 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romieu I, Gouveia N, Cifuentes LA, et al. Multicity study of air pollution and mortality in Latin America (the ESCALA study). Res Rep Health Eff Inst 2012;171;5–86. [PubMed] [Google Scholar]
- 9.Katsouyanni K, Samet JM, Anderson HR, et al. Air pollution and health: a European and North American approach (APHENA). Res Rep Health Eff Inst 2009;142:5–90. [PubMed] [Google Scholar]
- 10.Gasparrini A, Guo Y, Hashizume M, et al. Temporal variation in heat-mortality associations: a multicountry study. Environ Health Perspect 2015;123:1200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Y, Gasparrini A, Armstrong B, et al. Global variation in the effects of ambient temperature on mortality: a systematic evaluation. Epidemiology 2014;25;781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasparrini A, Guo Y, Hashizume M, et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 2015;386:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International statistical classification of diseases and related health problems. Geneva: World Health Organization, 2004. [Google Scholar]
- 14.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med 2000;343:1742–9. [DOI] [PubMed] [Google Scholar]
- 15.Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the National Morbidity, Mortality, and Air Pollution Study. Epidemiology 2005;16:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, Yin P, Meng X, et al. Fine particulate air pollution and daily mortality: a nationwide analysis in 272 Chinese cities. Am J Respir Crit Care Med 2017;196:73–81. [DOI] [PubMed] [Google Scholar]
- 17.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006;295:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah AS, Langrish JP, Nair H, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet 2013;382:1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samoli E, Analitis A, Touloumi G, et al. Estimating the exposure-response relationships between particulate matter and mortality within the APHEA multicity project. Environ Health Perspect 2005;113:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samoli E, Peng R, Ramsay T, et al. Acute effects of ambient particulate matter on mortality in Europe and North America: results from the APHENA study. Environ Health Perspect 2008;116:1480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominici F, Peng RD, Zeger SL, White RH, Samet JM. Particulate air pollution and mortality in the United States: did the risks change from 1987 to 2000? Am J Epidemiol 2007;166:880–8. [DOI] [PubMed] [Google Scholar]
- 22.Di Q, Dai L, Wang Y, et al. Association of short-term exposure to air pollution with mortality in older adults. JAMA 2017;318:2446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax 2014;69:660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KH, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ Int 2015;74:136–43. [DOI] [PubMed] [Google Scholar]
- 25.Chen R, Kan H, Chen B, et al. Association of particulate air pollution with daily mortality: the China Air Pollution and Health Effects Study. Am J Epidemiol 2012;175:1173–81. [DOI] [PubMed] [Google Scholar]
- 26.Martins MCH, Fatigati FL, Véspoli TC, et al. Influence of socioeconomic conditions on air pollution adverse health effects in elderly people: an analysis of six regions in São Paulo, Brazil. J Epidemiol Community Health 2004;58:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


