Abstract
Introduction: Reports on the neurotoxic and neuroprotective effects of cannabidiol (CBD) have not been in complete accord, showing different and somewhat contradictory results depending upon the brain cell types and experimental conditions employed. This work systematically examines the neuroprotective capability of CBD against oxidative stress (i.e., hydrogen peroxide [H2O2]) as well as its toxicity profile in the in vitro culture platform of primary hippocampal neurons.
Materials and Methods: The low cell-density (100 neurons per mm2) culture was used for analyzing the viability and morphology of neurons at a single-cell level with a confocal laser-scanning microscope (CLSM). Primary neurons were obtained from the hippocampal tissues of embryonic day-18 (E18) Sprague-Dawley rat pups and treated with CBD (0.1–100 μM) and/or H2O2 (0.1–50 μM) at 1 DIV (days in vitro).
Results: The lethal concentration 50 (LC50) value (the concentration causing 50% cell death) of CBD was calculated to be 9.85 μM after 24 h of incubation, and that of H2O2 was 2.46 μM under the same conditions. The neuroprotection ratio of CBD against H2O2 ([H2O2]=10 μM) was 2.40 with 5 μM of CBD, increasing the cell viability to 57% from 24%. The CLSM analysis suggested that the cell-death mechanisms were different for CBD and H2O2, and CBD did not completely rescue the morphological alterations of primary hippocampal neurons caused by H2O2, such as neurite degeneration, at least in the in vitro neuron culture.
Conclusion: Although CBD showed both neurotoxic and neuroprotective effects on hippocampal neurons in the in vitro setting, the use of low-concentrated (i.e., 5 μM) CBD, not causing toxic effects on the neurons, significantly rescued the neurons from the oxidative stress (H2O2), confirming its neuroprotection capability.
Keywords: cannabidiol, cell culture, hydrogen peroxide, neuroprotection, primary hippocampal neurons
Introduction
(−)-Cannabidiol (CBD, IUPAC name: 2-[(1R,6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol, C21H30O2), the main nonpsychoactive compound among 100+ phytocannabinoids extracted from the genus Cannabis, anecdotally—if not clinically—shows potential therapeutic benefits in various health care applications, including epilepsy, inflammation, cancer, and neurodegenerative diseases.1–5 The year of 2018 witnessed the first approval of Cannabis-derived CBD (Epidiolex®) for the treatment of epileptic seizures associated with the Lennox–Gastaut or Dravet syndrome in patients 2 years of age and older by the U.S. Food and Drug Administration (FDA).6–8
In addition to the therapeutic effect on epileptic seizures, previous reports suggest that CBD is neuroprotective. The earliest research involved the neuroprotective action of CBD in the rat cortical neuron culture exposed to toxic levels of glutamate and tert-butyl hydroperoxide.9 The bidirectional Ca2+ regulation capability of CBD for primary hippocampal cells (neuron:glia=2:1) was reported, indicating the regulatory role of CBD in Ca2+ homeostasis10–12: for example, the intracellular calcium concentration ([Ca2+]i) was reduced by about 25% of basal Ca2+ level in both neurons and glia by CBD application to a HEPES-based buffer solution with double K+ concentration, while CBD caused the [Ca2+]i elevation, with no significant cell death, by about 45% of basal Ca2+ level under physiological conditions. The neuroprotective properties of CBD against oligomycin, an inhibitor of ATP synthetase,10 and ammonium acetate and ethanol in the hippocampal neuron culture also have been reported.13
Oxidative stress is thought to cause pathological conditions in neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotropic lateral sclerosis.14,15 The cytoprotective effect of CBD against hydrogen peroxide (H2O2), a crucial metabolic molecule in the oxidative stress,16 was reported for cerebellar granule cells17 and oligodendrocyte progenitor cells,18 but there have been no reports on its effect against H2O2 in the neurons that are more relevant to the brain function, such as hippocampal neurons.19 H2O2 has been used as a model molecule to study oxidative stress-induced neural death and also to screen neuroprotective agents on the cultured neurons.16,20–25 As our on-going research program on neurochemistry and neurophysiology,26–31 this study investigates the neuroprotective effect of CBD against H2O2 in the hippocampal neuron culture.
In addition to the neuroprotective property, CBD has also been reported to show different toxicity profiles on different brain cell types. For example, even 0.1 μM of CBD increased [Ca2+]i and induced cell death in the case of optic nerve oligodendrocytes after 24 h of incubation, while 10 μM of CBD did not affect the viability of cortical neurons (CBD exposure time: 20–30 min).32 However, no cytotoxicity was observed with 1 μM of CBD for oligodendrocyte progenitor cells (CBD exposure time: 24 h).18 Furthermore, CBD of below 4 μM did not significantly increase hypodiploid apoptotic cells for primary microglial cells, but CBD of above 8 μM induced apoptosis in a time- and concentration-dependent manner, confirmed by increase in hypodiploid cells and DNA-strand breaks, and activation of capase-8 and -9 (CBD exposure time: 24 h).33 Considering the previous somewhat contradictory results, we first systematically evaluated the in vitro neurotoxic effect of CBD to hippocampal neurons with different CBD concentrations.
Materials and Methods
Chemicals and reagents
CBD (10-mM stock in dimethyl sulfoxide (DMSO), storage at −20°C) was obtained from Cayman Chemical Company. Neurobasal™ medium (Thermo Fisher Scientific), B-27™ serum-free supplement (50×; Thermo Fisher Scientific), GlutaMAX™ supplement (100×; Thermo Fisher Scientific), calcein acetoxymethyl ester (AM; Thermo Fisher Scientific), ethidium homodimer-1 (Thermo Fisher Scientific), CellTracker™ Red CMTPX Dye (Thermo Fisher Scientific), Hank's Balanced Salt Solution (HBSS; Welgene), phosphate-buffered saline (PBS, 10 mM, pH 7.4; Welgene), penicillin/streptomycin (5000 U/mL of penicillin and 5000 μg/mL of streptomycin; Welgene), L-glutamic acid (Sigma), poly-D-lysine hydrobromide (Sigma), paraformaldehyde (Sigma), Triton™ X-100 (Sigma), anti-β-tubulin III antibody produced in rabbit (Sigma), Alexa Fluor™ 488 phalloidin (Invitrogen), Alexa Fluor 594 anti-rabbit goat antibody (Invitrogen), DMSO (Junsei), H2O2 (Junsei), and Accumax™ (Stemcell Technologies) were used as received. Antifade mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) was obtained from Vector Laboratories.
Primary hippocampal neuron culture
Primary hippocampal neurons were dissected from embryonic day-18 (E18) Sprague-Dawley rat pups and treated with Accumax for 10 min to detach the cells from primary tissues. Hippocampi were washed four times with HBSS, suspended in HBSS, centrifuged at 1000 rpm for 3 min, and resuspended with Neurobasal medium containing B-27 serum-free supplement, 2-mM GlutaMAX, 12.5-μM L-glutamic acid, and 1% penicillin/streptomycin. After filtration using a cell strainer, the hippocampal neurons were seeded on glass coverslips coated with poly-D-lysine in a 24-well plate at density of 100 cells/mm2. The cultures were maintained at 37°C under a humidified atmosphere of 5% CO2. This study was approved by IACUC (Institutional Animal Care and Use Committee) of KAIST.
Cell viability assay
For neurotoxicity experiments, the culture media were replaced with the Neurobasal medium containing CBD (0.1–100 μM) or H2O2 (0.1–50 μM) at 1 DIV (days in vitro). In the case of H2O2, heat potentially accelerated the decomposition rate of H2O2, and, therefore, 0.1- or 1-mM H2O2 stock solution was prepared in the 4°C cold medium. The fresh Neurobasal medium was poured to the wells and diluted with a predetermined amount of the H2O2 stock solution at 4°C to minimize the decomposition of H2O2, instead of diluting the media at ambient temperature. For neuroprotection experiments, the culture media were replaced, at 1 DIV, with 990 μL of fresh Neurobasal medium containing CBD of various concentrations (0, 0.1, 1, 2, 3, or 5 μM), followed by the addition of 10 μL of 0.1-mM H2O2 stock solution. For the 0-μM case of CBD 0.1% DMSO was used. As another set, the neurons were also preincubated with 5 μM of CBD for 1 h at 1 DIV, before H2O2 treatment. After incubation for 24 h, the cells were treated with 2-μM calcein AM and 4-μM ethidium homodimer-1 in PBS for 20 min at 37°C. Images of live and dead cells were obtained with an LSM 700 confocal microscope (Carl Zeiss). Cell viability was estimated by using ImageJ software (NIH) and image analysis of ZEN software (Carl Zeiss), and expressed as %viability (mean±standard error) after at least three independent cultures with three replicates.
Fluorescent staining
Neurons were treated with the Neurobasal media containing CBD (1, 5, or 10 μM) or 0.1% DMSO at 1 DIV. After incubation for 24 h, the neurons were treated with Celltracker Red CMTPX Dye at 37°C for 30 min, fixed with 4% paraformaldehyde in PBS for 15 min at room temperature, and washed three times with PBS each for 5 min. The fixed samples were permeabilized with 0.1% Triton X-100 solution in PBS for 10 min at room temperature and washed three times with PBS each for 5 min. The samples were then treated with Alexa Fluor 488 phalloidin for visualizing F-actin or rabbit-produced β-tubulin Ⅲ primary antibody, incubated at 37°C, and washed with PBS three times each for 5 min. For visualizing β-tubulin Ⅲ, the samples were additionally treated with Alexa Fluor 594 goat anti-rabbit secondary antibody. Finally, the samples were mounted on slide glasses with the DAPI solution to stain nuclei for 30 min. Fluorescence images were obtained with an LSM 700 confocal microscope.
Results and Discussion
The primary hippocampal neurons, after dissociation from the hippocampal tissues of E18 Sprague-Dawley rat pups, were seeded on a poly-D-lysine-coated glass coverslip at density of 100 cells/mm2 in the Neurobasal medium, which was replaced with the medium containing CBD (0.1–100 μM) at 1 DIV. In the case of 1% DMSO, which was the DMSO concentration for preparation of 100-μM CBD solution, we did not observe any noticeable difference compared with the nontreated control in cell viability and morphology. Throughout this article, we used the value of %viability, which was defined as % of cell viability with the nontreated control as a reference (i.e., the observed viability divided by the viability of the nontreated cells). After incubation for 24 h (i.e., at 2 DIV), the cells were stained with calcein AM for live cells (green) and ethidium homodimer-1 for dead cells (red), and the confocal laser-scanning microscope (CLSM) images were taken for the viability analysis.
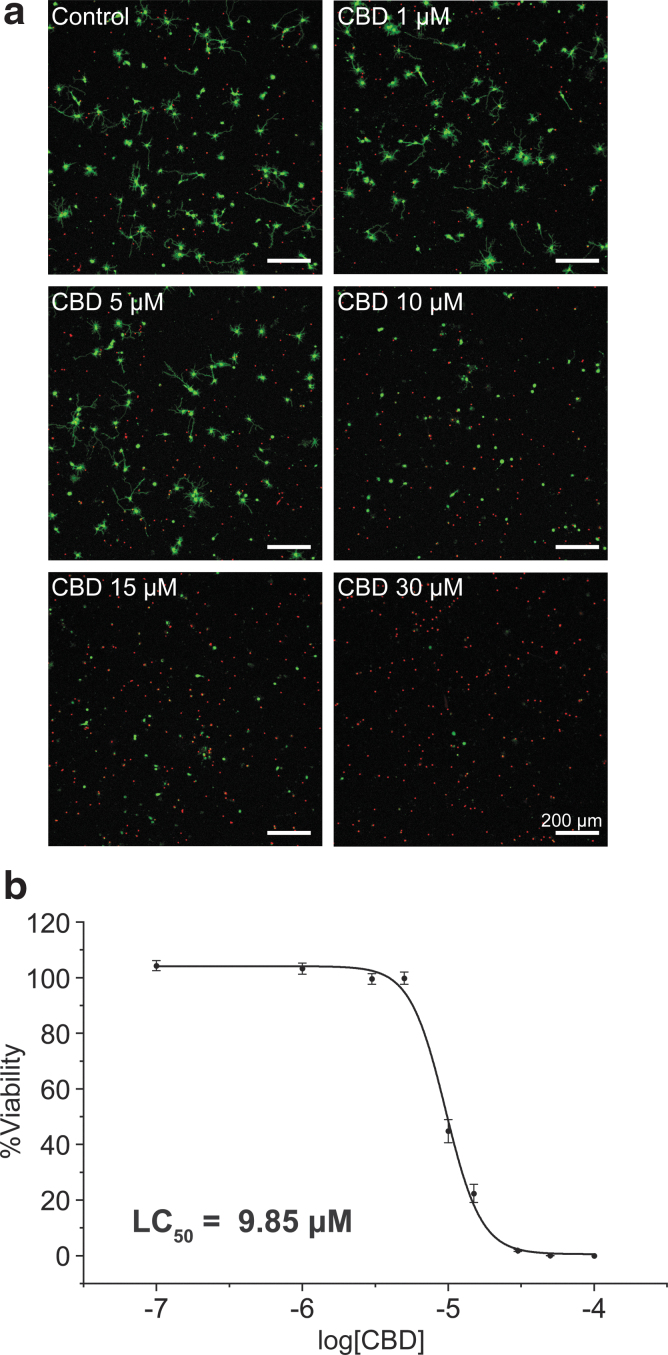
The CLSM images (Fig. 1a) arguably showed the concentration-dependent neurotoxicity of CBD in the hippocampal neuron culture. For example, while the cell death was not noticeable up to 5 μM of CBD, the red fluorescence in CLSM images indicated that most hippocampal neurons were dead with 30 μM of CBD. The quantitative analysis showed that the %viability decreased to 44.80% with 10 μM of CBD, and the lethal concentration 50 (LC50) value, the concentration at which the %viability was 50%, was calculated to be 9.85 μM (Fig. 1b). It is to be noted that this result is the first quantitative in vitro data, affording the LC50 value of CBD, for the hippocampal-neuron culture.
FIG. 1.
Neurotoxicity of CBD to primary hippocampal neurons. The neurons were treated with CBD (0, 0.1, 1, 3, 5, 10, 15, 30, 50, and 100 μM) at 1 DIV, and the CLSM images were taken after 24 h of incubation. (a) CLSM images of hippocampal neurons (green: live, red: dead). (b) A graph of %viability versus log[CBD]. LC50 was calculated as the concentration at which the %viability was 50%. Data are expressed as mean±SE of n=3, each with three replicates. CBD, cannabidiol; CLSM, confocal laser-scanning microscope; DIV, days in vitro; LC50, lethal concentration 50; SE, standard error.
The careful analysis of the CLSM images suggested that CBD might disrupt the cell adhesion. Some CLSM images had green-stained vestiges, and some dying cells, stained in both red and green, had partially detached neurites and cell body. We could frequently find the costained neurons with partial detachment as well as live neurons with no or few neurites, especially in the case of 10 μM of CBD.
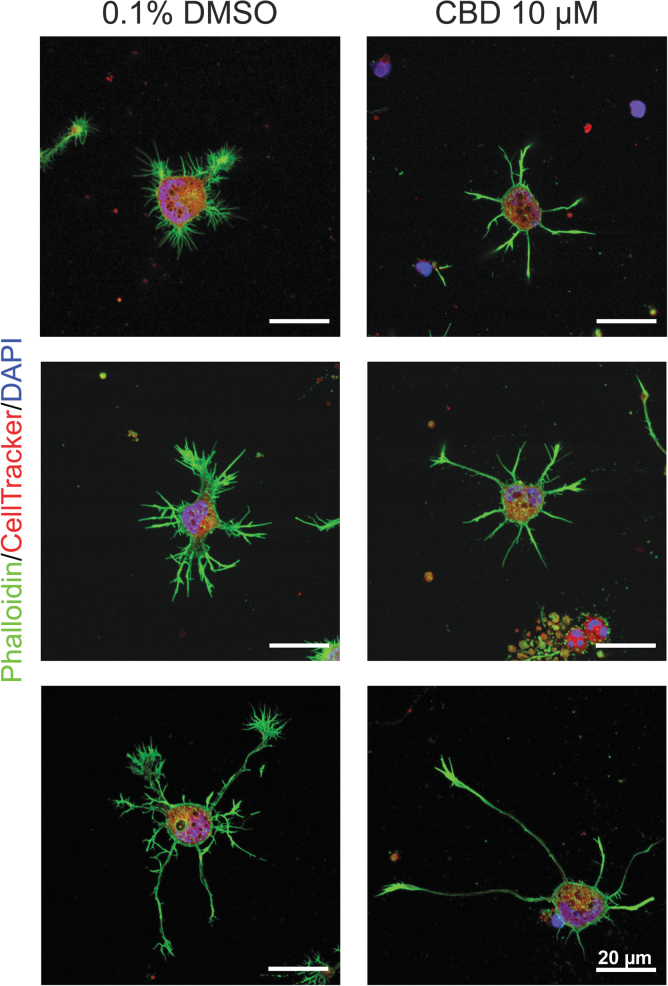
To get some insights into the death-related cell detachment, the cells that remained attached to the culture plate were stained, at 2 DIV, with phalloidin for F-actin (green), CellTracker Red CMTPX Dye for cytoplasm (red), and DAPI for nuclei (blue). The CLSM images (Fig. 2) clearly showed that the neurons did not develop properly in the CBD-containing medium ([CBD]=10 μM), although the quantitative analysis was not feasible due to the cell detachment.
FIG. 2.
Effects of CBD on cell adhesion and morphology. Representative CLSM images of primary hippocampal neurons in CBD (10 μM) or 0.1% DMSO, after staining F-actin (green), cytoplasm (red), and nuclei (blue). DMSO, dimethyl sulfoxide.
Although the developmental rate, judged by the Banker's classification of developmental stages,34 seemed not to be affected by CBD, it was apparent that the neurons presented few, if not no, filopodia and lamellipodia structures. For example, no axonal branches were observed, and the growth cones were shrunken in size noticeably. In addition, we observed some cellular debris, such as only nuclei (top image in Fig. 2), condensed chromatin, and ruptured cells (middle image in Fig. 2). Based on the observation, it was hypothesized that the neuronal death by CBD might be considered to be a kind of detachment-induced apoptosis/anoikis (i.e., apoptosis following the loss of cell anchorage, especially the loss of cell/matrix interaction).35,36
Detailed elucidation on the neurotoxic mechanism of CBD remains to be seen at this moment, but our results implied that the lipophilic CBD might interfere with the integrity of cell membranes, causing the cell rupture and death, at its high concentration. However, it is also to mention that our neurotoxicity results are not translated directly to the in vivo conditions. The brain is composed of neuronal and non-neuronal cells and others, and the in vivo tolerance of hippocampal neurons to CBD would be different from the in vitro conditions. For example, glial cells, such as astrocytes, play supporting roles in the brain. Relevant reports showed that the in vitro neurotoxicity of unnatural monosaccharides to primary hippocampal neurons was mitigated when a hippocampal tissue was used26,28 or a neuron/astrocyte coculture was employed.27 In this respect, it could be explained that the coculture system of neuron and glia (2:1) did not show the neurotoxicity of CBD.10–12
It is to note that the H2O2 neurotoxicity experiments should be performed with caution under the tightly controlled conditions to ensure the data reproducibility, because of the rapid decomposition of H2O2 at ambient temperature. Specifically, the H2O2 stock solution (0.1 or 1 mM) was to be prepared at the low temperature (4°C) and transferred to the culture medium right after preparation (within 5 min). It was also found to be critical that the dilution of the H2O2 stock solution in the culture medium was done at 4°C, not at ambient temperature.
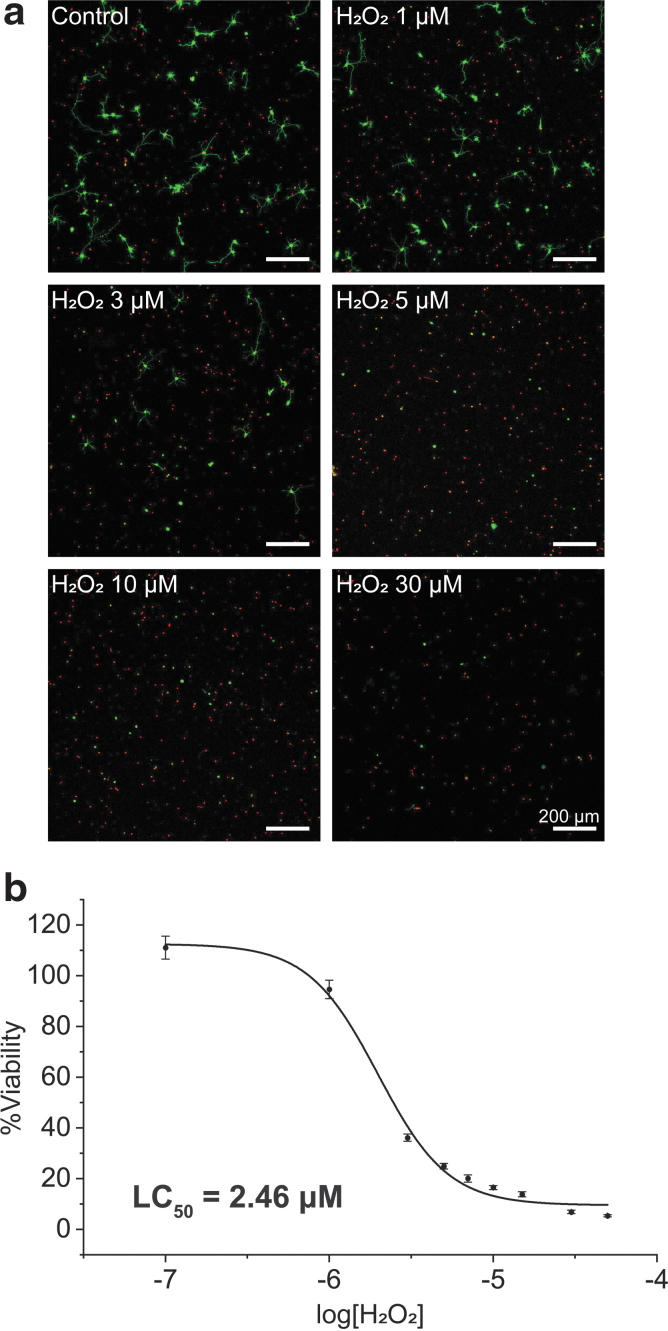
After 24 h of incubation at 37°C, the %viability was obtained by the analysis of the CLSM images (Fig. 3a). There was no significant difference in viability, compared with the control, up to 1 μM of H2O2, but the %viability decreased with H2O2 of 3 μM and above. The LC50 value of H2O2 for hippocampal neurons was calculated to be 2.46 μM, and the %viability decreased to 16.49% with 10 μM of H2O2 (Fig. 3b). The LC50 value of H2O2 has previously been reported to be about 40 μM at the cell density of 700 cells/mm2 and about 92 μM at that of 2000 cells/mm2, after 24 h of incubation, for cortical neurons.21 In comparison, we obtained a lower LC50 value (2.46 μM), which might be caused by the difference in cell type and/or the lower cell density (100 cells/mm2) used for our system.
FIG. 3.
Neurotoxicity of H2O2 to primary hippocampal neurons. The neurons were treated with H2O2 (0, 0.1, 1, 3, 5, 7, 10, 30, and 50 μM) at 1 DIV, and the CLSM images were taken after 24 h of incubation. (a) CLSM images of hippocampal neurons with different H2O2 concentrations. (b) A graph of %viability versus log[H2O2]. LC50 was calculated as the concentration at which the %viability was 50%. Data are expressed as mean±SE of n=3 each with three replicates. H2O2, hydrogen peroxide.
H2O2 is known as a trigger of oxidative stress-induced apoptosis.16,21 Accordingly, we observed the round neuronal morphology of neurons with no or few neurites, one of the major features of apoptosis, after the treatment of H2O2. In addition, the weak and broken degenerative neurites and cellular debris after degeneration in the CLSM images also suggested H2O2-induced apoptosis.37,38
CBD is known to be an antioxidant,39 with the similar oxidation potential to butylhydroxytoluene (BHT).9,40 CBD was reported to reduce the toxic levels of glutamate-induced neurotoxicity to about 40%, with its neuroprotective ability that was similar to BHT and more efficient than commercial antioxidants, such as ascorbate and α-tocopherol, for primary cortical neurons.9 Phenolic cannabinoids, including CBD, also have been shown to be the potent cannabinoid receptor 1-independent antioxidants, and protected the mouse hippocampal HT22 cell line up to ∼60% from oxidative stress induced by H2O2 ([CBD]=5 μM).17
In addition, CBD (10 μM) was reported to reduce the death of neuron-like PC12 cells from 39% to 12% against β-amyloid peptides, and decrease the reactive oxygen species production, lipid peroxidation, intracellular calcium, and caspase-3 levels.41 Several other compounds have also been studied for potential protection of cortical or hippocampal neurons against H2O2.42–44 Y-27632, a Rho-associated kinase (ROCK) inhibitor, increased the cell viability to 87% from 66.5% against H2O2 at maximum.42 N-Acetyl cysteine, inhibiting the oxidative stress by downregulating mitogen-activated protein kinase (MAPK) signal transduction and tau phosphorylation, was reported to be effective in the neuroprotection against H2O2.43 In the case of vitamin E, a fat-soluble antioxidant, also exhibited a neuroprotective capability, increasing the cell viability from 32% to 78% in the hippocampal neuron culture.44
In this work, we investigated the neuroprotective effect of CBD against H2O2 for primary hippocampal neurons. Specifically, we used 10 μM of H2O2 based on our result of the neurotoxicity effect of H2O2: this concentration of H2O2, inducing about 80% cell death, gave a suitable condition for neuroprotective studies. We also limited the CBD concentrations to less than 10 μM, considering the previous studies on the plasma and brain pharmacokinetic profile of CBD in rats and mice, indicating that the CBD concentrations of ≥10–20 μM in the in vitro studies would be supraphysiological.45,46
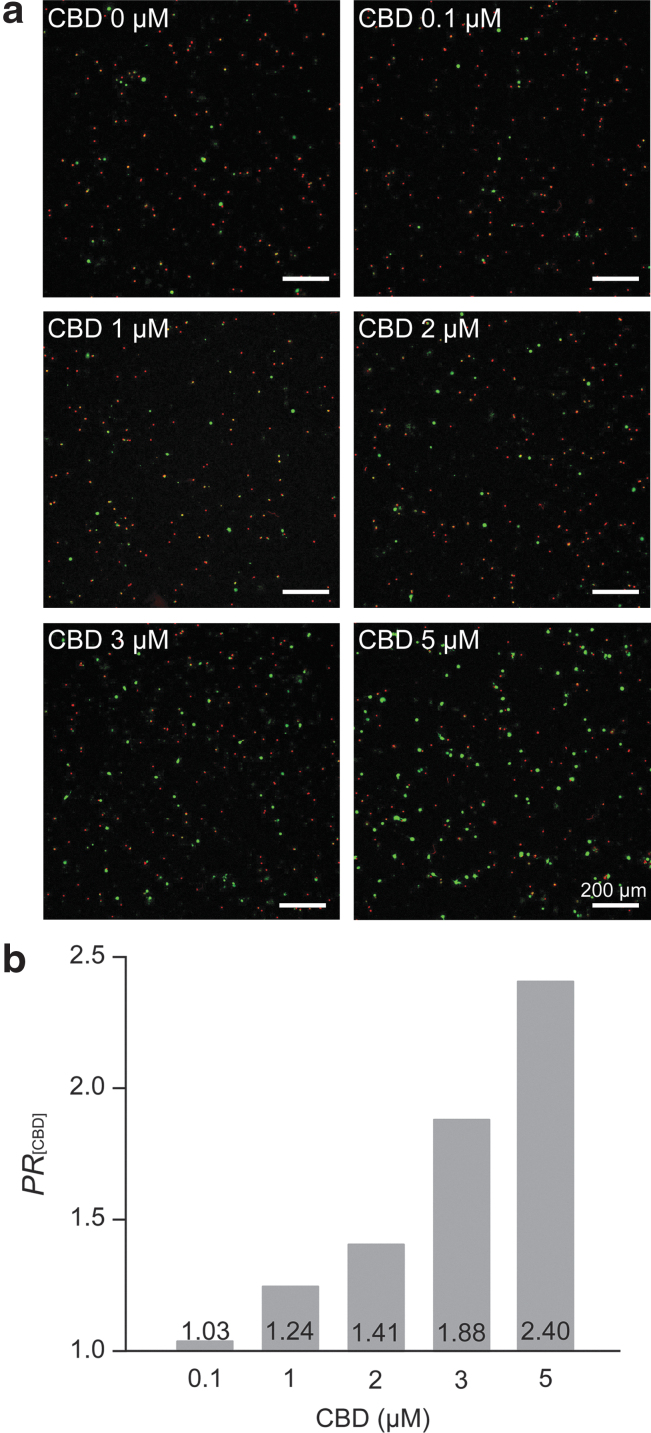
The CLSM images clearly showed that CBD was effective in the neuroprotection of primary hippocampal neurons against H2O2 (10 μM) (Fig. 4a). The quantitative analysis on the neuroprotection was performed by calculating the protection ratio of CBD, PR[CBD], by dividing the %viability values for [+H2O2][+CBD] cases by that for the [+H2O2][−CBD] case ([H2O2]=10 μM).
FIG. 4.
Neuroprotective effect of CBD against H2O2. Hippocampal neurons were treated with H2O2 (10 μM) and CBD (0, 0.1, 1, 2, 3, and 5 μM) at 1 DIV and incubated for 24 h. (a) CLSM images of primary hippocampal neurons after 24-h incubation with CBD and H2O2. (b) A graph of PR[CBD] versus the CBD concentration. Data are expressed as the mean %viabilities of the [+H2O2][+CBD] cases divided by the mean %viability of the [+H2O2][−CBD] case, repeated n=4 cultures, each with three replicates. The 0.1% DMSO was used for 0-μM CBD.
The bar graph of PR[CBD] versus [CBD] confirmed the concentration-dependent neuroprotective effects of CBD against H2O2 in the in vitro culture of primary hippocampal neurons (Fig. 4b). For example, the PR[CBD] value was 1.88 for 3 μM of CBD, which further increased to 2.40 for 5 μM of CBD. Interestingly, this PR[CBD] value was similar to the reported maximum PR value for vitamin E, 2.43,44 implying that the antioxidant property of CBD played an important role in the neuroprotection against H2O2. The preincubation of the neurons with CBD (5 μM, 1 h) did not lead to any noticeable neuroprotection against H2O2.
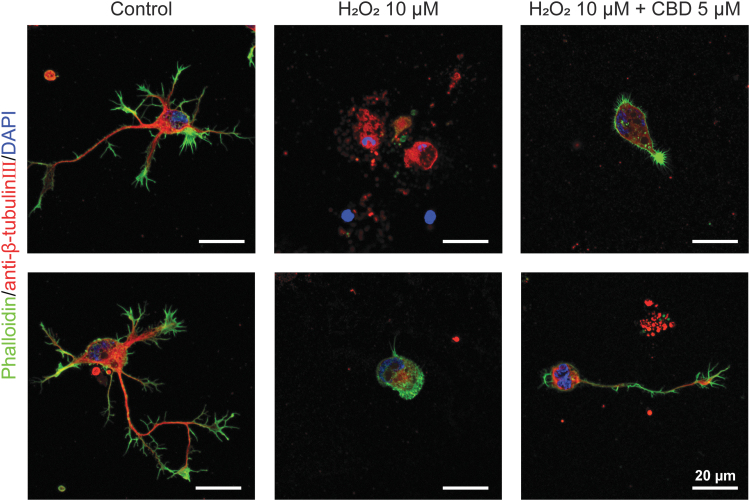
Although CBD increased the %viability against H2O2, showing its neuroprotective capability, the analysis of the high-resolution CLSM images indicated that CBD did not completely prevent the neurite degeneration in apoptotic process even at its concentration of 5 μM (Fig. 5). Therefore, we hypothesized that CBD acted as an antioxidant based on its phenolic structure and partially protected the hippocampal neurons from the initiation and progress of apoptosis caused by oxidative stress. It has been reported that CBD protected oligodendrocyte progenitor cells from apoptosis by decreasing the expression of the proapoptotic proteins or apoptotic effectors, such as Bax and caspase-12, and also increasing the antiapoptotic proteins, such as Bcl-2.18
FIG. 5.
High-magnification CLSM images of hippocampal neurons after 24-h treatment of (middle) H2O2 (10 μM), and (right) H2O2 (10 μM), and CBD (5 μM). The cells were stained in green (F-actin), red (β-tubulin III), and blue (nuclei).
Another possibility would be the regulation of [Ca2+]i.10–12 In the H2O2-induced apoptosis was reported the [Ca2+]i increase, by the disruption of Ca2+-storing organelles and also calcium influx through activation of several receptors and channels, such as N-methyl-D-aspartate (NMDA) subtype receptor and transient receptor potential melastatin 7 (TRPM7) channel.21,23–25 Previous reports also showed that the H2O2-induced cell death could be prevented by controlling the Ca2+ influx. For example, H2O2-induced neuronal apoptosis was inhibited by using the antagonist of NMDA receptors, such as MK801.25 The cytotoxicity of H2O2 was also attenuated by blocking the TRPM7 channel with the antagonist, 2-APB, or knocking down the TRPM7 channel expression.24
Another plausible mechanism on the neuroprotective effects of CBD against H2O2 might be activation of selective receptor mechanisms; H2O2 is reported to reversibly sulfenylate cysteines C201 and C208 and deactivate monoacylglycerol lipase, which cleaves 2-arachidonoyl-sn-glycerol, a ligand for cannabinoid receptor type 1 receptor.47
Conclusions
In summary, we used a low-density, neuron culture platform for evaluating both neurotoxic and neuroprotective effects of CBD on primary hippocampal neurons. Our in vitro study showed that CBD was neurotoxic, although its LC50 value was high (about 10 μM), suggesting that the detailed pharmacokinetic studies on the bioavailability of CBD be done with various administration methods, such as ingestion, inhalation, and transdermal or topical delivery. Biological modes of CBD action on neurons have not been elucidated fully, but our work clearly confirmed that CBD was highly effective in neutralizing the H2O2-induced neurotoxicity on primary hippocampal neurons, which is intimately involved in the onset and progress of neurodegenerative diseases.
Abbreviations Used
- AM
acetoxymethyl ester
- BHT
butylhydroxytoluene
- CBD
cannabidiol
- CLSM
confocal laser-scanning microscope
- DAPI
4′,6-diamidino-2-phenylindole
- DIV
days in vitro
- DMSO
dimethyl sulfoxide
- E18
embryonic day 18
- FDA
U.S. Food and Drug Administration
- H2O2
hydrogen peroxide
- HBSS
Hank's Balanced Salt Solution
- LC50
lethal concentration 50
- MAPK
mitogen-activated protein kinase
- NMDA
N-methyl-D-aspartate
- PBS
phosphate-buffered saline
- ROCK
Rho-associated kinase
- SE
standard error
- TRPM7
transient receptor potential melastatin 7
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by Cannabis Medical, Inc.
Cite this article as: Kim J, Choi JY, Seo J, Choi IS (2021) Neuroprotective effect of cannabidiol against hydrogen peroxide in hippocampal neuron culture, Cannabis and Cannabinoid Research 6:1, 40–47, DOI: 10.1089/can.2019.0102.
References
- 1. Reekie TA, Scott MP, Kassiou M. The evolving science of phytocannabinoids. Nat Rev Chem. 2017;2:0101 [Google Scholar]
- 2. Koppel BS, Brust JC, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurological disorders. Neurology. 2014;82:1556–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scuderi C, Filippis DD, Iuvone T, et al. Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders. Phytother Res. 2009;23:597–602 [DOI] [PubMed] [Google Scholar]
- 4. Iuvone T, Esposito G, Filippis DD, et al. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci Ther. 2009;15:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayakawa K, Mishima K, Fujiwara M. Therapeutic potential of non-psychotropic cannabidiol in ischemic stroke. Pharmaceuticals. 2010;3:2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–2020 [DOI] [PubMed] [Google Scholar]
- 7. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888–1897 [DOI] [PubMed] [Google Scholar]
- 8. GW Pharmaceuticals. EPIDIOLEX® (cannabidiol) oral solution – the first FDA-approved plant-derived cannabinoid medicine – now available by prescription in the U.S. http://ir.gwpharm.com/news-releases/news-release-details/epidiolexr-cannabidiol-oral-solution-first-fda-approved-plant.GW Pharmaceuticals. EPIDIOLEX® (cannabidiol) oral solution – the first FDA-approved plant-derived cannabinoid medicine – now available by prescription in the U.S. http://ir.gwpharm.com/news-releases/news-release-details/epidiolexr-cannabidiol-oral-solution-first-fda-approved-plant. (accessed March1, 2019)
- 9. Hampson AJ, Grimaldi M, Axelrod J, et al. Cannabidiol and (-)Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–8273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryan D, Drysdale AJ, Lagourcade C, et al. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci. 2009;29:2053–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drysdale AJ, Ryan D, Pertwee RG, et al. Cannabidiol-induced Ca2+ elevation in hippocampal cells. Neuropharmacology. 2006;50:621–631 [DOI] [PubMed] [Google Scholar]
- 12. Ryan D, Drysdale AJ, Pertwee RG, et al. Interactions of cannabidiol with endocannabinoid signaling in hippocampal tissue. Eur J Neurosci. 2007;25:2093–2102 [DOI] [PubMed] [Google Scholar]
- 13. Kinney WA, McDonnell ME, Zhong HM, et al. Discovery of KLS-13019, a cannabidiol-derived neuroprotective agent, with improved potency, safety, and permeability. ACS Med Chem Lett. 2016;7:424–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim GH, Kim JE, Rhie SJ, et al. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol. 2015;24:325–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barnham KJ, Master CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214 [DOI] [PubMed] [Google Scholar]
- 16. Ricart KC, Fiszman ML. Hydrogen peroxide-induced neurotoxicity in cultured cortical cells grown in serum-free and serum-containing media. Neurochem Res. 2001;26:801–808 [DOI] [PubMed] [Google Scholar]
- 17. Marsicano G, Moosmann B, Hermann H, et al. Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem. 2002;80:448–456 [DOI] [PubMed] [Google Scholar]
- 18. Mecha M, Torrao AS, Mestre L, et al. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis. 2012;3:e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lupia CR, Hu Y, Devinsky O, et al. Cannabinoids as hippocampal network administrators. Neuropharmacology. 2017;124:25–37 [DOI] [PubMed] [Google Scholar]
- 20. Whittemore ER, Loo DT, Cotman CW. Exposure to hydrogen peroxide induces cell death via apoptosis in cultured rat cortical neurons. Neuroreport. 1994;5:1485–1488 [DOI] [PubMed] [Google Scholar]
- 21. Whittemore ER, Loo DT, Watt JA, et al. A detailed analysis of hydrogen peroxide-induced cell death in primary neuronal culture. Neuroscience. 1995;67:921–932 [DOI] [PubMed] [Google Scholar]
- 22. Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci. 1996;16:2553–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoty KR, Gallagher AJ, Hastings TG, et al. Characterization of hydrogen peroxide toxicity in cultured rat forebrain neurons. Neurochem Res. 1997;22:333–340 [DOI] [PubMed] [Google Scholar]
- 24. Coombes E, Jiang J, Chu XP, et al. Pathophysiologically relevant levels of hydrogen peroxide induce glutamate-independent neurodegeneration that involves activation of transient receptor potential melastatin 7 channels. Antioxid Redox Signal. 2011;14:1815–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mailly F, Marin P, Israël M, et al. Increase in external glutamate and NMDA receptor activation contribute to H2O2-induced neuronal apoptosis. J Neurochem. 1999;73:1181–1188 [DOI] [PubMed] [Google Scholar]
- 26. Choi JY, Seo J, Park M, et al. Multiplexed metabolic labeling of glycoconjugates in polarized primary cerebral cortical neurons. Chem Asian J. 2018;13:3480–3484 [DOI] [PubMed] [Google Scholar]
- 27. Choi JY, Park M, Cho H, et al. Neuro-compatible metabolic glycan labeling of primary hippocampal neurons in non-contact, sandwich-type neuron-astrocyte co-culture. ACS Chem Neurosci. 2017;8:2607–2612 [DOI] [PubMed] [Google Scholar]
- 28. Kang K, Joo S, Choi JY, et al. Tissue-based metabolic labeling of polysialic acids in living primary hippocampal neurons. Proc Natl Acad Sci U S A. 2015;112:E241–E248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marcus M, Baranes K, Park M, et al. Interactions of neurons with physical environments. Adv Healthcare Mater. 2017;6:1700267. [DOI] [PubMed] [Google Scholar]
- 30. Kang K, Park YS, Park M, et al. Axon-first neuritogenesis on vertical nanowires. Nano Lett. 2016;16:675–680 [DOI] [PubMed] [Google Scholar]
- 31. Cho WK, Kang K, Kang G, et al. Pitch-dependent acceleration of neurite outgrowth on nanostructured anodized aluminum oxide substrates. Angew Chem Int Ed. 2010;49:10114–10118 [DOI] [PubMed] [Google Scholar]
- 32. Mato S, Sánchez-Gómez MV, Matute C. Cannabidiol induces intracellular calcium elevation and cytotoxicity in oligodendrocytes. Glia. 2010;58:1739–1747 [DOI] [PubMed] [Google Scholar]
- 33. Wu HY, Goble K, Mecha M, et al. Cannabidiol-induced apoptosis in murine microglial cells through lipid raft. Glia. 2012;60:1182–1190 [DOI] [PubMed] [Google Scholar]
- 34. Dotti CG, Sullivan CA, Banker, GA, The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grossmann J. Molecular mechanisms of “detachment-induced apoptosis-Anoikis.” Apoptosis. 2002;7:247–260 [DOI] [PubMed] [Google Scholar]
- 36. Bonfoco E, Chen W, Paul R, et al. Beta1 integrin antagonism on adherent, differentiated human neuroblastoma cells triggers an apoptotic signaling pathway. Neuroscience. 2000;101:1145–1152 [DOI] [PubMed] [Google Scholar]
- 37. Lesay A, Hickman JA, Gibson RM. Disruption of focal adhesions mediates detachment during neuronal apoptosis. Neuroreport. 2001;10:2111–2115 [DOI] [PubMed] [Google Scholar]
- 38. Gibson RM. Caspase activation is downstream of commitment to apoptosis of Ntera-2 neuronal cells. Exp Cell Res. 1999;251:203–212 [DOI] [PubMed] [Google Scholar]
- 39. Borges RS, Batista J Jr., Viana RB, et al. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules. 2013;18:12663–12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Babich H. Butylated hydroxytoluene (BHT): a review. Environ Res. 1982;29:1–29 [DOI] [PubMed] [Google Scholar]
- 41. Iuvone T, Esposito G, Esposito R, et al. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J Neurochem. 2004;89:134–141 [DOI] [PubMed] [Google Scholar]
- 42. Wang X, Wang B, Li Z, et al. Neuroprotection effect of Y-27632 against H2O2-induced cell apoptosis of primary cultured cortical neurons. RSC Adv. 2016;6:49187 [Google Scholar]
- 43. Wu W, Liu BH, Xie CL, et al. Neuroprotective effects of N-acetyl cysteine on primary hippocampus neurons against hydrogen peroxide-induced injury are mediated via inhibition of mitogen-activated protein kinases signal transduction and antioxidative action. Mol Med Rep. 2018;17:6647–6654 [DOI] [PubMed] [Google Scholar]
- 44. Lee HJ, Lee JH, Cho NS. Study on the effect of vitamin E on cultured hippocampal neurons damaged by hydrogen peroxide. Kor J Ori Med Physiol Pathol. 2003;17:447–450 [Google Scholar]
- 45. Deiana S, Watanabe A, Yamasaki Y, et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology. 2012;219:859–873 [DOI] [PubMed] [Google Scholar]
- 46. Bih CI, Chen T, Nunn AV, et al. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12:699–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dotsey EY, Jung KM, Basit A, et al. Peroxide-dependent MGL sulfenylation regulates 2-AG-mediated endocannabinoid signaling in brain neurons. Chem Biol. 2015;22:619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]