Abstract
Despite marked advances in the field of cartilage tissue engineering, it remains a challenge to engineer cartilage constructs with homogeneous properties. Moreover, for engineered cartilage to make it to the clinic, this homogeneous growth must occur in a time-efficient manner. In this study we investigated the potential of increased media volume to expedite the homogeneous maturation of mesenchymal stem cell (MSC) laden engineered constructs over time in vitro. We assessed the MSC-laden constructs after 4 and 8 weeks of chondrogenic culture using bulk mechanical, histological, and biochemical measures. These assays were performed on both the intact total constructs and the construct cores to elucidate region-dependent differences. In addition, local strain transfer was assessed to quantify depth-dependent mechanical properties throughout the constructs. Our findings suggest that increased media volume enhances matrix deposition early in culture and ameliorates unwanted regional heterogeneities at later time points. Taken together, these data support the use of higher media volumes during in vitro culture to hasten tissue maturation and increase the core strength of tissue constructs. These findings will forward the field of cartilage tissue engineering and the translation of tissue engineered constructs.
Impact statement
The translation of engineered cartilage therapeutics is hindered by our inability to grow mechanically robust tissue constructs with uniform properties. In most instances, the center regions of tissue constructs are mechanically inferior due to limitations in nutrient supply. This study demonstrates a simple and easily adoptable approach for growing homogeneous tissue constructs in a time-efficient manner through increased media volumes during in vitro culture.
Keywords: mesenchymal stem cells, engineered cartilage, three-dimensional culture, depth-dependent properties
Introduction
Articular cartilage is an avascular tissue with a notoriously poor healing capacity. Injury to this tissue, either traumatic or age related, often leads to osteoarthritis (OA), a debilitating joint disease. OA most commonly occurs in the knee, hip, ankle, or hand, limiting patient mobility and independence. In the United States alone, OA impacts upwards of 27 million people, costing an estimated 100 billion dollars.1,2 There is a clear need for therapeutics to repair damaged cartilage and prevent OA to relieve the socioeconomic burden of this disease. Unfortunately, the traditional treatment options (e.g., microfracture and chondroplasty) for OA fail to restore long-term function and relieve pain. Modern clinical treatments, such as autologous matrix-induced chondrogenesis and matrix-associated autologous chondrocyte implantation, show some success in early stage disease; however, these procedures are restricted to an “optimal” patient population or those with localized cartilage defects.3
Cartilage tissue engineering, especially whole or hemi-joint tissue engineering, is a promising approach that may one day enable functional restoration in patients with large, irregularly shaped defects that are otherwise not treatable. There are various techniques to create these large engineered cartilage tissues, including the modular assembly of smaller constructs, woven scaffolds, and mold-based tissue engineering.4–9 While these strategies have shown promise in vitro, few have been translated to preclinical animal models. This may be due to the fact that large tissue engineered constructs require a significant preculture period in vitro before in vivo implantation to achieve native-like tissue mechanics. This limits the commercial and clinical appeal of such approaches, given the long wait times and costs associated with extended preculture relative to traditional metal and plastic replacements, which are available as an “off the shelf” treatment. These synthetic joint replacements have a defined lifetime, however, and large engineered tissues have the potential to integrate with native tissue and outlast synthetic alternatives, motivating continued research in the field of whole or hemi-joint tissue engineering.
In an effort to lessen the preculture period required to produce mechanically competent engineered tissues, considerable work has been done to explore how components of the growth media and dosing regimens might expedite tissue formation.10–14 However, there is still no consensus on the optimal culture conditions to produce a functional tissue. This lack of consensus is, in part, due to the vast array of cartilage tissue engineering approaches. Differences in cell number and cell type may impact the nutritional demands of the construct.15,16 Furthermore, nutrient diffusion is influenced by the scaffold porosity and hydrophobicity, as well as the overall geometry of the sample, where large thick samples generally underperform smaller thinner constructs. For example, we recently showed that thick (>2.3 mm thickness) constructs composed of bovine mesenchymal stem cells (MSCs) were much weaker in the center in comparison to the outer regions of the tissue, even after an extended in vitro growth period.17,18 To improve the supply of culture media throughout tissue engineered constructs, researchers have created macroscopic channels throughout the samples.4,7,17,19,20 In conjunction with creating these additional routes for media exchange, bioreactors may be utilized to promote media flow.7 These strategies have improved the core strength of engineered constructs; however, it may be more challenging to incorporate symmetric macroscopic channels in complex anatomic noncylindrical constructs.
Based on the above challenges and limitations, the primary objective of this study was to accelerate homogeneous growth of thick MSC-seeded constructs without altering construct shape (i.e., adding macroscopic channels). To do so, we modulated the volume of chemically defined chondrogenic media provided to MSC-laden agarose constructs during culture. A volume of 1 mL (1V), 3 mL (3V), or 5 mL (5V) of media was supplied to each construct (∼565,000 cells), with media changed twice weekly for up to 8 weeks in culture. We evaluated the effect of this increased media volume on the depth-dependent construct viability, extracellular matrix content, and mechanics at short (4 weeks) and long (8 weeks) time points. Our findings indicate that increased media volume expedites construct maturation and ameliorates unwanted regional mechanical heterogeneities during long-term culture, supporting the use of an increased media volume for growing tissue engineered constructs in vitro.
Materials and Methods
Cell isolation, three-dimensional encapsulation, and culture
MSCs were isolated from juvenile bovine stifle joints (3 months old, Research 87, MA). MSCs were pooled from three donors for this study. MSCs were expanded in basal media consisting of Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher) supplemented with 10% fetal bovine serum (Thermo Fisher) and 1% penicillin/streptomycin/fungizone (PSF; Thermo Fisher). At passage 3, MSCs were trypsinized and washed with phosphate buffered saline (PBS; Thermo Fisher).
MSCs were encapsulated at 20 million cells/mL in a 2% w/v agarose solution. First, cells were suspended in a chemically defined media (CM) at 40 million cells/mL. This cell suspension was mixed at a 1:1 ratio with 4% w/v agarose (type VII, 49°C) in PBS and cast between two glass plates, 2.25 mm apart from each other.21,22 After 10 min, a dermal biopsy punch (4 mm diameter) was used to create MSC-seeded agarose constructs. All constructs were cultured in CM containing high-glucose DMEM, 1% PSF, 0.1 μM dexamethasone, 100 μg/mL sodium pyruvate, 40 μg/mL L-proline, 50 μg/mL ascorbate 2-phosphate, and ITS (6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 ng/mL selenous acid), 1.25 mg/mL bovine serum albumin, 5.35 mg/mL linoleic acid, and 10 ng/mL transforming growth factor-β3 (TGF-β3) (R&D Systems). Media was changed twice weekly, and care was taken to maintain the sidedness (top and bottom) of the constructs. Therefore, the constructs were not flipped throughout culture. Each construct (∼565,000 cells) received 1 mL (1V), 3 mL (3V), or 5 mL (5V) of media. Constructs were cultured up to 8 weeks.
Cell viability and histological analyses
Cell viability was assessed by diametrically halving the constructs (n = 3–4/group) and staining one half with Calcein-AM and ethidium homodimer (Live/Dead Kit; Invitrogen). Stained constructs were imaged under 10 × magnification in five regions: top, center, bottom, left, and right. A custom MATLAB (MathWorks, Inc.) script was used to count the number of live and dead cells in each image.18 The live and dead cell counts from each of the five images were summed to determine the total number of live and dead cells in each construct.
The opposing halves (n = 4) were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned (7 μm). Sections were stained with Alcian Blue (proteoglycan) and picrosirius red (PSR) (collagen). Immunohistochemistry was performed to identify the presence of collagen type II (II-II6B3 at 10 μg/mL; Developmental Studies Hybridoma Bank) as previously described.23
Analysis of bulk mechanical properties
To determine the bulk mechanical properties, constructs were tested in uniaxial unconfined compression using a custom-built device.22 Constructs were left either intact (n = 3–4/group, 4 weeks; n = 3–4/group, 8 weeks) or cored using a 3 mm dermal biopsy punch (n = 3–4/group, 8 weeks) before testing. Constructs were equilibrated under a static load of 0.02 N for 5 min and then subjected to a 10% strain at 0.05%/s, followed by a relaxation period of 600 s. The strain was calculated from the postcreep thickness of the construct. Equilibrium modulus was calculated from the equilibrium stress and sample geometry.
Regional biochemical analyses
After bulk mechanical testing, samples (n = 3–4/group, 4 weeks; n = 3–4/group, 8 weeks) were prepared for regional analyses of the glycosaminoglycan (GAG) and collagen content. Intact constructs were cored (using a 3 mm biopsy punch) and halved, creating 4 segments: annulus top (AT), center top (CT), annulus bottom (AB), and center bottom (CB). These segments were weighed (wet weights) before freezing and lyophilization. Lyophilized samples were digested overnight in 100 μg/mL Proteinase K (Roche) at 60°C. After digestion, GAG content was determined using the 1, 9 dimethylmethylene blue (DMMB) dye binding assay24 and collagen content using orthohydroxyproline (OHP) assay, with a 1:7.14 (OHP: collagen) ratio, as previously described.25 GAG and collagen are presented as percent of the construct wet weight.
Analysis of local mechanical properties
To determine the depth-dependent equilibrium modulus,26 1V and 5V constructs were tested in uniaxial unconfined compression using a custom-built device18 in conjunction with a fluorescent inverted microscope (Nikon Eclipse TE 2000-U, Nikon). Constructs were left either intact (n = 3–4/group, 8 weeks) or cored using a 3 mm biopsy punch (n = 3–4/group, 8 weeks) before testing. All intact and cored constructs were halved diametrically, and cell nuclei were labeled with Hoechst 33342 for 10 min. The cut surface of one half was placed in contact with the glass slide in the PBS-filled bath of the device, facing the microscope objective. A series of 4% compressive strain steps were applied through 20% total. After each strain step, constructs were equilibrated for 7 min, and then an image was captured using a 2 × objective. Load was recorded at each step after the equilibrium period. Images were analyzed using the Vic2D software (Correlated Solutions) to calculate the displacement fields of the cells. Local Lagrangian strain (Exx) was calculated from the displacement fields, where x is the axial direction of the cylindrical construct. MATLAB postprocessing calculated the average strain values in 10 regions from the top (region 1) to the bottom (region 10) of the construct. Local modulus was calculated at the 12% step strain using the resultant strain values and the equilibrium boundary stresses. The reported top, middle, and bottom equilibrium moduli are averages of regions 2–4, 5–7, and 8–10, respectively.18
Statistical analyses
Statistical analyses were performed using GraphPad Prism 8. Data are reported as mean and standard deviation, with significance set at p < 0.05. For the viability quantification, a one-way analysis of variance (ANOVA) was performed using media volume as the independent variable for each time point, cell type (live or dead), and construct region (total or center). For the bulk mechanical testing, two separate two-way ANOVAs were performed. The first two-way ANOVA used media volume and time point as independent variables, and the second two-way ANOVA used media volume and construct region (intact or core) as independent variables, for the 8-week data. For the regional biochemical analyses, a two-way ANOVA was performed with media volume and construct region (AT, CT, AB, CB) as independent variables. Finally, for the analysis of local mechanics, a two-way ANOVA was performed for each region (top, middle, bottom) using media volume and construct region (intact, core) as the independent variables. Post hoc Tukey's tests were performed to identify differences between groups.
Results
Overall MSC viability and matrix accumulation in constructs
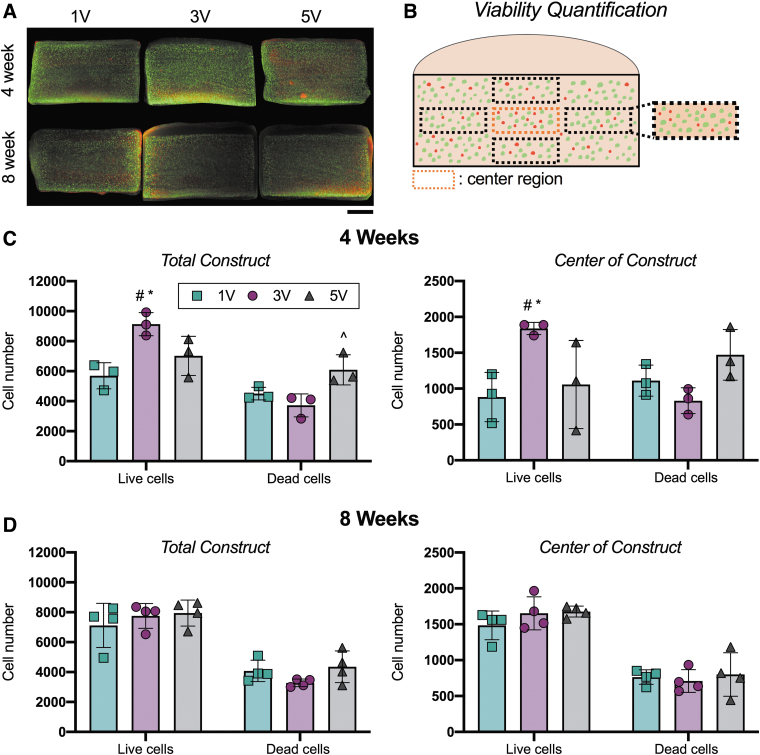
MSC-seeded agarose constructs showed little difference in cell viability between the culture media volume conditions (Fig. 1). Constructs cultured for 8 weeks all showed a similar number of live and dead cells in both the total construct and the center of the constructs (Fig. 1D).
FIG. 1.
Construct viability with different culture media volumes over time. (A) Representative live/dead images at 4 and 8 weeks. Scale bar = 1 mm. (B) Schematic of viability quantification. Five images were averaged for the total construct measure. Only the center region was reported for the center viability. (C, D) Live and dead cell number throughout the total constructs and in the center of the constructs after 4 and 8 weeks of culture. Mean ± standard deviation. #Versus 1V constructs within cell category [live, dead]; ^versus 3V within cell category; *versus 5V within cell category, p < 0.05. n = 3–4/group. Color images are available online.
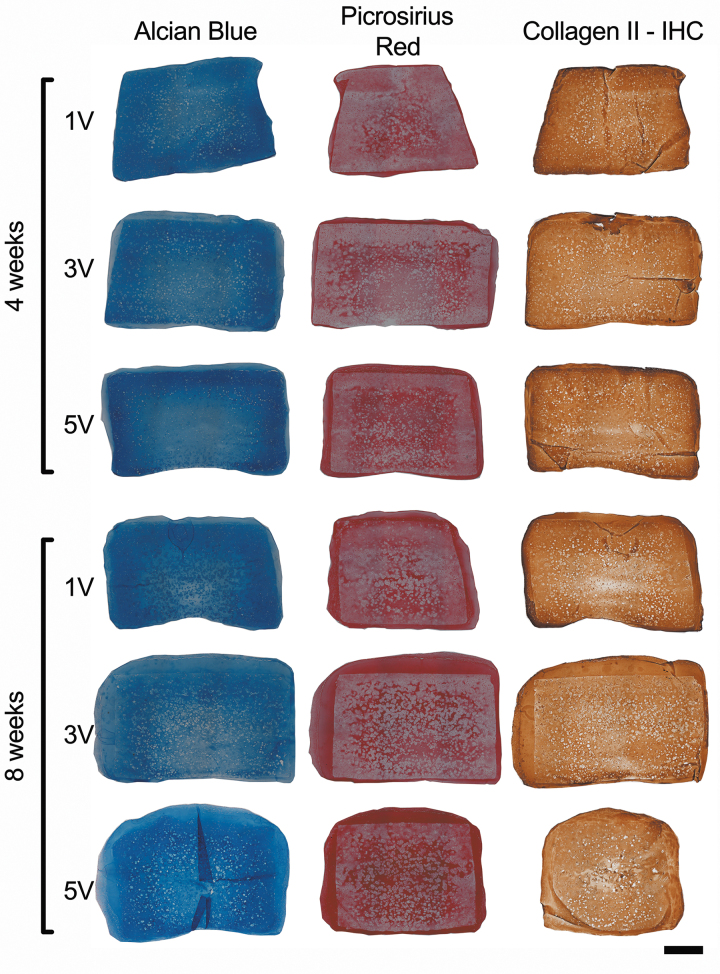
The distributions of proteoglycans and collagen in the constructs were assessed with histology and immunohistochemistry (Fig. 2). Alcian blue staining was more intense in the constructs cultured in 5 mL of media per construct (5V) after 8 weeks. The distribution of collagen, as determined by PSR staining, was similar to previous work with MSC-seeded agarose constructs cultured with TGF-β3 under free swelling conditions.18 There was intense PSR staining in the outer fibrous cap surrounding the constructs, which was very apparent after 8 weeks of culture. Constructs cultured under 5V conditions had the most uniform PSR staining. However, the innermost regions of all stained sections (PSR and AB) showed decreased extracellular matrix content. This trend was also noted in the sections immunostained for type II collagen.
FIG. 2.
Alcian blue staining for proteoglycans, picrosirius red staining for collagen, and Collagen Type II immunohistochemistry after 4 and 8 weeks of culture. Scale bar = 1 mm. Color images are available online.
Bulk mechanics of intact and cored constructs
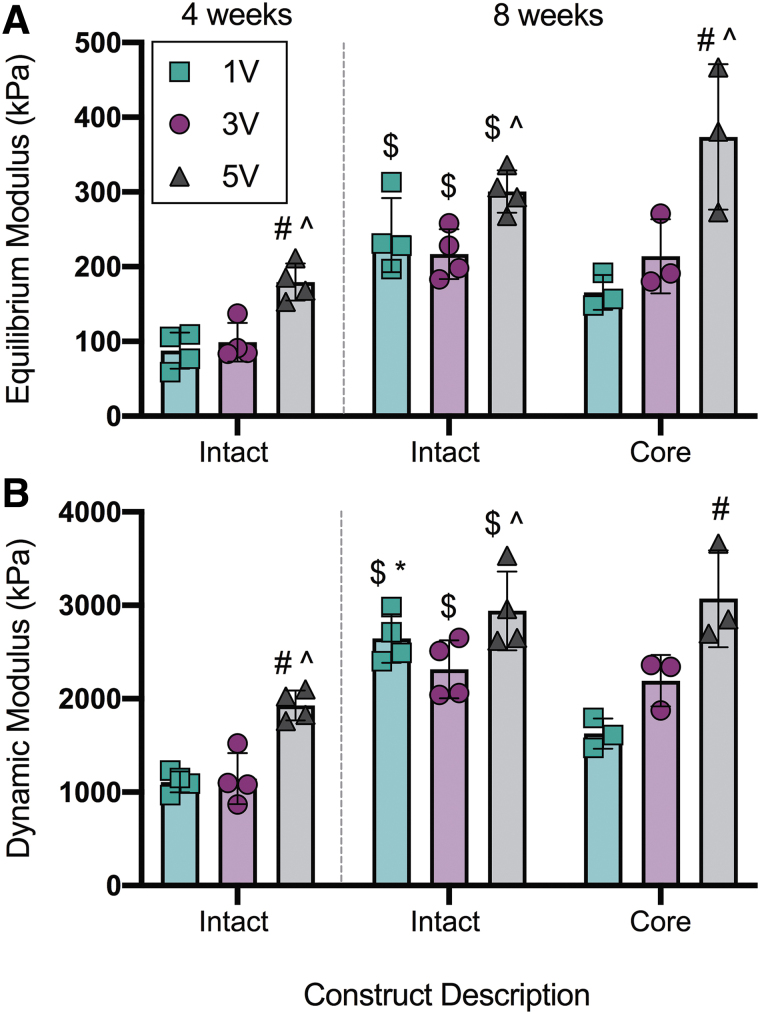
Mechanical properties were assessed for both the entire construct (intact) and for full thickness cores taken from the central region of the construct (core). The increased GAG and collagen content in 5V constructs at 4 weeks (Fig. 4) significantly enhanced the bulk mechanics of these constructs in comparison to 1V and 3V constructs (Fig. 3). Specifically, the bulk mechanics of the intact constructs were ∼2-fold higher in the 5V group (E5V = 179 ± 25 kPa, |G*|5V = 1929 ± 159 kPa), in comparison to the 1V (pE = 0.009, p|G*| = 0.006) and 3V (pE = 0.025, p|G*| = 0.009) groups, at this time point. All of the intact constructs showed enhanced mechanics with time in culture. After 8 weeks of culture, the equilibrium and dynamic moduli increased further for the 5V constructs, reaching E5V = 301 ± 28 kPa and |G*|5V = 2940 ± 422 kPa, respectively. At this 8 weeks time point, the core strength of the 5V constructs (E5V = 373 ± 97 kPa, |G*|5V = 3071 ± 518 kPa) was also significantly higher than the 1V (pE = 0.002, p|G*| = 0.001) and 3V (pE = 0.015) cores. Interestingly, increased media volume in the 3V and 5V groups resurrected the strength of their respective cores, whereas the 1V cores were significantly weaker in comparison to the total constructs (p|G*| = 0.015).
FIG. 4.
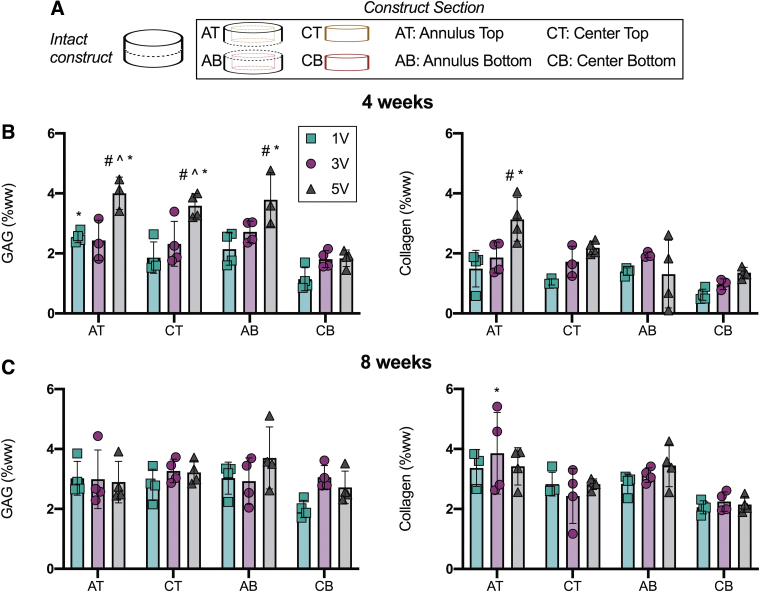
Regional biochemical analyses of MSC-seeded agarose constructs. (A) Constructs were sectioned into 4 regions: AT, CT, AB, CB. (B, C) GAG and collagen content normalized by wet weight (%wet weight) after 4 and 8 weeks of culture. Mean ± standard deviation. #Versus 1V constructs within region; ^versus 3V constructs region; *versus CB region within media volume, p < 0.05. n = 3–4/per group. AB, annulus bottom; CB, center bottom; CT, center top; MSC, mesenchymal stem cell. AT, annulus top; GAG, glycosaminoglycan. Color images are available online.
FIG. 3.
Bulk assessment of unconfined compressive properties of the intact constructs and construct cores. (A) Equilibrium modulus. (B) Dynamic modulus. Mean ± standard deviation. #Versus 1V constructs within time point and construct description; ^versus 3V constructs within time point and construct description; $versus intact constructs at 4 weeks and within media volume; *versus core within media volume, p < 0.05. n = 3–4/per group. Color images are available online.
Regional biochemical analyses of MSC-seeded constructs
Regional quantification of GAG and collagen content using DMMB and OHP assays (Fig. 4), respectively, supported the histological findings. Namely, we detected an increase in matrix content as a function of media volume, with the greatest amounts located in the outer edges of the constructs. For the regional biochemical analyses, the AT, CT, AB, and CB regions were tested in constructs from each media volume group (Fig. 4A). Differences in matrix content were most apparent after 4 weeks of culture (Fig. 4B) and plateaued after 8 weeks of culture (Fig. 4C). At the 4-week time point, within a construct region, GAG and collagen (%ww) trended upward with increasing media volume. The AT (p = 0.025), CT (p = 0.001), and AB (p = 0.007) regions of the 5V constructs had significantly more GAG than these same regions in constructs cultured on 1V conditions. In addition, the AT (p = 0.004) of the 5V constructs had significantly more collagen than the AT region of the 1V constructs. Of note, the CB region of the 1V constructs had the lowest matrix content (GAG: 1.1 ± 0.4%WW, collagen: 0.6 ± 0.2%WW). When constructs were cultured with 5V media volume for 8 weeks, the matrix content of the CB region increased over two-fold for both GAG and collagen (GAG: 2.7 ± 0.5%WW, collagen: 2.1 ± 0.3%WW), in comparison to the matrix content in the CB region of the 1V constructs at 4 weeks.
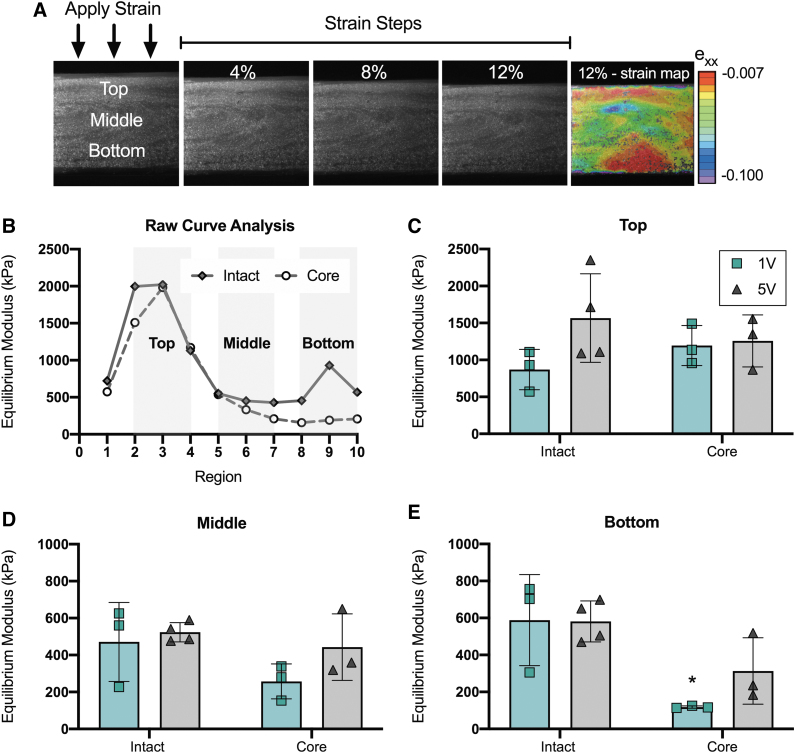
Depth-dependent local mechanics
Assessment of local equilibrium moduli of the constructs showed depth-dependent properties in both the axial (top to bottom) and radial (total vs. core) directions, and these differences were exacerbated by lower media volumes (Fig. 5). The top region of the constructs, defined by regions 2–4 (Fig. 5B), showed the highest equilibrium modulus across all media volumes after 8 weeks of culture (Fig. 5C). In this top region, there were no statistical differences between the intact constructs and cores across media volumes. In the middle of the constructs, defined by regions 5–7 (Fig. 5B), the equilibrium modulus dropped to ∼1/3rd of the values recorded for the top region; however, there were still no statistical differences between the total constructs and cores (Fig. 5D). Media volume had the greatest impact on the bottom of the constructs, defined by regions 8–10 (Fig. 5B). While the 1V cores (E: 118 ± 6 kPa) were significantly weaker (p = 0.011) than the total constructs (588 ± 246 kPa), the 5V cores were not statistically different than the total constructs (Fig. 5E).
FIG. 5.
Assessment of the local equilibrium modulus in the top, middle, and bottom regions of the intact constructs and construct cores after 8 weeks of culture. (A) Schematic of step-wise strain application and mapping throughout the depth of constructs. Equilibrium modulus was calculated from the 12% strain step. (B) Constructs were divided into 10 regions (1: very top, 10: very bottom). Regions 2–4, 5–7, 8–10 were averaged to report the equilibrium modulus in the top, middle, and bottom regions, respectively. (C–E) Equilibrium modulus of the top, middle, and bottom regions. Intact constructs and construct cores were tested separately for 1V and 5V constructs. *Versus intact within region, p < 0.05. n = 3–4/per group. Color images are available online.
Discussion
While the field of cartilage tissue engineering has progressed tremendously over the past 25 years, promotion of uniform construct growth and matrix deposition remains a challenge. This limits the translational potential of tissue engineered cartilage therapeutics. Recent data and theoretical models support that rapidly growing engineered constructs quickly deplete key nutrients from surrounding media, which may restrict further construct growth, compromise vitality of certain cell types or subpopulations, and exacerbate diffusion-based heterogeneities throughout the constructs.27 To combat this rapid depletion of nutrients, here, we increased the media volume, and therefore nutrient availability, in growing MSC-seeded agarose constructs. We investigated the potential of increased media volumes to (1) accelerate construct maturation and (2) ameliorate unwanted regional heterogeneities in engineered tissues. To do this, we generated cylindrical MSC-seeded agarose constructs (2.25 mm thick, 4 mm diameter) and exposed them to 1 mL (1V), 3 mL (3V), or 5 mL (5V) of chondrogenic media for up to 8 weeks of culture in vitro. Results from this work demonstrate that constructs cultured in 5 mL of media yielded tissue with the highest mechanical strength. Specifically, bulk properties of these constructs were two-fold higher than constructs cultured in lower media volumes after 4 weeks of culture. In addition, at this early time point, in each region of the constructs, GAG and collagen content trended upward with increasing media volume. These data support the use of higher media volumes to expedite the early construct growth in engineered cartilage.
After longer durations of culture (i.e., 8 weeks), differences in bulk mechanical properties were no longer apparent. However, regional analyses revealed sustained improvements with higher media volumes. Specifically, mechanical heterogeneities persisted in the 1V constructs, but were not observed or were significantly diminished in the 5V constructs. Similarities in the bulk mechanical properties between 1V, 3V, and 5V intact constructs are likely due to the robust matrix deposition seen in the outer portion of the constructs. During the bulk mechanical testing, this matrix-dense outer portion of the constructs likely shielded the matrix-sparse inner portion of the constructs. To confirm this hypothesis, we cored the 4 mm diameter constructs with a 3 mm biopsy punch to eliminate these outer structural supports. The dynamic modulus of the 1V cores was significantly lower compared with the 1V intact samples, as expected. Interestingly, for the constructs cultured in the highest media volume, 5 mL, the dynamic modulus of the cores was not different than the intact samples. These data support that, overall, the mechanical properties of the 5V constructs were more homogeneous than those of the 1V constructs. Interestingly, we also found that the equilibrium and dynamic moduli of the 5V construct cores were higher than the 1V cores, despite little to no differences in biochemical content in this region between groups. Differences in the mechanical properties of the construct cores may be due to the organization or crosslinking of the matrix within the constructs and not just the raw matrix content.
To further refine the spatial resolution of this mechanical analysis, we analyzed the local strains in the bottom region of the intact constructs and cores. Throughout culture, the bottom region of the constructs was in contact with the well plate, and therefore, this region is the most nutrient deprived during culture. Special care was taken to maintain this orientation, so as to mimic a scenario where a secondary bony material may be present beneath the engineered cartilage, restricting diffusion at this surface. This analysis showed that the equilibrium modulus in the bottom of the 1V constructs was significantly lower in the cores versus intact samples. However, increased media volume rescued the mechanical properties of this central bottom region, eliminating differences between the intact and cored samples for the 5V constructs.
The depth and axial dependent analyses performed in this study provide for a higher resolution understanding of the heterogeneities that emerge within engineered constructs and point to methods that may be used to overcome these limitations. With larger media volumes, we observed that the mechanical properties of the constructs were less heterogeneous and bringing this technology closer to the goal of homogeneous growth in a particular region. This may be especially important as new cell, growth factor, and material patterning strategies are developed that intentionally introduce local gradients into engineered constructs to better mimic native articular cartilage. Metabolic insufficiencies identified here may thwart such efforts,20,28–33 and the increased media volumes detailed here may help to overcome these limitations and enable the production of more sophisticated anisotropic tissues and heterogeneous tissues by design.
While these data should prove valuable for the field, there are several limitations of the current study that bear further investigation. First, we conducted these studies using juvenile bovine MSCs. These cells were chosen based on their established potential for rapidly generating cartilaginous tissue. Other work has shown profound species and cell-type specific differences in matrix content throughout engineered cartilage tissues.34 For example, tissue constructs fabricated using porcine MSCs have the opposite matrix distribution than the results of our study and work from others using bovine MSCs.34 Similarly, human MSCs show marked donor-to-donor variation.35 It will be important to establish optimal media volumes for each cell type utilized. Our choice of biomaterial also may impact the distribution of matrix within the constructs.23,36 Agarose is a simple hydrogel, but lacks adhesion moieties to promote cell health and/or differentiation.37–39 The media requirements for a given cell population may be influenced by the ligands they interact with in their immediate extracellular environment. Finally, it should also be pointed out that prolonged exposure to every component within these growth media may be less than ideal. For example, high levels of TGF, for a prolonged period of time, may ultimately drive unwanted hypertrophic conversion,10 and transient high levels of exposure may be sufficient for some nutrients.40,41 Finally, while the construct thicknesses investigated in this article match or exceed most cartilaginous surfaces in the human body, the extent to which this increased media volume could enable functional and homogeneous matrix deposition in very thick cartilage constructs has not yet been assessed. These will be important avenues of exploration in future studies.
While some limitations to this work certainly exist, these new data do support an exciting aspect of cartilage tissue engineering—patient-specific whole joint engineering. Advances in three-dimensional printing and additive manufacturing make this goal a more realistic possibility. One can readily convert a patient-specific computed tomography scan to a mold to then generate an anatomic tissue.6,42 However, the time required to mature mechanically competent tissues in vitro, as well as the marked heterogeneities in tissue deposition and corresponding material properties in these very large constructs may hinder their translation, as unwanted heterogeneities may compromise the load-bearing function of the biologic implant.18,43 Thus, the work presented here extends our understanding of media and nutrient requirements for cartilage tissue engineering efforts and underscores the need to increase nutrient availability to expedite construct growth and improve homogeneous tissue deposition. These data will further our work toward whole joint replacement with a functional, living engineered implant.
Conclusions
This study demonstrates that MSC-seeded agarose constructs (∼565,000 cells/construct) mature more quickly and more homogeneously in larger volumes (i.e., 5 mL) of chondrogenic culture media. This simple approach can easily be applied to other cartilage tissue engineering studies to expedite the in vitro preculture period before implanting the engineered constructs in vivo. In addition, this culture strategy can be implemented to boost the core strength of engineered cartilage, thereby eliminating unwanted regional heterogeneities commonly observed in engineered tissues. Future studies will investigate the impact of larger media volumes on the growth trajectory of anatomic engineered cartilage constructs.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by the National Institute of Health (R01 EB008722 and T32 AR050950) and the Department of Veterans Affairs (I01 RX00070, IK6 RX003416, and I01 RX003375). Additional support was provided by the Penn Center for Musculoskeletal Disorders (P30 AR069619).
References
- 1. Murphy, L., and Helmick, C.G.. The Impact of osteoarthritis in the United States: a population-health perspective: a population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthop Nurs 31, 85, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Jafarzadeh, S.R., and Felson, D.T.. Updated estimates suggest a much higher prevalence of arthritis in United States adults than previous ones. Arthritis Rheumatol 70, 185, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martín, A.R., Patel, J.M., Zlotnick, H.M., et al. . Emerging therapies for cartilage regeneration in currently excluded “red knee” populations. NPJ Regen Med 4, 12, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ford, A.C., Chui, W.F., Zeng, A.Y., et al. . A modular approach to creating large engineered cartilage surfaces. J Biomech 67, 177, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moutos, F.T., Glass, K.A., Compton, S.A., et al. . Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc Natl Acad Sci U S A 113, E4513, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saxena, V., Kim, M., Keah, N.M., et al. . Anatomic mesenchymal stem cell-based engineered cartilage constructs for biologic total joint replacement. Tissue Eng Part A 22, 386, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daly, A.C., Sathy, B.N., and Kelly, D.J.. Engineering large cartilage tissues using dynamic bioreactor culture at defined oxygen conditions. J Tissue Eng 9, 1, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li, F., Truong, V.X., Fisch, P., et al. . Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta Biomater 77, 48, 2018 [DOI] [PubMed] [Google Scholar]
- 9. Lee, C.H., Cook, J.L., Mendelson, A., et al. . Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 376, 440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albro, M.B., Nims, R.J., Durney, K.M., et al. . Heterogeneous engineered cartilage growth results from gradients of media-supplemented active TGF-β and is ameliorated by the alternative supplementation of latent TGF-β. Biomaterials 77, 173, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cigan, A.D., Nims, R.J., Albro, M.B., et al. . Insulin, ascorbate, and glucose have a much greater influence than transferrin and selenous acid on the in vitro growth of engineered cartilage in chondrogenic media. Tissue Eng Part A 19, 1941, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Theodoridis, K., Aggelidou, E., Manthou, M., et al. . Assessment of cartilage regeneration on 3D collagen-polycaprolactone scaffolds: evaluation of growth media in static and in perfusion bioreactor dynamic culture. Colloids Surf B Biointerfaces 183, 110403, 2019 [DOI] [PubMed] [Google Scholar]
- 13. Byers, B.A., Mauck, R.L., Chiang, I.E., et al. . Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechancial and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A 14, 1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kisiday, J.D., Kurz, B., DiMicco, M.A., et al. . Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng 11, 141, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Sengers, B.G., Van Donkelaar, C.C., Oomens, C.W.J., et al. Computational study of culture conditions and nutrient supply in cartilage tissue engineering. Biotechnol Prog 21, 1252, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Leddy, H.A., Awad, H.A., and Guilak, F.. Molecular diffusion in tissue-engineered cartilage constructs: effects of scaffold material, time, and culture conditions. J Biomed Mater Res B Appl Biomater 70, 397, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Bian, L., Angione, S.L., Ng, K.W., et al. . Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthr Cartil 17, 677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farrell, M., Comeau, E., and Mauck, R.. Mesenchymal stem cells produce functional cartilage matrix in three-dimensional culture in regions of optimal nutrient supply. Eur Cell Mater 23, 425, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Nims, R.J., Cigan, A.D., Albro, M.B., et al. . Matrix production in large engineered cartilage. Tissue Eng Part C Methods 21, 747, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim, M., Farrell, M.J., Steinberg, D.R., et al. . Enhanced nutrient transport improves the depth-dependent properties of tri-layered engineered cartilage constructs with zonal co-culture of chondrocytes and MSCs. Acta Biomater 58, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mauck, R.L., Wang, C.C.B., Oswald, E.S., et al. . The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthr Cartil 11, 879, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Mauck, R.L., Yuan, X., and Tuan, R.S.. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr Cartil 14, 179, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Erickson, I.E., Huang, A.H., Sengupta, S., et al. . Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthr Cartil 17, 1639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farndale, R.W., Buttle, D.J., and Barrett, A.J.. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883, 173, 1986 [DOI] [PubMed] [Google Scholar]
- 25. Neuman, R.E., and Logan, M.A.. The determination of hydroxyproline. J Biol Chem 184, 299, 1950 [PubMed] [Google Scholar]
- 26. Schinagl, R.M., Gurskis, D., Chen, A.C., et al. . Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res 15, 499, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Farrell, M.J., Shin, J.I., Smith, L.J., et al. . Functional consequences of glucose and oxygen deprivation onengineered mesenchymal stem cell-based cartilage constructs. Osteoarthr Cartil 23, 134, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li, C., Armstrong, J.P., Pence, I.J., et al. . Glycosylated superparamagnetic nanoparticle gradients for osteochondral tissue engineering. Biomaterials 176, 24, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li, C., Ouyang, L., Pence, I.J., et al. . Buoyancy-driven gradients for biomaterial fabrication and tissue engineering. Adv Mater e1900291, 31, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oh, S.H., Kim, T.H., and Lee, J.H.. Creating growth factor gradients in three dimensional porous matrix by centrifugation and surface immobilization. Biomaterials 32, 8254, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Steele, J.A.M., McCullen, S.D., Callanan, A., et al. . Combinatorial scaffold morphologies for zonal articular cartilage engineering. Acta Biomater 10, 2065, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Camarero-Espinosa, S., Rothen-Rutishauser, B., Weder, C., et al. . Directed cell growth in multi-zonal scaffolds for cartilage tissue engineering. Biomaterials 74, 42, 2016 [DOI] [PubMed] [Google Scholar]
- 33. Ng, K.W., Ateshian, G.A., and Hung, C.T.. Zonal chondrocytes seeded in a layered agarose hydrogel create engineered cartilage with depth-dependent cellular and mechanical inhomogeneity. Tissue Eng Part A 15, 2315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo, L., Thorpe, S.D., Buckley, C.T., et al. . The effects of dynamic compression on the development of cartilage grafts engineered using bone marrow and infrapatellar fat pad derived stem cells. Biomed Mater 10, 055011, 2015 [DOI] [PubMed] [Google Scholar]
- 35. Kim, M., Erickson, I.E., Huang, A.H., et al. . Donor variation and optimization of human mesenchymal stem cell chondrogenesis in hyaluronic acid. Tissue Eng Part A 24, 1693, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahearne, M., and Kelly, D.J.. A comparison of fibrin, agarose and gellan gum hydrogels as carriers of stem cells and growth factor delivery microspheres for cartilage regeneration. Biomed Mater 8, 035004, 2013 [DOI] [PubMed] [Google Scholar]
- 37. Kwon, M.Y., Vega, S.L., Gramlich, W.M., et al. . Dose and timing of N-cadherin mimetic peptides regulate MSC chondrogenesis within hydrogels. Adv Healthc Mater 7, 1, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwon, M.Y., Wang, C., Galarraga, J.H., et al. . Influence of hyaluronic acid modification on CD44 binding towards the design of hydrogel biomaterials. Biomaterials 222, 119451, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bian, L., Guvendiren, M., Mauck, R.L., et al. . Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A 110, 10117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang, A.H., Stein, A.S., Tuan, R.S., et al. . Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A 15, 3461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim, M., Erickson, I.E., Choudhury, M., et al. . Transient exposure to TGF-β3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J Mech Behav Biomed Mater 11, 92, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hung, C.T., Lima, E.G., Mauck, R.L., et al. . Anatomically shaped osteochondral constructs for articular cartilage repair. J. Biomech 36, 1853, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Albro, M.B., Bergholt, M.S., St-Pierre, J.P., et al. . Raman spectroscopic imaging for quantification of depth-dependent and local heterogeneities in native and engineered cartilage. NPJ Regen. Med 3, 3, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]