Abstract
Introduction: Treatment of traumatic brain injury (TBI) with granulocyte colony-stimulating factor (G-CSF) has been shown to enhance brain repair by direct neurotrophic actions on neural cells and by modulating the inflammatory response. Administration of cannabinoids after TBI has also been reported to enhance brain repair by similar mechanisms.
Objectives: The primary objective of this study was to test the hypothesis that G-CSF mediates brain repair by interacting with the endocannabinoid system.
Methods and Results: (i) Mice that underwent controlled cortical impact (CCI) were treated with G-CSF for 3 days either alone or in the presence of selective cannabinoid receptor 1 (CB1-R) or cannabinoid receptor 2 (CB2-R) agonists and antagonists. The trauma resulted in decreased expression of CB1-R and increased expression of CB2-R in the cortex, striatum, and hippocampus. Cortical and striatal levels of the major endocannabinoid ligand, 2-arachidonoyl-glycerol, were also increased by the CCI. Administration of the hematopoietic cytokine, G-CSF, following TBI, resulted in mitigation or reversal of trauma-induced CB1-R downregulation and CB2-R upregulation in the three brain regions. Treatment with CB1-R agonist (WIN55) or CB2-R agonist (HU308) mimicked the effects of G-CSF. (ii) Pharmacological blockade of CB1-R or CB2-R was not effective in preventing G-CSF's mitigation or reversal of trauma-induced alterations in these receptors.
Conclusions: These results suggest that cellular and molecular mechanisms that mediate subacute effects of G-CSF do not depend on activation of CB1 or CB2 receptors. Failure of selective CB receptor antagonists to prevent the effects of G-CSF in this model has to be accepted with caution. CB receptor antagonists can interact with other CB and non-CB receptors. Investigation of the role of CB receptors in this TBI model will require studies with CB1-R and in CB2-R knockout mice to avoid nonspecific interaction of CB receptor agents with other receptors.
Keywords: brain repair, cannabinoid receptors, endocannabinoids, mouse, traumatic brain injury
Background
The endocannabinoid system (eCBS) participates in the brain's complex cascade of responses to injury by decreasing vasoconstriction, gliosis, neuroinflammation, and excitotoxicity.1 The eCBS consists of ligands, N-arachidonoylethanolamine (AEA) and 2-arachidonoyl-glycerol (2-AG), receptors (CB1, CB2, and possibly also TRPV1 and GPR55), transporters, and enzymes, which are responsible for the synthesis and degradation of these lipid mediators.2,3 Two additional eCB ligands, N-eicosa-5Z,11Z,14Z-trienoyl-(sciadonoyl)-ethanolamide and 2-eicosa-5Z,11Z,14Z-trienoyl-(sciadonoyl)-glycerol, have been identified, which interact preferentially with the CB1 receptor.4 Novel eCB ligands (ω-3-derived endocannabinoid epoxides) formed from enzymatic oxidation of ω-3 endocannabinoids by cytochrome P450 epoxygenases have also been characterized recently.5 These dual functional ω-3 endocannabinoid epoxides exhibit preference toward binding to the CB2 receptor.
The CB1 receptor is the most abundant CB receptor in the brain and is responsible for mediating, among other actions, the psychoactive effects of cannabinoids. These receptors are localized on GABAergic interneurons and glutamatergic neurons.6–8 CB2 receptors are expressed predominantly in peripheral cells as well as on small subpopulations of neurons in the brainstem. Although considered to be located mostly in the immune system, CB2-R is now well recognized to be expressed by resident inflammatory cells within the central nervous system (CNS), on microglial and dendritic cells9,10 and on brain endothelial cells.11 Activation of CB2 receptors has also been reported to mediate brain repair and behavioral recovery from TBI in a mouse model by modulating recruitment of peripheral monocytes, promoting anti-inflammatory cytokine production, and improving blood flow to the site of injury.12
A recent report has shown that activation of CB1 and CB2 receptors by administration of the phytocannabinoid tetrahydrocannabinol (Δ9-THC) into mice greatly stimulated generation of myeloid-derived suppressor cells (MDSCs).13 These MDSCs comprise a heterogeneous population of immature macrophages, granulocytes, dendritic cells, and other myeloid cells, which exert immunosuppressive actions. Moreover, induction of MDSCs by THC administration was associated with a significant increase in granulocyte colony-stimulating factor (G-CSF) levels.13 Interestingly, G-CSF itself will also enhance proliferation of peripheral monocytes, promote recruitment of bone marrow-derived cells to the site of injury, promote microgliosis, and act directly in the brain as a neurotrophic factor to enhance recovery from injury.14,15
G-CSF has been identified as one of many cytokines that modulate the response to traumatic brain injury (TBI). Stab lesions of the hippocampus made by insertion and removal of an electrode or needle triggered an acute local rise in G-CSF and other cytokines released at the sites of insertion in the frontal cortex and hippocampus.16 In addition, hippocampal neurogenesis was shown to be increased by the microlesion. The peak level of G-CSF in the hippocampus was reached at 6 h with return to baseline by 24 h. In the frontal cortex, G-CSF levels peaked at 12 h and also returned to baseline by 24 h.16 From these results and the reports of others on the effects of G-CSF in stroke,17,18 it was postulated that G-CSF may play an important role in the brain's repair response to injury.
This hypothesis was tested and confirmed in mice by administration of G-CSF for 3 days after delivery of mild to moderate controlled cortical impact (CCI). G-CSF treatment significantly enhanced recovery in performance of the radial arm water maze (RAWM), a test of learning and memory.14,15 G-CSF treatment also modulated astrocytosis and microgliosis, increased expression of neurotrophic factors and anti-inflammatory cytokines, and generated new neurons in the hippocampus.
Although it is known that administration of THC will increase expression of G-CSF13 and that G-CSF administration will promote recovery from TBI, the impact of G-CSF administration on the eCBS has not been studied up till now. The overall objective of this research is to test the hypothesis that enhanced brain repair promoted by a course of G-CSF administration following TBI is mediated, in part, by the eCBS acting in both the brain and periphery. To test this hypothesis, we assessed the effects of G-CSF following TBI in mice treated with G-CSF in combination with selective CB1 and CB2 receptor agonists and antagonists.
Methods
Animals
This study was carried out in strict accordance with the National Institutes of Health Guide for the care and use of laboratory animals. The protocol was approved by the institutional animal care and use committee of the University of South Florida. Adult, 3-month-old, male C57BL/6J mice (25–30 g) were housed in standard laboratory cages and left undisturbed for 1 week after arrival at the animal facility. Animals had ad libitum access to water and laboratory chow and were maintained in a temperature- and humidity-controlled room on a 12-h light/12-h dark cycle with lights on at 7:00 AM. Twelve groups of C57BL/6J mice (n=6 per group) sustained CCI to the right frontal cortex. One group served as controls (sham surgery, no drugs). Two groups were treated for 3 days after CCI with daily subcutaneous (s.c.) injections of G-CSF (100 μg/kg) or vehicle. The other groups received cannabinoid receptor 1 (CB1-R) or cannabinoid receptor 2 (CB2-R) agonist or antagonists alone×3 days or with G-CSF×3 days. Animals were euthanized 3 days after CCI. Each brain was removed after perfusion with heparinized saline and dissected into three regions (cerebral cortex, corpus striatum, and hippocampus) for analyses.
Surgery and CCI
Animals underwent an experimental TBI with a CCI device (Pittsburgh Precision Instruments), as described previously.19 Animals initially received buprenorphine (0.05 mg/kg, s.c.) at the time of anesthesia induction (with 125 mg/kg ketamine and 12.5 mg/kg xylazine). After deep anesthesia had been achieved (verified by checking for pain reflexes), individual animals were fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). After exposing the skull, craniectomy (∼3 mm to accommodate the impactor tip) was performed over the right frontoparietal cortex (0.5 mm anteroposterior and 0.5 mm mediolateral to bregma). All mice received a mild TBI. The pneumatically operated TBI device (with a convex tip diameter of 2.5 mm) impacts the brain at a velocity of 6.0 m/sec, reaching a depth of 0.5 mm below the dura mater layer, and remains in the brain for 150 msec. The impactor rod was angled at 158° to the vertical to maintain a perpendicular position in reference to the tangential plane of the brain curvature at the impact surface. A linear variable displacement transducer (Macrosensors, Pennsauken, NJ) connected to the impactor measured velocity and duration to verify consistency. Bone wax was used to cover the craniectomized region, and the skin incision sutured thereafter. A computer-operated thermal blanket pad and a rectal thermometer allowed maintenance of body temperature within normal limits. All animals were closely monitored until recovery from anesthesia and over the next 3 days.
Drugs
G-CSF (Neupogen) was purchased from Amgen (Thousand Oaks, CA). The dose regimen of G-CSF (100 μg/kg) was chosen to replicate previous studies with this TBI model.14 Three days of treatment with 100 μg/kg G-CSF promoted recovery at 7 and 14 days after injury. In another study, a single high dose (300 μg/kg) administered 1 week after CCI improved motor performance when tested 8 weeks later.20 Other doses of G-CSF were not used in the present study because the aim was to reproduce the same profile of recovery with GCSF treatment as reported earlier. It should be noted that the dose of G-CSF (100 μg/kg×three doses) administered in the present study was lower than that reported to be effective in mobilizing the bone marrow in a rat model of stroke (300 μg/kg for 10 days), but higher than that utilized by others in rodent models of stroke.17,18,21
Cannabinoid drugs were purchased from TOCRIS, Inc. (Bio-Techne, Minneapolis, MN). These include WIN 55, 212-2 mesylate (selective CB1 agonist) Cat# 1038; AM251 (CB1 receptor antagonist) Cat# No. 1117; HU308 (selective CB2 receptor agonist) Cat# 3088; AM630 (CB2 receptor antagonist) Cat# No.1120; and FAAH inhibitor (arachidonoyl serotonin, dual FAAH inhibitor/TRPV1 antagonist) Cat# 2836. Doses utilized were chosen based on the supplier's references (https://tocris.com/pharmacology/cannabinoid-receptors).
Quantitative real-time PCR of CB receptors
The mouse brain tissue samples were homogenized, and total RNA was purified using the RNeasy Mini Kit (Qiagen, Germantown, MD). One microgram of total RNA was used to synthesize the complementary cDNA with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the manufacturer's protocol. The real-time PCR was performed using 2 μL of cDNA with 1 μL of 20×TaqMan Mouse CB1-R primer/probe set (assay ID number Mm01212171_s1) or CB2-R primer/probe set (assay ID number Mm02620087_s1) (Applied Biosystems, Foster City, CA), 10 μL of TaqMan® Fast Advanced Master Mix (Applied Biosystems), and 7 μL of nuclease-free water for a total volume of 20 μL. As an internal control, a second real-time PCR was performed using 2 μL of cDNA with 1 μL 20×TaqMan mouse GAPDH primer/probe set(Assay ID number Mm99999915_g1) (Applied Biosystems), 10 μL of 2×TaqMan Fast Advanced Master Mix, and 7 μL of nuclease-free water for a total volume of 20 μL. Thermocycling conditions were as follows: 50°C, 2 min; 95°C, 10 min; and 40 cycles at 95°C for 15 sec and 60°C for 1 min using the Applied BioSystems ViiA 7 System. Mouse CB1-R and CB2-R mRNA relative expression (RQ) to GAPDH was calculated. Each sample was run in duplicate.
Western blots
The brain tissue samples were homogenized in T-PER Tissue Protein Reagent (PI-#78510) with the protease and phosphatase inhibitor cocktail (PI-78443) by a homogenizer KZ-II (ServiceBio Company) and set at 60 Hz, 120 sec. After homogenizing, the sample was centrifuged at 11,000 rpm for 20 min at 4°C and the supernatant was transferred to a new tube. Each sample's protein concentration was measured by a BCA kit (Fisher Scientific; #23225). Total protein, 50 μg, was separated by a Novex XCell Surelock Electrophoresis Cell (Life Technologies) NuPAGE 4–12% Bis-Tris gel (Invitrogen), followed by transfer onto a nitrocellulose membrane (Bio-Rad; 0.45 μm). After transfer, the membrane was blocked in 5% bovine serum albumin in tris buffered saline with Tween 20 (TBST) buffer for 1 h, then membranes were probed with primary antibodies overnight at 4°C with gentle agitation.
The antibodies used were as follows:
Phospho-p44/42 mitogen-activated protein kinase (MAPK) (extracellular signal-regulated kinase 1/2 [Erk1/2]) (Thr202/Tyr204) (Cell Signaling 9101S) and p44/42 MAPK (9201S) and the second antibody was anti-rabbit immunoglobulin G horseradish peroxidase linked (Cell Signaling 7074S).
Levels of GAPDH in the same blots were measured by immunoblotting. Antibody 97166 (Cell Signaling).
The levels of phosphorylated p44/42 MAPK and total p44/42 MAPK or CB1 receptor were quantified by densitometry.
Mouse brain 2-AG enzyme-linked immunosorbent assay
Regions of brain were dissected as described above, and tissue was processed according to the protocol for the enzyme-linked immunosorbent assay (ELISA) kit for mouse 2-AG (Cat# CE0443Ge; Cloud-Clone Corp. Katy, TX).
Statistics
Data are expressed as mean±standard error of mean (n=3–4 mice per data point). Prism 7 (GraphPad Software, Inc., San Diego, CA) was used to perform one-way or two-way analysis of variance, followed by correction for multiple comparisons. p Values <0.05 were considered statistically significant.
Results
Effects of G-CSF treatment on expression of brain CB1 and CB2 receptor mRNA following TBI
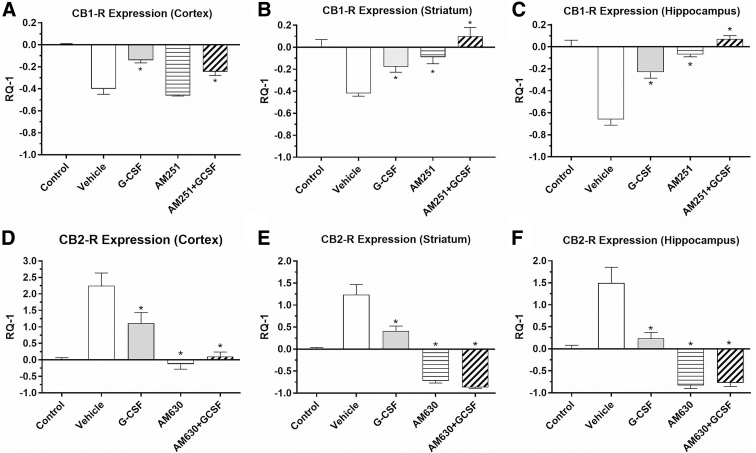
Mice treated with vehicle for three consecutive days after TBI exhibited significant changes in expression of both CB1-R and CB2-R mRNA on the side of the injury (Fig. 1). CB1-R mRNA expression was significantly decreased in three brain regions on the side (right) of the injury in vehicle-treated mice compared with controls without TBI. G-CSF treatment significantly mitigated or reversed downregulation of CB1-R to various extents in all three regions compared with vehicle treatment. By contrast, CB2-R expression was significantly upregulated in all three regions (Fig. 1D–F) compared with untreated control mice. Treatment with G-CSF significantly reversed the CB2 receptor upregulation to various extents in the three regions, p<0.05. (Fig. 1D–F).
FIG. 1.
Effects of TBI on expression of CB1-R and CB2-R in brain regions (in right hemisphere, the side of injury), following 3 days of treatment with vehicle or G-CSF and CB-R antagonists. Data are expressed as mean RQ-1±SEM. Vehicle and G-CSF treatment groups utilized n=4 mice; CB-R antagonists (with and without G-CSF) groups utilized n=4 mouse samples per Rx group. (A–C) CB1-R mRNA was significantly downregulated in the three brain regions in vehicle-treated mice compared with controls without TBI; G-CSF treatment significantly reversed the effect on CB1-R downregulation in all regions compared with vehicle Rx (*p<0.05). Treatment with AM251 alone (CB1 receptor inverse agonist), 1 mg/kg i.p.×3 days, did not reverse downregulation in the cortex (A), but did reverse downregulation in the striatum (B) and hippocampus (C). The combination of AM251 with G-CSF enhanced the expression of CB1-R, reversing the downregulation of CB1-R caused by the TBI in all three regions. (D–F) CB2-R expression was significantly upregulated in all three regions in vehicle-treated mice compared with untreated control mice; treatment with G-CSF significantly reversed CB2 receptor upregulation in all three regions *p<0.05). Administration of the CB2-R antagonist (AM630), 1.0 mg/kg i.p.×3 days, alone significantly reduced CB2-R expression in all three regions. Coadministration of G-CSF with AM630 enhanced downregulation of CB2-R expression elicited by G-CSF treatment alone in all three regions. Statistical significance in each brain region was determined by one-way ANOVA, followed by the Dunnett multiple comparison test (comparison with vehicle treatment). *p<0.05. i.p., intraperitoneal; ANOVA, analysis of variance; SEM, standard error of mean; CB1-R, cannabinoid receptor 1; CB2-R, cannabinoid receptor 2; G-CSF, granulocyte colony-stimulating factor; TBI, traumatic brain injury.
Treatment with the CB1 receptor inverse agonist (AM251, 1 mg/kg×3 days) did not change downregulation in the cortex (Fig. 1A), but did reverse downregulation in the striatum and hippocampus (Fig. 1B, C). The combination of AM251 with G-CSF enhanced the expression of CB1-R, reversing downregulation of CB1-R caused by the TBI in all three regions. Administration of the CB2-R antagonist (AM630), 1 mg/kg intraperitoneal×3 days, alone significantly reduced CB2-R expression in the three brain regions (Fig. 1D–F). Coadministration of G-CSF with AM630 enhanced the downregulation of CB2-R expression elicited by G-CSF treatment alone in all three regions. Although administration of G-CSF significantly altered both CB1-R and CB2-R expression levels in directions opposite that induced by the trauma, this reversal of the effect of trauma produced by G-CSF treatment could not be prevented by using CB-R antagonists. These data suggest that G-CSF effects on CB-R mRNA expression after injury do not require activation CB receptors.
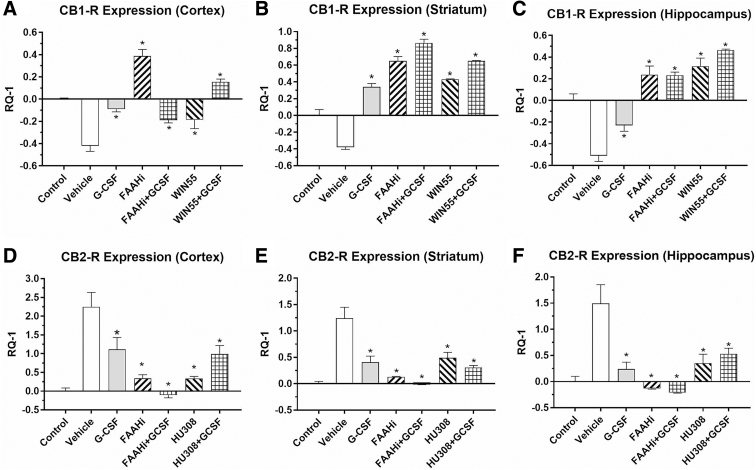
In a second set of experiments, the effect of administration of CB1-R agonist (WIN55), CB2-R agonist (HU308), or an inhibitor of FAAH (arachidonoyl serotonin) with and without G-CSF was studied. CB1-R expression was downregulated in the vehicle-treated animals compared with sham controls in the three brain regions (Fig. 2A–C). Treatment for 3 days after TBI with G-CSF or FAAH inhibition (and the combination of both) resulted in significant mitigation or reversal of the downregulation. Treatment with WIN55 (a selective CB1-R agonist) also resulted in significant reversal of CB1-R downregulation. CB2-R expression (Fig. 2D–F) was upregulated by the injury. G-CSF treatment alone resulted in reversal of the upregulation in all three brain regions. Notably, the FAAH inhibitor and HU308 (CB2 receptor agonist) reversed the upregulation significantly either when given alone or in combination with GCSF in all three regions. These data underscore the fact that G-CSF administration alone mimics the effects of selective CB-R agonists when administered after CCI. However, blockade of the CB1 and CB2 receptors did not prevent G-CSF regulation of CB receptor mRNA expression.
FIG. 2.
Effects of TBI on expression of CB1-R and CB2-R in brain regions (in right hemisphere, the side of injury), following 3 days of treatment with vehicle, G-CSF, FAAH inhibitor (5 mg/kg i.p.×3 days), the selective CB1 receptor agonist WIN55 (3 mg/kg i.p.×3 days), or the selective CB2 receptor agonist HU308 (3 mg/kg i.p.×3days). In the upper row (A–C), CB1-R expression was downregulated in the vehicle-treated animals compared with sham controls in the three brain regions. Treatment for 3 days after TBI with G-CSF and FAAH inhibition (and the combination of both) resulted in significant reversal of downregulation. Treatment with WIN55 (alone or in combination with G-CSF) also resulted in significant reversal of the CB1 receptor downregulation. In the lower row (D–F), CB2 receptor expression was upregulated by the injury. G-CSF alone significantly reversed the upregulation in the three brain regions. The FAAH inhibitor and HU308 (CB2 receptor agonist) each reversed the upregulation significantly, either when given alone or in combination with G-CSF (*p<0.05). One-way ANOVA followed by the Dunnett test for multiple comparisons against vehicle-treated mice (n=4 mice per treatment group).
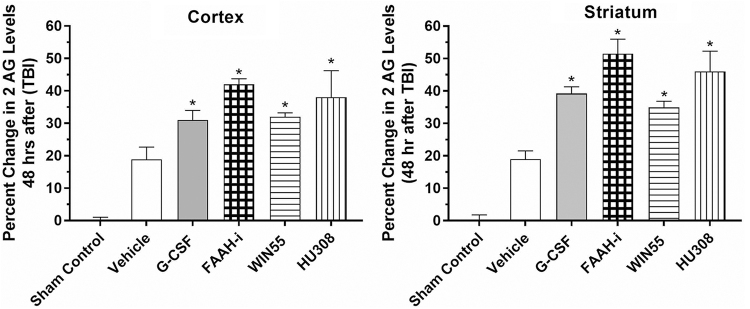
Effects of G-CSF on expression of the endogenous cannabinoid 2-AG
In this set of experiments, levels of the endogenous cannabinoid ligand 2-AG in right cerebral cortex and striatum were measured following CCI and treatment for 3 days with G-CSF, an inhibitor of FAAH, WIN55 (CB1-R agonist), and HU308 (CB2-R agonist). G-CSF treatment resulted in augmented levels of 2-AG, similar to those induced by CB1 and CB1 receptor ligand agonists (WIN55 and HU308, respectively (Fig. 3). In addition, inhibition of FAAH with arachidonoyl serotonin also increased levels of 2-AG. AEA levels, not shown, were below the level of detection by the ELISA. FAAH is the hydrolytic catabolic enzyme of the two major endocannabinoids (anandamide, AEA, and 2-AG, respectively) and is expected to increase levels of AEA and 2-AG. The FAAH inhibitor used here is also responsible for the enzymatic hydrolysis of other N-acyl-ethanolamines such as N-palmitoylethanolamine (PEA) and N-oleoylethanolamine (OEA),22,23 which are endocannabinoid-like compounds. These, in most cases, do not directly activate the two cannabinoid receptors (CB1 and CB2), but have other targets, which may play a role in the interaction of the eCB system with G-CSF.
FIG. 3.
Levels of the endogenous cannabinoid ligand 2-AG in the right cerebral cortex and striatum following controlled cortical impact and treatment for 3 days with G-CSF, an inhibitor of FAAH, WIN55 (CB1 receptor agonist), and a CB2 receptor agonist (HU308). Data are expressed as percent change in 2-AG levels from vehicle treatment levels (mean vehicle control levels were 1.76±0.05 ng/g in the cortex and 1.72±0.04 ng/g in the striatum). G-CSF as well as treatment with CB1 and CB2 agonists increased levels of 2-AG in both the cortex and striatum. In addition, inhibition of the major catabolic enzyme for endocannabinoids (FAAH) also increased levels of 2-AG. One-way ANOVA, followed by the Dunnett test for multiple comparisons, showed that all treatments significantly increased 2-AG levels compared with vehicle treatments after TBI (*p<0.05). 2-AG, 2-arachidonoyl-glycerol.
Effects on signal transduction
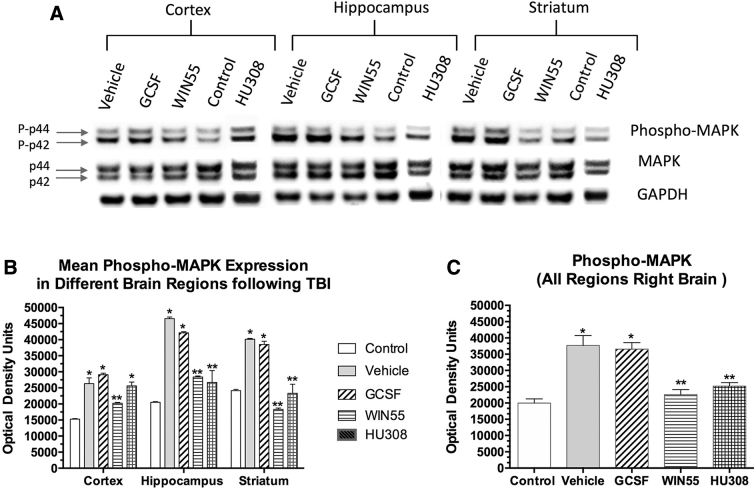
Signal transduction triggered by G-CSF and a ligand for the CB1-R (WIN55) and CB2-R (HU308) was studied in the three brain regions following CCI. (Fig. 4). The trauma alone triggered increased phospho-MAPK expression in all three brain regions. G-CSF treatment daily for 3 days after CCI did not modify signal transduction in any region. However, treatment with the CB1-R agonist (WIN55) or CB2-R agonist (HU308) for three consecutive days after injury significantly diminished phospho-MAPK in the three regions.
FIG. 4.
Effects of TBI followed by treatment with G-CSF and cannabinoids on signal transduction (phospho-MAPK protein expression). (A) Sample Western blot showing phospho-MAPK and GAPDH expression following various treatments. (B) Quantification (optical density) of normalized signal in three brain regions from three mice per treatment (from right hemisphere). Each bar is the mean (±SEM). Two-way ANOVA, followed by correction for multiple comparisons, revealed that both the brain region and treatment contributed significantly to total variance (p<0.0001); *p<0.001 compared with control (sham surgery, no treatment) and **p<0.01 compared with vehicle-treated samples. (C) Analysis of pooled data from the three regions of the right hemisphere (n=9; three samples from each of the three mice per treatment). MAPK, mitogen-activated protein kinase.
Discussion
The interaction of G-CSF with the eCBS in the context of brain injury is complex. Following CCI to the right frontal cortex, expression of CB1 receptors was downregulated in the cortex, striatum, and hippocampus. The CB2 receptors were upregulated in the three brain regions following CCI. The effects of CCI on expression of CB receptors are similar to those reported in a different rodent model of TBI.24 Using a weight-drop model of TBI, CB1 receptor expression (mRNA and protein) was decreased at 1 and 3 days after injury, but returned to normal by 2 weeks; the greater the decrease in CB1 receptors, the worse was the behavioral impairment and brain edema.24 In that same study, CB2 receptor mRNA expression was increased at 1, 3, and 14 days after injury and was associated with increased microglial activation and high neurological deficit.24
In the present study, administration of G-CSF for 3 days after CCI resulted in reversal of CB1-R downregulation in three brain regions, the cortex, striatum, and hippocampus. Coadministration of G-CSF with the CB1-R antagonist (AM251) did not prevent the reversal of downregulation of CB1-R in the three brain regions. This result fails to support the hypothesis proposed here—that G-CSF effects are mediated by activation of CB1 receptors in the brain repair cascade.
Earlier reporting from this laboratory has shown that 3 days of G-CSF treatment after CCI, using identical conditions as reported here, resulted in significantly enhanced behavioral recovery (performance in the RAWM). The recovery from injury was also associated with increased expression of neurotrophic factors and a spectrum of inflammatory and anti-inflammatory cytokines as well as increased microgliosis and astrocytosis 2 weeks after TBI.14 However, it is important to note that the increased microgliosis that was augmented by G-CSF treatment following CCI was not noted until 7 and 14 days after injury. In fact, 3 days after CCI, G-CSF treatment did not increase microglial activation and microgliosis in any of the three brain regions.4,5 Therefore, failure of the CB1-R blockade and CB2-R blockade to reverse the G-CSF-induced changes in CB-R expression 3 days after CCI can be hypothesized to require at least 1 or 2 weeks to observe the effect.
Moreover, prior work from our laboratory showed that blocking recruitment of peripheral monocytes to the site of brain injury (using a chemotactic receptor antagonist drug) did not prevent the beneficial actions of G-CSF.25 These results support the hypothesis that direct action of G-CSF on neural cells, independent of its hematopoietic effects, is primarily responsible for enhanced recovery from brain injury. Taken together with our present results, it is most likely that beneficial effects of G-CSF on recovery from TBI are due to direct actions on G-CSF receptors expressed by CNS neurons and glia and are not reliant on activation of CB1 or CB2 receptors.
Interestingly, a common response to G-CSF and to CB receptor agonists was to further increase cortical and striatal levels of the endocannabinoid ligand 2-AG beyond the elevated levels triggered by the TBI itself. Our findings are consistent with a previous report in which TBI resulted in significant increases in brain levels of the most abundant endocannabinoid 2-AG.1 In that study, administration of 2-AG was shown to decrease brain edema, inflammation, and infarct volume and improve clinical recovery. The role of CB1 in mediating these effects was demonstrated using selective antagonists or CB1 knockout mice.1
Common ground for the interaction of G-CSF and endocannabinoids was explored briefly in the context of intracellular signal transduction triggered by the G-CSF receptors and CB receptors. Intracellular MAPKs are activated by phosphorylation in response to trauma and a variety of mitogenic signals.26 Activation of G-CSF receptors has been reported to increase expression of phospho-MAPK27 and a similar signal transduction occurs when CB1-R is activated.28 The increased expression of phospho-MAPK in various brain regions following CCI was significantly downregulated by treatment for 3 days with the CB1-R agonist WIN55 as well as by the CB2 receptor agonist HU308. By contrast, G-CSF treatment did not downregulate phospho-MAPK expression compared with vehicle treatments. Hence, the mechanisms responsible for enhanced brain repair, following treatment with either G-CSF or cannabinoid drugs, do not appear to be explained by potential interaction of the intracellular MAPK pathways triggered by G-CSF or cannabinoids. However, intracellular signal transduction pathways triggered by activation of quantitative PCRs are complex. Activation of CB receptors involves other signaling cascades beyond the MAPK pathway, including ERK1/2, c-Jun N-terminal kinase, and p38, which are involved in regulation of cell proliferation, cell cycle control, and cell death.29 Mechanistically oriented molecular research will be required to determine the relationship between G-CSF and cannabinoid-triggered intracellular signaling pathways in mediating the actions of these agents.
In summary, administration of G-CSF alone results in many behavioral, cellular, and molecular effects similar to the beneficial effects produced by the phytocannabinoid THC in a model of TBI. However, pharmacological blockade of cannabinoid receptors did not block the effects of G-CSF in reversing effects of TBI on CB receptor expression. In particular, CB1-R blockade was not effective in preventing G-CSF's reversal of the trauma-induced downregulation of CB1 receptors in three brain regions studied. In fact, the CB1-R antagonist enhanced the effect of G-CSF in reversing trauma-induced downregulation of CB1-R. Moreover, CB2-R antagonists administered in combination with G-CSF also did not prevent reversal of receptor downregulation produced by G-CSF treatment alone.
Several caveats should be mentioned. The study is primarily observational and will require more mechanism-oriented research to better understand the interaction of the hematopoietic and neurotrophic cytokine G-CSF with the eCBS in the context of brain repair. Another limitation is that data reported here were obtained 3 days after CCI and did not measure changes at later time points. Future experiments will examine changes in the eCBS at 7 and 14 days after CCI. Another caveat is that ketamine, used to anesthetize the mice during the CCI, may have mitigated acute damage (triggered by glutamate release). However, the present work on subacute changes (hours to days) following TBI is well beyond the short half-life of ketamine. To be certain, this hypothesis would have to be tested by comparing other anesthetics with ketamine to determine the extent of injury and repair. Other anesthetics may also mitigate acute damage, but to induce TBI without anesthesia would be counter to the ethical principles of animal research. Finally, failure of CB receptor antagonists to prevent the effects of G-CSF in this model has to be accepted with caution. The results are based on the use of single doses of CB receptor drugs, without exploration of higher and lower doses. Moreover, CB receptor antagonists, especially at higher doses, can interact with other CB and non-CB receptors. To eliminate the problem of nonspecific interaction of CB receptor antagonists with other receptors, the experiments can be repeated in CB1-R and CB2-R knockout mice. Although these experiments with CB receptor knockout mice might provide interesting insights into mechanisms of action of G-CSF in the process of brain repair, it might be more productive to work on developing a combination treatment containing cannabis-derived medications and G-CSF to promote recovery from TBI.
Abbreviations Used
- 2-AG
2-arachidonoyl-glycerol
- AEA
N-arachidonoylethanolamine
- CB
cannabinoid
- CB1-R
cannabinoid receptor 1
- CB2-R
cannabinoid receptor 2
- CCI
controlled cortical impact
- CNS
central nervous system
- eCBS
endocannabinoid system
- ERK1/2
extracellular signal-regulated kinase 1/2
- G-CSF
granulocyte colony-stimulating factor
- i.p.
intraperitoneal
- MAPK
mitogen-activated protein kinase
- MDSC
myeloid-derived suppressor cell
- RAWM
radial arm water maze
- s.c.
subcutaneous
- TBI
traumatic brain injury
- THC
tetrahydrocannabinol
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by a Veterans Affairs (VA) Merit TBI Grant (BX004037-01A1) to S.S.
Cite this article as: Song S, Kong X, Borlongan C, Sava V, Sanchez-Ramos J (2021) Granulocyte colony-stimulating factor enhances brain repair following traumatic brain injury without requiring activation of cannabinoid receptors, Cannabis and Cannabinoid Research 6:1, 48–57, DOI: 10.1089/can.2019.0090.
References
- 1. Shohami E, Cohen-Yeshurun A, Magid L, et al. . Endocannabinoids and traumatic brain injury. Br J Pharmacol. 2011;163:1402–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884 [DOI] [PubMed] [Google Scholar]
- 3. Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122 [DOI] [PubMed] [Google Scholar]
- 4. Hammels I, Binczek E, Schmidt-Soltau I, et al. . Novel CB1-ligands maintain homeostasis of the endocannabinoid system in omega3- and omega6-long-chain-PUFA deficiency. J Lipid Res. 2019;60:1396–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Das A, Watson J, Carnevale L, et al. . Omega-3 endocannabinoid-epoxides are novel anti-inflammatory and anti-pain lipid metabolites. Curr Dev Nutr. 2019;3 [Epub ahead of print]; DOI: 10.1093/cdn/nzz031.FS15-01-19 [DOI] [Google Scholar]
- 6. Hajos N, Katona I, Naiem SS, et al. . Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249 [DOI] [PubMed] [Google Scholar]
- 7. Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4 [DOI] [PubMed] [Google Scholar]
- 8. Katona I, Rancz EA, Acsady L, et al. . Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maresz K, Carrier EJ, Ponomarev ED, et al. . Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445 [DOI] [PubMed] [Google Scholar]
- 10. Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol. 2008;13:147–159 [DOI] [PubMed] [Google Scholar]
- 11. Golech SA, McCarron RM, Chen Y, et al. . Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res. 2004;132:87–92 [DOI] [PubMed] [Google Scholar]
- 12. Braun M, Khan ZT, Khan MB, et al. . Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav Immun. 2018;68:224–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hegde VL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur J Immunol. 2010;40:3358–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song S, Kong X, Acosta S, et al. . Granulocyte colony-stimulating factor promotes behavioral recovery in a mouse model of traumatic brain injury. J Neurosci Res. 2016;94:409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song S, Kong X, Acosta S, et al. . Granulocyte-colony stimulating factor promotes brain repair following traumatic brain injury by recruitment of microglia and increasing neurotrophic factor expression. Restor Neurol Neurosci. 2016;34:415–431 [DOI] [PubMed] [Google Scholar]
- 16. Song S, Song SJ, Cao C, et al. . Hippocampal neurogenesis and the brain repair response to brief stereotaxic insertion of a microneedle. Stem Cells Int. 2013;2013:205878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Six I, Gasan G, Mura E, et al. . Beneficial effect of pharmacological mobilization of bone marrow in experimental cerebral ischemia. Eur J Pharmacol. 2003;458:327–328 [DOI] [PubMed] [Google Scholar]
- 18. Schabitz WR, Kollmar R, Schwaninger M, et al. . Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34:745–751 [DOI] [PubMed] [Google Scholar]
- 19. Yu S, Kaneko Y, Bae E, et al. . Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res. 2009;1287:157–163 [DOI] [PubMed] [Google Scholar]
- 20. Acosta SA, Tajiri N, Shinozuka K, et al. . Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS One. 2014;9:e90953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Solaroglu I, Cahill J, Jadhav V, et al. . A novel neuroprotectant granulocyte-colony stimulating factor. Stroke. 2006;37:1123–1128 [DOI] [PubMed] [Google Scholar]
- 22. Costa B, Bettoni I, Petrosino S, et al. . The dual fatty acid amide hydrolase/TRPV1 blocker, N-arachidonoyl-serotonin, relieves carrageenan-induced inflammation and hyperalgesia in mice. Pharmacol Res. 2010;61:537–546 [DOI] [PubMed] [Google Scholar]
- 23. Di Marzo V, Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. 2015;12:692–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lopez-Rodriguez AB, Acaz-Fonseca E, Viveros MP, et al. . Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PLoS One. 2015;10:e0128782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song S, Kong X, Acosta S, et al. . Effects of an inhibitor of monocyte recruitment on recovery from traumatic brain injury in mice treated with granulocyte colony-stimulating factor. Int J Mol Sci. 2017;18:1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neary JT. Protein kinase signaling cascades in CNS trauma. IUBMB Life. 2005;57:711–718 [DOI] [PubMed] [Google Scholar]
- 27. Marino VJ, Roguin LP. The granulocyte colony stimulating factor (G-CSF) activates Jak/STAT and MAPK pathways in a trophoblastic cell line. J Cell Biochem. 2008;103:1512–1523 [DOI] [PubMed] [Google Scholar]
- 28. Turu G, Hunyady L. Signal transduction of the CB1 cannabinoid receptor. J Mol Endocrinol. 2010;44:75–85 [DOI] [PubMed] [Google Scholar]
- 29. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19:833. [DOI] [PMC free article] [PubMed] [Google Scholar]