Abstract
Objectives: We investigated the time course of clinical response in the Treatment of Early Onset Schizophrenia Spectrum Disorders Study (TEOSS).
Methods: TEOSS randomized 119 predominantly outpatient youth ages 8–19 years with schizophrenia or schizoaffective disorder to 8 weeks of treatment with molindone, risperidone, or olanzapine. We used proportional hazards regression to determine whether these three antipsychotics differed in the time until clinical response, defined as the time from treatment initiation to the point of achieving a Clinical Global Impressions-Improvement (CGI-I) scale score of 1 (“very much improved”) or 2 (“much improved”) that was maintained until week 8.
Results: Of the 116 youth who initiated treatment, 56 (48%) achieved clinical response. Among clinical responders, the median (±interquartile range) time until clinical response was 4.0 (±4.0) weeks for olanzapine, 4.5 (±4.0) weeks for risperidone, and 6.0 (±4.0) weeks for molindone. There were no significant differences in time course for clinical response between medications (p = 0.84). Youth without symptom improvement (CGI-I ≥ 4) after 3 weeks were more likely to be clinical nonresponders at week 8 (relative risk ratio = 1.98, 95% confidence interval 1.29–3.05), compared with youth with at-least-minimal symptom improvement after 3 weeks when looking at all antipsychotics combined.
Conclusion: To our knowledge, our study is the first to investigate medication differences in treatment response timing in early onset schizophrenia spectrum disorders. Clinical response times for molindone, risperidone, and olanzapine were not significantly different. Furthermore, while lack of early improvement predicted clinical nonresponse, whether or not to continue antipsychotic treatment after 3 or more weeks without symptom improvement should be based on clinical judgment after weighing potential risks, benefits, and alternatives. ClinicalTrials.gov Identifier: NCT00053703
Keywords: psychosis, children and adolescents, pediatric, randomized controlled trial, early intervention, antipsychotic
Introduction
Schizophrenia spectrum disorders are caused by environmental and genetic factors and can be severely disabling (Cloutier et al. 2016; Taylor et al. 2019). When schizophrenia and schizoaffective disorders have an onset before age 18, they are considered early onset (McClellan et al. 2007), a subset of which are considered childhood onset (onset before age 13) (Driver et al. 2020). Evidence is mixed as to whether early onset schizophrenia spectrum disorders have a more severe presentation and worse prognosis compared with adult-onset schizophrenia spectrum disorders (Vourdas et al. 2003; Schimmelmann et al. 2007; Amminger et al. 2011; Driver et al. 2020). However, prior work suggests that the stratification of treatment response to different antipsychotic medications may differ in early onset compared with adult-onset schizophrenia and schizoaffective disorders (Kasoff et al. 2016; Driver et al. 2020).
The Treatment of Early Onset Schizophrenia Spectrum Disorders Study (TEOSS) is the largest double-blind randomized controlled trial in early onset schizophrenia (Frazier et al. 2007; Sikich et al. 2008) and has the potential to address longstanding questions about differential treatment response. TEOSS randomized 119 youth with early onset schizophrenia spectrum disorders to 8 weeks of acute treatment with molindone (a first-generation antipsychotic), risperidone (a second-generation antipsychotic), or olanzapine (a second-generation antipsychotic). TEOSS found that youth randomized to molindone had the highest rates of akathisia and that youth randomized to olanzapine had the greatest weight gain (Sikich et al. 2008; Taylor et al. 2018), but the response rates for molindone, risperidone, and olanzapine were similar (Sikich et al. 2008; Findling et al. 2010; Frazier et al. 2012; Gabriel et al. 2017). However, TEOSS did not investigate whether the time course of response differed between medications, which may further inform clinical decision making. Furthermore, in TEOSS the greatest improvement in symptoms was seen in the first 2 weeks (Sikich et al. 2008). However, whether symptom change in the first 2 weeks predicted subsequent response in TEOSS is unknown.
Evidence on how long antipsychotic response takes in children and adolescents is lacking, and as the largest randomized controlled trial in early onset schizophrenia spectrum disorders, TEOSS provides a unique opportunity to investigate clinical response time and optimal antipsychotic trial duration. Furthermore, the TEOSS study design allows us to determine whether there are differences between molindone, risperidone, and olanzapine in clinical response time course. We seek to expand the extant early onset psychosis literature by three interlinked analyses of the TEOSS data: (1) characterizing the time until clinical response across participants, (2) resolving whether this time course for clinical response differed between medications, and (3) determining whether and when lack of early symptom improvement predicted clinical nonresponse.
Methods
TEOSS overview
TEOSS methods have been detailed previously (Frazier et al. 2007; McClellan et al. 2007), and in this study, we provide a summary of the methods. TEOSS data are available through limited-access datasets of the National Institutes of Health. TEOSS was conducted at four academic sites in the United States—University of North Carolina at Chapel Hill, Harvard University, University of Washington, and Case Western Reserve University. The study was reviewed and approved by the Institutional Review Board at each site. TEOSS randomized 119 youth ages 8–19 years with schizophrenia, schizoaffective disorder, or schizophreniform disorder to 8 weeks of treatment with molindone, risperidone, or olanzapine in a 1:1:1 fashion. Participants who were randomized to molindone also received 1.0 mg of benztropine; all others received a placebo identical in appearance. Ultimately, fewer youth were randomized to olanzapine because randomization to olanzapine was stopped due to data safety monitoring board review of interim data showing increased metabolic side effects. Three participants left the study after randomization but before treatment initiation, such that 35 individuals were treated with olanzapine, 40 were treated with molindone, and 41 were treated with risperidone. The study was double blind. If participants were on an antipsychotic at the time of randomized treatment initiation, there was a crosstaper to the TEOSS antipsychotic. Typically, in the crosstaper, pre-enrollment antipsychotics were reduced to 67% of the initial dose on entry into the study on days 1–3, then 33% of the initial dose on days 4–6, and discontinued on day 7. During the study, symptoms were assessed weekly with the Clinical Global Impression-Improvement (CGI-I) scale (McClellan et al. 2007) and Positive and Negative Syndrome Scale (PANSS) (Kay et al. 1987).
A fixed-flexible dosing strategy (McClellan et al. 2007) based on participant age group (8–11 years old vs. 12–19 years old) was used and is summarized in Table 1. Dose increase was stopped if the participant was judged to be a 1 (“very much improved”) or 2 (“much improved”) on the CGI-I. After the first 2 days, all study medications were given in divided doses (i.e., taken twice daily). The mean endpoint daily doses were molindone 59.9 mg (±33.5), risperidone 2.8 mg (±1.4), and olanzapine 11.4 mg (±5.0) (Sikich et al. 2008).
Table 1.
Fixed-Flexible Dosing Goals (in Milligrams Per Day) by Age Group
| Starting dose | Fixed titration | Optional titration | |
|---|---|---|---|
| Ages 8–11 years | Day 0 | Day 11 | Day 50 |
| Molindone | 10 | 30 | 140 |
| Risperidone | 0.5 | 1.5 | 6 |
| Olanzapine | 2.5 | 7.5 | 20 |
| Ages 12–19 years | Day 0 | Day 11 | Day 29 |
| Molindone | 10 | 65 | 140 |
| Risperidone | 0.5 | 3 | 6 |
| Olanzapine | 2.5 | 12.5 | 20 |
Baseline demographic and clinical characteristics
Baseline demographic and clinical details have been reported previously (Frazier et al. 2007; Sikich et al. 2008). In brief, 66% of participants were male, 62% were white, and 31% were black, mean age was 13.8 ± 2.4 years, annual household income ranged from less than $20,000 to more than $100,000 with a median income bracket of $20,000–$60,000, diagnoses were 66% schizophrenia and 34% schizoaffective, and mean PANSS total score at baseline was 100.8, corresponding to a Clinical Global Impression Severity Scale score of “markedly ill” (score of 5 on a seven-point scale) (Leucht et al. 2005; Frazier et al. 2007). At the time of randomization, 93% were experiencing their first psychotic episode, 90% were outpatients, and 33% were antipsychotic naive (Sikich et al. 2008).
Outcome for current TEOSS analysis
Based on prior studies in schizophrenia (Leucht et al. 2009; Agid et al. 2011), we considered participants' clinical responders if they completed 8 weeks of randomized treatment and had a CGI-I of 1 (“very much improved”) or 2 (“much improved”) at week 8 compared with baseline. TEOSS used an anchored CGI-I that instructed raters to choose a CGI-I of 2 only when the patient had ≥25% reduction in psychotic symptom frequency or intensity and a clear improvement in functioning. Notably, “response” as defined by the original TEOSS investigators additionally required a ≥20% reduction in total symptoms on the PANSS (Sikich et al. 2008). Similar to other studies (Agid et al. 2011), we removed the requirement for a ≥20% reduction on the PANSS in our definition of “clinical response” so that our findings would be easily generalizable to clinical practice, in which completing the PANSS is often impractical due to time constraints. Furthermore, CGI-I and change in PANSS are highly correlated (Levine et al. 2008), and 91% of clinical responders had a ≥20% reduction in total symptoms on the PANSS and met the criteria for TEOSS investigator defined response. CGI-I is scored 1 (“very much improved”) to 7 (“very much worse”). Our outcome in the current analysis was time until clinical response, defined as the time from the point of treatment initiation to the point of achieving a CGI-I of 1 or 2 that was maintained until the end of the 8-week randomization period.
CGI-I of 3 (“minimally improved”) on the anchored CGI-I was described as the “level of functional impairment is not significantly different; psychotic symptoms are reduced in frequency or intensity by 5–25%.” We also investigated whether a CGI-I of 4 (“no change”) to 7 (“very much worse”) early in the course of treatment was useful for predicting clinical nonresponse.
Statistical analysis
We used survival analysis and multivariate Cox proportional hazards regression to determine whether the time course until clinical response differed between antipsychotic medications. We set the significance threshold as two-tailed p < 0.05 and the trend threshold for significance as two-tailed p < 0.10. We investigated age in years, sex, race (African American, European American, or Other), antipsychotic naive status, whether or not the participant was on an antipsychotic at the time of randomization, household income, final diagnosis (schizophrenia vs. schizoaffective), and baseline PANSS as potential confounders in univariate Cox regression analyses, and variables significant at the p < 0.10 level in univariate analyses were included in the multivariate Cox regression analysis along with antipsychotic. Among clinical responders, we used median and interquartile range to summarize the data as opposed to mean and standard deviation because time until sustained clinical response was not normally distributed based on the visual inspection of the Q-Q Plot and Shapiro–Wilk normality test (p < 0.001).
We calculated the relative risk of clinical nonresponse and 95% confidence intervals for youth who had no symptom improvement (CGI-I ≥ 4) at a given week compared with youth who had at-least-minimal symptom improvement (CGI-I < 4) at a given week and used Fisher's exact test to determine significance. We investigated the sensitivity, specificity, and positive predictive value for using no symptom improvement early in treatment as a predictor for ultimate clinical nonresponse. Analyses were conducted in R version 3.3.3.
Results
Time until clinical response
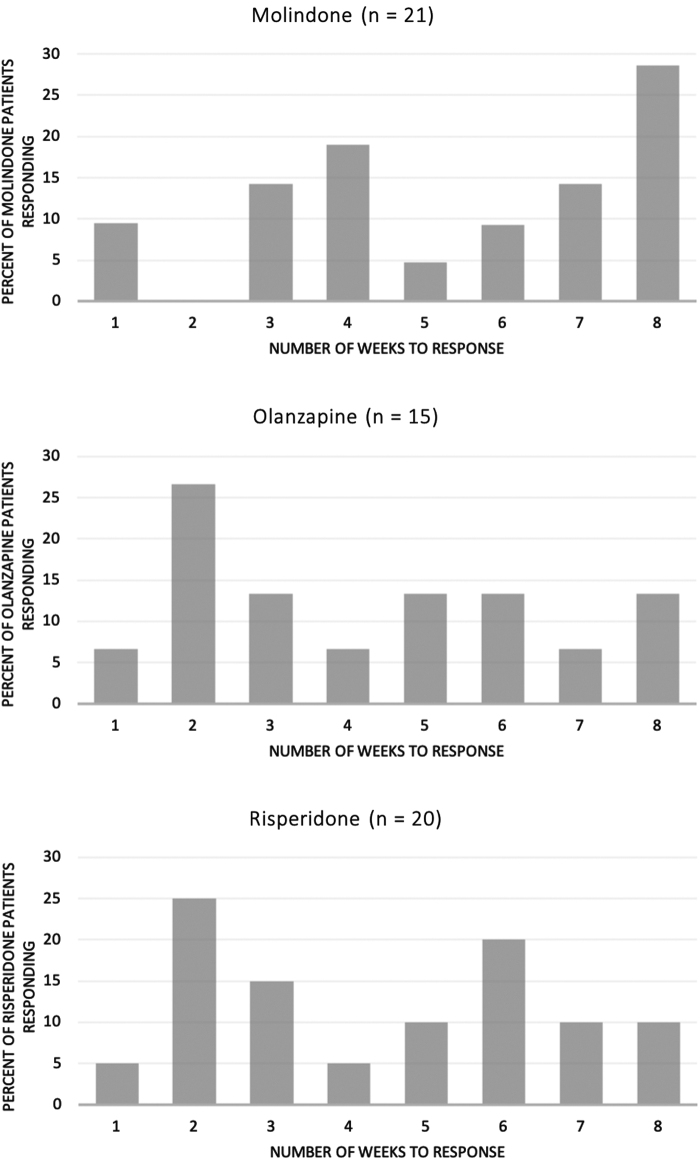
There were 56 (48%) clinical responders of the 116 youth who initiated treatment. Clinical response rates were similar for the three antipsychotics—43% (15 of 35) for olanzapine, 49% (20 of 41) for risperidone, and 53% (21 of 40) for molindone (three-sample test for equality of proportions p = 0.70). Among the 56 clinical responders, response times varied widely (Fig. 1). The range in time to clinical response for all three medications was 1–8 weeks, spanning the entire length of the trial. The median (±interquartile range) time until clinical response was 4.0 (±4.0) weeks for olanzapine, 4.5 (±4.0) weeks for risperidone, 6.0 (±4.0) weeks for molindone, and 4.0 (±4.0) weeks for olanzapine and risperidone combined. Moreover, 10 (18%) responders (2 olanzapine, 2 risperidone, and 6 molindone) did not achieve clinical response until week 8. All of the week 8 responders had demonstrated at-least-minimal improvement in symptoms by week 4.
FIG. 1.
Distribution of clinical response times for those who responded to molindone, olanzapine, and risperidone.
Figure 2 displays the Kaplan–Meier plot for time until clinical response, illustrating the similar time courses for clinical response for the three antipsychotics. In univariate proportional hazards regression, the time course for clinical response was similar for the three medications (p = 0.84) and did not vary based on sex, race, household income, antipsychotic naive status, whether the participant was on an antipsychotic at the time of randomization, final diagnosis, or baseline PANSS (all p > 0.13). The time courses for clinical response were also similar when second-generation antipsychotics, risperidone and olanzapine, were collapsed and compared with first-generation antipsychotic molindone (p = 0.76). However, there was a trend for younger individuals (hazard ratio [HR] = 0.91, standard error [SE] = 0.05, p = 0.08) to have shorter time courses for clinical response (Supplementary Fig. S1). In the multivariate proportional hazards regression analysis with antipsychotic and age as independent variables, younger age (HR = 0.90, SE = 0.06, p = 0.07) predicted a shorter time course for clinical response at the trend level, and antipsychotic differences remained nonsignificant (p > 0.46). In summary, there were no significant medication differences in time course for clinical response.
FIG. 2.
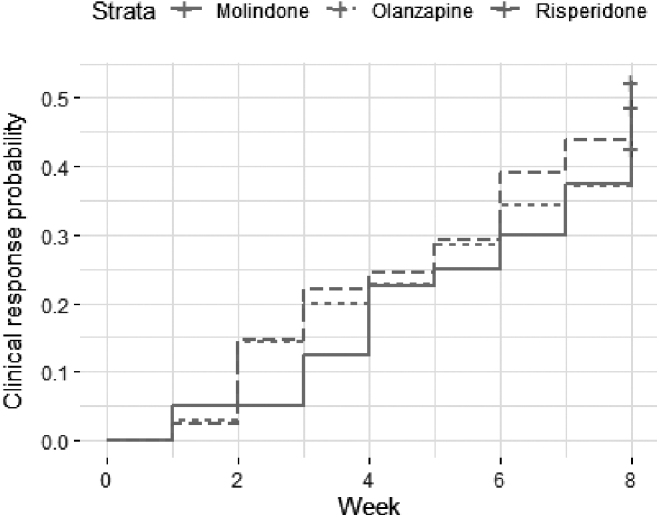
Kaplan–Meier plot showing likelihood of achieving clinical response by week.
Early symptom change as indicator of clinical nonresponse
When looking at all antipsychotics combined, the rates of at-least-minimal improvement (CGI-I < 4) were high for individuals who continued randomized treatment until the indicated week; at-least-minimal improvement rates were 47% at week 1, 80% at week 2, 87% at week 3, 89% at week 4, 92% at week 5, and 97% at weeks 6, 7, and 8. Very few (n < 5) participants had no symptom improvement at weeks 6 and 7, limiting the ability to draw inferences from the data regarding lack of symptom improvement at weeks 6 and 7. In terms of relative risk ratio (RR), having no improvement at week 1 (RR = 1.28, 95% confidence interval [CI] 0.90–1.84, p = 0.23) or week 2 (RR = 1.25, 95% CI 0.79–2.00, p = 0.52) did not significantly increase the likelihood of clinical nonresponse compared with individuals who had at-least-minimal improvement at the same week. However, having no improvement at week 3 (RR = 1.98, 95% CI 1.29–3.05, p = 0.03), week 4 (RR = 2.19, 95% CI 1.43–3.33, p = 0.02), and week 5 (RR = 3.00, 95% CI 1.79–5.03, p = 0.02) significantly increased the likelihood of clinical nonresponse compared with individuals who had at-least-minimal improvement at the same week.
Not having any symptom improvement at week 2 had 55% positive predictive value for clinical nonresponse (Table 2 and Supplementary Table S1). That is, if a participant did not have any symptom improvement by week 2, the likelihood of clinical nonresponse at week 8 was 55% (so the likelihood of clinical response was 45%). Not having any symptom improvement at weeks 3, 4, and 5 had higher positive predictive values for clinical nonresponse: 75%, 80%, and 83%, respectively.
Table 2.
Sensitivity, Specificity, and Positive Predictive Value of No Improvement at Given Week for Clinical Nonresponse at Week 8
| Week | Total N | N (%) with no improvement | Sensitivity (%) | Specificity (%) | PPV (%) |
|---|---|---|---|---|---|
| 1 | 115 | 60 (53) | 58 | 54 | 58 |
| 2 | 102 | 20 (20) | 23 | 84 | 55 |
| 3 | 94 | 12 (13) | 23 | 94 | 75 |
| 4 | 92 | 10 (11) | 21 | 96 | 80 |
| 5 | 78 | 6 (8) | 20 | 98 | 83 |
| 6a | 74 | 2 (3) | 5 | 98 | 50 |
| 7a | 73 | 2 (3) | 6 | 98 | 50 |
These results should not be generalized beyond the context of this trial because of the very small number of individuals without improvement who continued on their original treatment assignment at this point (n < 5).
PPV, positive predictive value.
Discussion
The National Institute for Health and Care Excellence (NICE) guidelines in the United Kingdom for “psychosis and schizophrenia in children and young people” recommend a 4–6-week medication trial at optimum dosage before switching medications (National Institute for Health and Care Excellence 2013). The most recent American Academy of Child and Adolescent Psychiatry (AACAP) Practice Parameters for schizophrenia state: “if insufficient effects are evident after a 6-week trial using adequate dosages, a different antipsychotic agent should be tried” (McClellan and Stock 2013). Despite these guidelines, it is unclear how often a full 6-week trial is utilized in clinical practice and whether a shorter trial may be justified in some cases.
Our main findings are that in TEOSS: (1) the time course for clinical response was similar for molindone, risperidone, and olanzapine; and (2) lack of symptom improvement after 3 weeks of treatment predicted clinical nonresponse, although lack of symptom improvement after 2 weeks of treatment did not predict clinical nonresponse.
Our finding that the time courses for clinical response were not significantly different between the three antipsychotics fits with the most recent AACAP Practice Parameter for schizophrenia, which recommends that antipsychotic choice be based on Food and Drug Administration (FDA) approval, side effects, patient and family preference, and cost—rather than perceived differences in efficacy (McClellan and Stock 2013; Pagsberg et al. 2017). Like prior work in older adolescents and adults with first-episode schizophrenia (Robinson et al. 2006, 2015), we found the clinical response times for the second-generation antipsychotics, risperidone (4.5 weeks) and olanzapine (4 weeks), were nearly identical. Importantly, the range in time to clinical response for all three medications was 1–8 weeks, spanning the entire length of the trial.
While there were no statistically significant differences between the three medications, the finding that risperidone and olanzapine responders had a median clinical response time that was 2 weeks shorter than the median response time for molindone (6 weeks) warrants further investigation in a larger study with greater power. It is important to keep in mind that molindone was dosed twice daily in TEOSS instead of three or four times daily as recommended on the FDA package insert. More frequent dosing of molindone may have improved outcomes for youth randomized to molindone and reduced the nonsignificant discrepancy between molindone and second-generation antipsychotic clinical response times.
Additionally, we found that one in six clinical responders did not achieve clinical response until week 8, and this was most common in the molindone arm (one in four). Notably, all patients who first achieved clinical response after 8 weeks had demonstrated at-least-minimal symptom improvement in the first 4 weeks of treatment. Our data suggest that waiting a full 8 weeks for clinical response may be prudent when partial symptom improvement is evident in the first 4 weeks. This is consistent with some schizophrenia guidelines, which state that when there is early symptom improvement, a longer antipsychotic trial of up to 10 weeks may be warranted (Hasan et al. 2012; Scottish Intercollegiate Guidelines Network 2013; Keating et al. 2017).
In contrast, when there is no improvement in symptoms early in treatment, guidelines vary markedly on how long to wait for response (Kreyenbuhl et al. 2009; Keating et al. 2017). The most recent AACAP Practice Parameter for schizophrenia (McClellan and Stock 2013) recommends changing antipsychotic after 6 weeks if the effects are “insufficient,” but does not include recommendations on what to do when there is no change in symptoms before the 6-week mark. Studies in children and adolescents regarding switching antipsychotic based on early symptom change are lacking, and findings in adults with schizophrenia are mixed. A study of 112 adults and older adolescents with first-episode psychosis found that degree of early symptom improvement at weeks 2, 4, and 8 did not sufficiently discriminate between those who would and would not respond by week 16 (Gallego et al. 2011). However, some evidence in adults with schizophrenia suggests that switching antipsychotic when there is no symptom improvement after 2 weeks may be reasonable because lack of symptom improvement after 2 weeks has predicted low response rates at 4–12 weeks in some studies (Correll et al. 2003; Leucht et al. 2007; Samara et al. 2015).
The current analysis found that lack of symptom improvement after 2 weeks did not predict clinical nonresponse at week 8. Even when there was no symptom improvement in the first 2 weeks, the likelihood of clinical response was 45%, which is similar to the likelihood of clinical response at antipsychotic initiation—48%. In contrast, we found that lack of symptom improvement after 3 weeks increased the likelihood of clinical nonresponse. However, 25% of individuals without symptom improvement after 3 weeks ultimately responded. The likelihood of clinical response was lowest (17%) when there was no symptom improvement after 5 weeks. Our findings support the clinical practice of recommending that patients continue their antipsychotic beyond 2 weeks even in the context of symptom persistence. When there is no symptom improvement after 3 or more weeks of treatment, clinicians can help patients and their families weigh the potential risks (e.g., inability to be certain that the current antipsychotic will not work for the patient if given more time) and benefits of switching to another antipsychotic. In terms of potential benefits of switching, a study in adults with schizophrenia spectrum disorders found that after 2 weeks of risperidone treatment, switching risperidone nonresponders to olanzapine resulted in a slightly greater reduction in psychotic and depressive symptoms compared with the early nonresponders who continued risperidone (Kinon et al. 2010).
Several methodological considerations about our study should be noted. First, clinical response was based on status after 8 weeks of treatment; however, prior work suggests that achieving response can take 16 weeks (Emsley et al. 2006; Gallego et al. 2011; Petrić et al. 2019). A longer study may have increased the response rate and the median response times. For instance, a 12-week study of individuals ages 15–40 with first-episode psychosis found a mean response time of 8 weeks for risperidone (Robinson et al. 2015) compared with the 4.5-week median clinical response time for risperidone in our analysis. Second, TEOSS was not powered to detect differences in clinical response times between antipsychotics, which would necessitate a larger study. A larger study would also enable investigation of whether lack of early symptom improvement at weeks 6 or 7 predicts clinical nonresponse. Third, our findings may not generalize to inpatient settings because only 10% of youth in TEOSS were inpatient when the study antipsychotic was initiated. Finally, psychosis exists on a spectrum (Alameda et al. 2019; Burton et al. 2019; Taylor et al. 2020), and because TEOSS only includes youth with schizophrenia, schizoaffective disorder, and schizophreniform disorder, our results may not generalize to nonschizophrenia/schizoaffective psychosis spectrum disorders, like unspecified psychotic disorder.
Conclusions
In conclusion, we found that the three antipsychotics in TEOSS did not significantly differ in clinical response times. Furthermore, even though the greatest symptom improvement occurred during the first 2 weeks in TEOSS (Sikich et al. 2008), lack of symptom improvement after 2 weeks did not predict clinical nonresponse. On the other hand, we found lack of symptom improvement after 3 weeks predicted clinical nonresponse, but we still found insufficient evidence for using lack of symptom improvement at any time point as a definitive sign that the antipsychotic should be changed.
Clinical Significance
Whether or not to continue antipsychotic treatment after 3 weeks without symptom improvement should be based on clinical judgment after weighing potential risks, benefits, and alternatives with the patient and family. To our knowledge, our study is the largest to investigate medication differences in treatment response timing in early onset schizophrenia spectrum disorders.
Disclaimer
This article reflects the views of the authors and may not reflect the opinions or views of the TEOSS Study Investigators or the NIH. The authors would like to thank NIH and the TEOSS Study Investigators for conducting an important and rigorous trial and making the resultant data easily available to eligible investigators.
Disclosures
M.H.B. receives research support from Therapix Biosciences, Neurocrine Biosciences, Janssen Pharmacueticals, and Biohaven Pharmaceuticals, and he serves on the scientific advisory boards of Therapix Biosciences and Teva Pharmaceuticals, none of whom provided support for the current study. There are no conflicts of interest and no relevant disclosures for any other authors.
Supplementary Material
Supplementary Material
References
- Agid O, Arenovich T, Sajeev G, Zipursky RB, Kapur S, Foussias G, Remington G: An algorithm-based approach to first-episode schizophrenia: Response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry 72:1439–1444, 2011 [DOI] [PubMed] [Google Scholar]
- Alameda L, Ashok A, Avery S, Bani-Fatemi A, Berkhout S, Best M, Bonfils K, Colizzi M, Dauvermann M, Plessis SD, Dwyer D, Eisner E, Ganesh S, Hernaus D, Ithal D, Kowalchuk C, Kristensen T, Lavigne K, Lee E, Lemmers-Jansen I, O'Donoghue B, Oliver L, Oluwoye O, Park MT, Di Carlo P, Joaquim HPG, Pinheiro A, Ramsay I, Rodriguez V, Sami M, Soni S, Sonnenschein S, Taylor J, Thomas M, Waterreus A, Wojtalik J, Yang Z, Emsley R, Kilian S: The 2019 Schizophrenia International Research Society Conference, 10–14 April, Orlando, Florida: A summary of topics and trends. Psychiatry Res 284:112672, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amminger GP, Henry LP, Harrigan SM, Harris MG, Alvarez-Jimenez M, Herrman H, Jackson HJ, McGorry PD: Outcome in early-onset schizophrenia revisited: Findings from the Early Psychosis Prevention and Intervention Centre long-term follow-up study. Schizophr Res 131:112–119, 2011 [DOI] [PubMed] [Google Scholar]
- Burton CZ, Tso IF, Carrión RE, Niendam T, Adelsheim S, Auther AM, Cornblatt BA, Carter CS, Melton R, Sale TG, McFarlane WR: Baseline psychopathology and relationship to longitudinal functional outcome in attenuated and early first episode psychosis. Schizophr Res 212:157–162, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier M, Aigbogun MS, Guerin A, Nitulescu R, Ramanakumar AV, Kamat SA, DeLucia M, Duffy R, Legacy SN, Henderson C, Francois C, Wu E: The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry 77:764–771, 2016 [DOI] [PubMed] [Google Scholar]
- Correll CU, Malhotra AK, Kaushik S, McMeniman M, Kane JM: Early prediction of antipsychotic response in schizophrenia. Am J Psychiatry 160:2063–2065, 2003 [DOI] [PubMed] [Google Scholar]
- Driver DI, Thomas S, Gogtay N, Rapoport JL: Childhood-onset schizophrenia and early-onset schizophrenia spectrum disorders: An update. Child Adolesc Psychiatr Clin 29:71–90, 2020 [DOI] [PubMed] [Google Scholar]
- Emsley R, Rabinowitz J, Medori R: Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry 163:743–745, 2006 [DOI] [PubMed] [Google Scholar]
- Findling RL, Johnson JL, McClellan J, Frazier JA, Vitiello B, Hamer RM, Lieberman JA, Ritz L, McNamara NK, Lingler J, Hlastala S, Pierson L, Puglia M, Maloney AE, Kaufman EM, Noyes N, Sikich L: Double-blind maintenance safety and effectiveness findings from the Treatment of Early-Onset Schizophrenia Spectrum (TEOSS) Study. J Am Acad Child Adolesc Psychiatry 49:583–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Giuliano AJ, Johnson JL, Yakutis L, Youngstrom EA, Breiger D, Sikich L., Findling RL, McClellan J, Hamer RM, Vitiello B, Lieberman JA, Hooper SR: Neurocognitive outcomes in the treatment of Early-Onset Schizophrenia Spectrum Disorders Study. J Am Acad Child Adolesc Psychiatry 51:496–505, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, McClellan Jon J, Findling RL, Vitiello B, Anderson R, Zablotsky B, Williams E, McNamara NK, Jackson JA, Ritz L, Hlastala SA, Pierson L, Varley JA, Puglia M, Maloney AE, Ambler D, Hunt-Harrison T, Hamer RM, Noyes N, Lieberman JA, Sikich L: Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS): Demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry 46:979–988, 2007 [DOI] [PubMed] [Google Scholar]
- Gabriel D, Jakubovski E, Taylor JH, Artukoglu BB, Bloch MH: Predictors of treatment response and drop out in the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) Study. Psychiatry Res 255:248–255, 2017 [DOI] [PubMed] [Google Scholar]
- Gallego JA, Robinson DG, Sevy SM, Napolitano B, McCormack J, Lesser ML, Kane JM: Time to treatment response in first episode schizophrenia: Should acute treatment trials last several months? J Clin Psychiatry 72:1691–1696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Möller H-J, World Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for Schizophrenia: World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: Update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry 13:318–378, 2012 [DOI] [PubMed] [Google Scholar]
- Kasoff LI, Ahn K, Gochman P, Broadnax DD, Rapoport JL: Strong treatment response and high maintenance rates of clozapine in childhood-onset schizophrenia. J Child Adolesc Psychopharmacol 26:428–435, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA: The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276, 1987 [DOI] [PubMed] [Google Scholar]
- Keating D, McWilliams S, Schneider I, Hynes C, Cousins G, Strawbridge J, Clarke M: Pharmacological guidelines for schizophrenia: A systematic review and comparison of recommendations for the first episode. BMJ Open 7:e013881, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinon BJ, Chen L, Ascher-Svanum H, Stauffer VL, Kollack-Walker S, Zhou W, Kapur S, Kane JM: Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology 35:581, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB: The schizophrenia patient outcomes research team (PORT): Updated treatment recommendations 2009. Schizophr Bull 36:94–103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Busch R, Kissling W, Kane JM: Early prediction of antipsychotic nonresponse among patients with schizophrenia. J Clin Psychiatry 68:352–360, 2007 [DOI] [PubMed] [Google Scholar]
- Leucht S, Davis JM, Engel RR, Kissling W, Kane JM: Definitions of response and remission in schizophrenia: Recommendations for their use and their presentation. Acta Psychiatr Scand 119:7–14, 2009 [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR: What does the PANSS mean? Schizophr Res 79:231–238, 2005 [DOI] [PubMed] [Google Scholar]
- Levine SZ, Rabinowitz J, Engel R, Etschel E, Leucht S: Extrapolation between measures of symptom severity and change: An examination of the PANSS and CGI. Schizophr Res 98:318–322, 2008 [DOI] [PubMed] [Google Scholar]
- McClellan J, Sikich L, Findling RL, Frazier JA, Vitiello B, Hlastala SA, Williams E, Ambler D, Hunt-Harrison T, Maloney AE: Treatment of early-onset schizophrenia spectrum disorders (TEOSS): Rationale, design, and methods. J Am Acad Child Adolesc Psychiatry 46:969–978, 2007 [DOI] [PubMed] [Google Scholar]
- McClellan J, Stock S: Practice parameter for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry 52:976–990, 2013 [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence: Psychosis and Schizophrenia in Children and Young People: Recognition and Management. London, England: Rcpysch Publiciations, 2013 [PubMed] [Google Scholar]
- Pagsberg AK, Tarp S, Glintborg D, Stenstrøm AD, Fink-Jensen A, Correll CU, Christensen R: Acute antipsychotic treatment of children and adolescents with schizophrenia-spectrum disorders: A systematic review and network meta-analysis. J Am Acad Child Adolesc Psychiatry 56:191–202, 2017 [DOI] [PubMed] [Google Scholar]
- Petrić D, Rački V, Gačo N, Kaštelan A, Graovac M: Retrospective analysis of the effectiveness and tolerability of long-acting paliperidone palmitate antipsychotic in adolescent first-episode schizophrenia patients. J Child Adolesc Psychopharmacol 29:197–204, 2019 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Gallego JA, John M, Petrides G, Hassoun Y, Zhang J-P, Lopez L, Braga RJ, Sevy SM, Addington J: A randomized comparison of aripiprazole and risperidone for the acute treatment of first-episode schizophrenia and related disorders: 3-Month outcomes. Schizophr Bull 41:1227–1236, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Woerner MG, Napolitano B, Patel RC, Sevy SM, Gunduz-Bruce H, Soto-Perello JM, Mendelowitz A, Khadivi A, Miller R: Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-Month outcomes. Am J Psychiatry 163:2096–2102, 2006 [DOI] [PubMed] [Google Scholar]
- Samara MT, Leucht C, Leeflang MM, Anghelescu I-G, Chung Y-C, Crespo-Facorro B, Elkis H, Hatta K, Giegling I, Kane JM: Early improvement as a predictor of later response to antipsychotics in schizophrenia: A diagnostic test review. Am J Psychiatry 172:617–629, 2015 [DOI] [PubMed] [Google Scholar]
- Schimmelmann BG, Conus P, Cotton S, McGorry PD, Lambert M: Pre-treatment, baseline, and outcome differences between early-onset and adult-onset psychosis in an epidemiological cohort of 636 first-episode patients. Schizophr Res 95:1–8, 2007 [DOI] [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network: Management of Schizophrenia: A National Clinical Guideline. Edinburgh, Scotland: SIGN, 2013 [Google Scholar]
- Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, Ritz L, Ambler D, Puglia M, Maloney AE, Michael E, De Jong S, Slifka K, Noyes N, Hlastala S, Pierson L, McNamara NK, Delporto-Bedoya D, Anderson R, Hamer RM, Lieberman JA: Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: Findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) Study. Am J Psychiatry 165:1420–1431, 2008 [DOI] [PubMed] [Google Scholar]
- Taylor JH, Asabere N, Calkins ME, Moore TM, Tang SX, Xavier RM, Merikangas A, Wolf DH, Almasy L, Gur RC, Gur RE: Characteristics of youth with reported family history of psychosis spectrum symptoms in the Philadelphia Neurodevelopmental Cohort. Schizophr Res 216:104–110, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Calkins ME, Gur RE: Markers of psychosis risk in the general population. Biol Psychiatry 2020. DOI: 10.1016/j.biopsych.2020.02.002 [DOI] [PubMed] [Google Scholar]
- Taylor JH, Jakubovski E, Gabriel D, Bloch MH: Predictors and moderators of antipsychotic-related weight gain in the Treatment of Early-Onset Schizophrenia Spectrum Disorders Study. J Child Adolesc Psychopharmacol 28:474–484, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vourdas A, Pipe R, Corrigall R, Frangou S: Increased developmental deviance and premorbid dysfunction in early onset schizophrenia. Schizophr Res 62:13–22, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.