Abstract
The immune system plays a critical role in directing tissue repair and regeneration outcomes. Tissue engineering technologies that are designed to promote new tissue growth will therefore be impacted by immune factors that are present in patients both locally at the site of intervention and systemically. The immune state of patients can be influenced by many factors, including infection, nutrition, and other disease comorbidities. As a result, the immune state is highly variable and may be a source of variability in tissue-engineered products in the clinic, which is not found in preclinical models. In this review, we will summarize key immune cells and evidence of their activity in tissue repair and potential in tissue engineering systems. We also discuss how clinical translation of tissue engineering strategies, in particular stem cells, helped elucidate the importance of the immune system. With increased understanding of the immune system's role in repair and tissue engineering systems, it will likely become a therapeutic target and component of future therapies.
Impact statement
Clinical translation of tissue-engineered products often yields variability in outcomes of repair. The immune system may be a major contributor to this variability. Each person's immune system is highly plastic and represents a living history of infections, diet, age, sex, and inherited genetic traits, as well as environmental factors. As we seek to design tissue-engineered products, we must consider the influence of the immune system on repair outcomes. Additionally, products can be designed to manipulate the immune system to skew toward a phenotype that promotes a desired repair outcome.
Keywords: biomaterials, immune response, immune state, immune variability, clinical translation, tissue engineering
The Immune System Orchestrates Wound Healing and Tissue Repair
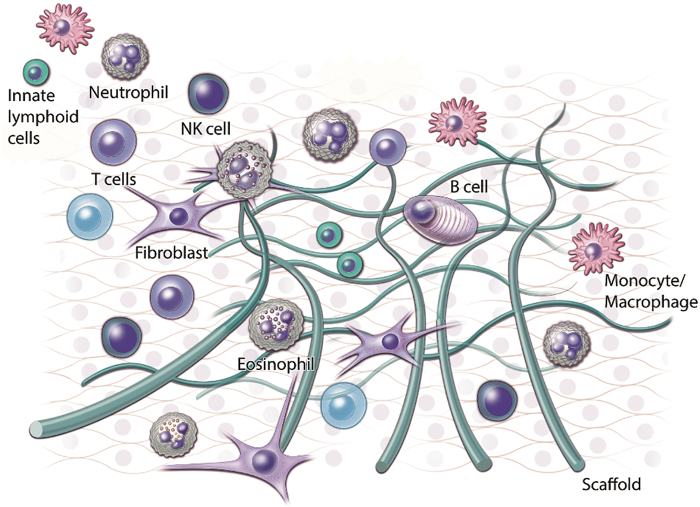
The immune system is capable of orchestrating tissue repair and healing. It is broadly categorized into two major components: innate and adaptive immune systems.1 In response to stimuli, the innate system mobilizes quickly with limited specificity. In parallel, the adaptive system activates more slowly with long-term memory and high specificity. The innate immune system comprises innate sensor cells, including epithelial cells, tissue-resident mast cells, macrophages, plasmacytoid dendritic cells, innate lymphocytes, and general/classical dendritic cells. Additional innate immune cells include basophils, natural killer (NK) cell types, granulocytes (including neutrophils, eosinophils, and basophils), and monocytes. Adaptive immune cells largely include T and B lymphocytes; however, additional cell types such as NKT cells and γδ T cells are of lymphoid origin despite their innate-like functional characteristics. Table 1 highlights the roles of these cells in tissue repair applications in both preclinical and clinical studies. Figure 1 demonstrates a graphical representation of the involvement of these cell types in wound healing.
Table 1.
Summary of Immune Cell Roles in Tissue Healing and Clinical Outcomes
| Role in healing (preclinical) | Clinical relevance | |
|---|---|---|
| Neutrophils | Neutrophil depletion delays/impairs healing2 Neutrophils enhanced angiogenesis in early wounds3 |
Dominance of neutrophil-derived proteases in recalcitrant venous ulcers, high levels in wound exudate associated with poor wound healing4 |
| Innate lymphoid cells (ILCs) | Barrier functions in tissue-dependent contexts dictate the roles of ILCs5,6 ILC1: proinflammatory through the secretion of IFNγ ILC2: innate immune response to intestinal parasitic worm clearance and ties to pathogenesis of asthma and allergy7 ILC3: intestinal barrier maintenance, promotion of fibrosis with synthetic biomaterials, or inflammation resolution in the lung8 |
Group 2 ILCs are enriched in atopic dermatitis (AD) skin lesions ILC2s in AD wounds may be a distinct population or in a different state of activation compared with ILC2s present in healthy skin9 RORγ+-CD127+CD3− ILC infiltration in human skin following wounding with punch biopsy enhances healing10 |
| NK cells | Defense against viral infection CD56dim and CD56bright NK cell classifications; CD56dim are cytotoxic, while CD56bright cells secrete mediating cytokines11 Clearance of senescent cells12 |
In patients undergoing endovascular aneurysm repair (EVAR), lower NK cell numbers correlated with negative long-term outcomes from EVAR (increased mortality)13 Sleep deprivation reduces NK cell number and NK cell activity14 |
| Eosinophils | Produce IL-4 and IL-13 direct stem cell fate in muscle regeneration15 Secrete IL-4 in liver injury to promote regeneration16 |
In burn wounds, high eosinophil infiltration is linked with increased wound healing. The absence of eosinophil infiltration leads to a poor prognosis and death in patients who did achieve a certain amount of eosinophils within 1 week following the burn17 However, aged patients with burn wounds present with increased eosinophil infiltration during early stages of healing, resulting in increased healing time compared with younger patients18 |
| Monocytes | CCR2hiCX3CR1low monocytes transition to CCR2lowCX3CR1hi to enter the injury site19 | CD33+CD14+CD11b+HLA-DRlow monocyte populations increased following hip surgery and an upregulation of STAT3 signaling correlated with surgical recovery20 |
| Macrophages | Required for salamander limb regeneration21; M2/alternatively activated macrophage phenotypes M1/proinflammatory macrophage phenotypes22,23 |
M1 macrophages, in chronic venous leg ulcers (CVUs), inhibited tissue repair24 M2 macrophages are also associated with accelerating bone healing in patients with traumatic brain injury25 |
| γδ T cells | Regulate macrophage homing Can induce IL-4 to regulate the macrophage phenotype (specific subsets of γδ T cells within injury)26,27 Modulate repair mechanisms in the wound environment through expression of IL-17 and IL-22 in CCl4, which induced liver fibrosis26,28 |
Psoriasis: γδ T cell frequency is increased compared with healthy controls, Psoriasis-associated γδs secrete higher levels of IL-17 than healthy control γδ− T cells29 |
| Immunologically activated fibroblasts | Secrete cytokines,30 orchestrate monocyte differentiation into macrophages31 Proangiogenic capabilities32 |
IFNγ, MCP-1, GM-CSF, and bFGF were overexpressed by wound fibroblasts in patients with chronic wounds compared with patients with well-healing wounds33 |
| CD8+ T cells | Depletion of CD8+ T cells results in wounds with increased mechanical toughness, linked to regulation fibroblasts and macrophages during wound healing34 | Elevated CD8+ T cells in the patient's blood and at the fracture site were associated with impaired bone repair35,36 |
| Tregs | Control effector T cell and neutrophil activity Regulate macrophage and monocyte phenotypes to anti-inflammatory states37–39 In muscle injury, they promote tissue regeneration through the expression of a proregenerative protein, amphiregulin40 |
CD161+ Tregs are enriched in patients with inflammatory bowel disease (Crohn's disease), suppress inflammation, and accelerate wound closure in the colon41 |
| CD4+ helper T cells | CD4+ T helper cells mediate immune response through cytokine secretion.1 Three types of CD4+ helper cells include the following: TH1 cells, which dominantly secrete interferon (IFN) and tumor necrosis factor (TNF) TH2 cells, which secrete IL-4, IL-5, and IL-13 TH17 cells, which secrete IL-17, IL-21, and IL-22 and follicular helper T cells TH2 CD4+ cells are IL-4-producing T cells that were responsible for enhancing neuronal protection and recovery following CNS injury42,43 |
TH17 cells are found near breast capsule implants and contribute to IL-17a secretion and fibrosis near the breast capsule implant44 TH17 cell frequency and plasma TH17-associated cytokine concentrations positively correlate with carotid artery plaques45 |
| B cells | Secrete IL-10 (intestinal injury)46; Enable skin wound closure through the soluble secretion of IL-6 and transforming growth factor beta47,48 |
In renal transplant patients, B cell-mediated immune regulation was characterized by the ratio of IL-10/TNFα expression Patients with poor graft outcomes had a low IL-10/TNFα ratio in transitional B cells49 |
HLA, human leukocyte antigen; IL, interleukin; NK, natural killer; STAT3, signal transducer and activator of transcription 3; TH2, T helper type 2; Treg, regulatory T cell.
FIG. 1.
Several immune cell populations orchestrate wound repair in the presence of a scaffold. This schematic highlights the immune cells known to play a role in clinical outcomes, including NK cells, innate lymphoid cells, T cells, fibroblasts, B cells, eosinophils, and monocytes/macrophages. NK, natural killer.
Stromal Cells Contribute to the Immune Environment in Wound Healing and Tissue Repair
The tissue stroma comprises fibroblasts and the connective tissue matrix. While the stroma has not historically been considered immunologically active, recent studies show that fibroblast subsets can secrete cytokines that interact with the immune system.30–32 Senescent cells and inflammatory fibroblasts are emerging as important regulators in tissue repair and also serve as a connection between traditional immune cells and the stroma. Senescent cells are characterized by cell cycle arrest and secretion of a senescence-associated secretory phenotype (SASP).50 Senescent cells develop after injury and their SASP is critical to tissue repair.51,52 Secreted SASP factors include interleukin (IL)-1, IL-6, MIP-1a, and IL-8, as well as extracellular matrix (ECM)-degrading enzymes. These cytokines can influence the local immune response and thus contribute to the immune environment and tissue repair.53,54
The phenotype of senescent cells and how they evolve during wound healing remain unknown. It is hypothesized that the SASP may benefit wound healing when present acutely as the inflammatory cytokines stimulate immune cell recruitment and surveillance that enable debris clearance and tissue remodeling. When senescent cells were cleared early after a cutaneous injury (using the p16-3MR mouse model), granulation tissue and blood vessel development decreased in the wound.51 In both muscle injury and liver fibrosis, the presence of senescent cells limited fibrosis or fibrogenesis and enhanced tissue repair.12,55 However, senescent cells are also found in atherosclerosis and osteoarthritis lesions and the removal of senescent cells reduces disease pathology.56,57 These studies suggest that injury-related accumulation of senescent cells can either retard or accelerate wound healing potentially depending on the phenotype and kinetics.52 Additional work is required to better understand and exploit the stroma and cellular senescence in tissue repair and tissue engineering.
Immunomodulatory Properties of Stem Cells
Mesenchymal stem cells (MSCs) and other adult stem cell types generated excitement in the field of tissue engineering for their ability to proliferate and differentiate into multiple tissue types. Insight from clinical trials using stem cells presented questions regarding their therapeutic mechanisms of action in tissue repair.58–60 According to Clinicaltrails.gov, there are currently over 5000 clinical trials utilizing stem cells as a therapeutic, with ∼1000 of those trials using MSCs specifically.60 MSCs are being tested for their ability to treat a wide range of clinical challenges, including bone and cartilage repair, neurodegeneration, cardiovascular regeneration, liver disease, diabetes, graft-versus-host disease, and some autoimmune diseases.60
While there is strong evidence of stem cell capacity to differentiate into multiple cell types in vitro, their activity following implantation into wound models is less clear. Previously, MSCs were thought to facilitate tissue healing by homing to damaged tissue and differentiating into a specific cell phenotype. However, several clinical trials suggested that the immunomodulatory capacity of stem cells was the primary therapeutic mechanism of action.61,62 Important considerations of cell delivery are viability and localization after injection. When injected in vivo, stem cells are primarily found in the lungs and spleen.63 Both organs, especially the spleen, are immunologically active and may respond to the immunologically active factors in dead cells. Apoptotic and necrotic cells emit damage signals that alter the wound microenvironment and stimulate innate and adaptive immune responses.64,65
MSCs reduce inflammation and promote tissue repair through the secretion of multiple factors, including prostaglandin E2, hepatocyte growth factor, and inducible nitric oxide synthase, which diminishes T cell proliferation.66–69 MSCs also reduced immunoglobulin production in plasma cells through CCL2 (a chemokine).70 In addition, MSC secretion of leukemia inhibitory factor promotes regulatory T cell (Treg) expansion.71 In clinical studies of systemic lupus erythematosus (SLE) and Crohn's disease, MSC treatment reduced autoantibody levels in the serum.72,73 These clinical trials suggest that successful therapeutic response in patients is not primarily due to the differentiation and tissue-forming capabilities of stem cells but rather a product of cross talk between these cells and the patient's immune system, further supporting the importance of the immune system in tissue engineering technologies.
Biomaterials Can Orchestrate Healing Through Immunomodulation
While stem cell therapies in the clinic suggested immunological therapeutic mechanisms of action in tissue repair, research in biomaterials also points to the immune system in tissue engineering. In clinical studies of the hydrogel system BST-CarGel® for cartilage repair, immunomodulation appeared to be a key mechanism of action.74–76 Hoemann and Buschmann demonstrated that chitosan hydrogels activated the complement system, an early component of the innate immune system's response, to improve cartilage repair when used in combination with surgical microfracture.77 It is also likely that poly(ethylene glycol) hydrogels used for cartilage repair also modulated the immune response in addition to concentrating and attracting progenitor cells after microfracture.78,79
Biological scaffolds derived from the ECM of tissues create a proregenerative environment. Importantly, research into this class of biomaterials established the importance of the macrophage phenotypes in regeneration outcomes, which have been applied in many clinical indications. Biological scaffolds are used clinically for abdominal repair, wound healing, and rotator cuff repair and are in clinical testing for muscle, cardiac, and soft tissue reconstruction.80–82 Brown et al. found that a biological scaffold derived from the small intestine submucosa–extracellular matrix (SIS-ECM) promoted invading and neighboring macrophages toward an alternatively activated macrophage phenotype.83,84 In this work, macrophages were described as either classical M1, proinflammatory, or alternative M2, protissue, healing phenotypes. However, cross-linking of the porcine-derived, SIS (CDI-SIS) biological scaffold with carbodiimide inhibited the proregenerative macrophage phenotype and instead induced an M1 phenotype and resulted in decreased repair compared with the SIS-ECM. Additional studies reinforced the link between the macrophage phenotype and tissue remodeling with the biological scaffold in the presence or absence of cells.83,85 When assessing 14, various, biologically derived surgical meshes, a correlation was found between a positive remodeling outcome and recruitment of higher numbers of M2 macrophages (also characterized by a higher M2/M1 macrophage ratio84). An M2 macrophage phenotype is now synonymous with biological scaffold efficacy and tissue regeneration.
Additional studies on biological scaffolds found that an adaptive immune response, specifically a type 2 immune response, is critical for scaffold efficacy and regenerative relevance.86–88 Following volumetric muscle loss and implantation of biological scaffolds, T helper type 2 (TH2) cells producing IL-4 infiltrated the wound site. Critically, TH2 cells aid in orchestrating healing and functional tissue repair; they promoted the M2 macrophage phenotype at the wound site and promoted systemic increases of IL-4 production.
Type 2 immune responses, characterized by IL-4 production, are important for healing in multiple tissues.43,89 Walsh et al. found that IL-4-producing T cells were responsible for enhancing neuronal protection and recovery, following both optic nerve crush injury and spinal cord contusive injury.42 Importantly, these T cells were stimulated toward a type 2 response independent of the antigen-presenting major histocompatibility complex II (MHC II). Damage-associated molecular patterns from damaged cells following injury were responsible for inducing IL-4-producing T cells. Chawla also found that eosinophils secreting IL-4 stimulate muscle and liver regeneration.15,16 In a review by Gieseck et al., the authors acknowledge that type 2 signatures are indeed beneficial to wound healing and that future therapeutic targeting against fibrosis should carefully aim to block the pathogenic features of sustained type 2 responses without losing its benefits for wound healing.90 However, it is critical to recognize that it is not known how the kinetics of a type 2 immune response in healing may or may not be correlated with a type 2 response in fibrosis or even allergic responses. Nevertheless, a type 2 immune response is tied to healing and thus must be considered within the context of regenerative engineering products.
The Human Immune State Is Inherently Variable
The immune state of an individual influences their therapeutic response. Variables that can impact the immune system include, but are not limited to, age; infection; history of infection; allergies; the presence of other injuries, disease, or cancer; and sex.
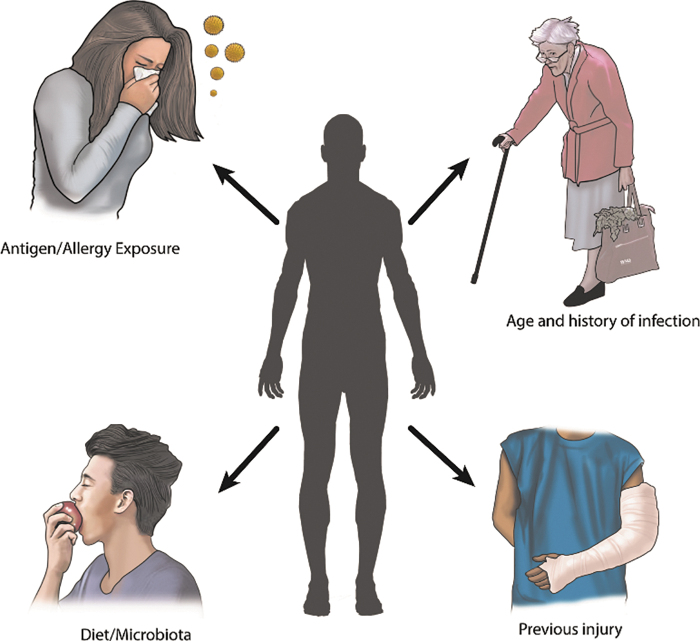
Variations in immune states are both heritable and nonheritable traits. Heritable traits account for a minority of the variation in a person's immune state91; specific examples include sex differences as well as MHC and non-MHC-associated genes. Sex- and hormone-based differences represent some of the most obvious heritable immune differences. Females, with estrogen, have an enhanced and protective humoral immunity response versus males, with testosterone, due to the ability to generate natural antibodies to bacterial infection.92 Research in MHC and human leukocyte antigen (HLA) complexes links disease presentation to inherited MHC or HLA alleles.93 Autoimmune diseases such as SLE, ankylosing spondylitis, and type 1 diabetes are all associated with heritable HLA domains and disease presentation.94 Vaccination and cancer recurrence also critically connect HLA alleles with cancer recurrence risk.95 It is unknown how HLA may impact the response to tissue trauma and tissue-engineered systems. Nonheritable influences, such as history of infections, antigen/allergen exposure, parasitic infections, and an individual's microbiota, play dominant roles in shaping immune states (Fig. 2). For example, smokers have increased leukocyte counts, reduced serum immunoglobulins, and an increased percentage of autoantibodies, linking the environmental exposure (smoking) to the immune state of the person.96
FIG. 2.
An individual's nonheritable immune influences include antigen/allergy exposure, age and history of infections, diet/microbiota, and previous injury. Together, nonheritable influences demonstrate significant impact on an individual's immune state.
Allergic disease pathways overlap with immunological characteristics of parasitic infections in terms of the influx of TH2 cells, heavy eosinophil activity, and high immunoglobulin E (IgE) production.97,98 Moreover, a person's established allergies or their predisposition to development of allergies can significantly influence their immune system response to infections and injuries. Hypersensitivity to otherwise nonharmful environmental agents can influence how a person's immune system may respond to clinical interventions postinjury and can limit therapeutic options. Biomaterials, especially metals used in orthopedic implants, can have a significantly higher failure rate if a person already possesses an established hypersensitivity or the propensity to develop sensitivity once an implant is in place.99 While genetic composition may determine the likelihood to develop an allergy, the hygiene hypothesis postulates that a person's early environmental exposure also helps to establish their likelihood of developing hypersensitivity to commonplace environmental agents. Intriguingly, multiple studies demonstrate the inverse association between parasitic infections and chronic or severe allergies.100 This proposed modification of the hygiene hypothesis proposes that the decline of helminth infections worldwide is associated with the increased prevalence of allergy-related diseases.101 However, these findings depended on the strain of the parasite as meta-analyses found that some parasitic strains reduced the incidence of asthma, while infection with other strains increased this risk. Regardless, having an established allergic sensitivity or having been previously infected by parasites has lasting effects on a person's subsequent immune responses and should be considered as noninherited aspects of what establishes an individual's immune state and response to injury and tissue engineering systems.
The microbiome and intestinal infections dramatically influence development of lymphoid tissue as well as interactions and phenotyping of the immune system in the gut.91,102 This is unsurprising as 20% of all lymphocytes reside in the gut. Factors such as obesity, malnutrition, increased caloric intake, and antibiotics influence immunity, specifically the memory T and B cells that develop with age.102,103 For example, gut microbiota in aged mice have increased clearance of hepatitis B virus (HBV) compared with young mice.104 Toll-like receptor activation, associated with lipopolysaccharide, allowed aged mice to promote HBV clearance. The history of microbiota in the gut is learned over time, altering each person's immunotype state.105 The influence of the microbiome on tissue-engineered products has not yet been investigated, but is likely to be a fruitful area for investigation given the role of the microbiome in regulating a patient's immune state.
Age alters the immune state in diverging ways.91,106–108 The loss of immune cells, reduced diversity of B and T cell clones, and response to the immunological challenge are all altered significantly with age. The variability of the immune state becomes more pronounced with age, indicating a cumulative effect of environmental (nonheritable) exposures. Additionally, the failure to maintain self-tolerance predisposes the aged population to autoimmune disease.109 Macrophages and dendritic cells display reduced phagocytotic function in aged populations, and immunosenescence occurs in T cells that still possess effector function, but do not proliferate after activation.110 Importantly, chronic low-grade inflammation occurs with aging, indicating the broad skewing of the immune system. The immunological changes that occur with aging likely impact the clinical responses to tissue-engineered products. Since most preclinical testing of tissue engineering technologies is performed in young animals, the variable performance in clinical studies may be due to the role of aging and variable aging phenotypes of patients.
The notion that each patient's medical history and environment play a large factor in their responses to tissue healing is widely accepted. Systemic factors in the immune system impact local immune responses and healing. After hip surgery in patients, upregulation of signal transducer and activator of transcription 3 (STAT3) in CD14+ monocyte populations correlated with surgical recovery (Table 1).20 Elevated CD8+ T cells in the patient's blood and at the fracture site correlated with reduced bone repair (Table 1).35,36 Both examples highlight the influence of the immune system in healing. Personalized medicine has become more widely accepted for drug development and cancer therapies, and future therapies that target the immune system will face high clinical variability due to each patient's personalized immune state and medical history. The immunological variability of each patient helps to explain why many therapeutics fail to achieve similar results in the clinic while achieving success in limited preclinical models.
Conclusion
While the contribution of a patient's medical history and environmental factors is generally viewed as an obvious set of factors, proregenerative and immunomodulatory therapy development by researchers traditionally does not take this into account. Just as a clinician must view each patient's medical history and previous environmental exposure as critical factors in determining treatment regimens, immunotherapy research and engineering product design can also take into consideration these factors. Thus, there is a need to further understand the impact of variability in immune states on tissue-engineered product efficacy. As we seek to advance regenerative engineering products, the immune state and any desired manipulation should inform product design.
Acknowledgments
The authors gratefully acknowledge funding from the National Science Foundation's Graduate Research Fellowship Program for D.M., DGE-1746891; the Rhines Rising Star Larry Hench Professorship that supported E.M.M.; and the Morton Goldberg Professorship Chair, the N.I.H. Pioneer Award, and the Bloomberg∼Kimmel Institute for Cancer Immunotherapy support for J.H.E.
Disclosure Statement
J.H.E. is an inventor on intellectual property related to biological scaffolds and inhibiting fibrosis. J.H.E. is a consultant to ACell and Unity Biotechnology and a founder of Aegeria.
Funding Information
This work was supported by the National Science Foundation (DGE-1746891), the National Institute of Health (Grant ID: DP1-AR076959) and the National Center of Advancement of Translational Science (Grant # 1KL2TROO1429).
References
- 1. Murphy, K., and Weaver, C.. Janeway's immunobiology. New York: Garland Science, 2016 [Google Scholar]
- 2. Nishio, N., Okawa, Y., Sakurai, H., and Isobe, K.I.. Neutrophil depletion delays wound repair in aged mice. Age (Omaha) 30, 11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang, Y., Li, L., Liu, Y., and Liu, Z.-R.L.. PKM2 released by neutrophi ls at wound site facilitates early wound healing by promoting angiogenesis. Wound Repair Regen 24, 328, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moor, A.N., Vachon, D.J., and Gould, L.J.. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen 17, 832, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Vivier, E., Artis, D., Colonna, M., et al. . Innate lymphoid cells: 10 years on. Cell 174, 1054, 2018 [DOI] [PubMed] [Google Scholar]
- 6. Castellanos, J.G., and Longman, R.S.. The balance of power: innate lymphoid cells in tissue inflammation and repair. J Clin Invest 130, 2640, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker, J.A., and McKenzie, A.N.. Development and function of group 2 innate lymphoid cells. Curr Opin Immunol 25, 148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taube, C., Tertilt, C., Gyülveszi, G., et al. . IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS One 6, e21799, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim, B.S., Siracusa, M.C., Saenz, S.A., Noti, M., Monticelli, L.A., and Sonnenberg, G.F.. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med 5, 170ra16, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li, Z., Hodgkinson, T., Gothard, E.J., et al. . Epidermal Notch1 recruits RORγ+ group 3 innate lymphoid cells to orchestrate normal skin repair. Nat Commun 7, 11394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mandal, A., and Viswanathan, C.. Natural killer cells: in health and disease. Hematol Oncol Stem Cell Ther 8, 47, 2015 [DOI] [PubMed] [Google Scholar]
- 12. Krizhanovsky, V., Yon, M., Dickins, R.A., et al. . Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Svetlikov, A.V., Gurevich, V., Ermakov, A., et al. . Role of natural killer cells and circulating endothelial cells in post-endovascular aneurysm repair long-term results. J Vasc Surg 67, e89, 2018 [Google Scholar]
- 14. Irwin, M., McClintick, J., Costlow, C., Fortner, M., White, J., and Gillin, J.C.. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J 10, 643, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Heredia, J.E., Mukundan, L., Chen, F.M., et al. . Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goh, Y.S., Henderson, N.C., Heredia, J.E., et al. . Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A 110, 9914, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wight, A., Raker, J.W., Merrington, W.R., and Cope, O.. The ebb and flood of the eosinophils in the burned patient and their use in the clinical management. Ann Surg 137, 175, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burns, B., Jackson, K., Farinas, A., et al. . Eosinophil infiltration of burn wounds in young and older burn patients. Burns 46, 1136, 2019 [DOI] [PubMed] [Google Scholar]
- 19. Dal-Secco, D., Wang, J., Zeng, Z., et al. . A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2 + monocytes at a site of sterile injury. J Exp Med 212, 447, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaudillière, B., Fragiadakis, G.K., Bruggner, R.V., et al. . Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med 6, 255ra131, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Godwin, J.W., Pinto, A.R., and Rosenthal, N.A.. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A 110, 9415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wynn, T.A.A., and Vannella, K.M.M.. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou, D., Huang, C., Lin, Z., et al. . Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal 26, 192, 2014 [DOI] [PubMed] [Google Scholar]
- 24. Sindrilaru, A., Peters, T., Wieschalka, S., et al. . An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in human and mice. Sindrilaru 2013. J Clin Invest 121, 985, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang, R., Liang, Y., and Wei, S.. M2 macrophages are closely associated with accelerated clavicle fracture healing in patients with traumatic brain injury: a retrospective cohort study. J Orthop Surg Res 13, 213, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. D'Alessio, F.R., Kurzhagen, J.T., and Rabb, H.. Reparative T lymphocytes in organ injury. J Clin Invest 129, 2608, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wehrmann, F., Lavelle, J.C., Collins, C.B., et al. . γδ T cells protect against LPS-induced lung injury. J Leukoc Biol 99, 373, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hammerich, L., Bangen, J.M., Govaere, O., et al. . Chemokine receptor CCR6-dependent accumulation of γδ T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology 59, 630, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cai, Y., Shen, X., Ding, C., et al. . Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35, 596, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haniffa, M.A., Wang, X.-N., Holtick, U., et al. . Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol 179, 595, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Hou, X., Chen, G., Bracamonte-Baran, W., et al. . The cardiac microenvironment instructs divergent monocyte fates and functions in myocarditis. Cell Rep 28, 172, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sarawati, S., Marrow, S.M.W., Watch, L.A., and Young, P.P.. Identification of a pro- angiogenic functional role for FSP1-positive fibroblast subtype in wound healing. Nat Commun 10, 3027, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwarz, F., Jennewein, M., Bubel, M., Holstein, J.H., Pohlemann, T., and Oberringer, M.. Soft tissue fibroblasts from well healing and chronic human wounds show different rates of myofibroblasts in vitro. Mol Biol Rep 40, 1721, 2013 [DOI] [PubMed] [Google Scholar]
- 34. Davis, P., Aspinall, R., and Wastell, C.. Effect of CD4+ and CD8+ cell depletion on wound healing. BJS 88, 298, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Reinke, S., Geissler, S., Taylor, W.R., et al. . Terminally differentiated CD8+ T cells negatively affect bone regeneration in humans. Sci Transl Med 5, 1, 2013 [DOI] [PubMed] [Google Scholar]
- 36. Schlundt, C., Schell, H., Goodman, S.B., Vunjak-Novakovic, G., Duda, G.N., and Schmidt-Bleek, K.. Immune modulation as a therapeutic strategy in bone regeneration. J Exp Orthop 2, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burzyn, D., Kuswanto, W., Kolodin, D., et al. . A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li, J., Tan, J., Martino, M.M., and Lui, K.O.. Regulatory T-cells: potential regulator of tissue repair and regeneration. Front Immunol 9, 1, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burzyn, D., Benoist, C., and Mathis, D.. Regulatory T cells in nonlymphoid tissues. Nat Immunol 14, 1007, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arpaia, N., Green, J.A., Moltedo, B., et al. . A distinct function of regulatory T cells in tissue protection. Cell 162, 1078, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Povoleri, G.A., Nova-Lamperti, E., Scottà, C., et al. . Human retinoic acid–regulated CD161+ regulatory T cells support wound repair in intestinal mucosa. Nat Immunol 19, 1403, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walsh, J.T., Hendrix, S., Boato, F., et al. . MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest 125, 2547, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Allen, J.E., and Wynn, T.A.. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog 7, 5, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wolfram, D., Rabensteiner, E., Grundtman, C., et al. . T regulatory cells and TH17 cells in peri–silicone implant capsular fibrosis. Plast Reconstr Surg 129, 327e, 2012 [DOI] [PubMed] [Google Scholar]
- 45. Liu, Z., Lu, F., Pan, H., et al. . Correlation of peripheral Th17 cells and Th17-associated cytokines to the severity of carotid artery plaque and its clinical implication. Atherosclerosis 221, 232, 2012 [DOI] [PubMed] [Google Scholar]
- 46. Yanaba, K., Yoshizaki, A., Asano, Y., Kadono, T., Tedder, T.F., and Sato, S.. IL-10- producing regulatory B10 cells inhibit intestinal injury in a mouse model. Am J Pathol 178, 735, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sîrbulescu, R.F., Boehm, C.K., Soon, E., et al. . Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions. Wound Repair Regen 25, 774, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iwata, Y., Yoshizaki, A., Komura, K., et al. . CD19, a response regulator of B lymphocytes, regulates wound healing through hyaluronan- induced TLR4 signaling. Am J Pathol 175, 649, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cherukuri, A., Rothstein, D.M., Clark, B., et al. . Immunologic human renal allograft injury associates with an altered IL-10/TNF-α expression ratio in regulatory B cells. J Am Soc Nephrol 25, 1575, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Deursen, J.M. The role of senescent cells in ageing. Nature 509, 439, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Demaria, M., Ohtani, N., Youssef, S.A., et al. . An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31, 722, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jeon, O.H., Kim, C., Laberge, R.-M., et al. . Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 23, 775, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sommerfeld, S.D., Cherry, C., Schwab, R.M., et al. . Interleukin-36y—producing macrophages drive IL-17—mediated fibrosis. Sci Immunol 4, eaax4783, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Finley, P.J., DeClue, C.E., Sell, S.A., DeBartolo, J.M., and Shornick, L.P.. Diabetic wounds exhibit decreased ym1 and arginase expression with increased expression of IL-17 and IL-20. Adv Wound Care 5, 486, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jun, J.-I., and Lau, L.F.. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12, 676, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Price, J.S., Waters, J.G., Darrah, C., et al. . The role of chondrocyte senescence in osteoarthritis. Aging Cell 1, 57, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Childs, B.G., Baker, D.J., Wijshake, T., Conover, C.A., Campisi, J., and Van Deursen, J.M.. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wong, K.L., Lee, K.B.L., Tai, B.C., Law, P., Lee, E.H., and Hui, J.H.P.. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years' follow-up. J Arthrosc Relat Surg 29, 2020, 2013 [DOI] [PubMed] [Google Scholar]
- 59. Trouson, A., Thanker, R., Lomax, G., and Gibbons, D.L.. Clinical Trials for stem cell therapies. BMC Med 9, 52, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Squillaro, T., Peluso, G., and Galderisi, U.. Clinical trials with mesenchymal stem cells: an update. Cell Transplant 25, 829, 2016 [DOI] [PubMed] [Google Scholar]
- 61. Aggarwal, S. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Ankrum, J.A., Ong, J.F., and Karp, J.M.. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 32, 252, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kurtz, A. Mesenchymal stem cell delivery routes and fate. Int J Stem Cells 1, 1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peng, Y.F., Martin, D.A., Kenkel, J., Zhang, K., Ogden, C.A., and Elkon, K.B.. Innate and adaptive immune response to apoptotic cells. J Autoimmun 29, 303, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kono, H., and Rock, K.L.. How dying cells alert the immune system to danger. Nat Rev Immunol 8, 279, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meirelles, S., Maria, A., Tadeu, D., and Caplan, A.I.. Cytokine & growth factor reviews mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 20, 419, 2009 [DOI] [PubMed] [Google Scholar]
- 67. Aggarwal, S., and Pittenger, M.F.. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Sato, K., Ozaki, K., Oh, I., et al. . Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109, 228, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213, 440, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Rafei, M., Hsieh, J., Fortier, S., et al. . Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood 112, 4991, 2019 [DOI] [PubMed] [Google Scholar]
- 71. Di lanni, M., Del Papa, B., De loanni, M., et al. . Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol 36, 309, 2008 [DOI] [PubMed] [Google Scholar]
- 72. Ciccocioppo, R., Gallia, A., Sgarella, A., Kruzliak, P., Gobbi, P.G., and Corazza, G.R.. Long- term follow-up of crohn disease fistulas after local injections of bone marrow-derived mesenchymal stem cells. Mayo Clin Proc 90, 747, 2015 [DOI] [PubMed] [Google Scholar]
- 73. Wang, D., Zhang, H., Liang, J., et al. . Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant 22, 2267, 2013 [DOI] [PubMed] [Google Scholar]
- 74. Shive, M.S., Hoemann, C.D., Restrepo, A., et al. . BST-CarGel: in situ chondro induction for cartilage repair. Oper Tech Orthop 16, 271, 2006 [Google Scholar]
- 75. Méthot, S., Changoor, A., Tran-Khanh, N., et al. . Osteochondral biopsy analysis demonstrates that BST-CarGel treatment improves structural and cellular characteristics of cartilage repair tissue compared with microfracture. Cartilage 7, 16, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hoemann, C.D., Sun, J., Légaré, A., McKee, M.D., and Buschmann, M.D.. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthr Cartil 13, 318, 2005 [DOI] [PubMed] [Google Scholar]
- 77. Chevrier, A., Hoemann, C.D., Sun, J., and Buschmann, M.D.. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthr Cartil 15, 316, 2007 [DOI] [PubMed] [Google Scholar]
- 78. Sharma, B., Fermanian, S., Gibson, M., et al. . Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med 5, 167ra6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wolf, M.T., Zhang, H., Sharma, B., et al. . Two-year follow-up and remodeling kinetics of ChonDux hydrogel for full-thickness cartilage defect repair in the knee. Cartilage 11, 447, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Costa, A., Adamo, S., Gossetti, F., et al. . Biological scaffolds for abdominal wall repair: future in clinical application? Materials (Basel) 12, 2375, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Badylak, S.F., Valentin, J.E., Ravindra, A.K., McCabe, G.P., and Stewart-Akers, A.M.. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14, 1835, 2008 [DOI] [PubMed] [Google Scholar]
- 82. Sicari, B.M., Rubin, J.P., Dearth, C.L., et al. . An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med 6, 234, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brown, B.N., Valentin, J.E., Stewart-Akers, A.M., McCabe, G.P., and Badylak, S.F.. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 30, 1482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brown, B.N., Londono, R., Tottey, S., et al. . Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 8, 978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sicari, B.M., Dziki, J.L., Siu, B.F., Medberry, C.J., Dearth, C.L., and Badylak, S.F.. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials 35, 8605, 2014 [DOI] [PubMed] [Google Scholar]
- 86. Sadtler, K., Estrellas, K., Allen, B.W., et al. . Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 352, 366, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Allman, A.J., McPherson, T.B., Badylak, S.F., et al. . Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation 71, 1631, 2001 [DOI] [PubMed] [Google Scholar]
- 88. Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 28, 3587, 2007 [DOI] [PubMed] [Google Scholar]
- 89. Gause, W.C., and Allen, J.E.. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev 13, 607, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gieseck III, R.L., Wilson, M.S., and Wynn, T.A.. Type 2 immunity in tissue repair and fibrosis Nat Rev Immunol 18, 62, 2018 [DOI] [PubMed] [Google Scholar]
- 91. Brodin, P., and Davis, M.M.. Human immune system variation. Nat Rev Immunol 17, 21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fischinger, S., Boudreau, C.M., Butler, A.L., Streeck, H., and Alter, G.. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol 41, 239, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dawkins, R.L., and Lloyd, S.S.. MHC genomics and disease: looking back to go forward. Cells 1, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Arango, M.T., Perricone, C., Kivity, S., et al. . HLA-DRB1 the notorious gene in the mosaic of autoimmunity. Immunol Res 65, 82, 2017 [DOI] [PubMed] [Google Scholar]
- 95. Gough, S.C.L., and Simmonds, M.J.. The HLA region and autoimmune disease: associations and mechanisms of action. Curr Genomics 8, 453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Semenzato, U., Turato, G., Bonato, M., et al. . Consistently low blood lymphocytes in smokers are associated with worse clinical outcomes. Eur Respir Soc 52, PA947, 2018 [Google Scholar]
- 97. Sokol, C.L., Barton, G.M., Farr, A.G., and Medzhitov, R.. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nature 9, 310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Valenta, R. The future of antigen-specific immunotherapy of allergy. Nat Rev Immunol 2, 446, 2002 [DOI] [PubMed] [Google Scholar]
- 99. Wawrzynski, J., Gil, J.A., Goodman, A.D., and Waryasz, G.R.. Hypersensitivity to orthopedic implants: a review of the literature. Rheumatol Ther 4, 45, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yazdanbakhsh, M., van den Biggelaar, A., and Maizels, R.M.. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol 22, 372, 2001 [DOI] [PubMed] [Google Scholar]
- 101. Yazdanbakhsh, M., Kremsner, P.G., and Van Ree, R.. Allergy, parasites, and the hygiene hypothesis. Science 296, 490, 2002 [DOI] [PubMed] [Google Scholar]
- 102. Kau, A.L., Ahern, P.P., Griffin, N.W., Goodman, A.L., and Gordon, J.I.. Human nutrition, the gut microbiome and the immune system. Nature 474, 7351, 327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gensollen, T., Iyer, S.S., Kasper, D.L., and Blumberg, R.S.. How colonization by microbiota in early life shapes the immune system. Science 352, 539, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chou, H.-H., Chien, W.-H., Wu, L.-L., et al. . Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc Natl Acad Sci U S A 112, 2175, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Buford, T.W (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome 5, 80, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mcmichael, A., Simon, A.K., and Hollander, G.A.. Evolution of the immune system in humans from infancy to old age. Proc R Soc B Biol Sci 282, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tang, Q., Koh, L.K., Jiang, D., and Schwarz, H.. CD137 ligand reverse signaling skews hematopoiesis towards myelopoiesis during aging. Aging (Albany. NY) 5, 643, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ahadi, S., Zhou, W., Rose, S.M.S-F., et al. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat Med 26, 83, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Goronzy, J.J., and Weyand, C.M.. T-cell senescence and contraction of T-cell repertoire diversity—catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther 5, 225, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lang, P.O., Govind, S., and Aspinall, R.. Reversing T cell immunosenescence: why, who, and how. Age (Omaha) 35, 609, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]