Abstract
Aldosterone is a mineralocorticoid hormone that controls body fluid and electrolyte balance. Excess aldosterone is associated with cardiovascular and metabolic diseases. Inflammation plays a critical role on vascular damage promoted by aldosterone and aggravates vascular abnormalities, including endothelial dysfunction, vascular remodeling, fibrosis and oxidative stress, and other manifestations of end-organ damage that are associated with hypertension, other forms of cardiovascular disease, and diabetes mellitus and the metabolic syndrome. Over the past few years, many studies have consistently shown that aldosterone activates cells of the innate and adaptive immune systems. Macrophages and T cells accumulate in the kidneys, heart, and vasculature in response to aldosterone, and infiltration of immune cells contributes to end-organ damage in cardiovascular and metabolic diseases. Aldosterone activates various subsets of innate immune cells such as dendritic cells and monocytes/macrophages, as well as adaptive immune cells such as T lymphocytes, and, by activation of mineralocorticoid receptors stimulates proinflammatory transcription factors and the production of adhesion molecules and inflammatory cytokines and chemokines. This review will briefly highlight some of the studies on the involvement of aldosterone in activation of innate and adaptive immune cells and its impact on the cardiovascular system. Since aldosterone plays a key role in many cardiovascular and metabolic diseases, these data will open up promising perspectives for the identification of novel biomarkers and therapeutic targets for prevention and treatment of diseases associated with increased levels of aldosterone, such as arterial hypertension, obesity, the metabolic syndrome, and heart failure.
Keywords: adaptive immune response, lymphocytes, blood pressure, dendritic cells, hypertension, inflammation, innate immune response, mineralocorticoid receptor, monocytes/macrophages
Graphical Abstract
Graphical Abstract.
Aldosterone is a mineralocorticoid hormone, discovered more than 60 years ago, involved in fluid and electrolyte balance. The effects of aldosterone on the regulation of body fluid ion concentration were first described in 1952 by James Tait, Sylvia Simpson, and Hilary Grundy.1 The structure of aldosterone was identified in 1954 by these same scientists with the support of Tadeus Reichestein.2–4 The principal site of production and secretion of aldosterone are the zona glomerulosa of adrenal cortex and several stimuli contribute to the production and release of aldosterone in physiological situations, including activation of the renin–angiotensin system,5–9 release of adrenocorticotropin,10,11 and increased concentration of potassium ion (K+).12,13 Aldosterone synthesis also occurs in extra-adrenal tissues, such as the central nervous system,14,15 the cardiovascular system (myocardium and vascular smooth muscle cells [VSMCs])7,16–18 and more recently, it has been demonstrated to occur in adipocytes.19 There is no direct evidence that immune cells produce aldosterone, although adrenal mast cells could promote cell proliferation and steroidogenesis in the zona glomerulosa.20,21 They could also release serotonin to activate mineralocorticoid production in concert with the renin–angiotensin system.20–22
The main effects of aldosterone are linked to the regulation of fluid and electrolyte balance. Aldosterone causes renal sodium (Na+) and water reabsorption, and K+ and hydrogen (H+) excretion at the level of principal cells of the distal tubule and collecting duct. Reabsorption of Na+ and water is considered the primary mechanism for an associated rise in blood pressure observed with activation of the mineralocorticoid receptor (MR). A few years after discovery of aldosterone, its excess was associated with elevated blood pressure. Jerome Conn23 described in 1955 the first case of primary aldosteronism characterized by hypokalemia, low plasma renin activity and high plasma and urinary aldosterone in patients with hypertension, which has been since shown to be a frequent cause of resistant hypertension.24 Enhanced concentrations of aldosterone were also reported at the very beginning of the aldosterone era in patients with primary hypertension, in absence of an adrenal tumor, suggesting that hypertension could be a state of mild chronic hyperaldosteronism.25
In 1957, Gross et al. demonstrated that high doses of aldosterone in rats, associated with unilateral nephrectomy and 1% of NaCl in the drinking water, increased blood pressure and produced hypertension. A similar effect was observed using an analog of aldosterone, deoxycorticosterone acetate (DOCA). Hypertension induced by aldosterone or DOCA produces vascular alterations and end-organ lesions such as vascular and interstitial fibrosis in the heart and in the kidneys, whereas the effects induced by DOCA were more severe.26
The cardiovascular effects of aldosterone are mediated by genomic and nongenomic mechanisms via activation of MR27 and G-protein-coupled estrogen receptors.28–30 MR antagonists lower blood pressure and have protective effects on cardiovascular disease, decreasing vascular and end-organ damage as well as substantially reducing the risk of both morbidity and mortality among patients with heart failure.31,32 The use of the MR antagonist spironolactone has also been demonstrated to be a very effective antihypertensive in a recent trial in patients with resistant hypertension, the PATHWAY-2 trial.33
Excess aldosterone is a cardiovascular risk factor not only for hypertension but also for stroke, coronary artery disease, congestive heart failure, and diabetes mellitus. Aldosterone induces vascular dysfunction and remodeling, increases generation of reactive oxygen species (ROS) and inflammation.28,34–38 Inflammation or activation of the immune system plays a critical role in the pathophysiology of hypertension and vascular damage promoted by aldosterone.39–42 From an evolutionary perspective it has been argued that aldosterone developed as part of a defense mechanism that through salt retention, vasoconstriction and inflammation protects from trauma and hemorrhage, leading to blood pressure homeostasis and wound healing. MR may have provided a critical evolutionary survival advantage in presence of trauma to control fluid loss following injury. However, modern lifestyle and sedentary behavior associated with an unhealthy diet rich in salt may have turned the aldosterone/MR defense mechanism into a harmful one that contributes to increase in blood pressure, cardiovascular disease, and events in populations with obesity and advanced age.43
From a therapeutic point of view, after the initial description of primary aldosteronism in the 50s, the Randomized ALdactone Evaluation Study (RALES) trial in 1999 demonstrated the therapeutic role for aldosterone antagonists in chronic severe (NYHA class III/IV) systolic heart failure. In 2003, the Eplerenone Post-myocardial infarction Heart failure Efficacy and Survival Study (EPHESUS) showed that benefit of aldosterone receptor antagonists in patients with an ejection fraction <40% after myocardium infarct, and after that TOPCAT showed moderate effects of spironolactone in heart failure with preserved ejection fraction with reduction in hospitalization for heart failure.44 More recently as already mentioned, the PATHWAY-2 trial showed the efficacy of blocking MR in resistant hypertension.33
This review will now focus on the important and complex interactions between aldosterone and the immune system, and how this impacts on the cardiovascular system. Some of the initial discoveries and the latest findings on aldosterone-induced activation of the immune system will be discussed in more detail.
ALDOSTERONE PRODUCTION OF INFLAMMATORY MEDIATORS, FIBROSIS, AND REMODELING
Aldosterone has been associated since its discovery with inflammation, fibrosis, vascular damage, and end-organ failure. It is important to mention that remodeling and fibrosis are a final process of inflammation. The first step to inflammation is production of inflammatory mediators that recruit immune cells that then contribute to local inflammation. In situations where the initial injury is not controlled, active inflammation occurs with infiltration of cells, tissue destruction and an attempt to repair the damage (healing). If not controlled, this can lead to remodeling of the organ, production of extracellular matrix and deposition of collagen resulting in characteristic tissue changes and fibrosis.45
How does aldosterone induce an inflammatory response by activating the immune system to affect the cardiovascular system? The first evidence that aldosterone stimulates an immune response was provided 1 year after its identification, in 1955. Experiments in rats that underwent bilateral adrenalectomy and were subjected to an inflammatory challenge, granuloma-pouches, showed that aldosterone inhibits the effects of cortisol and increases the exudate, and the weight of the spleen and thymus.46 However, the effects of aldosterone on inflammatory responses were not explored for the next 40 years, until Brilla et al. showed that hypertension induced by aldosterone causes cardiac inflammation with remodeling of the right and left ventricles.47 Myocardial interstitial and perivascular fibrosis, the final manifestation of an inflammatory process, was observed in aldosterone-infused rats. These effects were mediated by MR, since treatment with spironolactone, a MR antagonist, prevented hypertrophy of the left ventricle, and blunted the increase in the blood pressure.48
Subsequent studies have shown that aldosterone increased expression of inflammatory mediators and not only the final process of fibrosis.49 Aldosterone infusion increased the expression of adhesion molecules, such as intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), as well as infiltration of Cluster of Differentiation 68 (CD68)-positive cells (a marker of macrophages). Cardiac infiltration of inflammatory cells was preceded by increased expression of other inflammatory markers such as cyclooxygenase-2 (COX-2), monocyte chemoattractant protein-1 (MCP-1), and osteopontin (OPN). Furthermore, aldosterone infusion promoted severe coronary inflammatory lesions, characterized by monocyte/macrophage infiltration and focal ischemic and necrotic changes. The MR antagonist attenuated the expression of these proinflammatory markers in the rat heart and reduced vascular and myocardial damage.49 The association of aldosterone and inflammation with oxidative stress was also described in the heart of rats, confirming that aldosterone induces inflammation and oxidative stress in the cardiovascular system and in this manner contributes to cardiovascular disease.38
The other important fact that confirmed the involvement of aldosterone with inflammation was its association with transcription factors that induce expression of a large range of genes implicated in inflammation. In addition to triggering inflammatory responses in the heart and the vasculature, aldosterone stimulates macrophage infiltration and increases DNA-binding activity of transcription factors such as nuclear factor-κB (NF-κB)50 and activator protein-1 (AP-1) in rat kidneys.50,51 These processes were attenuated by a MR antagonist but also by BMS 182874, a selective endothelin (ET) type A receptor antagonist, indicating that renal damage in response to aldosterone is associated with inflammatory processes that are partially mediated by ET-1.51 The MR antagonist eplerenone also reduced systemic OPN, albuminuria, and the expression of proinflammatory genes such as MCP-1, interleukin (IL)-6, IL-1β, and OPN in the kidney.52 In addition, inflammatory cell infiltration (monocytes/macrophages), associated with focal ischemic and necrotic changes and vascular, myocardial, and renal damage, as well as associated inflammatory markers, were decreased by both ET type A receptor and MR antagonists.49,51
The treatment of hypertensive patients with eplerenone was associated with a reduction of proinflammatory mediators, including MCP-1, OPN, basic fibroblast growth factor (bFGF), and IL-8, and reduction of collagen/elastin ratio of the media and stiffness of small arteries. The anti-inflammatory cytokines IL-10 and IL-1Ra were also reduced by eplerenone, possibly as a consequence of countervailing mechanisms in response to the reduction of inflammatory markers.53
The angiotensin (Ang) type 1a receptor (AGTR1a or AT1a) has been shown to be required for aldosterone signaling in VSMCs for pathways that leads to increase in the expression of inflammatory markers such as extracellular signal-regulated kinase (ERK)1/2, c-Jun N-terminal protein kinase (JNK), and NF-κB signaling.54 The absence of this receptor in Agtr1a null mice protected these mice from aldosterone-induced dysfunction and vascular remodeling.55 Effects of aldosterone do not require elevated levels of plasma aldosterone, perhaps due to these interactions with other receptors intracellularly such as the AT1 receptor, or with other systems such as the ET system. Indeed, in a rodent model, the stroke prone spontaneously hypertensive rat, that has an activated renin–angiotensin system and ET system as demonstrated by the responses to their blockade, treatment with the selective MR antagonist eplerenone even in the presence of normal plasma aldosterone, reduced vascular remodeling and cardiac fibrosis.56 Another example of association of MR-mediated and AT1 receptor-mediated effects is found in a study of mice with VSMC-specific deletion of MR.57 These mice have lower blood pressure with aging despite absence of changes in sodium excretion, which excludes a role of the kidney in these effects. There is however a reduction in myogenic tone and Ang II-induced vascular constriction. The mechanism for this is reduced expression and activity of L-type Ca2+ channels. This suggests that MR in VSMC contributes to Ang II-induced vascular contraction and elevated blood pressure.
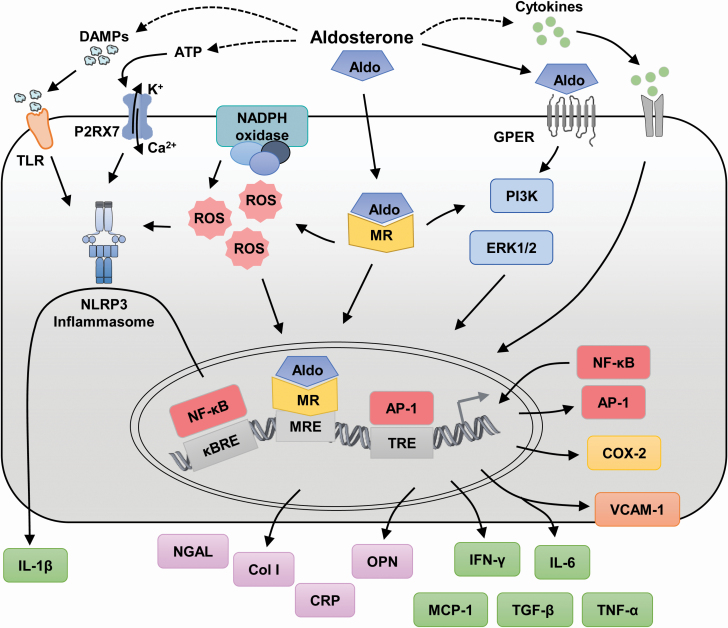
Aldosterone also induces C-reactive protein (CRP) expression in VSMCs in vitro and in vivo, which is abolished by MR antagonism.58 In addition, aldosterone stimulated generation of ROS and activated ERK1/2 phosphorylation, which leads to activation of intracellular pathways to increased expression of inflammatory markers, which was inhibited by the MR antagonist spironolactone. Aldosterone increased as well the expression of ICAM-1 in endothelial cells from human coronary arteries and the adherence of human monocytes.59 Aldosterone stimulation increased c-Src phosphorylation and trafficking to lipid rafts/caveolae in VSMCs, which contributed to induce the proinflammatory markers ICAM-1 and VCAM-1.60Figure 1 highlights different pathways involved in the inflammatory response to aldosterone. The production of inflammatory mediators via aldosterone could occur either through activation of MRs or as well via G-protein-coupled estrogen receptors that also respond to aldosterone.
Figure 1.
Aldosterone and production of inflammatory mediators. Aldosterone induces the production of inflammatory mediators either through activation of mineralocorticoid receptors (MRs) or G-protein-coupled estrogen receptors (GPERs). The dashed line arrows indicate mechanisms not depicted in the figure. Abbreviations: Aldo, aldosterone; AP-1, activator protein-1; ATP, adenosine triphosphate; Ca2+, calcium; Col I, Collagen type I; COX-2, cyclooxygenase-2; CRP, C-reactive protein; DAMPs, damage-associated molecular patterns; ERK, extracellular signal-regulated kinase; IFN, interferon; IL, interleukin; K+, potassium; κBRE, nuclear factor-κB (NF-κB) response element; MCP-1, macrophage chemoattractant protein-1; MRE, MR response element; NADPH, reduced nicotinamide adenine dinucleotide phosphate; NF-κB, nuclear factor-κB; NGAL, neutrophil gelatinase-associated lipocalin; NLRP3, NOD-like receptor pyrin-domain containing protein 3; OPN, osteopontin; P2RX7, P2X purinoceptor 7; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; TGF, transforming growth factor; TLR, Toll-like receptor; TNF, tumor necrosis factor; TRE, 12-O-tetradecanoylphorbol-13-acetate response element (AP-1 response element); VCAM-1, vascular cell adhesion molecule-1.
Chemokine receptors are also associated with vascular injury induced by aldosterone. DOCA-salt-induced hypertension increased C–C chemokine receptor type 2 (CCR2) expression, the CCR2 ligands chemokine C–C motif ligand (CCL) 2 (MCP-1), monocyte chemotactic protein 3 (CCL7), monocyte chemoattractant protein 2 (CCL8), and monocyte chemotactic protein 5 (CCL12), and macrophage infiltration into the vascular wall.61 The treatment with a CCR2 antagonist reversed DOCA/salt-induced increases in CCR2 expression and macrophage accumulation in the vascular wall, and reduced as well the blood pressure elevation. C-X3-C motif chemokine ligand 1 (CX3CL1 or fractalkine), a unique chemokine that is a leukocyte chemoattractant and an adhesion molecule, and its receptor, CX3CR1, were associated with interstitial fibrosis in the kidney of DOCA/salt mice.62 Mice lacking CX3CR1 displayed reduced fibrosis together with reduction in collagen deposition, transforming growth factor (TGF)-β1 expression and fewer monocyte/macrophages infiltrating the kidneys, interestingly without reduction of blood pressure elevation due to DOCA/salt.
In patients with mild to moderate stable chronic heart failure, plasma aldosterone is increased and is associated with enhanced levels of markers of oxidative stress (8-iso-PGF2a), inflammation (ICAM-1), and extracellular matrix turnover (TIMP-1), after adjusting for potential confounding factors (age, sex, race, diabetes, smoking, heart rate, left ventricular mass, and body mass index).63 However, no differences in OPN and CRP, biomarkers usually associated with increased aldosterone, were observed in these patients.
A recent study has shown that the levels of IL-6 and tumor necrosis factor-α (TNF-α) in perirenal adipose tissue are higher in patients with primary aldosteronism than in normotensive subjects or patients with essential hypertension.64 Expression of genes related to fibrosis such as fibronectin, collagen I, TGF-β and as well genes related to adipogenesis were increased in patients with primary aldosteronism compared with the other hypertensive patients. Chou et al. showed that patients with primary aldosteronism also displayed higher plasma IL-6 levels, left ventricular mass index, degree of myocardial fibrosis, and more impaired diastolic function than patients with essential hypertension.65 This study showed important correlations of plasma IL-6 levels with 24-hour urinary aldosterone and echocardiographic parameters. The intracellular pathway to stimulation of expression of IL-6 in human umbilical vein endothelial cells by aldosterone is via the MR/phosphoinositide 3-kinases (PI3K)/protein kinase B (Akt)/NF-κB pathway. IL-6 trans-signaling played a critical role in aldosterone-induced fibronectin and type 1 collagen expression. Inhibition of IL-6 trans-signaling by soluble IL-6 receptor or soluble gp130, also prevented myocardial fibrosis and cardiac hypertrophy induced by aldosterone infusion by blocking binding of IL-6 and IL-6 receptor to gp130 to mediate aldosterone effects on cardiac fibroblasts.65
VSMCs stimulated by aldosterone and pretreated with atorvastatin exhibited reduced ROS production.66 Atorvastatin inhibited Rac1/2 and p47phox translocation from the cytosol to the membrane and attenuated aldosterone-induced vascular inflammation and macrophage adhesion to VSMCs. A Rac1/2 inhibitor and a ROS scavenger were able to reduced macrophage adhesion in VSMCs, suggesting that in conditions associated with aldosterone-induced vascular damage, statins may have vasoprotective effects by inhibiting oxidative stress and inflammation.
The study of new molecules potentially playing roles as biomarkers or targets for therapy such as microRNA have been recently the subject of study in the aldosterone field regarding cardiovascular deleterious effects of this hormone. Syed et al. showed that aldosterone/salt upregulated miR-21 expression in the left ventricle of mice.67 The genetic ablation of miR-21 exacerbated aldosterone/salt-mediated cardiac hypertrophy, injury, dysfunction, and production of inflammatory markers as plasminogen activator inhibitor type 1 (PAI-1), MCP-1, OPN, and IL-6 in the left ventricle. These cardiac actions could be mediated, at least partially, by an upregulation of the miR-21 target gene Sprouty 2, suggesting that miR-21 plays a protective role on the cardiac pathology triggered by excess aldosterone.
ALDOSTERONE AND THE IMMUNE SYSTEM
The immune system coordinates several protective processes in response to pathogens, harmful substances from the environment and cell/tissue injury. A coordinated action of cells of the innate and adaptive immune systems together with inflammatory mediators regulates acute or chronic inflammation. Whereas the innate immune system provides a rapid, and nonspecific response, mediated by the activation of phagocytes (neutrophils, monocytes/macrophages, and natural killer cells), the adaptive immune system is activated later, relying on the coordination and expansion of B and T lymphocytes.45 B lymphocytes mediate humoral immune responses and produce antibodies. T lymphocytes, represented by subsets of T cells (CD8+ or cytotoxic T cells, γδ T cells, CD4+ or helper T cells [Th], which polarize into Th1, Th2, Th17, and T regulatory cells [Treg]), are involved in cell-mediated immune responses. Coordinated actions of both the innate and adaptive immune systems provide highly specialized responses that lead to long-lasting and enhanced reaction to subsequent invasions by pathogens or to cell damage.
Many mechanisms have been suggested to explain how immune cells may increase blood pressure: by inducing generation of ROS and a pro-oxidative environment; via the release of cytokines, which directly affects renal, cardiac, and vascular cell functions; by the activation of metalloproteinases and profibrotic factors and, consequently inducing tissue remodeling; and by changing adipose tissue function, which affects the function of adjacent tissues and organs.68 Inflammation in the perivascular adipose tissue (PVAT) induces vascular oxidative stress, decreases nitric oxide (NO) bioavailability, reduces adiponectin that is anti-inflammatory, and enhances expression of TNF-α, contributing to remodeling and endothelial dysfunction.
Over the last few years, many studies have consistently shown that aldosterone activates cells of the innate and adaptive immune systems. Macrophages and T cells accumulate in the kidneys, heart, and vasculature in response to aldosterone and the infiltration of these immune cells contributes to end-organ damage in cardiovascular and metabolic diseases. In the next topic, we will discuss some of the pioneering studies demonstrating involvement of aldosterone in the activation of cells of the innate and adaptive immune systems.
ALDOSTERONE AND INNATE IMMUNITY
Aldosterone and macrophages
Monocytes/macrophages, cells belonging to the innate immune system, are involved in aldosterone and DOCA/salt-induced blood pressure elevation and vascular injury. Homozygous osteopetrotic mice, deficient in macrophage colony-stimulating factor (M-CSF or CSF-1) and accordingly bearing dysfunctional monocytes/macrophages, when submitted to DOCA/salt-induced hypertension, exhibited reduced blood pressure elevation, blunted inflammation and oxidative stress, and less endothelial dysfunction and vascular remodeling, when compared with DOCA/salt-treated wild-type and heterozygote mice, suggesting for the first time that innate immune cells, monocytes/macrophages, contribute to inflammation and end-organ damage associated with DOCA/salt hypertension.39 Furthermore, endothelial dysfunction, collagen deposition, and ROS generation in aldosterone/salt-treated mice were also blunted in heterozygote osteopetrotic mice.69 Macrophage depletion in rats using liposome-encapsulated clodronate also reduced blood pressure and vascular ROS while restoring sympathetic nerve α 2-adrenergic receptor function in mesenteric arteries of DOCA/salt rats.70
Innate immune cells express receptors that recognize specific components associated to pathogens or cellular damage.45,71–73 These receptors are called pattern recognition receptors (PRRs) and they are activated (i) by pathogen-associated molecular patterns (PAMPs), which are molecules expressed by microbial pathogens, and (ii) by damage-associated molecular patterns (DAMPs), i.e., cell components that are released during cell damage or death. Once PRRs are activated, innate immune cells release cytokines and chemokines that recruit additional cells to the site of infection or injury. PRRs comprise the large families of Toll-like receptors (TLRs), C-type lectin receptors (CLRs), RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs). The NLRs are cytoplasmic proteins that recognize endogenous or microbial molecules. A subfamily of NLRs, called NACHT-LRR-PYD-containing protein or NLR pyrin-domain containing protein (NALP or NLRP), has gained increased attention in cardiovascular disease. NALP3 or NLRP3 (NACHT, LRR, and PYD domains-containing protein 3 or NLR pyrin-domain containing protein 3) is one of the best characterized members of the NLRs family. NLRP3 regulates the assembly of the inflammasome and leads to activation of caspase-1, which processes pro-interleukin-1β (IL-1β) and pro-interleukin-18 (IL-18), increasing IL-1β and IL-18 release. NLRP3 is activated by a variety of signals that are indicative of damage, including muramyl dipeptide, bacterial DNA, adenosine triphosphate (ATP), toxins, double stranded RNA, paramyxoviruses, uric acid and cholesterol crystals, environmental pollutants, mitochondria-derived ROS, low intracellular K+ concentrations, liposomes, urban particulate matter, and inorganic particles (titanium dioxide, silicon dioxide, and asbestos). The activation is a 2-step process where the first signal or priming process, leads to the expression of NLRP3 and pro-IL-1β through activation of NF-κB. Then, the second signal, indicative of damage, leads to NLRP3 activation and stimulates the assembly of the inflammasome.
Aldosterone and the NLRP3 inflammasome
In the last few years, the role of aldosterone as an important activator of the NLRP3 inflammasome has been extensively studied. Aldosterone increases the production of ROS37,74–77 and may lead to release of mitochondrial DAMPs. Doi et al. were one of the first to show that aldosterone- and salt-induced blood pressure increases and expression of inflammasome components in the kidney.78 Rats infused with aldosterone displayed increased macrophage and T lymphocyte infiltration in the kidney, as well as increased renal inflammatory markers such as MCP-1, TNF-α and inflammasome activation markers, IL-1β, caspase-1, and the NLRP3. Treatment of these animals with an immunosuppressive drug, mizoribine, attenuated renal damage and fibrosis, reduced the expression of inflammasome components, and reduced blood pressure in this animal model of hypertension.
Mice treated with DOCA/salt also showed increased blood pressure and renal expression of the components of the inflammasome. Mice deficient in an adapter protein of NLRP3, ASC (the adaptor molecule apoptosis-associated speck-like protein containing a CARD, also known as PYCARD), did not show increased blood pressure and expression of renal inflammatory mediators, or renal accumulation of macrophages and collagen.79 In addition, treatment with a NLRP3 receptor inhibitor, MCC950, reduced blood pressure values and inflammatory markers such as TNF-α, IL-6, OPN, and IL-17A in kidneys of in DOCA/salt-treated animals.
In view of the role of the components of the inflammasome in the development of arterial hypertension and kidney injury, the contribution of IL-1β was also assessed using treatment with anakinra, an IL-1 receptor antagonist.80 Anakinra is lowered blood pressure in mice treated with DOCA/salt, but it did not alter the renal immune cell infiltration, despite partially decreasing the expression of some of the renal inflammatory markers.
Bruder-Nascimento et al. showed that aldosterone directly activates the NLRP3 inflammasome, and highlighted the role of immune cells in this process.81 In bone-marrow-derived macrophages (BMDMs) from wild-type mice, aldosterone increased mitochondrial ROS and caspase-1 activation. Caspase-1 activation was blunted in aldosterone-stimulated BMDM from NLRP3 knockout mice. Aldosterone-induced NF-κB activation and increased NLRP3 and IL-1β gene expression, thus triggering the first signal for inflammasome activation. The use of a NF-κB inhibitor prevented aldosterone-induced NLRP3 and IL-1β gene expression. Aldosterone infusion in wild-type mice activated the NLRP3 inflammasome (it increased NLRP3 protein expression, caspase-1 activity, and mature IL-1β) in macrophages from the peritoneal cavity. Aldosterone also increased plasma IL-1β and induced vascular dysfunction (abnormal vascular reactivity, remodeling, and increased expressed of adhesion molecules). NLRP3 deletion almost completely prevented all effects of aldosterone: the changes in vascular reactivity, the increased expression of VCAM-1 and ICAM-1, and the adherence of macrophages to aortic segments; the vascular remodeling (increased cross-sectional area and increased wall to lumen ratio) and the increase in systolic blood pressure. The vascular and inflammatory changes induced by aldosterone were all attenuated in wild-type mice that received bone marrow transplantation from NLRP3-deficient mice, which suggests that NLRP3 inflammasome in the immune system has a very important role on vascular damage induced by aldosterone. Of importance, leukocytes from patients with hyperaldosteronism exhibit NLRP3 inflammasome activation (increased NLRP3 protein expression, caspase-1 activation, and mature IL-1β) and increased serum IL-1β levels in comparison to healthy human volunteers. Polymorphonuclear cells from hypertensive patients that do not exhibit increased aldosterone levels also presented increased NLRP3 inflammasome activity. These data support the evidence that the NLRP3 inflammasome plays a key role in aldosterone-induced inflammation and vascular remodeling. Some of these effects of activation of the inflammasome by aldosterone are also highlighted in Figure 1.
The use of a selective inhibitor of the NLRP3 receptor MCC950,82 reduced blood pressure and decreased inflammasome activation and inflammatory markers in DOCA/salt hypertension.83 Treatment with MCC950 reduced expression levels of collagen subunits types I, III, IV, and V, TGF-β and vimentin, and also myeloid cell (CD45+CD11b+) and macrophage (CD45+CD11b+F4/80+) infiltration in the kidney. Renal function, measured by saline intake, urine volume, urine osmolality, urine Na+, and albuminuria as well interstitial fibrosis, was improved after treatment with the NLRP3 receptor.
A recent study by Ferreira et al. has suggested that the NLRP3 inflammasome is also activated in other conditions in which aldosterone levels are increased, including diabetes and obesity.84 The vascular dysfunction and inflammation present in db/db mice are associated with MR activation, underlining the involvement of aldosterone in vascular changes in diabetes. Treatment of db/db mice with a MR antagonist and the selective NLRP3 receptor antagonist MCC950 prevented vascular dysfunction, inflammasome activation in macrophages and rise in plasma IL-1β. The effects of MCC950 treatment did not depend on changes in blood glucose or aldosterone levels.
ATP is released from intracellular storage pools in response to platelet aggregation, activation of neutrophils and many cell irritants, such as cell death or apoptosis, or inflammation. ATP can stimulate NLRP3 as mentioned above. ATP also binds to purinergic receptors such as the P2X purinoceptor 7 (P2RX7), which is an ATP-gated cation channel expressed in immune cells.85 Stimulation of this receptor leads to activation of the inflammasome and release of IL-1β from macrophages and dendritic cells. P2RX7 knockout (P2rx7−/−) hypertensive DOCA/salt mice present less renal injury and inflammation with attenuated hypertension and renal function compared with wild-type DOCA/salt mice.86 Immune cell infiltration as well IL-1β release by macrophages is also reduced in P2rx7−/− mice compared with the wild-type mice. The results discussed above support the involvement of P2RX7, and potentially of PRRs and the inflammasome in hypertension and renal injury induced by DOCA/salt.
Aldosterone and neutrophil gelatinase-associated lipocalin
Lipocalin 2 (LCN2, also identified as neutrophil gelatinase-associated lipocalin [NGAL]) that is a potent biomarker of renal injury has been shown to be a MR target.87 Interestingly, NGAL was also associated with aldosterone-induced vascular fibrosis.88Lcn2 knockout prevented vascular fibrosis in unilaterally nephrectomized mice treated with aldosterone/salt. Furthermore, NGAL produced by immune cells appears to play a pivotal role in cardiac damage induced by aldosterone. Unilaterally nephrectomized/aldosterone/salt-treated mice experienced recruitment of various immune cell populations such as granulocytes, B lymphocytes, and activated cytotoxic T lymphocytes to lymph nodes, in addition displaying augmentation of expression of NGAL in macrophages, dendritic cells, and peripheral blood mononuclear cells. Mice with Lcn2 knockout immune cells (achieved through bone marrow transplantation) were protected against aldosterone-induced cardiac remodeling and inflammation.89
Araos et al. demonstrated that NGAL can modulate the inflammatory response of dendritic cells induced by aldosterone.90 Dendritic cells are necessary for the development of cardiac hypertrophy and fibrosis. Ablation of these cells prevented the development of cardiac hypertrophy, perivascular fibrosis and overexpression of NGAL, brain natriuretic peptide increased levels, collagen 1A1 and connective tissue growth factor induced by excess aldosterone. The expression of NGAL is higher in macrophages and dendritic cells, and aldosterone was able to modulate the expression of this glycoprotein and IL-23 expression in dendritic cells. NGAL produced by dendritic cells may play a pivotal role in the activation of adaptive immunity. This occurs after stimulation of IL-23 leading to a lymphocyte Th17-driven response, which results in cardiovascular fibrosis as a consequence of aldosterone excess.
ALDOSTERONE AND ADAPTIVE IMMUNITY
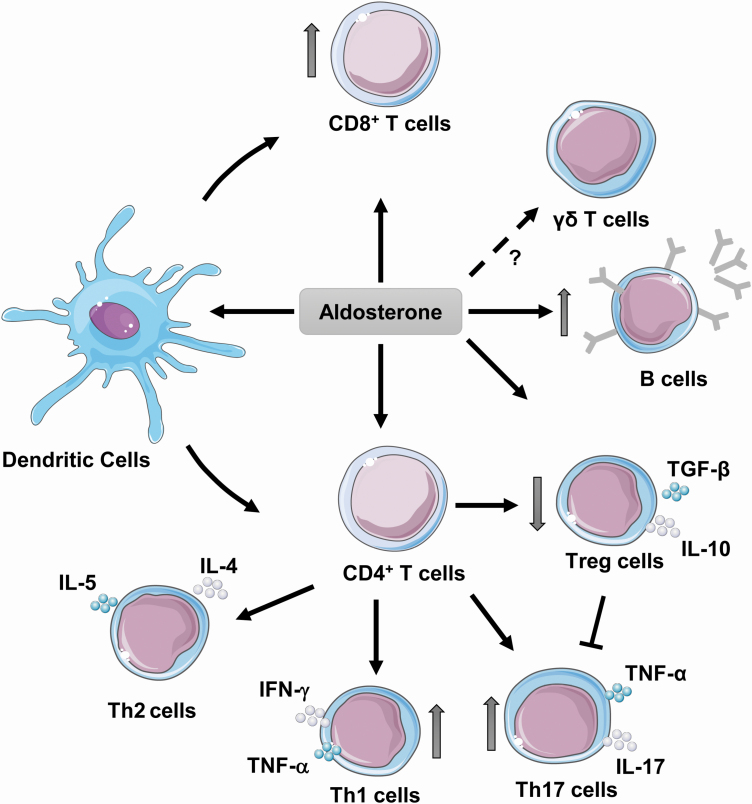
T cells play a role in the mechanisms leading to blood pressure elevation and vascular injury in hypertension.91 Many elegant studies have shown that aldosterone activates or modulated the action of T lymphocytes (Figure 2). The pioneering study of Guzik et al. in 2007 showed that mice lacking B and T lymphocytes due to recombinase-activating gene (Rag1) knockout, exhibit blunted blood pressure elevation, endothelial dysfunction, vascular remodeling, and superoxide production, which was restored by adoptive transfer of T but not B cells. Furthermore, they extended their observations to DOCA/salt-treated Rag1 null mice that presented blunted blood pressure elevation and increased vascular superoxide production.
Figure 2.
Aldosterone and activation of adaptive immune cells. Aldosterone induces activation of dendritic cells and increased polarization of CD4+ naive T cells into Th17, Th1, and decreased Treg cells. Aldosterone also increases recruitment of B lymphocytes and activation of CD8+ T cells. Abbreviations: IFN, interferon; IL, interleukin; TGF, transforming growth factor; Th, T helper cells; TNF, tumor necrosis factor; Treg, T regulatory cells.
T cells require 2 signals for activation, including interaction of the T-cell receptor with an antigen presented in the context of a major histocompatibility complex, and stimulation by costimulatory molecules on the antigen-presenting cell.45 Activation of naive T cells requires a costimulatory signal such as the binding of B7 ligands on the antigen-presenting cell to the CD28 and costimulatory receptor expressed in T cells. The use of drugs that block this costimulation, such as cytotoxic T lymphocyte-associated antigen-4-immunoglobulin (CTLA4-Ig, abatacept) that inhibits B7 ligands on antigen-presenting cells, blunts T-cell activation, vascular infiltration, and increases in blood pressure in both Ang II-induced and DOCA/salt hypertension.92 CTLA4-Ig treatment also abolished Ang II-induced production of TNF-α and interferon (IFN)-γ by T cells. Similar results were observed in B7 (CD80/CD86) knockout mice. These observations suggest that T-cell costimulation via B7 ligands is essential for development of hypertension induced by either angiotensin II or mineralocorticoids.
Herrada et al. have shown that aldosterone activates CD8+ T cells by mechanisms that rely on dendritic cells.93 Dendritic cells stimulated with aldosterone also polarize CD4+ T cells to a Th17 phenotype. An MR antagonist prevented activation or polarization induced by aldosterone. In addition, aldosterone blunted polarization and activation of suppressor forkhead box P3-positive Treg cells, which negatively modulate immune responses.91 Kasal et al. showed that aldosterone-induced augmentation of blood pressure, endothelial dysfunction, vascular remodeling, oxidative stress, and enhanced aortic and renal cortex macrophage and T-cell infiltration could all be prevented by adoptive transfer of Treg cells.41 Adoptive transfer of T cells of Scurfy mice, which are deficient in Treg cells, into Rag1 null mice, worsened Ang II-induced endothelial dysfunction and oxidative stress in PVAT of mesenteric arteries, as well as cell infiltration and proinflammatory polarization in PVAT and the renal cortex, and T-cell infiltration in the renal cortex compared with Rag1 null mice receiving wild-type T cells.94 Coinjection of Treg cells in Rag1 null mice adoptively transfer with Scurfy T cells blunted the Ang II detrimental effects. In contrast to other studies in which angiotensin II and aldosterone exert similar effects on immune cells, these studies have not been reproduced with aldosterone or DOCA, but similar results could be expected.
Further support to aldosterone-induced changes in the ratio of Th17/Treg cells was provided by studies in DOCA/salt hypertension. Vessels, heart, and kidneys of DOCA/salt-treated rats exhibit activation of Th17 cells and downregulation of Treg cells.95 Treatment of DOCA/salt rats with spironolactone prevented Th17 cell activation and downregulation of Treg cells. Blockade of IL-17 actions with anti-IL-17A antibodies reduced arterial hypertension and expression of profibrotic and proinflammatory mediators and collagen deposition in the heart and kidney in DOCA/salt rats.
Th17 cells produce IL-17 that is a proinflammatory cytokine secreted by innate and adaptive immune cells.96 Th17 cells require IL-23 for expansion and survival. IL-23 is released by activated dendritic cells and macrophages. Krebs et al. evaluated the involvement of IL-17/IL-23 axis in a severe hypertensive model with renal injury induced by cotreatment with DOCA and Ang II.97 IL-17-deficient mice displayed increased albuminuria and more glomerular injury and renal infiltration of γδ T cells than wild-type mice after 14 days of DOCA/Ang II. Similarly, increased albuminuria, glomerular injury, and γδ T-cell infiltration were found in IL-23p19-deficient mice with DOCA/Ang II-induced hypertension. IL-17/IL-23 deficiency accelerated DOCA/Ang II-induced albuminuria and renal damage, suggesting a protective role for the IL-17/IL-23 axis in this model of arterial hypertension.
γδ T cells are a small subset of T cells that behave as innate-like T cells and play a role in the initiation of the immune response in hypertension.91 Similar to CD4+ T cells, γδ T cells can produce IL-17 in response to IL-23. Ang II infusion increased blood pressure, vascular dysfunction, and γδ T-cell numbers and activation.98 In the same study, Ang II-induced hypertension, endothelial dysfunction, and T-cell activation were blunted in T-cell receptor (Tcr) δ knockout mice devoid of γδ T cells, and in mice injected with γδ T cell-depleting antibodies. The most interesting findings have shown an increase in this population in IL-17-deficient mice exposed to Ang II and DOCA/salt.97 These cells seem to have an important role in the development of injury in hypertension, but have not been studied in details in relation to effects of aldosterone. Furthermore, using a humanized mouse model in which the murine immune system is replaced by the human immune system, Itani et al. showed that Ang II activates human T cells, which then invade critical end-organ tissues in hypertension.99 Although these events were not directly observed/studied with aldosterone, one needs to consider that aldosterone is secreted in response to and mediates some of the actions of Ang II, including hypertension, oxidative stress, and vascular inflammation.100
Channels, transporters, and receptors in immune cells and aldosterone
The latest findings on aldosterone-induced activation of the immune system relate to intrinsic proteins in the development of cardiovascular damage induced by aldosterone. Glucocorticoid-regulated kinase 1-dependent (SGK1-dependent) signaling in T cells promotes hypertension and contributes to end-organ damage induced by Ang II and DOCA/salt.101 Loss of SGK1 in T cells blunts hypertension, abrogates renal and vascular inflammation, and protects against hypertensive renal and vascular injury compared with control mice. Furthermore, the expression of several Na+ channels and sodium transporters was detected on T lymphocytes, and Na+-K+-2Cl− cotransporter 1 (NKCC1) was upregulated in Th17 cells, and was necessary for the salt-induced increase in SGK1 and IL-23 receptor.
Liu et al. showed the involvement of Na–Cl cotransporter (NCC) and CD8+ T cells in the development of hypertension in a salt-sensitive model. Kidney cells from the distal convoluted tubule cocultured with CD8+ T cells upregulated NCC via ROS-induced activation of Src kinase, upregulation of the K+ channel, and stimulation of the Cl− channel.102 This last event increased Cl− efflux, leading to compensatory Cl− influx via NCC activation and Na+ retention. Adaptive immunity is involved in the kidney defect in Na+ handling and the pathogenesis of salt-sensitive hypertension. These studies demonstrate that T cells and this cotransporter may be novel therapeutic targets for the treatment of hypertension associated with excess aldosterone and salt.
Aldosterone appears to require an intact sympathetic drive to the spleen for priming of immunity and increases in blood pressure. The coupling of the nervous system and immune cell activation in the splenic marginal zone is established through sympathetic-mediated placental growth factor (PIGF) release.103 DOCA/salt hypertensive mice after splenectomy or left celiac ganglionectomy were unable to exhibit increased blood pressure.104 PIGF expression in the spleen was increased in DOCA/salt mice if neurosplenic sympathetic drive was intact. Pigf knockout mice were protected from the increased blood pressure induced by DOCA/salt and from T-cell costimulation and infiltration in the kidney. These data support the hypothesis that coupling of brain-to-spleen is a neuroimmune pathway that enhances the effect of aldosterone on the immune response necessary to develop elevated blood pressure.
The absence of MR in T cells decreased both systolic and diastolic blood pressure and attenuated renal and vascular damage induced by Ang II.105 T-cell MR knockout prevented the accumulation of IFN-γ-producing T cells, particularly the CD8+ population, in both kidneys and aorta. Similarly, the selective MR antagonist eplerenone attenuated Ang II-induced elevation of blood pressure and accumulation of IFN-γ-producing T cells in wild-type mice. In cultured CD8+ T cells, T-cell MR knockout suppressed IFN-γ expression whereas T-cell MR overexpression and aldosterone both enhanced IFN-γ expression. In addition, T-cell MR overexpressing mice presented higher blood pressure compared with control mice after Ang II infusion, which was abolished by IFN-γ-neutralizing antibodies. MR may interact with nuclear factor of activated T cells 1 (NFAT-1) and AP-1 to regulate IFN-γ production in T cells and modulate target organ damage and control blood pressure. Targeting MR specifically in T cells may be an effective novel approach for treatment of hypertension.
Shao et al. showed that MR modulates the activation of Treg cells in congestive heart failure.106 Advanced congestive heart failure activates Treg cell proliferation via the upregulation of Kv1.3 K+ channel, which promotes cardiac fibrosis by stimulating secretion of TGF-β. Coincubation of cardiac fibroblasts with Treg cells increased proliferation and levels of collagen I, III, and matrix metalloproteinase (MMP)-2. Intracellular TGF-β was increased in Treg cells, and the Kv1.3 current was higher in patients and rats with congestive heart failure compared with healthy volunteers and control rats. Eplerenone antagonized Kv1.3 channels in Treg cells and promoted suppression of Treg cell activation/proliferation. Eplerenone could play a role alleviating cardiac fibrosis in late stages of congestive heart failure.
Future developments
A recent study has revealed that sex could play an important role in the effects of aldosterone on inflammation. Belanger et al. showed that female rats displayed lower blood pressure than male rats after 3 weeks of DOCA/salt treatment, although the renal injury was comparable between the sexes.107 Female mice treated with DOCA/salt had more suppressive Treg cells than male mice. However, the increase in proinflammatory T cells in the kidney was similar in both sexes. Depletion of Treg cells with anti-CD25 antibodies in DOCA/salt-treated mice increased blood pressure only in females, therefore abolishing the sex difference in DOCA/salt-induced blood pressure elevation. This further supports the idea that Treg cells protect against the development of hypertension, and could be particularly important for the control of blood pressure in females.
CONCLUSION AND PERSPECTIVES
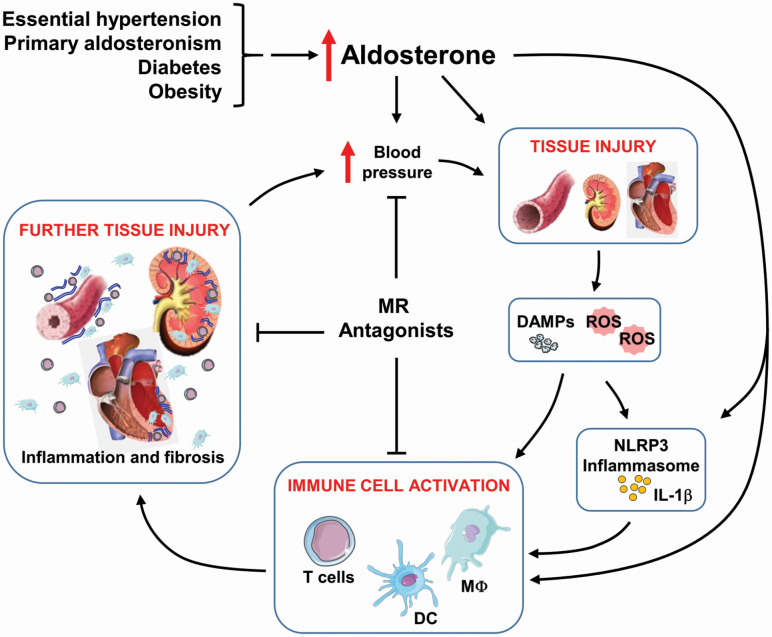
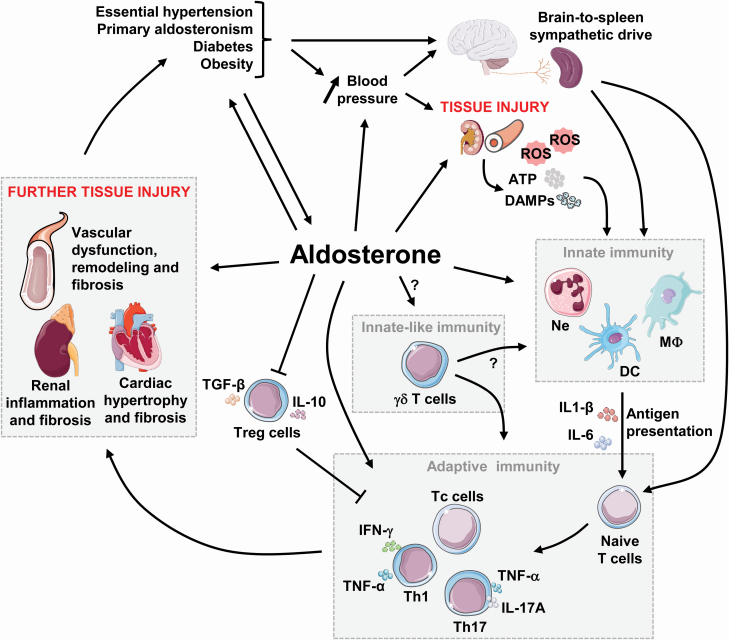
Together all these elegant and complex studies show that excess aldosterone modulates many components of the immune system driving inflammation that contributes to vascular, cardiac and renal damage, leading to aggravation of end-organ injury in cardiovascular and metabolic diseases, as highlighted in Figure 3. The use of MR antagonists, as well as selective blockers or antibodies against specific receptors or cytokines, provides beneficial effects in the prevention and treatment of hypertension. However, the mechanisms by which aldosterone interacts with the immune system remain unknown, and more studies are needed to provide a better understanding of the mechanisms causing hypertension and vascular injury induced by aldosterone.
Figure 3.
Aldosterone and immune responses in cardiovascular diseases. Excess aldosterone production occurring in diseases such as essential hypertension, primary aldosteronism, diabetes mellitus, and obesity contributes to increase blood pressure (BP). Over time, elevated BP, and/or aldosterone cause renal and vascular injury, which activates the innate and adaptive immune systems causing further tissue injury and thereafter exacerbating the detrimental effects of the initial disease. Abbreviations: ATP, adenosine triphosphate; DAMPs, damage-associated molecular patterns; DCs, dendritic cells; IFN, interferon; IL, interleukin; MΦ, macrophage; Ne, neutrophil; ROS, reactive oxygen species; Tc, cytotoxic T cells; TGF, transforming growth factor; Th, T helper cells; TNF, tumor necrosis factor; Treg, T regulatory cells.
Some of the gaps in our knowledge include the role of immune cells in the production of aldosterone, and further investigation on γδ T cells and Th17 cells in vascular damage in hypertension induced by aldosterone. The depletion of MR receptors in specific subtypes of inflammatory cells, knockout of channels and different proteins involved in mediating effects of aldosterone could also be important approaches to develop novel therapeutic targets to treat hyperaldosteronism or resistant hypertension. The use of MR antagonists associated with inhibition of the immune system opens up promising perspectives to reveal novel biomarkers and therapeutic targets for the prevention and treatment of cardiovascular disease that could be especially important in conditions associated with increased levels of aldosterone, such as obesity, metabolic syndrome, high blood pressure, and heart failure.
FUNDING
The work of NSF, PP, and ELS was supported by Canadian Institutes of Health Research (CIHR) grants 102606 and 123465, and Canadian Institutes of Health Research First Pilot Foundation grant 143348, a Tier 1 Canada Research Chair (CRC) on Hypertension and Vascular Research by the Canada Research Chairs/CIHR Program, and by the Canada Fund for Innovation, all to ELS. RT was supported by the São Paulo Research Foundation (FAPESP, grant no. 2013/08216-2 to the Center for Research in Inflammatory Diseases).
DISCLOSURE
The authors declared no conflict of interest.
This manuscript was sent to Guest Editor, Charles T. Stier, PhD for editorial handling and final disposition.
REFERENCES
- 1. Tait JF, Simpson SA, Grundy HM. The effect of adrenal extract on mineral metabolism. Lancet 1952; 1:122–124. [DOI] [PubMed] [Google Scholar]
- 2. Hobbiger F, Simpson SA, Tait JF. The hydrolysis of cortisone acetate by enzymes of human blood. Biochem J 1954; 58 (330th Meeting):X. [PubMed] [Google Scholar]
- 3. Simpson SA, Tait JF, Wettstein A, Neher R, Von Euw J, Schindler O, Reichstein T. [Constitution of aldosterone, a new mineralocorticoid]. Experientia 1954; 10:132–133. [DOI] [PubMed] [Google Scholar]
- 4. Speirs RS, Simpson SA, Tait JF. Certain biological activities of crystalline electrocortin. Endocrinology 1954; 55:233–236. [DOI] [PubMed] [Google Scholar]
- 5. Biron P, Koiw E, Nowaczynski W, Brouillet J, Genest J. The effects of intravenous infusions of valine-5 angiotensin II and other pressor agents on urinary electrolytes and corticosteroids, including aldosterone. J Clin Invest 1961; 40:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carpenter CC, Davis JO, Ayers CR. Relation of renin, angiotensin II, and experimental renal hypertension to aldosterone secretion. J Clin Invest 1961; 40:2026–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takeda Y, Miyamori I, Yoneda T, Hatakeyama H, Inaba S, Furukawa K, Mabuchi H, Takeda R. Regulation of aldosterone synthase in human vascular endothelial cells by angiotensin II and adrenocorticotropin. J Clin Endocrinol Metab 1996; 81:2797–2800. [DOI] [PubMed] [Google Scholar]
- 8. Davis JO, Carpenter CC, Ayers CR, Holman JE, Bahn RC. Evidence for secretion of an aldosterone-stimulating hormone by the kidney. J Clin Invest 1961; 40:684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mulrow PJ, Ganong WF. Stimulation of aldosterone secretion by angiotensisn. II. A preliminary report. Yale J Biol Med 1961; 33:386–395. [PMC free article] [PubMed] [Google Scholar]
- 10. Farrell GL, Banks RC, Koletsky S. The effect of corticosteroid injection on aldosterone secretion. Endocrinology 1956; 58:104–108. [DOI] [PubMed] [Google Scholar]
- 11. Bartter FC The role of aldosterone in the regulation of body fluid volume and composition. Scand J Clin Lab Invest 1958; 10(Suppl 31):50–61. [PubMed] [Google Scholar]
- 12. Ganong WF, Mulrow PJ. Rate of change in sodium and potassium excretion after injection of aldosterone into the aorta and renal artery of the dog. Am J Physiol 1958; 195:337–342. [DOI] [PubMed] [Google Scholar]
- 13. Dluhy RG, Axelrod L, Underwood RH, Williams GH. Studies of the control of plasma aldosterone concentration in normal man. II. Effect of dietary potassium and acute potassium infusion. J Clin Invest 1972; 51:1950–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mackenzie SM, Clark CJ, Fraser R, Gómez-Sánchez CE, Connell JM, Davies E. Expression of 11beta-hydroxylase and aldosterone synthase genes in the rat brain. J Mol Endocrinol 2000; 24:321–328. [DOI] [PubMed] [Google Scholar]
- 15. Strömstedt M, Waterman MR. Messenger RNAs encoding steroidogenic enzymes are expressed in rodent brain. Brain Res Mol Brain Res 1995; 34:75–88. [DOI] [PubMed] [Google Scholar]
- 16. Lombès M Various actions of aldosterone: the kidney and beyond. Ann Endocrinol (Paris) 2009; 70:173–175. [DOI] [PubMed] [Google Scholar]
- 17. Ye P, Kenyon CJ, Mackenzie SM, Jong AS, Miller C, Gray GA, Wallace A, Ryding AS, Mullins JJ, Mcbride MW, Graham D, Fraser R, Connell JM, Davies E. The aldosterone synthase (CYP11B2) and 11beta-hydroxylase (CYP11B1) genes are not expressed in the rat heart. Endocrinology 2005; 146:5287–5293. [DOI] [PubMed] [Google Scholar]
- 18. Takeda Y, Miyamori I, Yoneda T, Iki K, Hatakeyama H, Blair IA, Hsieh FY, Takeda R. Production of aldosterone in isolated rat blood vessels. Hypertension 1995; 25:170–173. [DOI] [PubMed] [Google Scholar]
- 19. Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Corrêa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012; 59:1069–1078. [DOI] [PubMed] [Google Scholar]
- 20. Lopez AG, Duparc C, Naccache A, Castanet M, Lefebvre H, Louiset E. Role of mast cells in the control of aldosterone secretion. Horm Metab Res 2020; 52:412–420. [DOI] [PubMed] [Google Scholar]
- 21. Boyer HG, Wils J, Renouf S, Arabo A, Duparc C, Boutelet I, Lefebvre H, Louiset E. Dysregulation of aldosterone secretion in mast cell-deficient mice. Hypertension 2017; 70:1256–1263. [DOI] [PubMed] [Google Scholar]
- 22. Lefebvre H, Contesse V, Delarue C, Soubrane C, Legrand A, Kuhn JM, Wolf LM, Vaudry H. Effect of the serotonin-4 receptor agonist zacopride on aldosterone secretion from the human adrenal cortex: in vivo and in vitro studies. J Clin Endocrinol Metab 1993; 77:1662–1666. [DOI] [PubMed] [Google Scholar]
- 23. Conn JW Primary aldosteronism. J Lab Clin Med 1955; 45:661–664. [PubMed] [Google Scholar]
- 24. Kline GA, Prebtani APH, Leung AA, Schiffrin EL. Primary aldosteronism: a common cause of resistant hypertension. CMAJ 2017; 189:E773–E778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Genest J, Lemieux G, Davignon A, Koiw E, Nowaczynski W, Steyermark P. Human arterial hypertension: a state of mild chronic hyperaldosteronism? Science 1956; 123:503–505. [DOI] [PubMed] [Google Scholar]
- 26. Gross F, Loustalot P, Meier R. Production of experimental hypertension by aldosterone. Acta Endocrinol (Copenh) 1957; 26:417–423. [DOI] [PubMed] [Google Scholar]
- 27. Mcfarlane SI, Sowers JR. Cardiovascular endocrinology 1: aldosterone function in diabetes mellitus: effects on cardiovascular and renal disease. J Clin Endocrinol Metab 2003; 88:516–523. [DOI] [PubMed] [Google Scholar]
- 28. Ferreira NS, Cau SB, Silva MA, Manzato CP, Mestriner FL, Matsumoto T, Carneiro FS, Tostes RC. Diabetes impairs the vascular effects of aldosterone mediated by G protein-coupled estrogen receptor activation. Front Pharmacol 2015; 6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gros R, Ding Q, Liu B, Chorazyczewski J, Feldman RD. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. Am J Physiol Cell Physiol 2013; 304:C532–C540. [DOI] [PubMed] [Google Scholar]
- 30. Gros R, Ding Q, Sklar LA, Prossnitz EE, Arterburn JB, Chorazyczewski J, Feldman RD. GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension 2011; 57:442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 32. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717. [DOI] [PubMed] [Google Scholar]
- 33. Williams B, Macdonald TM, Morant S, Webb DJ, Sever P, Mcinnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society’s PATHWAY Studies Group Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Briet M, Schiffrin EL. Vascular actions of aldosterone. J Vasc Res 2013; 50:89–99. [DOI] [PubMed] [Google Scholar]
- 35. Schiffrin EL Effects of aldosterone on the vasculature. Hypertension 2006; 47:312–318. [DOI] [PubMed] [Google Scholar]
- 36. Bruder-Nascimento T, Da Silva MA, Tostes RC. The involvement of aldosterone on vascular insulin resistance: implications in obesity and type 2 diabetes. Diabetol Metab Syndr 2014; 6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silva MA, Bruder-Nascimento T, Cau SB, Lopes RA, Mestriner FL, Fais RS, Touyz RM, Tostes RC. Spironolactone treatment attenuates vascular dysfunction in type 2 diabetic mice by decreasing oxidative stress and restoring NO/GC signaling. Front Physiol 2015; 6:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol 2002; 161:1773–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ko EA, Amiri F, Pandey NR, Javeshghani D, Leibovitz E, Touyz RM, Schiffrin EL. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol 2007; 292:H1789–H1795. [DOI] [PubMed] [Google Scholar]
- 40. Brown NJ Aldosterone and vascular inflammation. Hypertension 2008; 51:161–167. [DOI] [PubMed] [Google Scholar]
- 41. Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 2012; 59:324–330. [DOI] [PubMed] [Google Scholar]
- 42. Guzik TJ, Hoch NE, Brown KA, Mccann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007; 204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Biwer LA, Wallingford MC, Jaffe IZ. Vascular mineralocorticoid receptor: evolutionary mediator of wound healing turned harmful by our modern lifestyle. Am J Hypertens 2019; 32:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ferrario CM, Schiffrin EL. Role of mineralocorticoid receptor antagonists in cardiovascular disease. Circ Res 2015; 116:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murphy K, Weaver C. Janeway’s Immunology, 9th edn. Garland Science: New York, NY, 2016. [Google Scholar]
- 46. Selye H Anticortisol action of aldosterone. Science 1955; 121:368–369. [DOI] [PubMed] [Google Scholar]
- 47. Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res 1990; 67:1355–1364. [DOI] [PubMed] [Google Scholar]
- 48. Brilla CG, Matsubara LS, Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol 1993; 25:563–575. [DOI] [PubMed] [Google Scholar]
- 49. Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, Mcmahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol 2002; 283:H1802–H1810. [DOI] [PubMed] [Google Scholar]
- 50. Terada Y, Ueda S, Hamada K, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Kagawa T, Horino T, Takao T. Aldosterone stimulates nuclear factor-kappa B activity and transcription of intercellular adhesion molecule-1 and connective tissue growth factor in rat mesangial cells via serum- and glucocorticoid-inducible protein kinase-1. Clin Exp Nephrol 2012; 16:81–88. [DOI] [PubMed] [Google Scholar]
- 51. Tostes RC, Touyz RM, He G, Chen X, Schiffrin EL. Contribution of endothelin-1 to renal activator protein-1 activation and macrophage infiltration in aldosterone-induced hypertension. Clin Sci (Lond) 2002; 103(Suppl 48):25S–30S. [DOI] [PubMed] [Google Scholar]
- 52. Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, Mcmahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int 2003; 63:1791–1800. [DOI] [PubMed] [Google Scholar]
- 53. Savoia C, Touyz RM, Amiri F, Schiffrin EL. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension 2008; 51:432–439. [DOI] [PubMed] [Google Scholar]
- 54. Lemarié CA, Simeone SM, Nikonova A, Ebrahimian T, Deschênes ME, Coffman TM, Paradis P, Schiffrin EL. Aldosterone-induced activation of signaling pathways requires activity of angiotensin type 1a receptors. Circ Res 2009; 105:852–859. [DOI] [PubMed] [Google Scholar]
- 55. Briet M, Barhoumi T, Mian MOR, Coelho SC, Ouerd S, Rautureau Y, Coffman TM, Paradis P, Schiffrin EL. Aldosterone-induced vascular remodeling and endothelial dysfunction require functional angiotensin type 1a receptors. Hypertension 2016; 67:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Endemann DH, Touyz RM, Iglarz M, Savoia C, Schiffrin EL. Eplerenone prevents salt-induced vascular remodeling and cardiac fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 2004; 43:1252–1257. [DOI] [PubMed] [Google Scholar]
- 57. Mccurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 2012; 18:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang X, Liu J, Pang X, Zhao J, Wang S, Wu D. Aldosterone induces C-reactive protein expression via MR-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Mol Cell Endocrinol 2014; 395:61–68. [DOI] [PubMed] [Google Scholar]
- 59. Caprio M, Newfell BG, La Sala A, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res 2008; 102:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Callera GE, Yogi A, Briones AM, Montezano AC, He Y, Tostes RC, Schiffrin EL, Touyz RM. Vascular proinflammatory responses by aldosterone are mediated via c-Src trafficking to cholesterol-rich microdomains: role of PDGFR. Cardiovasc Res 2011; 91:720–731. [DOI] [PubMed] [Google Scholar]
- 61. Chan CT, Moore JP, Budzyn K, Guida E, Diep H, Vinh A, Jones ES, Widdop RE, Armitage JA, Sakkal S, Ricardo SD, Sobey CG, Drummond GR. Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension 2012; 60:1207–1212. [DOI] [PubMed] [Google Scholar]
- 62. Shimizu K, Furuichi K, Sakai N, Kitagawa K, Matsushima K, Mukaida N, Kaneko S, Wada T. Fractalkine and its receptor, CX3CR1, promote hypertensive interstitial fibrosis in the kidney. Hypertens Res 2011; 34:747–752. [DOI] [PubMed] [Google Scholar]
- 63. Kotlyar E, Vita JA, Winter MR, Awtry EH, Siwik DA, Keaney JF Jr, Sawyer DB, Cupples LA, Colucci WS, Sam F. The relationship between aldosterone, oxidative stress, and inflammation in chronic, stable human heart failure. J Card Fail 2006; 12:122–127. [DOI] [PubMed] [Google Scholar]
- 64. Wu C, Zhang H, Zhang J, Xie C, Fan C, Zhang H, Wu P, Wei Q, Tan W, Xu L, Wang L, Xue Y, Guan M. Inflammation and fibrosis in perirenal adipose tissue of patients with aldosterone-producing adenoma. Endocrinology 2018; 159:227–237. [DOI] [PubMed] [Google Scholar]
- 65. Chou CH, Hung CS, Liao CW, Wei LH, Chen CW, Shun CT, Wen WF, Wan CH, Wu XM, Chang YY, Wu VC, Wu KD, Lin YH; TAIPAI Study Group IL-6 trans-signalling contributes to aldosterone-induced cardiac fibrosis. Cardiovasc Res 2018; 114:690–702. [DOI] [PubMed] [Google Scholar]
- 66. Bruder-Nascimento T, Callera GE, Montezano AC, Belin De Chantemele EJ, Tostes RC, Touyz RM. Atorvastatin inhibits pro-inflammatory actions of aldosterone in vascular smooth muscle cells by reducing oxidative stress. Life Sci 2019; 221:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Syed M, Ball JP, Mathis KW, Hall ME, Ryan MJ, Rothenberg ME, Yanes Cardozo LL, Romero DG. MicroRNA-21 ablation exacerbates aldosterone-mediated cardiac injury, remodeling, and dysfunction. Am J Physiol Endocrinol Metab 2018; 315:E1154–E1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 2009; 54:1384–1392. [DOI] [PubMed] [Google Scholar]
- 69. Leibovitz E, Ebrahimian T, Paradis P, Schiffrin EL. Aldosterone induces arterial stiffness in absence of oxidative stress and endothelial dysfunction. J Hypertens 2009; 27:2192–2200. [DOI] [PubMed] [Google Scholar]
- 70. Thang LV, Demel SL, Crawford R, Kaminski NE, Swain GM, Van Rooijen N, Galligan JJ. Macrophage depletion lowers blood pressure and restores sympathetic nerve α2-adrenergic receptor function in mesenteric arteries of DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 2015; 309:H1186–H1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martinon F Signaling by ROS drives inflammasome activation. Eur J Immunol 2010; 40:616–619. [DOI] [PubMed] [Google Scholar]
- 72. Tschopp J Mitochondria: sovereign of inflammation? Eur J Immunol 2011; 41:1196–1202. [DOI] [PubMed] [Google Scholar]
- 73. Wenceslau CF, Mccarthy CG, Szasz T, Spitler K, Goulopoulou S, Webb RC; Working Group on DAMPs in Cardiovascular Disease Mitochondrial damage-associated molecular patterns and vascular function. Eur Heart J 2014; 35:1172–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology 2007; 148:3773–3780. [DOI] [PubMed] [Google Scholar]
- 75. Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 2007; 293:H2009–H2023. [DOI] [PubMed] [Google Scholar]
- 76. Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 2006; 20:1546–1548. [DOI] [PubMed] [Google Scholar]
- 77. Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 2001; 38:1107–1111. [DOI] [PubMed] [Google Scholar]
- 78. Doi T, Doi S, Nakashima A, Ueno T, Yokoyama Y, Kohno N, Masaki T. Mizoribine ameliorates renal injury and hypertension along with the attenuation of renal caspase-1 expression in aldosterone-salt-treated rats. PLoS One 2014; 9:e93513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Krishnan SM, Dowling JK, Ling YH, Diep H, Chan CT, Ferens D, Kett MM, Pinar A, Samuel CS, Vinh A, Arumugam TV, Hewitson TD, Kemp-Harper BK, Robertson AA, Cooper MA, Latz E, Mansell A, Sobey CG, Drummond GR. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br J Pharmacol 2016; 173:752–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ling YH, Krishnan SM, Chan CT, Diep H, Ferens D, Chin-Dusting J, Kemp-Harper BK, Samuel CS, Hewitson TD, Latz E, Mansell A, Sobey CG, Drummond GR. Anakinra reduces blood pressure and renal fibrosis in one kidney/DOCA/salt-induced hypertension. Pharmacol Res 2017; 116:77–86. [DOI] [PubMed] [Google Scholar]
- 81. Bruder-Nascimento T, Ferreira NS, Zanotto CZ, Ramalho F, Pequeno IO, Olivon VC, Neves KB, Alves-Lopes R, Campos E, Silva CA, Fazan R, Carlos D, Mestriner FL, Prado D, Pereira FV, Braga T, Luiz JP, Cau SB, Elias PC, Moreira AC, Câmara NO, Zamboni DS, Alves-Filho JC, Tostes RC. NLRP3 inflammasome mediates aldosterone-induced vascular damage. Circulation 2016; 134:1866–1880. [DOI] [PubMed] [Google Scholar]
- 82. Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015; 21:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Krishnan SM, Ling YH, Huuskes BM, Ferens DM, Saini N, Chan CT, Diep H, Kett MM, Samuel CS, Kemp-Harper BK, Robertson AAB, Cooper MA, Peter K, Latz E, Mansell AS, Sobey CG, Drummond GR, Vinh A. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc Res 2019; 115:776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ferreira NS, Bruder-Nascimento T, Pereira CA, Zanotto CZ, Prado DS, Silva JF, Rassi DM, Foss-Freitas MC, Alves-Filho JC, Carlos D, Tostes RC. NLRP3 inflammasome and mineralocorticoid receptors are associated with vascular dysfunction in type 2 diabetes mellitus. Cells 2019; 8:1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996; 272:735–738. [DOI] [PubMed] [Google Scholar]
- 86. Ji X, Naito Y, Weng H, Endo K, Ma X, Iwai N. P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am J Physiol Renal Physiol 2012; 303:F1207–F1215. [DOI] [PubMed] [Google Scholar]
- 87. Latouche C, El Moghrabi S, Messaoudi S, Nguyen Dinh Cat A, Hernandez-Diaz I, Alvarez De La Rosa D, Perret C, López Andrés N, Rossignol P, Zannad F, Farman N, Jaisser F. Neutrophil gelatinase-associated lipocalin is a novel mineralocorticoid target in the cardiovascular system. Hypertension 2012; 59:966–972. [DOI] [PubMed] [Google Scholar]
- 88. Tarjus A, Martínez-Martínez E, Amador C, Latouche C, El Moghrabi S, Berger T, Mak TW, Fay R, Farman N, Rossignol P, Zannad F, López-Andrés N, Jaisser F. Neutrophil gelatinase-associated lipocalin, a novel mineralocorticoid biotarget, mediates vascular profibrotic effects of mineralocorticoids. Hypertension 2015; 66:158–166. [DOI] [PubMed] [Google Scholar]
- 89. Buonafine M, Martínez-Martínez E, Amador C, Gravez B, Ibarrola J, Fernández-Celis A, El Moghrabi S, Rossignol P, López-Andrés N, Jaisser F. Neutrophil Gelatinase-Associated Lipocalin from immune cells is mandatory for aldosterone-induced cardiac remodeling and inflammation. J Mol Cell Cardiol 2018; 115:32–38. [DOI] [PubMed] [Google Scholar]
- 90. Araos P, Prado C, Lozano M, Figueroa S, Espinoza A, Berger T, Mak TW, Jaisser F, Pacheco R, Michea L, Amador CA. Dendritic cells are crucial for cardiovascular remodeling and modulate neutrophil gelatinase-associated lipocalin expression upon mineralocorticoid receptor activation. J Hypertens 2019; 37:1482–1492. [DOI] [PubMed] [Google Scholar]
- 91. Caillon A, Paradis P, Schiffrin EL. Role of immune cells in hypertension. Br J Pharmacol 2019; 176:1818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 2010; 122:2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Herrada AA, Contreras FJ, Marini NP, Amador CA, González PA, Cortés CM, Riedel CA, Carvajal CA, Figueroa F, Michea LF, Fardella CE, Kalergis AM. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol 2010; 184:191–202. [DOI] [PubMed] [Google Scholar]
- 94. Mian MO, Barhoumi T, Briet M, Paradis P, Schiffrin EL. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J Hypertens 2016; 34:97–108. [DOI] [PubMed] [Google Scholar]
- 95. Amador CA, Barrientos V, Peña J, Herrada AA, González M, Valdés S, Carrasco L, Alzamora R, Figueroa F, Kalergis AM, Michea L. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 2014; 63:797–803. [DOI] [PubMed] [Google Scholar]
- 96. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005; 6:1123–1132. [DOI] [PubMed] [Google Scholar]
- 97. Krebs CF, Lange S, Niemann G, Rosendahl A, Lehners A, Meyer-Schwesinger C, Stahl RA, Benndorf RA, Velden J, Paust HJ, Panzer U, Ehmke H, Wenzel UO. Deficiency of the interleukin 17/23 axis accelerates renal injury in mice with deoxycorticosterone acetate + angiotensin II-induced hypertension. Hypertension 2014; 63:565–571. [DOI] [PubMed] [Google Scholar]
- 98. Caillon A, Mian MOR, Fraulob-Aquino JC, Huo KG, Barhoumi T, Ouerd S, Sinnaeve PR, Paradis P, Schiffrin EL. γδ T cells mediate angiotensin II-induced hypertension and vascular injury. Circulation 2017; 135:2155–2162. [DOI] [PubMed] [Google Scholar]
- 99. Itani HA, Mcmaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 2016; 68:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension 2002; 40:504–510. [DOI] [PubMed] [Google Scholar]
- 101. Norlander AE, Saleh MA, Pandey AK, Itani HA, Wu J, Xiao L, Kang J, Dale BL, Goleva SB, Laroumanie F, Du L, Harrison DG, Madhur MS. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight 2017; 2:e92801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liu Y, Rafferty TM, Rhee SW, Webber JS, Song L, Ko B, Hoover RS, He B, Mu S. CD8+ T cells stimulate Na-Cl co-transporter NCC in distal convoluted tubules leading to salt-sensitive hypertension. Nat Commun 2017; 8:14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M, Cifelli G, De Lucia M, Lembo G. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity 2014; 41:737–752. [DOI] [PubMed] [Google Scholar]
- 104. Perrotta M, Lori A, Carnevale L, Fardella S, Cifelli G, Iacobucci R, Mastroiacovo F, Iodice D, Pallante F, Storto M, Lembo G, Carnevale D. Deoxycorticosterone acetate-salt hypertension activates placental growth factor in the spleen to couple sympathetic drive and immune system activation. Cardiovasc Res 2018; 114:456–467. [DOI] [PubMed] [Google Scholar]
- 105. Sun XN, Li C, Liu Y, Du LJ, Zeng MR, Zheng XJ, Zhang WC, Liu Y, Zhu M, Kong D, Zhou L, Lu L, Shen ZX, Yi Y, Du L, Qin M, Liu X, Hua Z, Sun S, Yin H, Zhou B, Yu Y, Zhang Z, Duan SZ. T-cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ Res 2017; 120:1584–1597. [DOI] [PubMed] [Google Scholar]
- 106. Shao PP, Liu CJ, Xu Q, Zhang B, Li SH, Wu Y, Sun Z, Cheng LF. Eplerenone reverses cardiac fibrosis via the suppression of tregs by inhibition of Kv1.3 channel. Front Physiol 2018; 9:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Belanger KM, Crislip GR, Gillis EE, Abdelbary M, Musall JB, Mohamed R, Baban B, Elmarakby A, Brands MW, Sullivan JC. Greater T regulatory cells in females attenuate DOCA-salt-induced increases in blood pressure versus males. Hypertension 2020; 75:1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]