Abstract
BACKGROUND
Anterior cervical corpectomy and fusion (ACCF) is a treatment option for several cervical pathologies. Various graft materials such as autografts, titanium mesh cages (TMC), or poly-ether-ether-ketone (PEEK) cages are used. Additional posterior fixation (PF) to provide extra support and improve stability is sometimes performed initially, or later as supplementary treatment.
OBJECTIVE
To describe our retrospective study of 119 consecutive cases of ACCF with synthetic grafts, in 3 cohorts of cervical spondylotic myelopathy (CSM), infectious and neoplastic processes, and trauma, with special focus on need for supplementary PF.
METHODS
A total of 135 adult patients treated with ACCF between January 2005 and January 2018 were identified. Patients lost to follow-up were excluded, and 119 remaining patients were included for retrospective clinical and radiological assessment.
RESULTS
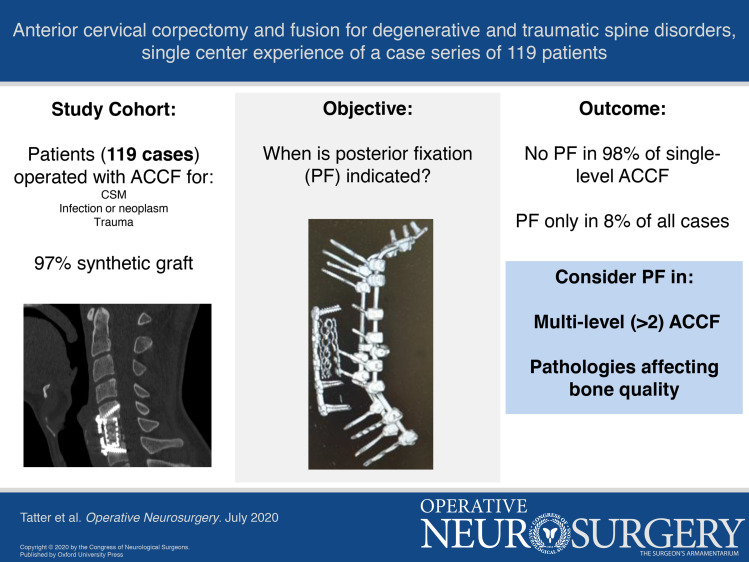
Synthetic grafts were used in 116 (97%) cases. Only 9 (8%) ACCF cases required later supplementary PF, where 7 (78%) cases were multilevel. There was a statistically significant difference in revision rate with PF for single-level compared to multilevel ACCFs (P = .001). Revision rates with PF were 2%, 29%, and 7% in CSM, infectious and neoplastic processes, and trauma cohorts, respectively.
CONCLUSION
The results indicate that ACCF is a safe and effective treatment for degenerative and traumatic cervical spine disorders, with low complication and revision rates. Single-level ACCF can be performed without additional PF. Multilevel ACCF (n > 2) and pathologies affecting bone quality seem to be risk factors for material subsidence and instability. In these cases, additional PF should be considered.
Keywords: Anterior cervical corpectomy, Cervical spine, Fusion, Radiological outcome, Spondylodiscitis, Subaxial cervical spine injuries, Surgical outcome
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- ACCF
anterior cervical corpectomy and fusion
- CSM
cervical spondylotic myelopathy
- CT
computed tomography
- MRI
magnetic resonance imaging
- PEEK
poly-ether-ether-ketone
- PF
posterior fixation
- TMC
titanium mesh cage
Anterior cervical corpectomy and fusion (ACCF) is a treatment option for degenerative cervical pathologies with anterior spinal cord compression and traumatic subaxial injuries.1-6 In short, the cervical spine is accessed by an anterior Smith and Robinson approach.7 After discectomy, the middle part of the vertebral body is resected. The defect is filled with a graft to maintain height and stability followed by anterior plating.
Various graft materials are used for ACCF. Autografts (usually harvested from iliac crest or fibula) have an advantage in higher fusion rates but a disadvantage in potential donor site complications.8 Synthetic grafts can be used and include poly-ether-ether-ketone (PEEK) cages or titanium mesh cages (TMC), usually filled with resected bone from the vertebra. Studies presenting radiographic follow-up have shown a high rate of solid bone fusion using TMC while avoiding donor site complications associated with autografts.9 However, there are known complications, in form of material subsidence at follow-up, for all types of graft material.1,10-16
ACCF may be the treatment of choice for several cervical conditions.1,2,8,13,14,17 While ACCF effectively decompresses the spinal cord, it is debated whether it can provide enough cervical stability, especially in multilevel ACCFs, or whether an additional posterior fixation (PF) is required.2,3,12,14,18-20 Different pathologies may require different considerations when ACCF is performed.
The aim of this study was to describe our experience of 119 consecutive cases of ACCF in 3 different cohorts: cervical spondylotic myelopathy (CSM), infectious and neoplastic processes, and trauma. Focusing on the need for supplementary PF, we compare and discuss our experiences from the 3 cohorts.
METHODS
Patient Selection
The study was approved by the Regional Ethical Review Board (Dnr: 2016/1708-31/4) that waived the need for informed consent. The study hospital is a publicly funded tertiary care center serving a region of approximately 2 million inhabitants. It is the region's only level 1 trauma center and handles, with very few exceptions, all spine trauma cases and the majority of the degenerative myelopathic cases in the region. Patients were identified through the surgical management software Orbit (Evry Healthcare Systems, Solna, Sweden). Medical records and imaging data from digital hospital charts were retrospectively reviewed using the health record software TakeCare (Compu Group Medical Sweden AB, Farsta, Sweden).
A total of 135 adult patients treated with ACCF at the Department of Neurosurgery during the period of January 2005 to January 2018 were identified. Sixteen patients were excluded since they were lost to follow-up. Fourteen of these, all trauma cases, were patients living in other regions or countries. The remaining 119 patients were included in the study.
Patients were divided into 3 cohorts, depending on underlying pathology: CSM, infectious or neoplastic processes, and trauma including cases of traumatic subaxial injury with cervical instability.
Preoperative diagnostic imaging in the first 2 cohorts included a magnetic resonance imaging (MRI) and, in most cases, a computed tomography (CT) scan. A detailed preoperative neurological examination was performed. In the trauma cohort, an initial trauma CT scan was performed followed by an MRI. Trauma patients routinely underwent a preoperative clinical evaluation. However, in many cases, they were unconscious, sedated, or otherwise unable to cooperate.
Surgical Technique
Surgeries were performed by either of 3 primary attending senior neurosurgeons.
For ACCF, a standard right-sided Smith-Robinson approach was performed. Following discectomy above and below the intended vertebrae, corpectomy was performed using a high-speed drill. The posterior longitudinal ligament was typically removed. When using TMC, a cage of appropriate dimensions was chosen, filled with the salvaged bone from the vertebra, and placed in the corpectomy defect. Expandable PEEK cages were otherwise used except for 3 trauma cases where iliac crest autograft was used. In the infectious and neoplastic cohort, mainly empty TMC or expandable cages were used. Adequate position of the graft was confirmed by fluoroscopy. An anterior plate was then positioned, bridging the vertebrae above and below the graft and stabilized with bicortical screws under fluoroscopic guidance (Figure 1).
FIGURE 1.
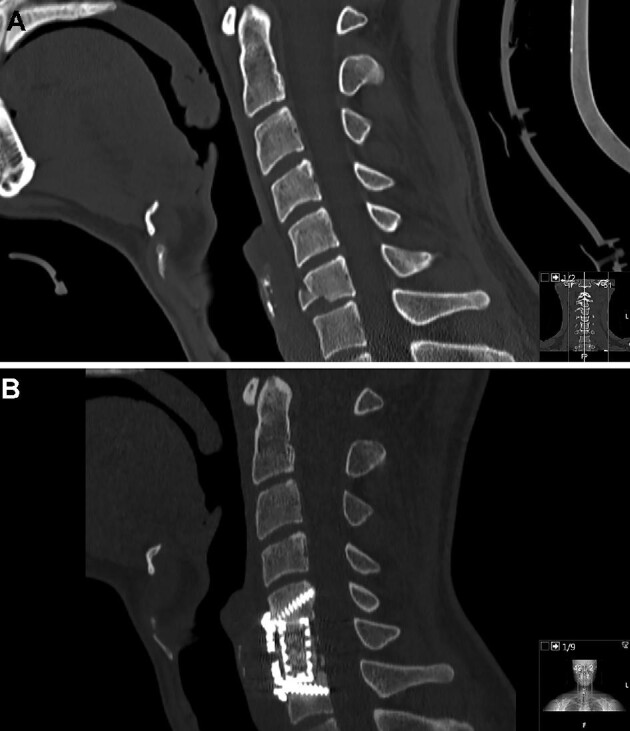
One-level ACCF for traumatic C6 fracture, before surgery A and after ACCF B.
Four cases were treated with a primary combined ACCF plus PF. In these cases, ACCF was performed first, followed by turning the patient to the prone position, head fixed in a Mayfield clamp, and performing a midline incision to expose the posterior aspects of the spine. Lateral mass screws were placed, typically 2 levels above and below the resected vertebrae. When needed, fixations were extended to the upper thoracic levels, where pedicle screws were placed. Rods were placed and cross-links were used when fixations extended 4 levels or more.
Postoperative Follow-up
Patients were mobilized without collars after surgery. Postoperative clinical controls were usually performed within the first 24 h. In adherence with routine protocols, all patients underwent a postoperative CT scan and follow-up CT scans 4 wk and 3 mo postsurgery. All patients were clinically evaluated by their surgeon after 3 mo. Additional imaging, in selected cases, was performed when clinically indicated.
Statistical Analysis
For descriptive purposes, continuous data are presented as means ± standard deviations and as medians [range]. McNemar's test was used to compare the functional outcome of patients before and after intervention. Pearson's chi-squared test was used to compare surgical revision rates. The statistical program SPSS (IBM SPSS Statistics for Macintosh, version 25) was used and a P-value <.05 was considered as significant.
RESULTS
Patient Cohorts and Functional Outcome
Cervical Spondylotic Myelopathy
Among the 50 patients in the CSM cohort, the most common preoperative symptom was pain, followed by motor or sensory deficit, balance impairment, and decreased bladder and/or sphincter function. Five patients in the cohort were scheduled for ACCF due to insufficient result of previous ACDF for CSM. Preoperative MRI revealed myelomalacia on T2-weighted images in 29 (58%) patients (Table 1). There were 28 (56%) cases of single-level, 17 (34%) cases of 2-level, and 5 (10%) cases of 3-level ACCFs (Figure 2). TMC were used in 38 (76%) cases and PEEK cages in 12 (24%) cases.
TABLE 1.
Patient Data
| Variable | CSM (n = 50) | Infectious and neoplastic processes (n = 14) | Trauma (n = 55) |
|---|---|---|---|
| Male gender | 21 (42%) | 6 (43%) | 41 (75%) |
| Age | 58 (30-76) | 65 (43-79) | 34 (16-79) |
| Preoperative symptoms | Pain: 36 (72%)Motor deficit: 34 (68%)Sensory deficit: 34 (68%)Decreased bladder and/or sphincter function: 2 (4%)Balance impairment: 25 (50%) | Pain: 10 (71%)Motor deficit: 10 (71%)Sensory deficit: 9 (64%)Decreased bladder and/or sphincter function: 1 (7%)Balance impairment: 4 (29%) | |
| Spinal level of fracture | C4: 2 (4%)C5: 16 (29%)C6: 19 (35%)C7: 14 (25%)C4-C5: 1 (2%)C5-C6: 3 (5%) | ||
| Myelomalacia | 29 (58%) | 5 (36%) | 29 (53%) |
| Preoperative spinal surgery | 5 (10%) | 0 | 1 (2%) |
CSM = cervical spondylotic myelopathy.
Variables are presented as count (%) or median (range).
FIGURE 2.
Distribution of single- and multilevel ACCFs in the 3 cohorts of CSM, infectious and neoplastic processes, and trauma.
At 3-mo follow-up, there was a significant improvement in prevalence of patient reported pain compared to preoperative findings (P = .016). No statistically significant improvements in motor or sensory deficit, balance impairment, or bladder/sphincter dysfunction were observed. The 3-mo mortality rate was 4% (n = 2). Cause of death was pneumonia in one patient with muscular dystrophia, while a second patient, with severe anorexia, refused nutritional support and did not manage the recovery period (Table 2).
TABLE 2.
Onset Symptoms and Functional Outcome
| Variable | Preop | Three-month follow-up |
|---|---|---|
| CSM | 50 | 48 |
| Pain | 36 (72%) | 28 (58%)a |
| Motor deficit | 34 (68%) | 27 (56%) |
| Sensory deficit | 34 (68%) | 30 (63%) |
| Balance impairment | 25 (50%) | 19 (40%) |
| Decreased bladder and/or sphincter function | 2 (4%) | 2 (4%) |
| Infectious and neoplastic processes | 14 | 12 |
| Pain | 10 (71%) | 6 (50%) |
| Motor deficit | 10 (71%) | 7 (58%) |
| Sensory deficit | 9 (64%) | 7 (58%) |
| Balance impairment | 4 (29%) | 3 (25%) |
| Decreased bladder and/or sphincter function | 1 (7%) | 0 |
| Three-month mortality (related to surgery) | ||
| CSM | 0 | |
| Infectious and neoplastic processes | 0 | |
| Trauma | 0 | |
| Three-month mortality (not related to surgery) | ||
| CSM | 2 (4%) | |
| Infectious and neoplastic processes | 2 (14%) | |
| Trauma | 2 (4%) |
CSM = cervical spondylotic myelopathy.
Variables are presented as count (%).
aP = .016.
Infectious and Neoplastic Processes
Fourteen patients were included. Preoperative symptoms were similar to the CSM cohort. The pathology found was infectious (n = 8, 57%) and neoplastic processes (n = 6, 43%). All included patients underwent preoperative MRI where 5 (36%) cases presented with myelomalacia on T2-weighted images (Table 1). There were 6 cases of single-level, 4 cases of 2-level, 3 cases of 3-level, and 1 case of 4-level ACCFs (Figure 2). TMC were used in 10 (71%) cases and PEEK cages in 4 (29%) cases. Three cases were treated with a combined ACCF and PF at index operation. At 3-mo follow-up, no statistically significant improvements in clinical parameters were observed. The 3-mo mortality rate was 14% (n = 2). Cause of death in both cases was related to primary pulmonary cancer (Table 2).
Trauma Cohort
All 55 included patients in the trauma cohort had cervical instability due to fractured vertebral bodies confirmed by preoperative CT scans. The most common site of fracture was C6 followed by C5 and C7 (Table 1). All patients except 3 underwent preoperative MRI where 38 (73%) cases presented with myelomalacia on T2-weighted images (Table 1). There were 51 (93%) cases of single-level and 4 (7%) cases of 2-level ACCFs (Figure 2). TMC were used in 45 (82%) cases, PEEK cages in 7 (13%) cases, and iliac crest autograft in 3 (5%) cases. One case (a patient previously treated with ACDF 1 wk earlier for bilateral facet-joint luxation) was treated with a combined ACCF and PF.
Odom's recovery scale was used to evaluate the recovery of patients with clinical myelopathy (n = 28). Six (20%) patients classified as good recovery, 11 (38%) as fair recovery, 11 (38%) had a poor recovery, and none got worse after treatment. The 3-mo mortality rate was 4% (n = 2). Cause of death was multiple organ failure in one, and sepsis following extracorporeal membrane oxygenation treatment for cardiac arrest in the other case (Table 2).
Radiographic and Surgical Outcome
Cervical Spondylotic Myelopathy
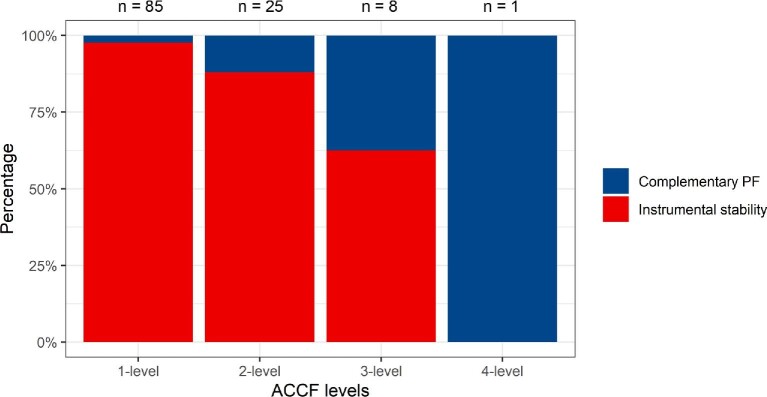
Postoperative CT scans within the first 24 h showed good material positioning in 48 examined patients. In 2 cases, 24-h CT scans were not performed; however, neither showed instability nor need for repeat surgery on later examinations. At 3-mo follow-up, 40 of the CSM patients showed no changes in material positioning compared to postoperative scans. Seven patients showed minor material migration without clinical significance. Two patients died within 3 mo after surgery. Of 50 patients in the CSM cohort, only 1 patient (2%) was revised with PF. It was a 3-level ACCF (C4-C6) with an expandable PEEK cage and an anterior plate C3-C7 with bicortical screws. Routine control CT after 4 wk showed fractures in C7 along the screws and dislocation of the anterior plate. There was no neurological deterioration or kyphosis. The case was revised with corpectomy of C7, anterior plate elongation C3-Th1, and PF of C4-Th3 (Figure 3, Table 3). Long-term follow-up scans were available in 24 patients at a median of 63 (7-124) mo. None of them showed new signs of instability (Table 4).
FIGURE 3.
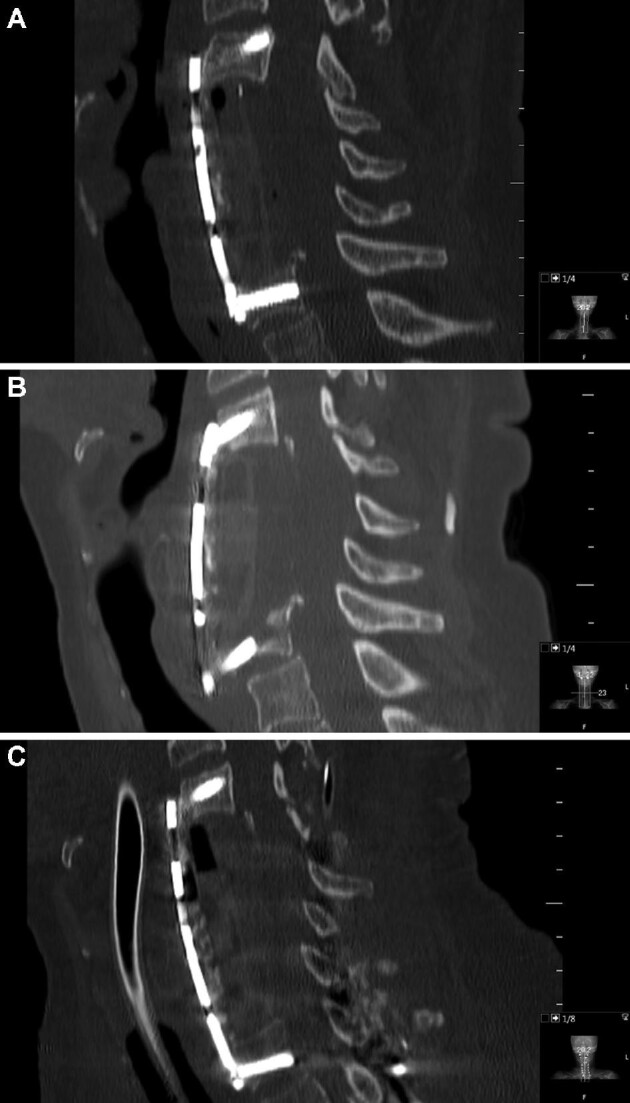
Three-level ACCF (C4-C6) with an expandable PEEK cage and anterior plate C3-C7. Postoperative CT scan A. CT scan after 4 wk showing fracture in C7 and dislocation of the anterior plate B. Revision surgery with corpectomy of C7, anterior plate elongation C3-Th1, and supplementary posterior fixation C4-Th3 C.
TABLE 3.
Surgical Revision With Posterior Fixation (PF)
| Surgical indication | ACCF level | Graft material and plating | Radiographic findings | Clinical findings | Repeat surgery | Time between index surgery and reoperation (d) |
|---|---|---|---|---|---|---|
| CSM | C4, C5, C6 | PEEK cage. Anterior plateC3-C7 | C7 fracture and material dislocation | – | Corpectomy C7, anterior plate C3-Th1 and PFC4-Th3 | 43 |
| Osteomyelitis and kyphosis | C4, C5, C6 | TMC. Anterior plateC3-C7. PF C3-Th1 | Degenerative signs C1-C2 | Kyphosis and cervicalgia | Elongation of PFC0-Th1 | 542 |
| Metastasis | C7, Th1 | PEEK cage. Anterior plate | Osteolytic changes Th2 | – | PF | 294 |
| C6-Th2 | C5-Th4 | |||||
| Cervical abscess and pathological fractures | C4, C5, C6, C7 | TMC. Anterior plate | Anterior plate dislocation and resorption zones around screws | – | PF | 94 |
| C3-Th1 | C2-Th2 | |||||
| Spondylodiscitis | C4, C5, C6 | TMC. Anterior plate | Material dislocation | – | PF | 32 |
| C3-C7 | C3-Th1 | |||||
| Burst fracture C5 and cleavage fracture C4 | C4, C5 | TMC. Anterior plate | Material dislocation and kyphosis | – | PF | 24 |
| C3-C6 | C3-C7 | |||||
| Epidural hematoma and intervertebral disc damage | C6 | TMC. Anterior plate | Subsidence of TMC into C7 and dislocation of anterior plate | – | PF | 37 |
| C6-C7 | C5-C7 | C4-C7 | ||||
| Flexion teardrop fracture C5 and compression fracture C6 | C5, C6 | TMC. Anterior plate | – | C5-radiculopathy | PF and laminectomy C4-C7 | 33 |
| C4-C7 | ||||||
| Flexion teardrop fracture and rupture of ligaments. Luxation of facet joints | C5 | TMC. Anterior plate | Insufficient surgical result | – | PF | 7 |
| C5-C6 | C4-C6 | C3-C7 |
CSM = cervical spondylotic myelopathy, TMC = titanium mesh cage, PEEK = poly-ether-ether-ketone.
TABLE 4.
Radiographic Outcome
| Postop control | Three-month follow-up | Long-term follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | CSM (n = 48) | Infectious and neoplastic processesa (n = 14) | Traumab (n = 55) | CSM (n = 48) | Infectious and neoplastic processes (n = 12) | Trauma (n = 53) | CSM (n = 24) | Infectious and neoplastic processes (n = 8) | Trauma (n = 10) |
| Good material positioning | 48 (100%) | 14 (100%) | 54 (98%) | 40 (83%) | 8 (67%) | 41 (77%) | 18 (75%) | 6 (75%) | 6 (50%) |
| Minor material migration | 0 | 0 | 0 | 7 (15%) | 2 (17%) | 9 (15%) | 6 (25%) | 0 | 4 (40%) |
| New sign of instability leading to PF | 0 | 0 | 1 (2%) | 1 (2%) | 2 (17%) | 3 (6%) | 0 | 2 (25%) | 0 |
ACCF, anterior cervical corpectomy and fusion; CSM = cervical spondylotic myelopathy, PF = posterior fixation.
aThree of these patients were treated with ACCF + PF at index surgery.
bOne of these patients was treated with ACCF + PF at index surgery.
Variables are presented as count (%).
Infectious and Neoplastic Processes
Postoperative CT scans within the first 24 hours showed good material positioning in all 14 patients. At 3-mo follow-up, 8 patients showed no changes in material positioning compared to postoperative scans and 2 patients showed minor material migration without clinical significance. Two patients died within 3 mo after surgery. The remaining 2 patients showed signs of instability and were treated with PF within the 3-mo follow-up period. One of these was a case of 3-level ACCF for spondylodiscitis and the other, a 4-level ACCF due to cervical abscess (candida) with pathological fracture.
Among 8 patients who were investigated with additional late follow-up scans, median 20 (6-84) mo, due to clinical complaints, 2 more underwent PF. One was a 2-level ACCF due to cervical metastasis where signs of instability, attributed to radiotherapy treatment, were found 9 mo postop. The other was initially treated with a 3-level ACCF, C4-C6 and PF, C3-Th1 for osteomyelitis. The patient was re-referred due to new symptoms from levels above index surgery, after more than a year and the PF was elongated to C0-Th1. In total, 6 (43%) cases in this cohort were treated with PF (Tables 3 and 4).
Trauma Cohort
Postoperative CT scans within the first 24 hours showed good material positioning in 54 patients. The remaining one, a single-level ACCF operated due to bilateral facet joint luxation, showed insufficient repositioning on postoperative CT and repeat surgery with PF was performed the following week. At 3-mo follow-up, 41 patients showed no changes in material positioning compared to postoperative scans. Two patients died within 3 mo after surgery. Nine patients showed minor material migration without clinical significance. Three patients were revised with PF within the 3-mo follow-up period. Two of them, a single- and a 2-level ACCF were due to development of kyphosis discovered on the 4-wk follow-up scan. The third case had no new radiographic findings but a C5-radiculopathy (Table 3).
Long-term follow-up was available in 10 patients at a median of 12 (4-85) mo. None of them showed new signs of instability. In total, 4 (7%) cases in this cohort were treated with PF (Tables 3 and 4).
ACCF Range and Revision With PF
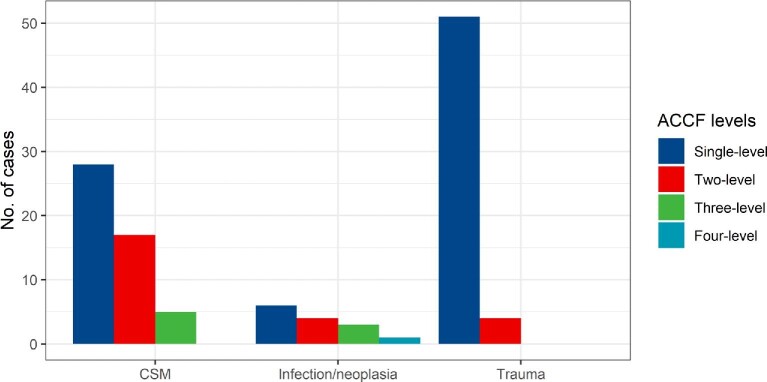
In total, 9 (8%) of the 115 patients in this study with initial only ACCF later underwent supplementary PF. Single-level ACCFs needed supplementary PF in 2% (n = 2/85), 2-level ACCFs in 12% (n = 3/25), and 3-level ACCFs in 38% (n = 3/8) of the cases. The only case treated with a 4-level ACCF was later revised with PF (Figure 4). There was a statistically significant difference when comparing revision rates with PF for single-level compared to multilevel ACCFs (P = .001). There were no cases of neurological deterioration in the time between the initial ACCF and the supplementary PF.
FIGURE 4.
Achieved stability with ACCF alone and need for supplementary posterior fixation in single- and multilevel ACCFs.
Complications
In the CSM cohort, there was one case of prevertebral hematoma that required evacuation, and one case of C5-root palsy that was resolved by the time of 3-mo follow-up.
In the infectious and neoplastic cohort, one patient had a peroperative left vertebral artery injury due to altered anatomy. It was occluded during surgery without any postoperative complication. There were no postoperative hematomas.
In the trauma cohort, 2 patients had recurrent laryngeal nerve palsy and 2 patients C5 root palsy. One patient with a history of liver cirrhosis and hepatitis due to alcohol abuse developed a prevertebral abscess 2 wk after surgery and was treated with a combination of antibiotics and hyperbaric oxygen therapy (Table 5).
TABLE 5.
Complications
| Variable | Total (n = 119) | CSM (n = 50) | Infectious and neoplastic processes (n = 14) | Trauma (n = 55) |
|---|---|---|---|---|
| Complication rate | 8% | 8% | 7% | 9% |
| Hematoma | 2 (2%) | 2 (4%) | 0 | 0 |
| Recurrent laryngeal nerve palsy | 2 (2%) | 0 | 0 | 2 (4%) |
| C5-root palsy | 4 (3%) | 2 (4%) | 0 | 2 (4%) |
| Surgical wound infection | 1 (1%) | 0 | 0 | 1 (2%) |
| Vertebral artery injury | 1 (1%) | 0 | 1 (7%) | 0 |
Variables are presented as count (%).
DISCUSSION
Background and Rationale
An anterior approach with ACCF is widely considered a safe and effective surgical treatment to achieve spinal cord decompression and high fusion rates in degenerative pathologies as well as traumatic spinal injuries.1,2,15,21 However, whether ACCF provides sufficient stability on its own, especially in multilevel ACCFs, remains controversial. Additional surgery with PF can provide extra support to improve cervical stability and is sometimes performed at the index operation or later as supplementary surgery due to material failure or instability.18,19,22 The aim of this study was to analyze and evaluate the outcome of 119 cases of ACCF in 3 different cohorts, CSM (n = 50), infectious and neoplastic processes (n = 14), and trauma (n = 55), and to examine to which extent supplementary PF was required to achieve stability.
Key Results
Of the 119 treated ACCF cases in this study (4 of them treated with an ACCF + PF at index surgery), 106 (92%) provided enough stability without need for supplementary PF. TMC was used as graft in most of the cases (78%). Earlier studies have shown high rates of subsidence correlated to the use of TMC grafts in ACCF.1 However, a recent meta-analysis from 2016 showed no significant differences in graft subsidence when comparing ACCF with ACDF.15 Also, more recently developed types of TMC have shown lower rates of subsidence compared to older types.1,14 Our results demonstrate that TMC is a good choice for ACCF, avoiding donor-site complications of autografts and providing high fusion rates and biomechanical stability.1
While instrument failures and severe subsidence may lead to spinal cord injury and neurological deterioration, minor material movements seem to be of little clinical relevance,1,15,18 which is supported by the findings of this study (Table 4). Most cases of minor material migration were not progressive and were not considered clinically relevant. There are, however, data suggesting that cage subsidence is a sign of biomechanical instability and may impair the construct immobilization and increase the stresses on the anterior plating, risking instrument-related complications.23 In a finite element study, Liu et al demonstrated that a cage with enlarged adaptive ends, aligning with the endplates, induced lower stress peaks in the screw-bone and screw-plate interfaces resulting in decreased risks of screw loosening, screw breakage, and plate extrusion, while cage subsidence significantly increased the stress loads on the fixation system.24 The risk of subsidence is directly dependent on bone quality.25 In line with this, we find the majority of our cases of supplementary PF in the cohort of infectious and neoplastic processes.
There's been a variety of study results concerning the correlation between range of ACCF and stability. A study of 300 ACCF surgeries showed 2-level ACCFs being more susceptible to severe subsidence and related complications compared to single-level ACCFs.26 A more recent study showed no stability-related complications when analyzing the outcome of 23 patients treated with 2-level ACCF for subaxial injuries,2 but the presented radiographic follow-up data were limited. A study of 21 patients with CSM treated with ACCF showed instability in 33% of the cases and recommended a combination with PF for ≥2-level surgeries.19 Another study with 33 patients treated with 2-level cervical ACCF and 7 patients with 3-level ACCF due to degenerative pathologies showed reconstructive failures in 2 and 5 cases, respectively. The authors concluded that a 2-level construct was reliable while a 3-level construct was not.27
In our study, only one case treated with single-level ACCF needed supplementary surgery with PF due to subsidence and anterior plate dislocation. The second revised single-level case was due to insufficient repositioning of luxation and is not to be considered construct failure. Thus, we find a very low risk (1%) of construct failure and instability in single-level cervical ACCF for degenerative and traumatic disorders. Two-level ACCFs had surgical revision with PF in 12% of the cases, while 88% provided sufficient stability with ACCF alone. Multilevel ACCFs also had higher revision rates with PF: 38% (n = 3) for 3-level and 100% (n = 1) for 4-level ACCFs. This is in accordance with the current literature.18,26 An observation is that the only case in the CSM cohort which needed surgical revision with PF was a 3-level ACCF. The overall revision rate with PF was 2% for CSM, 29% for infections and neoplasms, and 7% for trauma indicating a higher risk for revision surgery in pathologies affecting bone quality in the surgical area. In contrast to the CSM and trauma cohorts, 14% of the infectious and neoplastic cohort required PF already at index surgery. Thus, a total of 43% of this cohort needed PF, indicating that it is much more prone to instability.
Concerning nonmaterial-related complications, there was an overall complication rate of 8%. Prevertebral hematomas, recurrent laryngeal nerve palsy, and postsurgical infections were rarely occurring.
Each patient was scheduled for, and usually underwent 3 routine follow-up CT scans. One within the first 24 hr after surgery and then additional scans after 4 wk and 3 mo followed by an appointment at the outpatient clinic. This follow-up protocol identified 7 out of 9 cases that needed surgical revision. One of the 2 remaining had additional follow-up by the oncology department 9 mo after surgery and the other one was re-referred after one and a half years because of symptoms from another level. Thus, a 12-wk follow-up period seems to be adequate for this type of surgery.
Limitations
Study limitations are sample size and retrospective analysis, as well as the fact that our 3 cohorts represent 3 distinct types of pathologies that are treated with a similar surgical technique. A randomized study of specific subtypes of spinal pathology and a larger sample size would achieve higher clinical evidence.
CONCLUSION
The results of this study show that ACCF, with synthetic grafts, is a safe and effective treatment for degenerative and traumatic cervical spine disorders when indicated, with low complication and reoperation rates. Overall only 9 of the 119 cases needed surgical revision with PF for stability purposes. Single-level ACCFs showed sufficient stability in 98% of the cases without additional support. Multilevel ACCF and pathologies affecting bone quality, like infections and neoplasms, seem to be risk factors for construct failure and instability. In these cases, supplementary PF should be considered already at the index surgery.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
Charles Tatter, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden.
Oscar Persson, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden.
Gustav Burström, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden.
Erik Edström, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden.
Adrian Elmi-Terander, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden.
Operative Neurosurgery Speaks! Audio abstracts available for this article at www.operativeneurosurgery-online.com.
COMMENT
The authors present a notably large single-center series on the application of anterior cervical corpectomy for decompression and reconstruction of the cervical spine. The authors describe 3 cohorts, including patients with cervical myelopathy, infectious/neoplastic disease, and trauma. The report considers the need for supplemental posterior hardware as well as revision rates with different pathologies.
The authors should be applauded for this series. Anterior cervical corpectomy may offer a quite powerful technique for spinal decompression. For pathologies with direct ventral compression of the cord, corpectomy directly removes the compressive pathology. Further, it allows for excellent spinal re-alignment, improving cervical lordosis at the site of disease.
The current report shows that the majority of cases in this series did not require supplemental posterior fixation hardware. Of those that did, the vast majority (78%) were multi-level cases. This is an important point and one that may be instructive to spine surgeons. The biomechanics of a single level corpectomy likely do not demand further dorsal support. However, as additional levels are added, the spine surgeon may be wise to consider supplementing an anterior construct. Further, for infections, tumor, and sometimes trauma, a more robust construct may also be needed.
This report does consider multiple pathologies. Given the inherent differences between the pathologies noted here, this is a shortcoming of this series. Cervical spondylosis presents very different challenges from trauma or spinal infection. Therefore, it may be wise to consider the results of the individual cohorts as much as the series as a whole. In either case, this is a large and well-articulated series that may be instructive to practicing spine surgeons.
Zachary A. Smith
Oklahoma City, Oklahoma
Operative Neurosurgery Speaks (Audio Abstracts)
Listen to audio translations of this paper's abstract into select languages by choosing from one of the selections below.
REFERENCES
- 1.Wen Z, Lu T, Wang Y, Liang H, Gao Z, He X. Anterior cervical corpectomy and fusion and anterior cervical discectomy and fusion using titanium mesh cages for treatment of degenerative cervical pathologies: a literature review. Med Sci Monit. 2018;24:6398-6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain V, Madan A, Thakur M, Thakur A. Functional outcomes of subaxial spine injuries managed with 2-level anterior cervical corpectomy and fusion: a prospective study. Neurospine. 2018;15(4):368-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann S, Tschugg A, Obernauer J, Neururer S, Petr O, Thome C. Cervical corpectomies: results of a survey and review of the literature on diagnosis, indications, and surgical technique. Acta Neurochir (Wien). 2016;158(10):1859-1867. [DOI] [PubMed] [Google Scholar]
- 4.Theologis AA, Lansdown D, McClellan RT, Chou D, Pekmezci M. Multilevel corpectomy with anterior column reconstruction and plating for subaxial cervical osteomyelitis. Spine (Phila Pa 1976). 2016;41(18):E1088-E1095. [DOI] [PubMed] [Google Scholar]
- 5.Wei-bing X, Wun-Jer S, Gang L, Yue Z, Ming-xi J, Lian-shun J. Reconstructive techniques study after anterior decompression of multilevel cervical spondylotic myelopathy. J Spinal Disord Tech. 2009;22(7):511-515. [DOI] [PubMed] [Google Scholar]
- 6.Shamji MF, Massicotte EM, Traynelis VC, Norvell DC, Hermsmeyer JT, Fehlings MG. Comparison of anterior surgical options for the treatment of multilevel cervical spondylotic myelopathy: a systematic review. Spine (Phila Pa 1976). 2013;38(22 suppl 1):S195-S209. [DOI] [PubMed] [Google Scholar]
- 7.Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40(3):607-624. [PubMed] [Google Scholar]
- 8.Hwang SL, Lee KS, Su YFet al.. Anterior corpectomy with iliac bone fusion or discectomy with interbody titanium cage fusion for multilevel cervical degenerated disc disease. J Spinal Disord Tech. 2007;20(8):565-570. [DOI] [PubMed] [Google Scholar]
- 9.Burkett CJ, Baaj AA, Dakwar E, Uribe JS. Use of titanium expandable vertebral cages in cervical corpectomy. J Clin Neurosci. 2012;19(3):402-405. [DOI] [PubMed] [Google Scholar]
- 10.Seaman S, Kerezoudis P, Bydon M, Torner JC, Hitchon PW. Titanium vs. polyetheretherketone (PEEK) interbody fusion: meta-analysis and review of the literature. J Clin Neurosci. 2017;44:23-29. [DOI] [PubMed] [Google Scholar]
- 11.Wu WJ, Jiang LS, Liang Y, Dai LY. Cage subsidence does not, but cervical lordosis improvement does affect the long-term results of anterior cervical fusion with stand-alone cage for degenerative cervical disc disease: a retrospective study. Eur Spine J. 2012;21(7):1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayr MT, Subach BR, Comey CH, Rodts GE, Haid RW Jr.. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction, and instrumentation. J Neurosurg. 2002;96(1 suppl):10-16. [DOI] [PubMed] [Google Scholar]
- 13.Thalgott JS, Xiongsheng C, Giuffre JM. Single stage anterior cervical reconstruction with titanium mesh cages, local bone graft, and anterior plating. Spine J. 2003;3(4):294-300. [DOI] [PubMed] [Google Scholar]
- 14.Acosta FL Jr, Aryan HE, Chou D, Ames CP. Long-term biomechanical stability and clinical improvement after extended multilevel corpectomy and circumferential reconstruction of the cervical spine using titanium mesh cages. J Spinal Disord Tech. 2008;21(3):165-174. [DOI] [PubMed] [Google Scholar]
- 15.Zhao CM, Chen Q, Zhang Y, Huang AB, Ding WY, Zhang W. Anterior cervical discectomy and fusion versus hybrid surgery in multilevel cervical spondylotic myelopathy: a meta-analysis. Medicine (Baltimore). 2018;97(34):e11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrini P, Gambacciani C, Martini C, Montemurro N, Lepori P. Anterior cervical corpectomy for cervical spondylotic myelopathy: reconstruction with expandable cylindrical cage versus iliac crest autograft. A retrospective study. Clin Neurol Neurosurg. 2015;139:258-263. [DOI] [PubMed] [Google Scholar]
- 17.Niedzielak TR, Palmer J, Malloy JP. Clinical comparison of surgical constructs for anterior cervical corpectomy and fusion in patients with cervical spondylotic myelopathy or ossified posterior longitudinal ligament: a systematic review and meta-analysis. Clin Spine Surg. 2018;31(6):247-260. [DOI] [PubMed] [Google Scholar]
- 18.Oni P, Schultheiss R, Scheufler KM, Roberg J, Harati A. Radiological and clinical outcome after multilevel anterior cervical discectomy and/or corpectomy and fixation. J Clin Med. 2018;7(12):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayerl SH, Pohlmann F, Finger T, Prinz V, Vajkoczy P. Two-level cervical corpectomy-long-term follow-up reveals the high rate of material failure in patients, who received an anterior approach only. Neurosurg Rev. 2019;42(2):511-518. [DOI] [PubMed] [Google Scholar]
- 20.Koller H, Hempfing A, Ferraris L, Maier O, Hitzl W, Metz-Stavenhagen P. 4- and 5-level anterior fusions of the cervical spine: review of literature and clinical results. Eur Spine J. 2007;16(12):2055-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkhardt BW, Muller SJ, Wagner AC, Oertel JM. Anterior cervical spine surgery for the treatment of subaxial cervical spondylodiscitis: a report of 30 consecutive patients. Neurosurg Focus. 2019;46(1):E6. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence BD, Jacobs WB, Norvell DC, Hermsmeyer JT, Chapman JR, Brodke DS. Anterior versus posterior approach for treatment of cervical spondylotic myelopathy: a systematic review. Spine (Phila Pa 1976). 2013;38(22 suppl 1):S173-S182. [DOI] [PubMed] [Google Scholar]
- 23.Daubs MD. Early failures following cervical corpectomy reconstruction with titanium mesh cages and anterior plating. Spine. 2005;30(12):1402-1406. [DOI] [PubMed] [Google Scholar]
- 24.Liu N, Lu T, Wang YB, Sun ZW, Li JL, He XJ. Effects of new cage profiles on the improvement in biomechanical performance of multilevel anterior cervical corpectomy and fusion: a finite element analysis. World Neurosurg. 2019;129:E87-E96. [DOI] [PubMed] [Google Scholar]
- 25.Ullrich BW, Schenk P, Spiegl UJ, Mendel T, Hofmann GO. Hounsfield units as predictor for cage subsidence and loss of reduction: following posterior-anterior stabilization in thoracolumbar spine fractures. Eur Spine J. 2018;27(12):3034-3042. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Chen D, Guo Yet al.. Subsidence of titanium mesh cage: a study based on 300 cases. J Spinal Disord Tech. 2008;21(7):489-492. [DOI] [PubMed] [Google Scholar]
- 27.Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine (Phila Pa 1976). 2003;28(2):140-142. [DOI] [PubMed] [Google Scholar]