Abstract
Humans with salt-sensitive (SS) hypertension demonstrate increased morbidity, increased mortality, and renal end-organ damage when compared with normotensive subjects or those with salt-resistant hypertension. Increasing evidence indicates that immune mechanisms play an important role in the full development of SS hypertension and associated renal damage. Recent experimental advances and studies in animal models have permitted a greater understanding of the mechanisms of activation and action of immunity in this disease process. Evidence favors a role of both innate and adaptive immune mechanisms that are triggered by initial, immune-independent alterations in blood pressure, sympathetic activity, or tissue damage. Activation of immunity, which can be enhanced by a high-salt intake or by alterations in other components of the diet, leads to the release of cytokines, free radicals, or other factors that amplify renal damage and hypertension and mediate malignant disease.
Keywords: blood pressure, hypertension, immune cells, kidney
Hypertension, or high blood pressure, is a risk factor for cardiovascular, cerebrovascular, renal, and related diseases,1 and is a major contributing factor to morbidity and mortality throughout the world.2,3 Over 45% of adults in the United States can be characterized as hypertensive,4,5 and cardiovascular diseases comprise the leading cause of death in the United States.4 A form of hypertension which exhibits particularly high morbidity and mortality is salt (NaCl)-sensitive hypertension.6,7 Salt-sensitive (SS) hypertension, which is prevalent among African-American subjects,8,9 is subjectively defined as a 10 mm Hg increase in blood pressure following an elevation in sodium intake,10,11 and is present in approximately 30–50% of hypertensive subjects.10,11
The increased morbidity and mortality in SS hypertensives are understood, yet the cause of this form of hypertension remains unclear. One potential mediator/modifier of SS hypertension and associated end-organ damage are mechanisms related to activation of inflammation and immunity. Studies in experimental animals and observations in humans have implicated immune mechanisms in the etiology of hypertension12–22 but the causal interrelationships between sodium intake, immune mechanisms, hypertension, and renal end-organ damage are not clear. This brief review will describe newly elucidated mechanisms linking sodium intake with immune activation in the kidney and the amplification of hypertension. A summary of recent data indicating that environmental factors can modulate the ability of immune mechanisms to amplify SS hypertension concludes the review.
IMMUNITY IN HYPERTENSION AND RENAL END-ORGAN DAMAGE
Histological, functional, and genetic data indicate that abnormalities in immune function may participate in human hypertension and renal disease. Examination of kidneys from hypertensive subjects has demonstrated the presence of lymphocytes,23 other mononuclear cells,24 and the deposition of immunoglobulins and complement proteins25 in the renal interstitium adjacent to damaged renal tubules and glomeruli. Comparisons that are more detailed have demonstrated that hypertensive subjects, compared with normotensive humans, exhibit renal damage (glomerulosclerosis and renal fibrosis) as well as increased numbers of macrophages and T lymphocytes in the renal interstitital space.12 These correlative observations are supported by functional evidence demonstrating that immunotherapeutic modulation alters blood pressure in patients treated for other diseases. Treatment of HIV-positive men with highly active retroviral therapy, which led to increased CD4+ T lymphocytes in the blood, was associated with an increased prevalence of hypertension.26 Conversely, patients with psoriasis or rheumatoid arthritis who were treated with immunosuppressive agents demonstrated a reversible reduction in blood pressure in response to the immunotherapy.27 Lending further support to this concept, genetic association studies also suggest a role of the immune system in hypertension. Markers in the regions of several genes important in immune signaling (Sh2b3, CD247, and CD14) have been associated with hypertension or kidney disease in genome-wide association studies and other genetic association studies.28–32
These observations in human subjects document the importance of immunity in human hypertension. Parallel experimental studies, primarily performed in rats15,17–19,33–36 and mice,37–41 have employed numerous models of genetic or experimentally induced hypertension since the early 1960s to illustrate the mechanistic basis of immunity in hypertension and renal disease. These studies have been thoroughly reviewed elsewhere.14,16,18,20,37,42 The present review will describe the role of immune mechanisms in the development of SS hypertension and renal end-organ damage. The evidence presented has been largely obtained from work in experimental animals, but an emphasis has been placed, where possible, to reference supportive data obtained from human hypertension to provide a translational context to the experimental findings.
T CELLS IN SS HYPERTENSION
Immune mechanisms have been implicated in the development of hypertension in experimental models of SS hypertension.13–22 Our group has focused upon the mechanisms of hypertension and renal damage in the Dahl SS rat, a genetic model of salt-dependent hypertension and renal damage.13,18,43,44 Similar to humans with SS hypertension, the Dahl SS rat develops hypertension and end-organ damage when fed high salt.44–48 Moreover, SS humans develop albuminuria when compared with subjects with salt-resistant hypertension.49 This phenotype also closely parallels that of the Dahl SS rat which develops renal histological damage and albuminuria when fed a diet with high-salt content.13,43
The kidney of Dahl SS rats fed high salt has increased numbers of macrophages, T cells, and B cells which correlate with the degree of hypertension and renal damage when compared with Dahl SS maintained on a low-salt diet.13,43,44,50 In contrast, no changes are observed in infiltrating immune cells in the kidneys of rats that do not develop hypertension or renal damage when fed an elevated sodium diet.50 The infiltrating cells surround regions of damaged glomeruli and tubular structures in the kidney,13,43,44 indicating that they may play a role in the development of kidney damage. In support of these observations, SS hypertension and kidney damage also correlate with immune cell infiltration in the kidney in other experimental rodent models.15,36,51–53 The distribution of macrophages and T cells near damaged renal tubules and glomeruli in the SS rat is similar to that observed in hypertensive subjects.12 The potential role of individual cell types as mediators of damage in different tissue types is not known. To evaluate immune mechanisms in the development of SS hypertension, experiments demonstrated that treatment with immunosuppressive agents during a period of high NaCl intake prevented infiltration of T cells in the kidney and attenuated SS hypertension and renal damage in Dahl SS rats.43,44,54,55 Similar protective effects of immunosuppression were observed in other rodent models of hypertension including the spontaneously hypertensive rat, a genetic model exhibiting hypertension that is not sensitive to sodium intake, and rodents with experimental hypertension induced by treatment with nitric oxide inhibitors or angiotensin II,15,35,36,53,56–58 treatments which can induce sodium-sensitive hypertension.
To target individual immune cell types in experimental models of SS hypertension, genetic editing strategies59,60 were utilized in Dahl SS rats to generate null mutations in genes critical for the development of individual immune cells or in immune cell signaling.61–63 A null mutation in the Rag1 gene in Dahl SS (SSRag1−/−) resulted in a significant reduction in T and B cells in the circulation and spleen.62 Though differences in disease phenotypes were not observed in the SSRag1−/− when fed a low-salt diet, the development of hypertension and renal damage following high-salt intake was significantly blunted in those rats compared with Dahl SS controls with an intact lymphocyte population.62 Results of these experiments illustrate the importance of T and B cells in the full development of SS hypertension and related kidney damage.
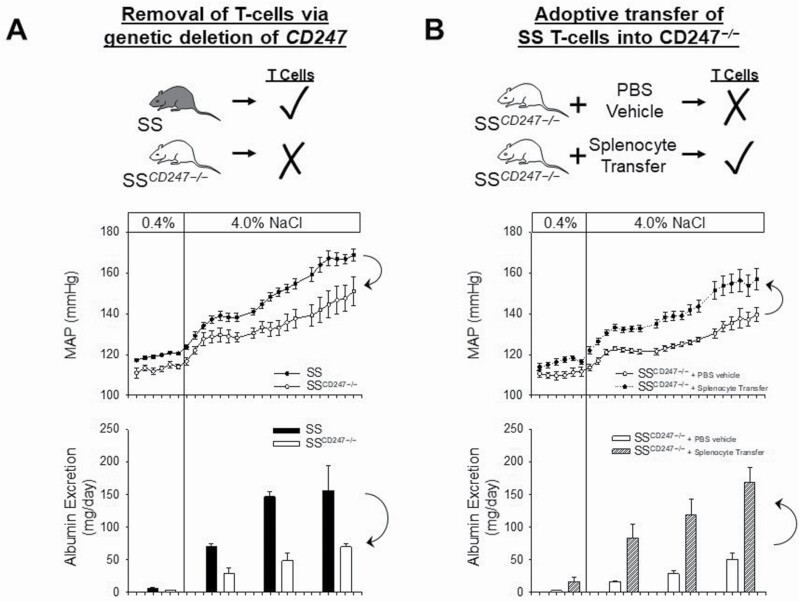
To specifically address the role of T cells in SS hypertension, Dahl SS lacking T cells were generated.61 The SSCD247−/− rats contain a null mutation in CD247, a gene which encodes the CD3 zeta chain.64–66 The SSCD247−/− rats demonstrate a >99% reduction in circulating T cells with no change in B cells compared with wild-type SS littermate controls.61 Similar to the SSRag1−/− which lacks T and B cells,62 no differences in arterial pressure or renal damage were observed between animals with and without T cells when fed low salt. After high-salt feeding, however, the severity of hypertension and renal damage was attenuated in the SSCD247−/− which lack T cells.61 To confirm the functional role of T cells in SS hypertension, a reconstitution study was recently performed in the SSCD247−/− rats.67 As depicted in Figure 1a, the genetic deletion of T cells in the Dahl SS background blunted SS hypertension and associated renal damage as assessed by urinary albumin excretion. The subsequent replacement of T cells (via splenocyte transfer) in the SSCD247−/− rats lacking endogenous T cells led to a recapitulation of the SS disease phenotype as both blood pressure and albuminuria returned to levels observed in the wild-type Dahl SS rats (Figure 1b). Not shown is the parallel attenuation of renal histological damage in SSCD247−/− rats and the recapitulation of albuminuria and kidney damage in SSCD247−/− that received a splenocyte transfer. These observations are in agreement with reports demonstrating the critical role of T cells for the full development of Ang II-mediated hypertension in mice.39 It is important to note, though, that recent data have demonstrated that the attenuation of Ang II hypertension in mice lacking T and B cells has not been universally observed.68 To complicate the issue, other recent studies have demonstrated an important role of B cells in Ang II-induced hypertension. Activation of B cells and increased IgG production is increased in Ang II-induced hypertension while B-cell deficiency blunted the hypertensive response to Ang II.69 It will be important to confirm the results observed in the SSRag1−/− and the SSCD247−/− rats in other laboratories under differing environmental conditions.
Figure 1.
Importance of T lymphocytes in the development of salt-sensitive (SS) hypertension and renal end-organ damage. (a) The development of SS hypertension and albuminuria (as a surrogate for renal damage) is attenuated in Dahl SS rats lacking T cells due to a null mutation in the CD247 gene (SSCD247−/−). (b) Adoptive transfer of splenocytes into SSCD247−/−, which increased CD4+ T cells in the circulation and kidney, was sufficient to restore hypertension and albuminuria in SSCD247−/−. Abbreviations: MAP, mean arterial pressure; PBS, phosphate buffered saline. Redrawn from Fehrenbach et al.67 with permission.
Adaptive immune mechanisms, likely mediated by T lymphocytes, therefore participate in the amplification of hypertension and kidney damage in Dahl SS rats.13,43,44,54,55,61–63 This conclusion is generally consistent with other experimental reports indicating that the infiltration or activation of immune cells in the kidney or other target organs participates in the development of hypertension and/or kidney disease.13,20,37,38,70–72 This conclusion is also consistent with reports from the human literature.12,23–27
MECHANISMS DRIVING IMMUNE CELL INFILTRATION AND ACTIVATION
The mechanisms leading to infiltration and activation of immune cells in target organs in SS hypertension are not well understood. Studies have implicated sympathetic nervous stimulation, elevations in renal perfusion pressure, exposure of antigens or neoantigens, and intake of sodium chloride or other dietary factors as activators or modulators of the immune response in hypertension.
Sympathetic nervous stimulation, which plays a significant role in human and experimental hypertension73,74 has been shown to activate immunity in hypertension.75 Signals from the central and sympathetic nervous system were demonstrated to be important for the expression of the early T-cell activation marker CD69 and the full induction of Ang II-induced hypertension in mice.76 It was further demonstrated that placental growth factor (PIGF) in the spleen was induced by increased sympathetic output in Ang II hypertension in mice.77 As splenic PIGF stimulates T-cell mobilization in target organs, PIGF may serve as a link connecting the sympathetic nervous system with the splenic immune system. More recently, studies have addressed the role of renal sympathetic nerves as mediators of inflammation in the kidney. Studies performed in deoxycorticosterone acetate (DOCA)-salt hypertensive rats demonstrated that renal denervation modulated inflammation, as assessed by alterations in cytokines, chemokines, and macrophage infiltration in the kidney.78 A similar set of studies was performed in Dahl SS fed high salt; no changes in the absolute number of infiltrating immune cells were observed with renal denervation in this model, but an assessment of cytokines and other immunomodulatory molecules was not performed.79 Interactions between the sympathetic nervous system and immunity appear to be important in hypertension, though the potential neuroimmune link remains to be fully explored and understood in the context of SS hypertension.
As described above, it was previously been observed that SS hypertension, renal damage, and infiltration of immune cells in the kidney of the Dahl SS occur in parallel.13,18 Interestingly, immunohistochemical studies indicated that infiltration of macrophages/monocytes into the kidney of SS rats as well as the renal histological damage is largely dependent upon an elevation of blood pressure.80 Using a servocontrol technique, experiments were thus undertaken to explore the role of elevated perfusion pressure to the kidney as the stimulus for immune cell infiltration in Dahl SS rats.81 Renal perfusion pressure to the left kidney was maintained constant during a 7-day period of high-salt intake through the use of an inflatable occluder placed around the aorta between the renal arteries. This preparation in conscious animals maintained perfusion pressure to the left kidney at control levels while the right kidney was exposed to the increased blood pressure resulting from increased salt intake. Interestingly, after 7 days of high-salt intake, the numbers of total T cells, B cells, monocytes, and macrophages were all lower in the left kidney than observed in the right kidney that experienced elevated perfusion and had increased renal histological damage. Though the mechanism is not clear, the effects of elevated perfusion pressure on renal damage and immune cell infiltration may be related to the transmission of elevated hydrostatic pressure to the glomerular capillaries with resultant tissue damage. Studies in hypertensive subjects82–84 and experimental animals83,85,86 indicate that renal vascular autoregulatory mechanisms are impaired in SS hypertension. When renal perfusion pressure is elevated, the transmission of elevated hydrostatic pressure to the renal vasculature may enhance the susceptibility to renal damage82,83 and lead to activation of immune mechanisms which amplify the disease process.
The elevated perfusion pressure and accompanying tissue damage likely lead to the release of chemokines, a family of small, secreted proteins. There are approximately 50 known chemokines that bind to around 20 chemokine receptors87,88; the primary chemokine function is to direct leukocyte trafficking to sites of injury. Tissue expression of multiple chemokines is elevated in clinical and experimental hypertension.89 The chemokines CCL2 and CCL5 as well as those of the CXC family have been implicated in hypertension. Interestingly, these cytokines have divergent effects on blood pressure and tissue injury. In experimental models of SS hypertension, CCR2, a receptor for CCL2, has been implicated as an important factor in the infiltration of immune cells into the kidney and in the development of hypertension and end-organ injury.90–92
Increased renal sympathetic nerve activation and elevated renal perfusion pressure may serve as primary stimuli leading to inflammation and immune activation, but classical cellular immune responses appear critical in this T-cell-dependent process. To activate T cells, the major histocompatibility complex on dendritic and other antigen presenting cells presents antigens to the T-cell receptor, and costimulation, involving CD28 on T cells and CD80 and CD86 (B7 ligands) on antigen presenting cells, is also required. In the kidney, dendritic cells are dispersed in the interstitial space93–95 to recognize foreign molecules. Patrolling monocytes, adherent to the glomerular microvasculature, may also participate in antigen presentation and subsequent inflammation in the kidney.96–98 Inhibition of the B7–CD28 interaction was shown to block T-cell activation, cytokine production, and migration and attenuated Ang II-dependent and DOCA-salt hypertension.99 In SS hypertension, deletion of the CD3 zeta chain of the T-cell receptor attenuated Dahl SS hypertension and related renal damage.61 The activation of the T-cell receptor and the B7/CD28 interaction is therefore a critical step mediating immune responses in hypertension.
The identity of the antigens or neoantigens that trigger immune responses in hypertension is unclear but remains a subject of intense study. Experimental data have suggested that HSP70 may serve as a common antigen triggering immune reactions in the kidney of SS animals and humans.71,100 Another potential antigenic signal may arise from antigenic peptides generated by the proteolytic modification of albumin by proximal tubules.101 Since albumin is filtered in damaged glomeruli, increased delivery of albumin to proximal tubule cells could serve as a trigger leading to an adaptive immune response localized in the kidney. A potential neoantigen, which was demonstrated in Ang II and DOCA-salt mouse models, is the formation of isoketal adducts via lipid peroxidation in dendritic cells.102 The isoketal adducts may serve as neoantigens to activate dendritic cells since scavenging of isoketals prevented the immunoreactivity associated with Ang II-induced hypertension and renal damage.102 Interestingly, adoptive transfer of activated dendritic cells from Ang II-treated animals induced T-cell activation, survival, proliferation and cytokine production, and promoted hypertension.102 These studies demonstrate that a number of different antigens and neoantigens may provide the stimulus mediating localized inflammatory responses in the kidney and other target organs in hypertension.
INFLUENCE OF DIET AND ENVIRONMENTAL FACTORS ON IMMUNITY IN SS HYPERTENSION
Recent interesting findings have indicated that high-salt intake or alterations in other dietary factors can also alter immune cell activation. It was demonstrated that elevated sodium concentration induced TH type 17 (TH17) cell polarization from naïve mouse or human T cells in a process dependent upon serum and glucocorticoid-inducible kinase-1.103,104 Additional studies demonstrated that high-salt feeding alters the gut microbiome and modulates the induction of TH17 cells in mice and humans.105 As described below, Interleukin (IL)-17, released from TH17 cells, has prohypertensive effects. Increased skin sodium content, which has been demonstrated to occur in response to a high-salt diet,106 has been reported to activate macrophages.107 Evidence indicates that large amounts of sodium can be stored in the skin.106 Further studies suggest that macrophages in the skin, in response to osmotic stress associated with elevated sodium, release vascular endothelial growth factor C by a TonEBP/NFAT5-dependent mechanism which lead to hyperplasia of the cutaneous lymph capillary network. The macrophages thus serve a homeostatic mechanism for skin storage, by mechanisms which remain to be elucidated.
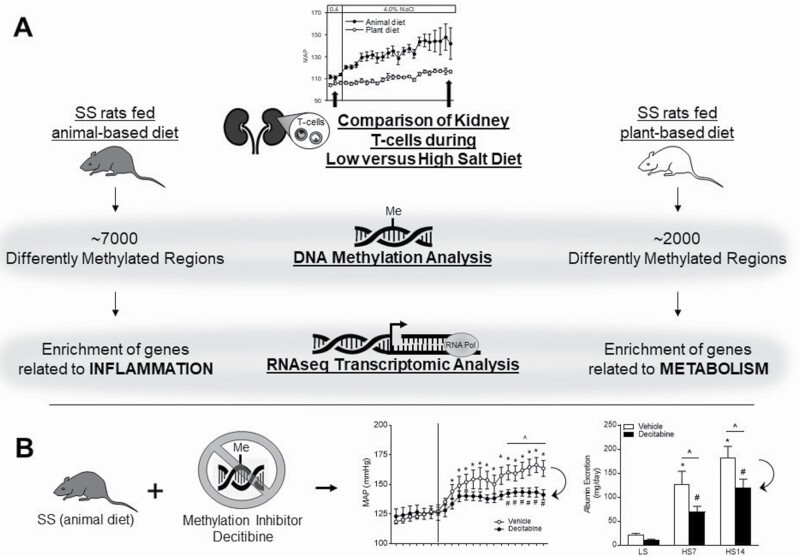
A more recent report from our group indicated that non-sodium components of the diet can also influence immune function with consequent effects on the hypertensive phenotype. A transcriptomic analysis of T cells isolated from the kidney demonstrated a shift in expression from genes related to inflammation in the Dahl SS rats fed a prohypertensive, animal-based diet to genes related to metabolism in SS fed a protective, grain-based diet which was associated with a diminished degree of SS hypertension and renal damage (Figure 2a).108 Further studies then investigated potential mechanisms leading to the transcriptomic effects of environment/diet on T-cell gene expression by examining the epigenetic profile of T cells isolated from the periphery and the kidney.109 In response to a high-salt challenge, the methylome of T cells isolated from the kidney of SS rats fed the prohypertensive diet exhibited a significant increase in differentially methylated regions with a preference for hypermethylation compared with the T cells isolated from the kidneys of rats fed the protective, grain diet. Furthermore, by utilizing transcriptomic data from T cells isolated from the same animals upon which the DNA methylation analysis was performed, a predominant negative correlation was observed between gene expression and DNA methylation. Finally, the inhibition of DNA methyltransferases blunted salt-induced hypertension and renal damage in the SS rats fed the prohypertensive diet, providing a functional role for DNA methylation (Figure 2b). These studies demonstrated the influence of environment- and diet-induced epigenetic modifications to alter immune cell function and ultimately disease severity.
Figure 2.
Influence of non-sodium dietary factors on immune responses in salt-sensitive (SS) hypertension. (a) A transcriptomic analysis of T cells isolated from the kidney demonstrated a shift in expression from genes related to inflammation in the Dahl SS rats fed a prohypertensive, animal-based diet to genes related to metabolism in Dahl SS fed a protective, grain-based diet which was associated with a diminished degree of SS hypertension and renal damage. In response to a high-salt challenge, the methylome of T cells isolated from the kidney of SS rats fed the prohypertensive diet exhibited a significant increase in differentially methylated regions with a preference for hypermethylation compared with the T cells isolated from the kidneys of rats fed the protective, grain diet. (b) The inhibition of DNA methyltransferases blunted salt-induced hypertension and renal damage in the SS rats fed the prohypertensive diet. *P < 0.05 vs. vehicle low salt (LS); #P < 0.05 vs. decitabine LS; ^P < 0.05 vs. vehicle. HS indicates high salt. Redrawn from Abais-Battad et al.108 and Dasinger et al.109 with permission.
To place these animal studies into a translational context, a longitudinal examination of healthy subjects indicated that a long-term reduction in sodium intake was associated with a decrease in total numbers of peripheral monocytes.110 The level of IL-6 and IL-23 also decreased with reduced salt intake, whereas levels of IL-10 increased.110 Together, the results of these studies in animals and humans indicate that changes in sodium intake as well as alterations in non-sodium components of the diet may have profound effects on the microbiome, DNA methylation, gene expression, the activation of immune cells, the production of cytokines and other factors, and the development of SS hypertension and related disease phenotypes.
DOWNSTREAM EFFECTS OF IMMUNE CELL ACTIVATION
Immune cells in the kidney contribute to elevated levels of arterial blood pressure and kidney damage by a variety of mechanisms including the production of cytokines,40,41,61,111–116 free radicals,15,36,39,54,102 and other substances.15,35,43,57,117 Studies in animal models have demonstrated the importance of many of these factors including IL-1β,118–120 IL-6,114,121–123 IL-17,40,41,99,112,124,125 interferon (IFN)-γ,40,41,126 tumor necrosis factor (TNF),127,128 and free radicals15,39,54,102 in the development of hypertension.
A number of cytokines have been demonstrated to influence tubular sodium transport. As an example, the cytokine IL-17A which is produced by TH17 cells increased sodium transporter expression in human proximal and mouse distal tubular cells111 and mice lacking IL-17A demonstrate blunted Ang II-induced hypertension.113 Mice lacking IFN-γ also exhibit blunted Ang II-dependent hypertension accompanied by a reduction in the phosphorylated forms of the Na–Cl cotransporter and the Na–K–2Cl cotransporter during Ang II administration, indicating a role of IFN-γ in stimulation of epithelial sodium transport.113 Moreover, kidney levels of IL-1 are increased in Ang II-induced hypertension129 and pharmacological blockade or genetic deletion of the IL-1 receptor (IL-1R1) mitigated increased epithelial sodium reabsorption by the Na–K–2Cl transporter and attenuated Ang II-induced elevation of blood pressure in mice.120
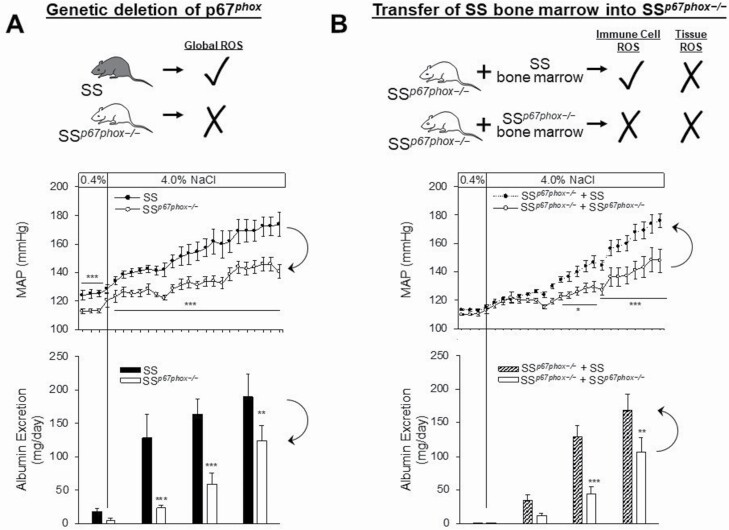
Other factors released by immune cells may contribute to altered vascular resistance. For example immune cells in the kidney can serve as a source of reactive oxygen species (ROS),15,36,39,54,102 which can have profound effects that result in an increase in vascular resistance and the development of hypertension.130,131 The importance of ROS production in the pathogenesis of Dahl SS hypertension and renal damage has been well recognized.132,133 To assess the role of ROS in immune cells, Dahl SS rats lacking the p67phox subunit of NADPH Oxidase 2 (NOX2),134 which demonstrate a blunted SS hypertension and renal damage (Figure 3a), underwent total body irradiation and received bone marrow transfer from either wild-type rats or rats lacking p67phox.135 This experiment was therefore able to specifically address the role of ROS released from NOX2 in cells of hematopoietic origin in SS hypertension. Interestingly, after a 3-week high-salt challenge, there was an exacerbated increase in mean arterial pressure in rats which received the bone marrow transfer from rats with intact p67phox while the rats receiving bone marrow from rats lacking p67phox had a blunted SS hypertension and renal damage response to high-salt intake (Figure 3b). These data altogether demonstrate that immune cell production of NOX2-derived ROS is sufficient to exacerbate SS hypertension, renal damage, and renal inflammation.
Figure 3.
Importance of free radical production by immune cells in the development of salt-sensitive (SS) hypertension and renal end-organ damage. (a) The development of SS hypertension and albuminuria (as a surrogate for renal damage) is attenuated in Dahl SS rats with a whole body null mutation in the p67phox subunit of NOX2 (SSp67phox−/−). (b) Total body irradiation of SSp67phox−/− followed by bone marrow transfer from SS or SSp67phox−/− demonstrated that intact p67phox (and thus active NOX2) in cells of hematopoietic origin is sufficient to restore the full SS disease phenotype to SS rats lacking p67phox in parenchymal cells. *P < 0.05, **P < 0.005, ***P < 0.001 vs. SS. Redrawn from Abais-Battad et al.135 with permission.
PERSPECTIVES AND CONCLUSIONS
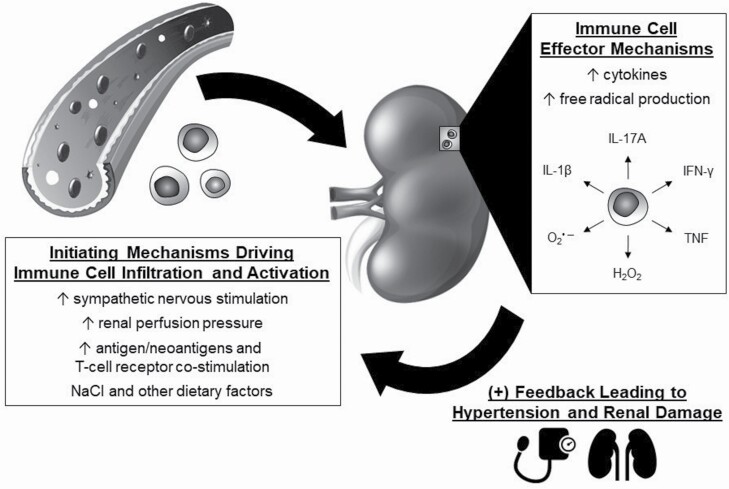
A hypothesized scheme whereby immune mechanisms participate in SS hypertension is illustrated in Figure 4. Observations made in Dahl SS rats genetically edited to be deficient in T cells61 or both T cells and B cells62 demonstrate an initial phase of SS hypertension and kidney damage that is largely immune-independent. We speculate that this initial phase, which occurs in response to elevated salt intake, is mediated by numerous mechanisms including aberrant regulation of hormonal, neural, paracrine, or autocrine mechanisms.13,18 These changes lead to increased vascular resistance in the kidney or systemic circulation and/or enhanced renal tubular sodium reabsorption.13,18 This increase in blood pressure results in elevated renal perfusion pressure that is transmitted to the kidney, an effect that is pronounced when renal vascular autoregulatory mechanisms are impaired as observed in SS hypertension.82–86 As described above, other initiating mechanisms include increased sympathetic nerve activity,75–79 antigens and neoantigens,71,100–102 and environmental modifiers of immune cell activation.103–105,107–109
Figure 4.
Hypothesized role of immune mechanisms in the development of salt-sensitive hypertension. Elevations in sympathetic nerve stimulation, elevated renal perfusion pressure, increased antigens or neoantigens, and environmental factors have all been demonstrated to activate immune cells in hypertension. The immune cells infiltrate target organs, including the kidney, and release free radicals, cytokines, and other molecules that mediate tissue damage and alter physiological function. As a result, the effector actions lead to the further development of hypertension and tissue damage.
We further hypothesize, based upon data obtained in Dahl SS rats,80,81 that the transmission of elevated blood pressure to the renal vasculature results in kidney damage sufficient to trigger an inappropriate immune response. The details of these mechanisms, including the site within the kidney, the signal transduction mechanisms, and the molecular machinery that is engaged are unclear, but it is likely that this insult triggers an innate and adaptive immune response. This immune response results in the migration of innate and adaptive immune cells in the regions surrounding damaged renal tubules and blood vessels. The newly infiltrating cells then release cytokines, free radicals, and/or other molecules that amplify the development of hypertension by increasing sodium reabsorption and constricting the vasculature while also mediating further tissue damage.
Though much work has advanced this field, many questions remain to be addressed. Broadly speaking, the mechanisms transducing activation of innate and adaptive immune mechanisms in hypertension and end-organ damage are unclear. The role of multiple immune cell types and their effector molecules to alter blood pressure and end-organ damage has been described, but the influence of specific immune cell types on different tissue compartments remains to be determined. Similarly, progress has been made to identify antigens triggering adaptive immune responses, but work remains to identify those antigens in experimental animals and in humans. Recent work has demonstrated that the influence of environmental factors (i.e., dietary intake, alterations in the microbiome, etc.) on immune activation are quite profound but remain little understood. Though these challenges remain, this field provides great potential for the development of improved therapy for this life-threatening disease.
To summarize, substantial evidence from humans and animals has demonstrated the importance of immune mechanisms in hypertension. Evidence presented in this review provides support for the view that immunity and inflammation serve to amplify a primary elevation of blood pressure and lead to malignant disease accompanied by end-organ damage. Subjects with hypertension, particularly those with SS hypertension, also show an associated increase in renal end-organ damage. Moreover, renal damage in the setting of hypertension, in both humans and experimental animals, is accompanied by an increased number of macrophages and T cells in the kidney. The increased number of immune cells is primarily adjacent to sites of tissue damage and the degree of infiltration correlates with the severity of tissue damage. Moreover, the effects of pharmacological treatment in patients with hypertension and correlative genetic data support a role for immune mechanisms as a causative factor in human hypertensive disease.
FUNDING
The authors’ work is supported by National Institutes of Health grants HL137748, HL116264, HL143832, American Heart Association grant 19CDA34660184 and the Georgia Research Alliance.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223. [DOI] [PubMed] [Google Scholar]
- 2. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet 2015; 386:801–812. [DOI] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018; 137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 5. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 1997; 350:1734–1737. [DOI] [PubMed] [Google Scholar]
- 7. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001; 37:429–432. [DOI] [PubMed] [Google Scholar]
- 8. Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL; American Heart Association Professional and Public Education Committee of the Council on Hypertension; Council on Functional Genomics and Translational Biology; and Stroke Council . Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension 2016; 68:e7–e46. [DOI] [PubMed] [Google Scholar]
- 9. Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 1986; 8:II127–II134. [DOI] [PubMed] [Google Scholar]
- 10. Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med 1978; 64:193–198. [DOI] [PubMed] [Google Scholar]
- 11. Kotchen TA, Cowley AW Jr, Frohlich ED. Salt in health and disease—a delicate balance. N Engl J Med 2013; 368:1229–1237. [DOI] [PubMed] [Google Scholar]
- 12. Hughson MD, Gobe GC, Hoy WE, Manning RD Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and Whites. Am J Kidney Dis 2008; 52:18–28. [DOI] [PubMed] [Google Scholar]
- 13. Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 2014; 307:F499–F508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caillon A, Paradis P, Schiffrin EL. Role of immune cells in hypertension. Br J Pharmacol 2019; 176:1818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodríguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 2004; 286:F606–F616. [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez-Iturbe B, Pons H, Johnson RJ. Role of the immune system in hypertension. Physiol Rev 2017; 97:1127–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson RJ, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Feig DI, Herrera-Acosta J. Subtle renal injury is likely a common mechanism for salt-sensitive essential hypertension. Hypertension 2005; 45:326–330. [DOI] [PubMed] [Google Scholar]
- 18. Mattson DL. Immune mechanisms of salt-sensitive hypertension and renal end-organ damage. Nat Rev Nephrol 2019; 15:290–300. [DOI] [PubMed] [Google Scholar]
- 19. Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodríguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med 2002; 346:913–923. [DOI] [PubMed] [Google Scholar]
- 20. Schiffrin EL. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens 2010; 19:181–186. [DOI] [PubMed] [Google Scholar]
- 21. Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int 2005; 68:2180–2188. [DOI] [PubMed] [Google Scholar]
- 22. Rodríguez-Iturbe B, Pons H, Quiroz Y, Lanaspa MA, Johnson RJ. Autoimmunity in the pathogenesis of hypertension. Nat Rev Nephrol 2014; 10:56–62. [DOI] [PubMed] [Google Scholar]
- 23. Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol 1958; 34:685–715. [PMC free article] [PubMed] [Google Scholar]
- 24. Olsen F. Inflammatory cellular reaction in hypertensive vascular disease in man. Acta Pathol Microbiol Scand A 1972; 80:253–256. [PubMed] [Google Scholar]
- 25. Paronetto F. Immunocytochemical observations on the vascular necrosis and renal glomerular lesions of malignant nephrosclerosis. Am J Pathol 1965; 46:901–915. [PMC free article] [PubMed] [Google Scholar]
- 26. Seaberg EC, Muñoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP; Multicenter AIDS Cohort Study . Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 2005; 19:953–960. [DOI] [PubMed] [Google Scholar]
- 27. Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 2006; 17:S218–S225. [DOI] [PubMed] [Google Scholar]
- 28. Ehret GB, O’Connor AA, Weder A, Cooper RS, Chakravarti A. Follow-up of a major linkage peak on chromosome 1 reveals suggestive QTLs associated with essential hypertension: GenNet study. Eur J Hum Genet 2009; 17:1650–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fox ER, Young JH, Li Y, Dreisbach AW, Keating BJ, Musani SK, Liu K, Morrison AC, Ganesh S, Kutlar A, Ramachandran VS, Polak JF, Fabsitz RR, Dries DL, Farlow DN, Redline S, Adeyemo A, Hirschorn JN, Sun YV, Wyatt SB, Penman AD, Palmas W, Rotter JI, Townsend RR, Doumatey AP, Tayo BO, Mosley TH Jr, Lyon HN, Kang SJ, Rotimi CN, Cooper RS, Franceschini N, Curb JD, Martin LW, Eaton CB, Kardia SL, Taylor HA, Caulfield MJ, Ehret GB, Johnson T, Chakravarti A, Zhu X, Levy D; International Consortium for Blood Pressure Genome-wide Association Studies (ICBP-GWAS) . Association of genetic variation with systolic and diastolic blood pressure among African Americans: the Candidate Gene Association Resource study. Hum Mol Genet 2011; 20:2273–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009; 41:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shinzawa M, Yamamoto R, Nagasawa Y, Shoji T, Obi Y, Namba T, Kitamura H, Kaneko T, Okada N, Iwatani H, Yamauchi A, Tsubakihara Y, Imai E, Isaka Y, Rakugi H. Gene polymorphisms contributing to hypertension in immunoglobulin A nephropathy. Clin Exp Nephrol 2012; 16:250–258. [DOI] [PubMed] [Google Scholar]
- 32. Poesen R, Ramezani A, Claes K, Augustijns P, Kuypers D, Barrows IR, Muralidharan J, Evenepoel P, Meijers B, Raj DS. Associations of soluble CD14 and endotoxin with mortality, cardiovascular disease, and progression of kidney disease among patients with CKD. Clin J Am Soc Nephrol 2015; 10:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodríguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol 2007; 293:F616–F623. [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez-Iturbe B, Johnson RJ. Role of inflammatory cells in the kidney in the induction and maintenance of hypertension. Nephrol Dial Transplant 2006; 21:260–263. [DOI] [PubMed] [Google Scholar]
- 35. Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 2001; 59:2222–2232. [DOI] [PubMed] [Google Scholar]
- 36. Rodríguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chávez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 2002; 282:F191–F201. [DOI] [PubMed] [Google Scholar]
- 37. Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension 2011; 57:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Madhur MS, Harrison DG. Synapses, signals, CDs, and cytokines: interactions of the autonomic nervous system and immunity in hypertension. Circ Res 2012; 111:1113–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007; 204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 2016; 68:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE, Harrison DG. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ Res 2016; 118:1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med 2018; 215:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Miguel C, Das S, Lund H, Mattson DL. T-lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol 2010; 298:R1136–R1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 2006; 48:149–156. [DOI] [PubMed] [Google Scholar]
- 45. Cowley AW Jr, Roman RJ. The role of the kidney in hypertension. J Am Med Assoc 1996; 275:1581–1589. [PubMed] [Google Scholar]
- 46. Feldman HI, Klag MJ, Chiapella AP, Whelton PK. End-stage renal disease in US minority groups. Am J Kidney Dis 1992; 19:397–410. [DOI] [PubMed] [Google Scholar]
- 47. Grim CE, Wilson TW, Nicholson GD, Hassell TA, Fraser HS, Grim CM, Wilson DM. Blood pressure in blacks. Hypertension 1990; 15:803–809. [DOI] [PubMed] [Google Scholar]
- 48. Lackland DT, Keil JE. Epidemiology of hypertension in African Americans. Semin Nephrol 1996; 16:63–70. [PubMed] [Google Scholar]
- 49. Bigazzi R, Bianchi S, Baldari D, Sgherri G, Baldari G, Campese VM. Microalbuminuria in salt-sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension 1994; 23:195–199. [DOI] [PubMed] [Google Scholar]
- 50. Fehrenbach DJ, Abais-Battad JM, Dasinger JH, Lund H, Mattson DL. Salt-sensitive increase in macrophages in the kidneys of Dahl SS rats. Am J Physiol Renal Physiol 2019; 317:F361–F374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol 2007; 292:F330–F339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mai M, Geiger H, Hilgers KF, Veelken R, Mann JF, Dämmrich J, Luft FC. Early interstitial changes in hypertension-induced renal injury. Hypertension 1993; 22:754–765. [DOI] [PubMed] [Google Scholar]
- 53. Pechman KR, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 2008; 294:R1234–R1239. [DOI] [PubMed] [Google Scholar]
- 54. De Miguel C, Lund H, Di F, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and lead to hypertension and renal disease. Am J Physiol 2011; 300:F734–F742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 2011; 57:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alvarez V, Quiroz Y, Nava M, Pons H, Rodríguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol Renal Physiol 2002; 283:F1132–F1141. [DOI] [PubMed] [Google Scholar]
- 57. Quiroz Y, Pons H, Gordon KL, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ, Rodríguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthase inhibition. Am J Physiol 2001; 281:F38–F47. [DOI] [PubMed] [Google Scholar]
- 58. Bendich A, Belisle EH, Strausser HR. Immune system modulation and its effect on the blood pressure of the spontaneously hypertensive male and female rat. Biochem Biophys Res Commun 1981; 99:600–607. [DOI] [PubMed] [Google Scholar]
- 59. Geurts AM, Cost GJ, Rémy S, Cui X, Tesson L, Usal C, Ménoret S, Jacob HJ, Anegon I, Buelow R. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol Biol 2010; 597:211–225. [DOI] [PubMed] [Google Scholar]
- 60. Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Ménoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 2009; 325:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL; PhysGen Knockout Program . CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 2014; 63:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt sensitive rats attenuates hypertension and renal damage. Am J Physiol 2013; 304:R407–R414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rudemiller NP, Lund H, Priestley JR, Endres BT, Prokop JW, Jacob HJ, Geurts AM, Cohen EP, Mattson DL. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension 2015; 65:1111–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Irving BA, Chan AC, Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor zeta chain. J Exp Med 1993; 177:1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Itoh Y, Matsuura A, Kinebuchi M, Honda R, Takayama S, Ichimiya S, Kon S, Kikuchi K. Structural analysis of the CD3 zeta/eta locus of the rat. Expression of zeta but not eta transcripts by rat T cells. J Immunol 1993; 151:4705–4717. [PubMed] [Google Scholar]
- 66. Sussman JJ, Bonifacino JS, Lippincott-Schwartz J, Weissman AM, Saito T, Klausner RD, Ashwell JD. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell 1988; 52:85–95. [DOI] [PubMed] [Google Scholar]
- 67. Fehrenbach DJ, Dasinger JH, Lund H, Zemaj J, Mattson DL. Splenocyte transfer exacerbates salt-sensitive hypertension in rats. Exp Physiol 2020; 105:864–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Seniuk A, Thiele JL, Stubbe A, Oser P, Rosendahl A, Bode M, Meyer-Schwesinger C, Wenzel UO, Ehmke H. B6.Rag1 knockout mice generated at the Jackson Laboratory in 2009 show a robust wild-type hypertensive phenotype in response to Ang II (Angiotensin II). Hypertension 2020; 75:1110–1116. [DOI] [PubMed] [Google Scholar]
- 69. Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, Kim HA, Krishnan SM, Lewis CV, Salimova E, Tipping P, Vinh A, Samuel CS, Peter K, Guzik TJ, Kyaw TS, Toh BH, Bobik A, Drummond GR. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 2015; 66:1023–1033. [DOI] [PubMed] [Google Scholar]
- 70. Rodriguez-Iturbe B. Renal infiltration of immunocompetent cells: cause and effect of sodium-sensitive hypertension. Clin Exp Nephrol 2010; 14:105–111. [DOI] [PubMed] [Google Scholar]
- 71. Rodriguez-Iturbe B. Autoimmunity in the pathogenesis of hypertension. Hypertension 2016; 67:477–483. [DOI] [PubMed] [Google Scholar]
- 72. Ryan MJ. An update on immune system activation in the pathogenesis of hypertension. Hypertension 2013; 62:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 2006; 7:335–346. [DOI] [PubMed] [Google Scholar]
- 74. Maranon RO, Lima R, Spradley FT, do Carmo JM, Zhang H, Smith AD, Bui E, Thomas RL, Moulana M, Hall JE, Granger JP, Reckelhoff JF. Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol 2015; 308:R708–R713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med 2012; 209:1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res 2010; 107:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M, Cifelli G, De Lucia M, Lembo G. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity 2014; 41:737–752. [DOI] [PubMed] [Google Scholar]
- 78. Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 2016; 68:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Alsheikh AJ, Lund H, Dasinger JH, Abais-Battad JM, Fehrenbach DJ, Mattson DL. Renal nerves and leukocyte infiltration in the kidney during salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 2019; 317:R182–R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW Jr. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol 2008; 19:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased perfusion pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension 2017; 70:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 2009; 54:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 2014; 12:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Campese VM, Parise M, Karubian F, Bigazzi R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension 1991; 18:805–812. [DOI] [PubMed] [Google Scholar]
- 85. Karlsen FM, Andersen CB, Leyssac PP, Holstein-Rathlou NH. Dynamic autoregulation and renal injury in Dahl rats. Hypertension 1997; 30:975–983. [DOI] [PubMed] [Google Scholar]
- 86. Takenaka T, Forster H, De Micheli A, Epstein M. Impaired myogenic responsiveness of renal microvessels in Dahl salt-sensitive rats. Circ Res 1992; 71:471–480. [DOI] [PubMed] [Google Scholar]
- 87. Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J 2018; 285:2944–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Roy I, Evans DB, Dwinell MB. Chemokines and chemokine receptors: update on utility and challenges for the clinician. Surgery 2014; 155:961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rudemiller NP, Crowley SD. The role of chemokines in hypertension and consequent target organ damage. Pharmacol Res 2017; 119:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD. Chemokine receptor 2b inhibition provides renal protection in angiotensin II—salt hypertension. Hypertension 2007; 50:1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chan CT, Moore JP, Budzyn K, Guida E, Diep H, Vinh A, Jones ES, Widdop RE, Armitage JA, Sakkal S, Ricardo SD, Sobey CG, Drummond GR. Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension 2012; 60:1207–1212. [DOI] [PubMed] [Google Scholar]
- 92. Alsheikh AJ, Dasinger JH, Abais-Battad JM, Fehrenbach DJ, Yang C, Cowley AW Jr, Mattson DL. CCL2 mediates early renal leukocyte infiltration during salt-sensitive hypertension. Am J Physiol Renal Physiol 2020; 318:F982–F993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 2006; 70:591–596. [DOI] [PubMed] [Google Scholar]
- 94. Woltman AM, de Fijter JW, Zuidwijk K, Vlug AG, Bajema IM, van der Kooij SW, van Ham V, van Kooten C. Quantification of dendritic cell subsets in human renal tissue under normal and pathological conditions. Kidney Int 2007; 71:1001–1008. [DOI] [PubMed] [Google Scholar]
- 95. John R, Nelson PJ. Dendritic cells in the kidney. J Am Soc Nephrol 2007; 18:2628–2635. [DOI] [PubMed] [Google Scholar]
- 96. Westhorpe CLV, Norman MU, Hall P, Snelgrove SL, Finsterbusch M, Li A, Lo C, Tan ZH, Li S, Nilsson SK, Kitching AR, Hickey MJ. Effector CD4+ T cells recognize intravascular antigen presented by patrolling monocytes. Nat Commun 2018; 9:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Devi S, Li A, Westhorpe CL, Lo CY, Abeynaike LD, Snelgrove SL, Hall P, Ooi JD, Sobey CG, Kitching AR, Hickey MJ. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med 2013; 19:107–112. [DOI] [PubMed] [Google Scholar]
- 98. Finsterbusch M, Hall P, Li A, Devi S, Westhorpe CL, Kitching AR, Hickey MJ. Patrolling monocytes promote intravascular neutrophil activation and glomerular injury in the acutely inflamed glomerulus. Proc Natl Acad Sci USA 2016; 113:E5172–E5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 2010; 122:2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pons H, Ferrebuz A, Quiroz Y, Romero-Vasquez F, Parra G, Johnson RJ, Rodriguez-Iturbe B. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am J Physiol Renal Physiol 2013; 304:F289–F299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Macconi D, Chiabrando C, Schiarea S, Aiello S, Cassis L, Gagliardini E, Noris M, Buelli S, Zoja C, Corna D, Mele C, Fanelli R, Remuzzi G, Benigni A. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J Am Soc Nephrol 2009; 20:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J 2nd, Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 2014; 124:4642–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013; 496:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013; 496:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Frätzer C, Krannich A, Gollasch M, Grohme DA, Côrte-Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ, Müller DN. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017; 551:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Titze J. Sodium balance is not just a renal affair. Curr Opin Nephrol Hypertens 2014; 23:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jantsch J, Schatz V, Friedrich D, Schröder A, Kopp C, Siegert I, Maronna A, Wendelborn D, Linz P, Binger KJ, Gebhardt M, Heinig M, Neubert P, Fischer F, Teufel S, David JP, Neufert C, Cavallaro A, Rakova N, Küper C, Beck FX, Neuhofer W, Muller DN, Schuler G, Uder M, Bogdan C, Luft FC, Titze J. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab 2015; 21:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Abais-Battad JM, Alsheikh AJ, Pan X, Fehrenbach DJ, Dasinger JH, Lund H, Roberts M, Kriegel AJ, Cowley AW, Kidambi S, Kotchen TA, Liu P, Liang M, Mattson DL. Transcriptomic analysis in renal T lymphocytes exposes sodium-independent dietary differences in Dahl SS rats. Hypertension 2019; 44:854–863. [Google Scholar]
- 109. Dasinger JH, Alsheikh AJ, Abais-Battad JM, Pan X, Fehrenbach DJ, Lund H, Roberts ML, Cowley AW Jr, Kidambi S, Kotchen TA, Liu P, Liang M, Mattson DL. Epigenetic modifications in T cells: the role of DNA methylation in salt-sensitive hypertension. Hypertension 2020; 75:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yi B, Titze J, Rykova M, Feuerecker M, Vassilieva G, Nichiporuk I, Schelling G, Morukov B, Choukèr A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res 2015; 166:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, Dale BL, Iwakura Y, Hoover RS, McDonough AA, Madhur MS. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension 2016; 68:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010; 55:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ −/− and interleukin-17A−/− mice. Hypertension 2015; 65:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. O’Leary, R., Penrose, H., Miyata, K. & Satou, R. Macrophage-derived IL-6 contributes to AngII-mediated angiotensinogen stimulation in renal proximal tubular cells. Am J Physiol 2016; 310:F1000–F1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wade B, Petrova G, Mattson DL. Role of immune factors in angiotensin II-induced hypertension and renal damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 2018; 314:R323–R333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Norlander AE, Madhur MS. Inflammatory cytokines regulate renal sodium transporters: how, where, and why? Am J Physiol Renal Physiol 2017; 313:F141–F144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Franco M, Martínez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodríguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 2007; 293:R251–R256. [DOI] [PubMed] [Google Scholar]
- 118. Southcombe JH, Redman CW, Sargent IL, Granne I. Interleukin-1 family cytokines and their regulatory proteins in normal pregnancy and pre-eclampsia. Clin Exp Immunol 2015; 181:480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Qi J, Zhao XF, Yu XJ, Yi QY, Shi XL, Tan H, Fan XY, Gao HL, Yue LY, Feng ZP, Kang YM. Targeting interleukin-1 beta to suppress sympathoexcitation in hypothalamic paraventricular nucleus in Dahl salt-sensitive hypertensive rats. Cardiovasc Toxicol 2016; 16:298–306. [DOI] [PubMed] [Google Scholar]
- 120. Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD, Crowley SD. Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin II-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab 2016; 23:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Crosswhite P, Sun Z. Ribonucleic acid interference knockdown of interleukin 6 attenuates cold-induced hypertension. Hypertension 2010; 55:1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lee DL, Sturgis LC, Labazi H, Osborne JB Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 2006; 290:H935–H940. [DOI] [PubMed] [Google Scholar]
- 123. Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 2016; 311:F555–F561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Norlander AE, Saleh MA, Pandey AK, Itani HA, Wu J, Xiao L, Kang J, Dale BL, Goleva SB, Laroumanie F, Du L, Harrison DG, Madhur MS. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight 2017; 2:e92801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Saleh MA, Norlander AE, Madhur MS. Inhibition of interleukin 17-A but not interleukin-17F signaling lowers blood pressure and reduces end-organ inflammation in angiotensin II-induced hypertension. JACC Basic Transl Sci 2016; 1:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sun XN, Li C, Liu Y, Du LJ, Zeng MR, Zheng XJ, Zhang WC, Liu Y, Zhu M, Kong D, Zhou L, Lu L, Shen ZX, Yi Y, Du L, Qin M, Liu X, Hua Z, Sun S, Yin H, Zhou B, Yu Y, Zhang Z, Duan SZ. T-cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ Res 2017; 120:1584–1597. [DOI] [PubMed] [Google Scholar]
- 127. Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension 2010; 56:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, Crowley SD. Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension 2014; 64:1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Crowley SD, Song YS, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux PL, Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension 2010; 55:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cowley AW Jr, Abe M, Mori T, O’Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Renal Physiol 2015; 308:F179–F197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Imig JD, Ryan MJ. Immune and inflammatory role in renal disease. Compr Physiol 2013; 3:957–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Makino A, Skelton MM, Zou AP, Cowley AW Jr. Increased renal medullary H2O2 leads to hypertension. Hypertension 2003; 42:25–30. [DOI] [PubMed] [Google Scholar]
- 133. Taylor NE, Cowley AW Jr. Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 2005; 289:R1573–R1579. [DOI] [PubMed] [Google Scholar]
- 134. Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O’Connor PM, Cowley AW Jr. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 2012; 15:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Abais-Battad JM, Lund H, Dasinger JH, Fehrenbach DJ, Cowley AW Jr, Mattson DL. NOX2-derived reactive oxygen species in immune cells exacerbates salt-sensitive hypertension. Free Radic Biol Med 2020; 146:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]