Abstract
Background
It was recently demonstrated that the detection of atrial fibrillation based on heart rate tracking by optical sensors is feasible and reliable using the Apple Watch and the corresponding application. There are already a number of smartwatches and other wearable devices alongside the Apple Watch that can additionally record a single-lead electrocardiogram (ECG) and it is reasonable to expect this technology to become a standard feature, as is already the case with automated heart rate tracking. This could potentially have enormous impact regarding the early diagnosis of several cardiac diseases.
Case summary
A 61-year-old male patient without previously known coronary artery disease was admitted with subacute ST-elevation myocardial infarction (STEMI) caused by occlusion of left anterior descending artery. Due to mildness of symptoms, the patient did only seek medical attention due to morphological changes in the single-lead ECG tracing acquired on his Apple Watch 5. The ECG recording of his smartwatch clearly showed ST-elevation, QRS widening, R-wave loss, and T-wave inversion. Coronary angiography revealed occlusion of the left anterior descending and recanalization was performed. The patient recovered without any complications and was discharged from the hospital 4 days after admission.
Discussion
While the potential of ECG recordings by smartwatches to detect atrial fibrillation is currently under scientific investigation, this case highlights the possible potential of these devices to detect STEMI.
Keywords: Case report, ST-elevation, Myocardial infarction, Apple Watch, ECG
Learning points
In our selected case an Apple Watch electrocardiogram (ECG) application recognized morphological ECG aberrations caused by ST-elevation infarction.
The use of an Apple Watch in the early diagnosis of ST-elevation myocardial infarction needs to be evaluated in large prospective trials.
Introduction
Wearable devices such as smartwatches and activity trackers are increasingly used in the general population without medical indication and can detect irregular pulses by optical sensors. A large scale clinical trial recently demonstrated that the detection of atrial fibrillation based on this data is feasible, using the Apple Watch and the corresponding application.1 Additionally, the Apple Watch Series 4 and 5 (Apple Inc., Cupertino, CA, USA) are among the first devices to offer complementary single-lead electrocardiogram (ECG) tracing. Recently, the Apple Watch received FDA clearance for both ECG and irregular rhythm notification functions. However, this ECG tracing is not intended to detect signs of myocardial ischaemia and therefore the clinical utility of this new monitoring approach for this setting is unknown. However, millions of people currently own an Apple Watch and the immediate accessibility of an ECG tracing could potentially shorten the time to diagnosis and revascularization, especially in patients with ST-elevation myocardial infarction (STEMI), where earlier treatment reduces morbidity and mortality.2 Here, we present a case of STEMI, where the diagnosis was driven by ECG changes detected by an Apple Watch.
Timeline
| Day 1 | Patient experiences chest pain symptoms for the first time and repeatedly records Apple Watch electrocardiogram (ECG) strips. |
| Day 3 | Patient presents to his primary care physician due to repeated warnings generated by the Apple Watch ECG Application. There, 12-lead ECG confirms ST-elevations and patient is immediately referred to chest pain unit. |
| Coronary angiography reveals occlusion of left anterior descending artery. After successful recanalization patient is referred to intensive care unit. | |
| Day 4 | Patient remains stable and is transferred to regular ward. |
| Day 7 | Patient is discharged home in good condition. |
| Follow-up (3 months) | Patient presents asymptomatic with unrestricted exercise capacity. |
Case presentation
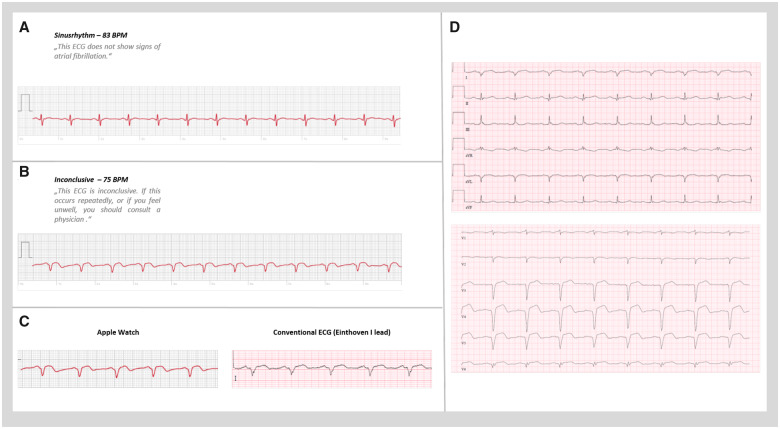
A 61-year-old male patient without previously known coronary artery disease was admitted for subacute STEMI. The patients cardiovascular risk factors included arterial hypertension and dyslipidaemia. The patient reported mild angina and dyspnoea beginning 3 days prior hospital admission. Symptoms were initially interpreted as muscular in origin by the patient, therefore he did not seek immediate medical attention. However, upon persistence of symptoms the patient performed serial ECG recordings on his Apple Watch, where he noticed a change in QRS-complex morphology himself. Figure 1A and B depicts the corresponding single-lead ECG tracings acquired by the patient’s Apple Watch a week before (A) and a day after (B) the start of chest pain, together with the specific recommendation generated by the applications internal algorithm. After onset of chest pain, the ECG tracing clearly showed an ST-elevation, QRS widening, R-wave loss, and T-wave inversion. The ECG lead acquired was corresponding to the Einthoven I lead, since the patient wore his smartwatch on the right wrist. These alterations were new in comparison to the previous ECG tracings before onset of angina and moreover, they were clearly reproducible in repeated tracings, which were acquired by the patient in the course of the following 3 days preceding hospitalization. Importantly, after onset of chest pain, the device algorithm noted an inconclusive ECG and suggested consultation of a doctor if the patient experienced any symptoms (Figure 1B and C), while the ECG tracing before was diagnosed as non-pathological by the device (Figure 1A). Angina and dyspnoea symptoms persisted during the following 3 days, but since the patient did not consider symptoms to be of cardiac origin, it was this Apple Watch alert which prompted the patient to arrange an appointment with a primary care cardiologist, where a 12-lead ECG showed ST-elevation of the anterior wall (I, aVL, V3–V6) (Figure 1D). The patient was immediately transferred to our chest pain unit. Physical examination showed rhythmic, normofrequent heart rate, blood pressure of 140/85 mmHg. Auscultation of heart and lung was inconspicuous. Transthoracic echocardiography on admission showed moderately impaired global left ventricular ejection fraction with akinesia of the apex and significant hypokinesia of the anterior left ventricular wall (representative video clips are included in the Video 1). Initial troponin (T-hs) was 5.29 ng/mL (ref: <0.014 ng/mL) and increased to a maximum of 6.22 ng/mL, CK-MB activity was 70 U/L (ref: <24 U/L) and lactatedehydrogenase upon admission was 806 U/L (ref: <249 U/L). Despite the subacute clinical course, persistence of angina symptoms upon hospitalization together with ST-elevations triggered the performance of an urgent coronary angiography, which revealed coronary artery disease with thrombotic occlusion of the middle segment of the left anterior descending artery (LAD). Successful recanalization of LAD was achieved by thrombus-aspiration and placement of one drug-eluting stent (Xience/3.5 mm × 28 mm) (representative video clips are included in the Video 1). The patient’s cardiovascular risk factors were arterial hypertension and hypercholesterinaemia of which only hypertension was previously treated. Peak creatine kinase reached 980 U/L (ref: <189 U/L) and normalized after 3 days. Angina symptoms ceased completely immediately after the coronary intervention. The patient was discharged from our intensive care unit after 24 h and left the hospital 4 days after admission. Upon follow-up after 3 months, the patient reported absence of any symptoms, with good exercise capacity.
Figure 1.
(A) Single-lead electrocardiogram tracings acquired by the patient’s Apple Watch a week before and (B) a day after the onset of chest pain symptoms, combined with the specific recommendations generated by the applications algorithm (smartwatch lead correspond to Einthoven I). (C) Direct comparison between Apple watch electrocardiogram and corresponding standard electrocardiogram lead (Einthoven I). (D) Standard 12-lead electrocardiogram recorded upon hospitalization, shows ST-elevations corresponding to the culprit lesion.
Discussion
Contemporary cardiac implanted electrical devices are dedicated to detection of cardiac arrhythmias. Although specific algorithms for detection of ST-segment elevation of the intracardiac ECG exist in some implantable cardioverter-defibrillator devices, there is so far no proven benefit in the detection of myocardial ischaemia or infarction.3 In contrast to implanted cardiac monitors and cardiac rhythm management devices, ECG monitoring by smartwatches is used unselectively without medical indication and the possibilities of mobile digital health solutions are rapidly increasing, with potentially millions of people gaining access to patient-generated continuous heart rate monitoring and rhythm surveillance by single-lead ECG recording. This has the potential to disrupt traditional health care delivery in cardiovascular medicine. Among currently commercially available smartwatches, the Apple Watch Series 4 and 5 are able to record a single-lead bipolar ECG, which is analysed by the devices algorithm. However, there have been recent reports, which describe the acquisition of Einthoven leads with the Apple Watch 4 as feasible and reproducible4 and obtaining these additional leads merely requires alternative positioning of the Apple Watch. Recording of Einthoven lead I, II, and III can be performed between the left arm wrist, the right index finger, the left lower abdominal region, and the left index finger, respectively.4,5 Additionally, Samol et al.5 recently reported the recording of three Wilson-like ECG leads Wr (right), Wm (medial), and Wl (left), which correspond with leads V1, V4, and V6 of a regular 12-lead ECG. Although there is not yet a standardized protocol to record these additional leads, this could be implemented fairly easily and offer an enormous diagnostic potential. Importantly, Avila4 and Samol et al.,5 retrospectively compared smartwatch ECG recordings in two cases and one case of STEMI patients, respectively, with 12-lead ECG after diagnosis and demonstrated good comparability. Our case, however, is the first report and proof of concept, demonstrating that an out of hospital, self-acquired single-lead ECG tracing can adequately detect ST-segment elevation and trigger the correct diagnosis. Our patient wore his Apple Watch on his left arm wrist and therefore recorded the ECG with his right index finger, which means that the recorded lead corresponds the Einthoven lead I. When compared with the Einthoven I lead acquired in the 12-channel standard ECG upon hospitalization, the key alterations in the Apple Watch ECG, namely ST-level elevation, QRS widening, R-wave loss, and T-wave inversion are strikingly comparable (Figure 1E). It might be highly beneficial if such ECG alterations or symptoms of angina would trigger automated programs for acquisition of multi-channel ECG recordings by differential smartwatch positioning, as described above. We believe that this could potentially shorten the time to correct diagnosis of myocardial infarction and revascularization, or lead to medical consultation of patients who would otherwise not do so, as is the case for our patient. Recent studies, as well as the recent European Society of Cardiology (ESC) guidelines emphasize shortening maximum delay between ‘onset of symptoms’ to ‘first medical contact’ and coronary intervention in STEMI patients.6,7 While there have been substantial improvements regarding the delay in treatment of STEMI within the medical system, the patient-related reasons for delays are harder to ameliorate especially in cases of silent myocardial infarction. Clinical predictors of patient-related reasons for delays in STEMI-treatment include for example female gender, type II diabetes, anterior infarction, or living alone.8,9 Especially for these patient groups self-acquired mobile device ECG recordings might significantly improve outcome and prevent late presentation.
However, the implementation of medical diagnostic tools in commercially available devices without standardized follow-up by physicians is potentially problematic. This could lead to uncertainty of consumers and additionally could result in thousands of false positive alerts, with an enormous burden on primary care physicians and emergency medicine services. With our report, we hope to highlight the need to closely follow these developments as medical community and implement the devices and the data acquired in high-quality scientific studies. While there is already good evidence in regards to detection of atrial fibrillation by these devices, clinical trials evaluating the potential of smartwatch ECG recordings to detect myocardial ischaemia are still lacking and will be of dire need in order to allow an evidence-based handling of patient acquired reports, presented to physicians. In the currently ongoing ‘ST LEUIS International Multicenter Study’, a standard 12-lead ECG and a smartphone ‘12-lead equivalent’ ECG will be recorded simultaneously, using the AliveCor™ Heart Monitor in patients presenting with chest pain for which the STEMI protocol was activated.6 Overall these developments have the potential to accelerate the diagnosis of myocardial ischaemia, while allowing not only sensitive but also specific detection of myocardial ischaemia in STEMI.
Ethical standards
All ethical standards were met in writing and submitting this article.
Lead author biography

Dr Martin Orban works since 2012 at the University Hospital Klinikum der Universitaet Munich. He went to medical school at the Ludwig-Maximilians-Universitaet Munich. From 2007 to 2012, he underwent his specialist training for cardiology at the German Heart Center in Munich. He is specialized in interventional cardiology and intensive care medicine therapy. Dr Orban is currently head of the cardiac intensive care unit of the University Hospital Klinikum der Universitaet Munich.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: M.O. has received speaker honoraria from Sedana Medical and AstraZeneca, outside the submitted work. All other authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T. et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, De Lemos JA. et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 3. Forleo GB, Tesauro M, Panattoni G, Della Rocca DG, Papavasileiou LP, Sergi D. et al. Impact of continuous intracardiac ST-segment monitoring on mid-term outcomes of ICD-implanted patients with coronary artery disease. Early results of a prospective comparison with conventional ICD outcomes. Heart 2012;98:402–407. [DOI] [PubMed] [Google Scholar]
- 4. Avila CO. Novel use of Apple Watch 4 to obtain 3-lead electrocardiogram and detect cardiac ischemia. Perm J 2019;23:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Samol A, Bischof K, Luani B, Pascut D, Wiemer M, Kaese S.. Single-lead ECG recordings including Einthoven and Wilson leads by a smartwatch: a new era of patient directed early ECG differential diagnosis of cardiac diseases? Sensors 2019;19:4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barbagelata A, Bethea CF, Severance HW, Mentz RJ, Albert D, Barsness GW. et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS International Multicenter Study. J Electrocardiol 2018;51:260–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.