Abstract
Background
A systemic right ventricle (RV) after atrial switch in transposition of the great arteries (TGA) or congenitally corrected TGA (ccTGA) often results in advanced heart failure in adulthood.
Case summary
Four patients with INTERMACS Class III underwent durable ventricular assist device (VAD) implantation for a systemic RV. Two patients were diagnosed with ccTGA and underwent tricuspid valve replacement, and two were diagnosed with TGA in childhood and underwent Mustard repair. The two patients with ccTGA received an EVAHEART (Sun Medical, Nagano, Japan) and HeartMate 3 (Abbott Laboratories, Abbott Park, IL, USA) at the age of 56 years and 34 years, respectively. Of the patients with TGA, one received a Heartmate II at age 40 years, and one received a HeartMate 3 at age 40 years. All patients were weaned from cardiopulmonary bypass without subpulmonic VAD support and transferred to the intensive care unit with optimum VAD support. No in-hospital deaths, cerebrovascular accidents, or other major complications occurred. The post-VAD right heart catheter study showed a remarkable reduction in pulmonary capillary wedge pressure in all patients.
Discussion
The indications for and surgical technique of durable VAD implantation for a systemic RV after atrial switch of TGA or ccTGA have not been fully established. A durable VAD, including the HeartMate 3, was successfully implanted in four such patients in this study. Pre-operative three-dimensional computed tomography images and intraoperative transoesophageal echocardiography guidance helped to determine the positions of the inflow and pump.
Keywords: Transposition of the great arteries, Congenitally corrected transposition of the great arteries, Heart failure, Ventricular assist device, HeartMate 3, Case series
Learning points
Developing advanced heart failure due to systemic right ventricle (RV) failure is a great problem for patients who have undergone atrial switch of transposition of the great arteries (TGA) or congenitally corrected TGA.
A durable ventricular assist device including HeartMate 3 can be successfully implanted in patients with systemic RV failure.
Pre-operative three-dimensional computed tomography images and intraoperative transoesophageal echocardiography guidance are useful to determine the position of the inflow and pump.
Introduction
A systemic right ventricle (RV) after atrial switch for transposition of the great arteries (TGA) or congenitally corrected TGA (ccTGA) often results in functional deterioration in adolescence or beyond because the morphological RV cannot tolerate the long-term systemic pressure overload.1–4 Peng et al.3 reported successful implantation of a durable ventricular assist device (VAD), the HeartWare HVAD (Medtronic, Minneapolis, MN, USA), into the failing systemic RV, which is a small inflow pump that attaches to the surface of the RV free wall.3
Other durable VADs, such as the HeartMate II and HeartMate 3 (Abbott Laboratories, Abbott Park, IL, USA), reportedly show similar or better long-term outcomes than the HeartWare HVAD in patients with advanced systemic left ventricular (LV) failure. However, the feasibility, safety, and effectiveness of the HeartMate II and HeartMate 3 for patients with a failing systemic RV remain unclear.5 We report four cases of durable VAD implantation for a systemic RV after atrial switch for TGA and ccTGA.
Timeline
| Patients |
||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Diagnosis | Congenitally corrected transposition of the great arteries (ccTGA) | ccTGA | Transposition of the great arteries (TGA) | TGA, ventricular septal defect (VSD) |
| Age at ventricular assist device (VAD) (years) | 56 | 34 | 40 | 40 |
| Sex | Male | Male | Male | Male |
| Body surface area (m2) | 1.53 | 1.72 | 1.50 | 1.87 |
| Previous surgical procedure (age) | Tricuspid valve replacement (38 years) | Tricuspid valve replacement (2 years) | Blalock–Hanlon (infant) | Balloon atrial septostomy (1 month) |
| Re-tricuspid valve replacement (16 years) | Mustard (1.5 years) | Mustard, VSD closure (4 months) | ||
|
Pulmonary artery banding (22 years) |
||||
| VAD type | EVAHEART | HeartMate 3 | HeartMate II | HeartMate 3 |
| VAD implant year | 2012 | 2020 | 2017 | 2020 |
| Length on VAD (days) | 1258 | 42 | 929 | 70 |
| Outcome | Transplanted | On VAD | On VAD | On VAD |
TGA, transposition of the great arteries.
Case presentation
Four patients underwent durable VAD implantation for a systemic RV in our hospital from August 2012 to March 2020. Data were collected in April 2020. Two patients had ccTGA, and two had undergone atrial switch procedure for TGA. All patients provided written informed consent for surgery and the use of their data for diagnostic and research purposes. This study was approved by our institutional review board (M30-026). All patients were categorized as Interagency Registry for Mechanically Assisted Circulatory Support Class III, and the VAD was implanted as a bridge to transplantation. Table 1 summarizes the right heart catheterization data and echocardiographic data before and after surgery. All patients took carvedilol and anti-aldosterone drugs before and after surgery. Amiodarone was used for atrial arrhythmias (atrial tachycardia, atrioventricular nodal reentrant tachycardia) in Patients 2 and 4 and for ventricular tachycardia in Patient 1, and the drug was discontinued post-operatively. Angiotensin-converting enzyme inhibitors were used in only Patients 2 and 3 before surgery, but in all patients after surgery because of improved renal function. They had no other past medical history or drug history.
Table 1.
Pre- and post-ventricular assist device catheterization and echocardiographic studies
| Patients | 1 |
2 |
3 |
4 |
||||
|---|---|---|---|---|---|---|---|---|
| Pre-VAD | Post-VAD | Pre-VAD | Post-VAD | Pre-VAD | Post-VAD | Pre-VAD | Post-VAD | |
| VAD speed (rpm) | — | 1900 | — | 5300 | — | 9200 | — | 5000 |
| Catheterization data | — | 5 months | — | 2 months | — | 3 months | — | 2 months |
| Pulmonary capillary wedge pressure (mmHg) | 17 | 10 | 22 | 10 | 24 | 6 | 35 | 11 |
| Pulmonary arterial pressure (mmHg) | 29/14 | 19/10 | 45/24 | 20/14 | 45/22 | 31/9 | 81/42 | 26/16 |
| Subpulmonic LV pressure (mmHg) | 29/12 | 19/5 | 52/24 | 25/6 | 40/7 | 28/6 | 92/12 | 32/11 |
| Right atrial pressure (mmHg) | 11 | 8 | 21 | 12 | 8 | 6 | 12 | 11 |
| Pulmonary artery pulsatility index | 1.36 | 1.12 | 1.0 | 1.58 | 2.87 | 3.66 | 3.25 | 1.6 |
| Subaortic RV pressure (mmHg) | 82/10 | — | 100/21 | — | 89/15 | — | 91/16 | — |
| Subaortic RVEDV (mL) | 123.5 | — | 142 | — | 386.8 | — | 425.7 | — |
| Subaortic RVEF (%) | 25 | — | 19 | — | 25 | — | 33 | — |
| Pulmonary vascular resistance, unit) | 2.29 | 0.91 | 1.51 | 0.97 | 2.50 | 2.03 | 9.22 | 1.9 |
| Cardiac output (c-Fick) | 2.9 | 4.2 | 3.6 | 6.2 | 4.3 | 4.5 | 2.7 | 4.7 |
| Cardia index (c-Fick) | 1.9 | 2.7 | 2.1 | 3.6 | 2.8 | 2.9 | 1.4 | 2.4 |
| Echocardiographic data | ||||||||
| Systemic atrioventricular valve regurgitation | Nonea | Nonea | Nonea | Nonea | Severe | trivial | severe | trivial |
| Aortic regurgitation | Trivial | Trivial | Trivial | Trivial | No AR | No AR | No AR | No AR |
Patients underwent mechanical tricuspid valve replacement.
AR, aortic regurgitation; LV, left ventricle; RV, right ventricle; RVEDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; VAD, ventricular assist device.
Pulmonary artery pulsatility index was calculated as [(Systolic pulmonary artery pressure − diastolic pulmonary artery pressure)/right atrial pressure].
Patient 1
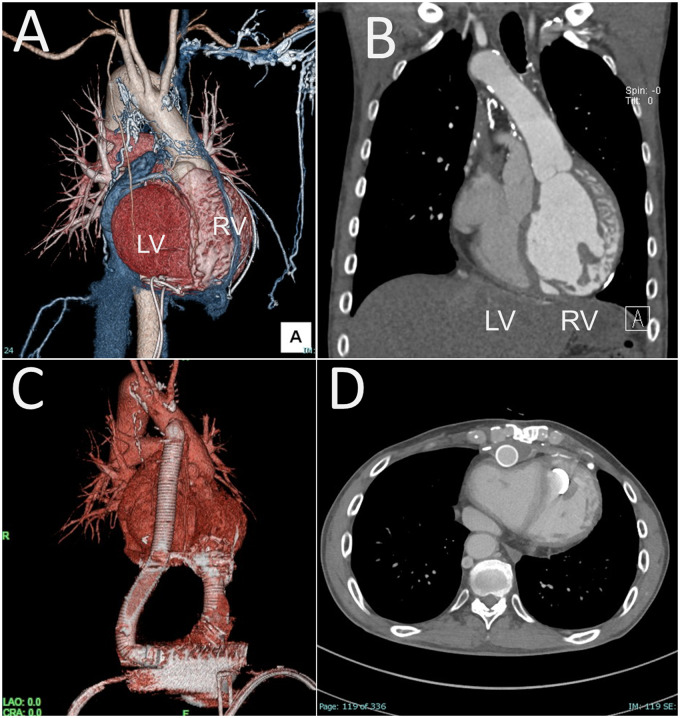
A 54-year-old man with ccTGA developed advanced heart failure requiring 2.5 mcg/kg/min of dobutamine support. He was first diagnosed with ccTGA at 37 years of age, when he developed congestive heart failure related to severe tricuspid regurgitation and complete atrioventricular block. At 38 years of age, he underwent tricuspid valve replacement with a mechanical valve, followed by optimum medical therapy and DDD pacemaker support. Morphologically, he exhibited mesocardia. While the RV was left-sided and anteriorly located, the ascending aorta was anteriorly located (Figure 1).
Figure 1.
Pre- and post-ventricular assist device implantation for a patient with congenitally corrected transposition of the great arteries (Patient 1). (A) The patient exhibited mesocardia. (B) The aorta arose from the morphologic right ventricle. (C, D) Post-operative computed tomography scan.
He underwent implantation of an EVAHEART (Sun Medical, Nagano, Japan), which has a flexible tube between the inflow cannula and the pump. Under cardiopulmonary bypass, the RV apex was cored for the inflow cuff of the EVAHEART. The outflow graft was sutured to the right side of the ascending aorta.
Patient 2
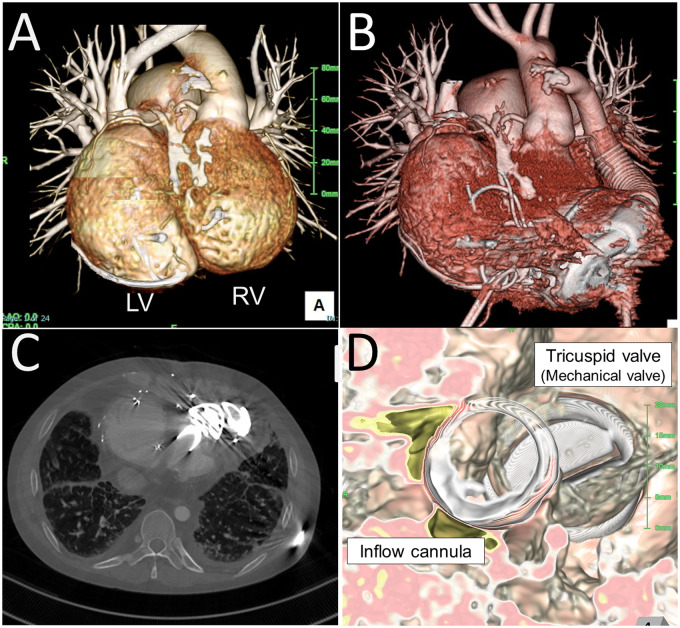
A 34-year-old man with ccTGA developed congestive heart failure requiring 2.0 mcg/kg/min of dobutamine and 0.3 mcg/kg/min of milrinone support related to poor systemic RV function. He was first diagnosed with ccTGA at 2 years of age, when he underwent tricuspid valve replacement with a mechanical valve for severe tricuspid regurgitation. The mechanical valve was upgraded at 16 years of age. He subsequently underwent 12 in-hospital treatments for congestive heart failure related to poor RV function. Morphologically, he exhibited mesocardia. The LV and RV were side-by-side; the RV was left-sided, and the tricuspid valve was located posteriorly (Figure 2). The ascending aorta was left-sided and anteriorly located.
Figure 2.
Pre- and post-HeartMate 3 implantation for a patient with congenitally corrected transposition of the great arteries (Patient 2). (A) The patient exhibited mesocardia. (B, C) Post-operative computed tomography scan. (D) The inflow cannula was directed toward the tricuspid valve.
He underwent HeartMate 3 implantation. Under cardiopulmonary bypass, the anterior side of the RV apex was cored under transoesophageal echocardiography (TOE) guidance, by which the inflow cannula was directed toward the tricuspid valve. Because the original sewing ring of the HeartMate 3 was too large for this small RV, the outer metal ring was removed to reshape the cuff one size smaller. This ‘mini-cuff’ was fixed by the sew-and-cut technique. The outflow graft was sutured to the left side of the ascending aorta (Figure 2, Video S1).
Patient 3
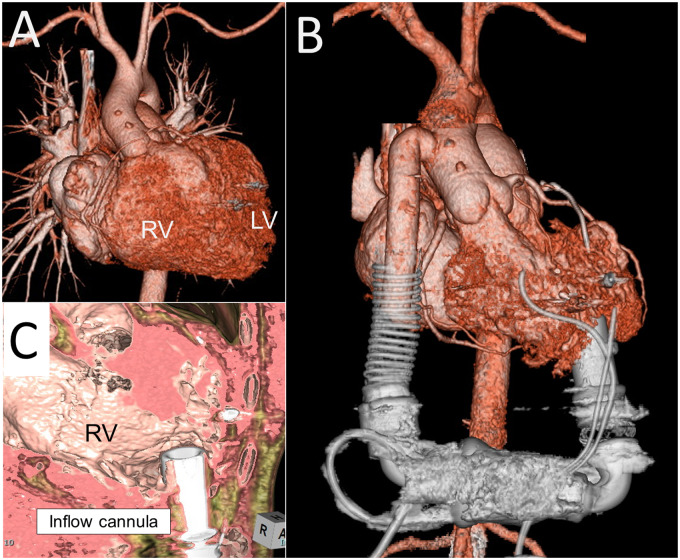
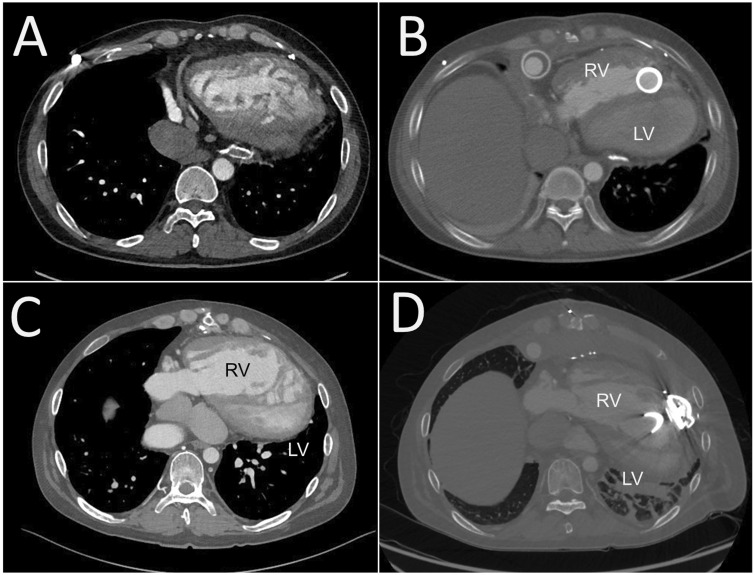
A 40-year-old man who had undergone atrial switch for TGA developed advanced heart failure requiring 5 mcg/kg/min of dobutamine support. He was first diagnosed with TGA immediately after birth, when he underwent the Blalock–Hanlon procedure. He subsequently underwent Mustard repair at 18 months of age. Since his first hospitalization for congestive heart failure at 29 years of age, he had been repeatedly hospitalized. Morphologically, he exhibited levocardia. Both the RV and ascending aorta were right-sided and anteriorly located (Figure 3).
Figure 3.
(A) Pre- and (B) post-HeartMate II implantation for a patient with transposition of the great arteries (Patient 3). (C) The inflow cannula was directed toward the lateral wall of the right ventricle.
He underwent HeartMate II implantation. After redo sternotomy, the left and right subcostal and preperitoneal spaces were largely dissected for the pump pocket. The short ascending aorta and post-Mustard venous return prompted femoral artery and vein cannulation for cardiopulmonary bypass. Innominate vein cannulation was subsequently added for full bypass. The posterior apex of the RV was cored under TOE guidance for the inflow cuff, which was attached by the cut-and-sew technique. The outflow graft was sutured to the right side of the ascending aorta.
Patient 4
A 40-year-old man who had undergone atrial switch for TGA developed advanced heart failure requiring 0.3 mcg/kg/min of milrinone support. He was first diagnosed with TGA with a ventricular septal defect soon after birth, and he underwent Mustard repair and ventricular septal defect closure at 4 months of age. He first developed congestive heart failure and was treated in-hospital at 22 years of age, when he underwent pulmonary artery banding for severe tricuspid regurgitation. Morphologically, he exhibited levocardia. The RV was left-sided and anteriorly located, and the ascending aorta was anteriorly located (Figure 4).
Figure 4.
(A) Pre- and (B) post-HeartMate 3 implantation for a patient with transposition of the great arteries (Patient 4).
He underwent HeartMate 3 implantation. Cardiopulmonary bypass was initiated by femoral artery and vein cannulation with the addition of innominate vein cannulation. The anterior apex of the RV was cored under TOE guidance for the inflow cuff, where the ‘mini-cuff’ was attached by the sew-and-cut technique. The outflow graft was sutured to the right side of the ascending aorta.
Post-operative anticoagulation protocol
When surgical bleeding had subsided (<1.0 mL/kg/h for 4 consecutive hours), a heparin infusion was started at 500 U/h and titrated to achieve an activated partial thromboplastin time of 60–80 s within 72 h post-operatively in all patients. Warfarin was commenced on post-operative Day 1, with a target international normalized ratio of 2–3. The heparin infusion was stopped when the international normalized ratio exceeded 2. When the platelet count exceeded 10 000/μL, aspirin (100 mg/day) was started on post-operative Day 1 or on post-operative Day 3, even if the platelet count was < 10 000/μL. Aspirin was continued until the last follow-up.
In-hospital outcomes
All patients were weaned from cardiopulmonary bypass without subpulmonic left-ventricular assist device (LVAD) support and transferred to the intensive care unit (ICU) with optimum systemic VAD support. No in-hospital deaths, cerebrovascular accidents or other major complications (bleeding, infection, or acute kidney injury) occurred. Patient 3, who previously underwent surgery for a pituitary adenoma, developed a seizure post-operatively but fully recovered 17 days after ICU arrival. The median ICU stay was 5 days (range 4–17 days).
Long-term outcomes
While Patient 1 was transplanted 1258 days post-VAD implantation, Patients 2, 3, and 4 remained on VAD support at the latest follow-up (42 929 and 70 days post-VAD implantation, respectively).6 A cerebrovascular accident occurred in Patient 1 prior to the transplant, and he developed haemorrhagic stroke and thrombo-embolic stroke at 10 and 31 months post-VAD implantation, respectively, with full neurologic recovery. The only other complication was a driveline infection in Patient 2, which was treated by antibiotics, local lavage, debridement, and driveline relocation. The characteristics of each VAD are shown in Table 2.
Table 2.
Comparison of EVAHEART, HeartMate II, and HeartMate 3 Devices
| EVAHEART | HeartMate II | HeartMate 3 | |
|---|---|---|---|
| Rotor design | Centrifugal | Axial | Centrifugal |
| Bearing design | Hydrodynamic levitation | Mechanical/pivot | Magnetic levitation |
| Pump position | Extratrathoracic (pump pocket) | Extratrathoracic (pump pocket) | Intrapericardial |
| Artificial pulse | None | None | Yes |
| Pump speed | 800–3000 rpm | 3000–9000 rpm | 6000–15 000 rpm |
| Pump size | 76 mm × 58 mm | 81 mm × 43 mm | 50.3 mm × 33.8 mm |
| Pump weight | 420 g | 281.3 g | 200 g |
| Positive point |
|
|
|
| Negative point |
|
|
Haemodynamic outcome
All patients underwent a right heart catheter study 1 month before VAD implantation and from 1 month after VAD implantation to immediately before hospital discharge (Table 1). All patients showed a substantial reduction in pulmonary capillary wedge pressure (PCWP), pulmonary arterial pressure, and pulmonary vascular resistance (PVR) after VAD implantation. Cardiac output and cardiac index were optimized, and PCWP and PVR decreased in all patients post-operatively. All patients also underwent transthoracic echocardiography before and after VAD implantation. The degree of tricuspid regurgitation decreased from severe to trace in Patients 3 and 4 after VAD implantation. No patients developed mild or worse de novo aortic regurgitation.
Discussion
The indications for durable VAD implantation for a systemic RV after atrial switch for TGA or ccTGA are not fully established. The major cause of congestive heart failure in these patients is poor systolic RV function and elevated RV end-diastolic pressure, which is qualified by other factors such as RV diastolic dysfunction, constrictive pericarditis, high PVR, or poor LV function. Therefore, a right heart catheter study provides important information regarding the haemodynamics of this pathology. High PCWP under optimum medical therapy is the most important indicator of durable VAD implantation, as seen in this study.
Although destination therapy by durable VAD has been developed in some western countries, many countries have not launched this program because of financial and social issues. Heart failure related to adult congenital heart disease (ACHD) is a worldwide epidemic. However, surgical interventions for ACHD-associated heart failure, such as VAD implantation or heart transplantation, are challenging because of anatomical variations of the heart and great vessels. In this study, we reported the feasibility and safety of modern durable VAD implantation for ACHD. Long-term VAD therapy is potentially warranted for this complex pathology.
Anticoagulation therapy is the key to success in durable VAD therapy, especially in patients with complex ACHD. First, VAD implantation into the systemic RV, as in all four cases in this study, is more likely to form a wedge thrombus associated with developed trabeculae, compared with implantation into the systemic LV. Second, VAD implantation into a small systemic ventricle, as in Patients 1 and 2 in this study, would also induce wedge thrombus formation. Finally, a previously implanted mechanical valve prosthesis or reduced left atrial function would cause thrombus formation without intensive anticoagulation therapy.
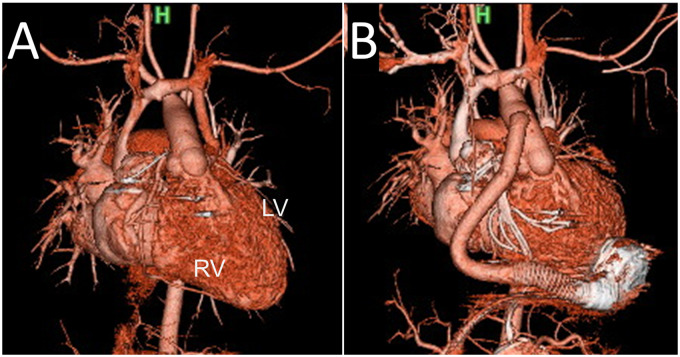
Selecting the VAD type and implantation procedure are keys to successful mechanical support for poor systemic RV function after atrial switch for TGA or ccTGA.7–9 In TGA, the RV apex, where the VAD is implanted, is located on the left side, and the RV is large relative to the tricuspid valve regurgitation.10 We selected the HeartMate 3 for Patient 4; this rotated the heart clockwise and deformed the subpulmonic LV structure (Figure 5), but preserved LV haemodynamics. This LV deformity may be a long-term limiting factor of the HeartMate 3 for patients who undergo atrial switch for TGA. In contrast, the two patients with ccTGA in this study had a small RV relative to the TGA after atrial switch. Additionally, the two patients’ ventricles were positioned side-by-side with additional supra-inferior obliquity. The ventricular mass was abnormally orientated relative to the thorax, with the apex pointing toward the right, as with the usual atrial arrangement.10 Therefore, the VAD insertion and pump placement positions were closer to the midline than usual. We selected the HeartMate 3 for Patient 2; positioned anteriorly and directed toward the tricuspid valve (Figure 2). The HeartMate 3 pump is bulkier than that of the HVAD; however, implanting the HeartMate 3 to the systemic RV is feasible after atrial switch.
Figure 5.
Effect of ventricular assist device implantation for patients with transposition of the great arteries. (A) Pre- and (B) post-HeartMate II implantation (Patient 3). (C) Pre- and (D) post-HeartMate 3 implantation (Patient 4). The left ventricle is deformed (heart rotates clockwise) by the HeartMate 3.
In conclusion, a durable VAD, including the HeartMate 3, was successfully implanted in patients with systemic RV failure related to atrial switch for TGA or ccTGA. Pre-operative three-dimensional computed tomography images and intraoperative TOE guidance were useful to determine the inflow and pump positions.
Lead author biography

Naoki Tadokoro is a consultant cardiac surgeon in National Cerebral and Cardiovascular Center, Osaka, Japan. He graduated in Medicine at Jikei University School of Medicine, Tokyo, Japan in 2012 and completed the surgical training in 2018 at the National Cerebral and Cardiovascular Center. He is also enrolled in PhD program of Keio University School of Medicine Cardiovascular Surgery in 2019. He is a Board Surgeon of Japanese Association of Cardiovascular Surgery and a certified surgeon of ventricular assist device implantation.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
We thank Norihide Fukushima, Masanobu Yanase, Osamu Seguchi, Seiko Nakajima, Kensuke Kuroda, Takuya Watanbe, Yorihiko Matsumoto, and Heima Sakaguchi, who are members of the Heart Team.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patients in line with COPE guidelines.
Conflict of interest: none declared.
References
- 1. Warnes CA. Transposition of the great arteries. Circulation 2006;114:2699–2709. [DOI] [PubMed] [Google Scholar]
- 2. Wallis GA, Debich-Spicer D, Anderson RH.. Congenitally corrected transposition. Orphanet J Rare Dis 2011;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peng E, O’Sullivan JJ, Griselli M, Roysam C, Crossland D, Chaudhari M. et al. Durable ventricular assist device support for failing systemic morphologic right ventricle: early results. Ann Thorac Surg 2014;98:2122–2129. [DOI] [PubMed] [Google Scholar]
- 4. Filippov AA, Del Nido PJ, Vasilyev NV.. Management of systemic right ventricular failure in patients with congenitally corrected transposition of the great arteries. Circulation 2016;134:1293–1302. [DOI] [PubMed] [Google Scholar]
- 5. Riggs KW, Fukushima S, Fujita T, Rizwan R, Morales DLS.. Mechanical support for patients with congenitally corrected transposition of the great arteries and end-stage ventricular dysfunction. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2019;22:66–73. [DOI] [PubMed] [Google Scholar]
- 6. Fujita T, Fukushima S, Fukushima N, Shiraishi I, Kobayashi J.. Three-dimensional replica of corrected transposition of the great arteries for successful heart transplantation. J Artif Organs 2017;20:289–291. [DOI] [PubMed] [Google Scholar]
- 7. Mohite PN, Popov AF, Garcia D, Hards R, Zych B, Khaghani A. et al. Ventricular assist device outflow graft in congenitally corrected transposition of great arteries - a surgical challenge. J Cardiothorac Surg 2012;7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joyce DL, Crow SS, John R, St Louis JD, Braunlin EA, Pyles LA. et al. Mechanical circulatory support in patients with heart failure secondary to transposition of the great arteries. J. Heart Lung Transplant 2010;29:1302–1305. [DOI] [PubMed] [Google Scholar]
- 9. Yajima S, Toda K, Tsukiya T, Sawa Y.. Three-dimensional simulation for left ventricular assist device implantation in a small patient with chest wall deformity. Eur J Cardiothorac Surg 2019;55:788–789. [DOI] [PubMed] [Google Scholar]
- 10. Morcos M, Kilner PJ, Sahn DJ, Litt HI, Valsangiacomo-Buechel ER, Sheehan FH.. Comparison of systemic right ventricular function in transposition of the great arteries after atrial switch and congenitally corrected transposition of the great arteries. Int J Cardiovasc Imaging 2017;33:1993–2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.