Abstract
Background
Eosinophilic myocarditis is a rare form of myocardial inflammatory disease. Eosinophilic infiltration of the myocardium is often the consequence of a systemic disorder but can remain unexplained in up to a third of patients. The disease course can range from mild to fulminant myocarditis and mortality remains high for fulminant cases.
Case summary
A 42-year-old male was admitted for cardiogenic shock. He presented in another hospital with fever, low blood pressure, diffuse electrocardiogram-abnormalities, and elevated troponin T (4.5 µg/L; reference <0.013 µg/L) levels. Coronary angiography was unremarkable. Mechanical circulatory support with the ImpellaTM CP device was initiated. Since fulminant myocarditis was suspected and magnetic resonance imaging was not feasible in urgency, an endomyocardial biopsy was performed. He transiently developed right ventricular failure after ImpellaTM implantation, requiring the re-institution of an inotropic agent. Biopsy showed eosinophilic myocarditis, even though there was no increase in the peripheral blood eosinophil count. Methylprednisone and Ramipril were initiated to which he responded well. No systemic disease or parasitic infection was found during further work-up. Left ventricular ejection fraction rapidly improved and was completely normalized at discharge.
Discussion
This case demonstrates the usefulness of myocardial biopsy in fulminant myocarditis since the only histopathology guided us towards the diagnosis of eosinophilic myocarditis. Treatment with methylprednisone and an angiotensin-converting enzyme-inhibitor resulted in rapid improvement. Awake mechanical circulatory support with the ImpellaTM device proved feasible and might have helped by unloading the left ventricle, as was reflected in an immediate decrease in troponin levels, even before methylprednisone initiation.
Keywords: Eosinophilic myocarditis, Cardiogenic shock, Mechanical circulatory support, Unloading, Case report
Learning points
Endocardial biopsies remain important in cases of fulminant myocarditis since systemic clues to the underlying mechanism causing myocardial inflammation could be absent (i.e. normal peripheral blood eosinophil count in this case and no other sign of eosinophilic disease).
Understanding this underlying mechanism enables physicians to start disease-specific therapy in some subtypes of myocarditis, such as eosinophilic myocarditis, giant cell myocarditis, sarcoidosis, …
Awake patients can be successfully supported with the ImpellaTM CP device, potentially offering the advantage of unloading the left ventricle and avoiding mechanical ventilation
Right ventricular (RV) function should be monitored closely during left ImpellaTM treatment since the RV remains responsible for the transmission of venous return (preload) to the left heart and the device; making the RV the Achilles heel of the left ImpellaTM supported circulation.
Even when it is suspected, eosinophilic myocarditis should be promptly treated with steroids.
Introduction
Eosinophilic myocarditis is a rare condition of myocardial inflammatory disease. Eosinophilic infiltration of the myocardium is often the consequence of a systemic disorder such as primary hypereosinophilic syndrome, granulomatosis with polyangiitis (eGPA), a parasitic infection, or drug reaction but can also remain unexplained in up to a third of patients.1 The disease course can range from mild to fulminant myocarditis and includes eosinophilic restrictive cardiomyopathy (Löffler syndrome). Mortality remains high for fulminant cases and therapy is based on little evidence.1
We describe a cardiogenic shock (CS) patient treated with awake ImpellaTM CP circulatory support and corticosteroids in biopsy-confirmed eosinophilic myocarditis, leading to full recovery of the left ventricle.
| Day 4 | Fever and flu-like illness for 3 days; spontaneous remission |
| Day 0 | Admission at emergency department with low blood pressure, elevated serum lactate (2.7 mmol/L) and left ventricular dysfunction [left ventricular ejection fraction (LVEF) 25%]. Diagnosis of cardiogenic shock and referral to our unit |
| Day 0 + 2 h | Coronary angiography, implantation of ImpellaTM CP device, and right ventricular biopsy |
| Day 0 + 4 h | Increased central venous pressure and signs of right ventricular (RV) failure on echocardiography (decreased tricuspid annular plane systolic excursion, RV dilation, and D-shaping of interventricular septum) for which milrinone was initiated at 0.3 mcg/kg/min |
| Day 2 | Biopsy result: eosinophilic infiltration. Methylprednisone was started at 2 mg/kg/day. |
| Day 3 | Initiation of Ramipril at 2.5 mg/day, gradual increase in dose. |
| Day 5 | Partial recuperation of cardiac function on echocardiography. Successful weaning trial (30 min at minimal flow level) of ImpellaTM followed by device removal. |
| Day 7 | Further uptitration of Ramipril to 5 mg/day and further clinical improvement. Intensive care unit discharge. |
| Day 14 | Normalized LVEF (52%) on cardiac magnetic resonance imaging. |
| Day 16 | Discharge from hospital; still on Ramipril 5 mg/day and methylprednisone 24 mg/day. |
| 4 months | Stop of methylprednisone. Stable (normalized) cardiac function on echocardiography. |
Timeline
Case presentation
A 42-year-old Caucasian male with unremarkable medical history was referred to our cardiac intensive care unit for suspected CS. He presented in another hospital with fever, low blood pressure (BP), diffuse electrocardiogram (ECG)-abnormalities, and elevated troponin (4.930 µg/L; normal range <0.013 µg/L) levels.
At admission, he was hypotensive [blood pressure (BP) 85/47 mmHg] with tachycardia (97–110 b.p.m.) and signs of impaired peripheral circulation (cold, prolonged capillary refill, and confusion). Central venous oxygen saturation was only 37% and serum lactate was 2.7 mmol/L. His ECG showed sinus rhythm, biatrial dilation, small QRS voltage with right axis, and non-specific repolarization abnormality. Echocardiography was performed and showed severe left ventricular dysfunction. The right ventricular (RV) function was mild to moderately impaired, but without dilation (Video 1). There was also a small pericardial effusion, but without signs of tamponade. An urgent coronary angiogram was performed and it was completely normal. Left ventricular end-diastolic pressure was 22 mmHg despite low BP. Since haemodynamic conditions rapidly deteriorated, an ImpellaTM CP device was implanted whilst being awake. Right ventricular myocardial biopsies were obtained once he was stable and a Swan-Ganz catheter was inserted per institutional ImpellaTM protocol.
The patient never required intubation. Soon after implantation of the device, suction events (automatic ImpellaTM flow reductions based on motor current changes indicating contact between the pump inlet and ventricular structures) occurred and the ImpellaTM flow had to be reduced to only minimal support. Pulmonary artery wedge pressure was decreasing while central venous pressure (CVP) had risen to 20 mmHg and pulmonary artery (PA) pulsatility index [(systolic PA pressure – diastolic PA pressure)/CVP] was only 0.45 (reference >1), indicating RV failure. Urgent transthoracic echocardiography complemented invasive measurements by showing severe RV dysfunction (Video 2) and confirmed an adequate ImpellaTM position. A low dose of milrinone (0.3 mcg/kg/min) was started, leading to an increase in the cardiac output, a decrease in CVP, and a successful uptitration of the ImpellaTM flow level to 3.5 L/min. Milrinone was chosen because it has pulmonary vasodilator properties in addition to the inotropic effects. Lactate levels rapidly decreased, and the patient was clinically well perfused.
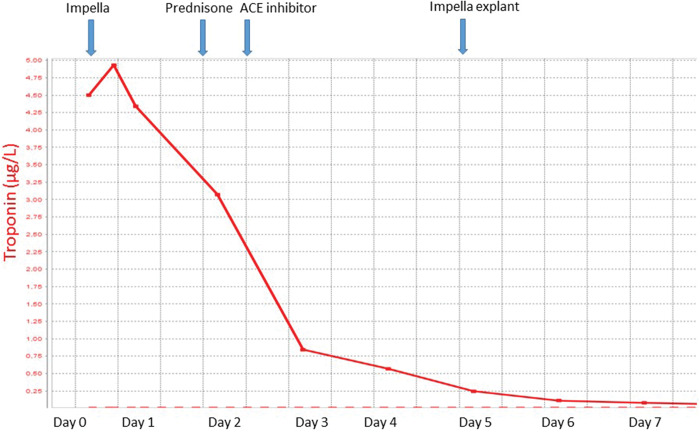
Pathological staining of the RV sample (Figure 1) showed a high load of infiltration with inflammatory cells, mainly eosinophils, compatible with eosinophilic myocarditis. Serum eosinophilic count was surprisingly within the normal range (Table 1). The serum anti-cytoplasmic antibody test was negative, as were serum antinuclear antibody (ANA) tests, and screening for parasites. Repetitive blood cultures remained sterile. There was also no recent drug exposure. We presumed idiopathic eosinophilic myocarditis and high dose methylprednisone was started at 2 mg/kg during the first week. C-reactive protein and troponin levels (Figure 2) decreased soon after unloading with the ImpellaTM was initiated and rapidly normalized after methylprednisone treatment. Renal function normalized after 24 h of ImpellaTM support. Left as well as right ventricular function started to recover on a daily echocardiogram. Angiotensin-converting enzyme (ACE) inhibition was initiated on Day 3. Over the next couple of days, cardiac function recovered and ImpellaTM-explantation was successful at Day 5. On Day 14, his cardiac function had completely recovered on magnetic resonance imaging, which showed some residual inflammation. On Day 16, the patient was discharged on an ACE-inhibitor and methylprednisone 24 mg, which was tapered by 4 mg every week. At follow-up visit after 4 months and complete steroid taper, his cardiac function remained completely normalized.
Figure 1.
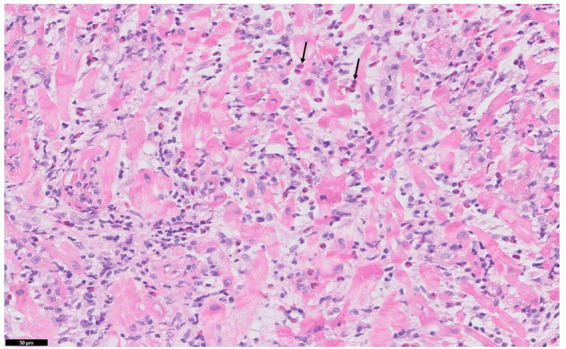
Biopsy from myocardium showing inflammatory cell infiltration with high number of eosinophils (arrow), myocyte destruction, and oedema.
Table 1.
Lab results and reference values at admission
| Test | Result | Reference value |
|---|---|---|
| Haemoglobin | 13 g/dL | 13.5–16.9 g/dL |
| White blood cell count | 13.9 × 109/L | 3.9–10.9 × 109/L |
| % Neutrophils | 86% | 41–71% |
| % Eosinophils | 0% | 1–8% |
| % Lymphocytes | 7% | 19–48% |
| % Monocytes | 6% | 5–15% |
| C-reactive protein | 105 mg/L | <5 mg/L |
| Urea | 101 mg/dL | <49 mg/dL |
| Creatinine | 1.18 mg/dL | 0.67–1.17 mg/dL |
| Troponin T hs | 4.930 µg/L | <0.013 µg/L |
| NT-pro-BNP | 2310 ng/L | <172 ng/L |
| Lactate (arterial) | 2.7 mmol/L | 0.5–2 mmol/L |
| Central venous oxygen saturation (SvO2) | 37% | >65% |
NT-pro-BNP, N-terminal prohormone of brain natriuretic peptide.
Figure 2.
Evolution of troponin levels during patient treatment, showing first decrease in troponin after unloading with ImpellaTM and subsequent further decrease after the start of methylprednisone.
Discussion
Fulminant eosinophilic myocarditis is a rare but often treatable disease. Diagnosis requires a myocardial biopsy, as was performed in this case. There was a normal peripheral blood eosinophil count in this case and there were no other clinical indications to the underlying diagnosis for acute heart failure. Indeed, peripheral blood eosinophilia was found to be absent in 14.1% of the histologically confirmed cases of a large retrospective study.1 Here, we were only able to identify the underlying diagnosis due to an early performed endomyocardial biopsy, standard of care at our institution in every case of fulminant myocarditis without an obvious explanation. We could not isolate a specific cause for the suppression of peripheral blood eosinophilia at presentation, but excluded bacteraemia with blood cultures, systemic lupus erythematosus by ANA blood test and performed peripheral blood smear which was normal. This case stresses the importance of early myocardial biopsy in cases of fulminant myocarditis even when specific indications for rare diseases are absent. We must emphasize that biopsies were taken in the RV as per hospital protocol but can also be taken in the left ventricle. Most data suggest a higher diagnostic yield and safety for left ventricular (or biventricular) biopsies in myocarditis.2,3
Eosinophilic myocarditis is mostly not a final diagnosis. Since prognosis and treatment are directly related to the underlying cause, further work-up for underlying aetiologies such as eGPA, hypereosinophilic syndrome, parasitic infection, or drug reaction is needed.1 We did not find evidence for any of these diagnoses; clinically there were no signs of hypersensitivity, nor signs of vasculitis, or parasite infections. This is not uncommon, since the idiopathic disease is reported in 36% of the total eosinophilic myocarditis case volume.1
Our patient presented with cardiogenic shock and was treated with an ImpellaTM percutaneous ventricular assist device. This is a micro-axial continuous flow pump mounted on a catheter. The advantage of the device is the smaller diameter (compared to most circulatory support devices) and the fact that preload as well as afterload are reduced by the continuous removal of blood out of the left ventricle. This effect is termed unloading and is associated with advantageous effects in the context of myocarditis.4 Also in our case troponin levels (previously reported to reflect disease activity in eosinophilic myocarditis) started to decrease after unloading was initiated, even before methylprednisone was administered to the patient. However, one of the major culprits of the left ImpellaTM device is the fact that it offers univentricular support, making all preload dependent on RV function. In the context of myocarditis, RV failure is a major risk since the RV remains responsible for preload to the device in the left heart. Also in our case, RV dysfunction limited left device function until a low dose of an inotrope supported the RV enough to allow full ImpellaTM flow. The fact that early treatment allowed avoidance of intubation might have also helped by evading the negative impact of positive pressure ventilation on RV function.5 Alternatives for circulatory support in the myocarditis that provide biventricular support are veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and biventricular ImpellaTM, with the second option remaining rare.6,7 The ImpellaTM device is used more and more as a secondary strategy to unload the left ventricle during VA-ECMO.8
Generally, prednisone is advised, initiated at 1–2 mg/kg daily; however, we lack data on dosing strategy.1 In our case, 2 mg/kg was initiated but tapered over 4 months based on experiences from idiopathic hypereosinophilic syndrome.9
Lead author biography

Tim Balthazar obtained his MD in 2012 and first completed cardiology residency, followed by formal intensive care training. He is currently working as consultant at the University Hospitals Leuven (Belgium) as cardiac intensivist. He has a special interest in medical intensive care, cardiogenic shock, and mechanical circulatory support.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
We thank Dr Hendrik Celen for referring the patient and being so kind to share the video clips of the initial echocardiography that he performed at the emergency department at the Heilig Hart hospital, Leuven, Belgium. We are also thankful to Prof. Dr Birgit Weynand and Dr Lise Waumans (University Hospitals, Leuven, Belgium) for providing us with the histological images.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidelines.
Conflict of interest: T.B.A., T.A., and C.V.D.B. have received a research grant from Abiomed. W.D. has no conflicts of interest to declare.
Funding: A research grant from the Frans Van De Werf fund to T.B.; a grant from university hospitals Leuven (Klinische onderzoeks- en opleidingsraad) to C.V.
References
- 1. Brambatti M, Matassini MV, Adler ED, Klingel K, Camici PG, Ammirati E.. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol 2017;70:2363–2375. [DOI] [PubMed] [Google Scholar]
- 2. Chimenti C, Frustaci A.. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies: a retrospective study over a 28-year period. Circulation 2013;128:1531–1541. [DOI] [PubMed] [Google Scholar]
- 3. Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A. et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation 2010;122:900–909. [DOI] [PubMed] [Google Scholar]
- 4. Tschöpe C, Van Linthout S, Klein O, Mairinger T, Krackhardt F, Potapov EV. et al. Mechanical unloading by fulminant myocarditis: LV-IMPELLA, ECMELLA, BI-PELLA, and PROPELLA concepts. J Cardiovasc Trans Res 2019;12:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lampert BC, Teuteberg JJ.. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant 2015;34:1123–1130. [DOI] [PubMed] [Google Scholar]
- 6. Asaumi Y, Yasuda S, Morii I, Kakuchi H, Otsuka Y, Kawamura A. et al. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J 2005;26:2185–2192. [DOI] [PubMed] [Google Scholar]
- 7. Kuchibhotla S, Esposito ML, Breton C, Pedicini R, Mullin A, O'Kelly R. et al. Acute biventricular mechanical circulatory support for cardiogenic shock. J Am Heart Assoc 2017;6:e006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G. et al. Concomitant implantation of Impella. Eur J Heart Fail 2017;19:404–412. [DOI] [PubMed] [Google Scholar]
- 9. Klion AD. How I treat hypereosinophilic syndromes. Blood 2015;126:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.