Abstract
Aims
Histological analysis of brain tissue samples provides valuable information about the pathological processes leading to common neurodegenerative disorders. In this context, the development of novel high‐resolution imaging approaches is a current challenge in neuroscience.
Methods
To this end, we used a recent super‐resolution imaging technique called STochastic Optical Reconstruction Microscopy (STORM) to analyse human brain sections. We combined STORM cell imaging protocols with neuropathological techniques to image cryopreserved brain samples from control subjects and patients with neurodegenerative diseases.
Results
This approach allowed us to perform 2D‐, 3D‐ and two‐colour‐STORM in neocortex, white matter and brainstem samples. STORM proved to be particularly effective at visualizing the organization of dense protein inclusions and we imaged with a <50 nm resolution pathological aggregates within the central nervous system of patients with Alzheimer’s disease, Parkinson’s disease, Lewy body dementia and fronto‐temporal lobar degeneration. Aggregated Aβ branches appeared reticulated and cross‐linked in the extracellular matrix, with widths from 60 to 240 nm. Intraneuronal Tau and TDP‐43 inclusions were denser, with a honeycomb pattern in the soma and a filamentous organization in the axons. Finally, STORM imaging of α‐synuclein pathology revealed the internal organization of Lewy bodies that could not be observed by conventional fluorescence microscopy.
Conclusions
STORM imaging of human brain samples opens further gates to a more comprehensive understanding of common neurological disorders. The convenience of this technique should open a straightforward extension of its application for super‐resolution imaging of the human brain, with promising avenues to current challenges in neuroscience.
Keywords: amyloid‐β, neuropathology, STORM, tau, TDP‐43, α‐synuclein
Abbreviations
- Aβ
Amyloid‐β
- AD
Alzheimer’s disease
- AF
Alexa Fluor
- ALS
amyotrophic lateral sclerosis
- BSA
bovine serum albumin
- CLB
cortical Lewy bodies
- CLEM
correlative light and electron microscopy
- DLB
dementia with Lewy bodies
- DN
dystrophic neuritis
- EMCCD
electron multiplying charge coupled device
- FTLD
fronto‐temporal lobar degeneration
- LB
Lewy bodies
- LN
Lewy neuritis
- MBP
myelin basic protein
- NCI
neuronal cytoplasmic inclusion
- NF
neurofilaments
- NFT
neurofibrillary tangles
- p.α‐syn
phosphorylated α‐synuclein
- p.Tau
hyperphosphorylated tau protein
- PBS
phosphate‐buffered saline
- PD
Parkinson's disease
- PSF
point spread function
- RT
room temperature
- SELFI
self‐interferences
- STORM
STochastic Optical Reconstruction Microscopy
- TDP‐43
Transactive response DNA‐binding protein 43
- TEM
transmission electron microscopy
- TIRF
total internal reflection fluorescence
- TLB
typical Lewy bodies
Introduction
Advances in neuroscience are closely linked to the progresses made in cell imaging. The invention of the microscope in the 17th century first allowed the characterization of the human brain at the cellular level and the emergence of clinical neurology, neurosurgery and psychiatry in the 18th and 19th centuries. Likewise, the understanding and classification of neurodegenerative disorders benefited from the development of immunostaining techniques and multichannel fluorescence imaging in the second part of the 20th century.
However, despite these advances, the diffraction barrier remains a resolution limit for conventional light microscopes, hindering the precise characterization of subcellular structures. Indeed, due to the diffraction of light, each fluorescent molecule appears as a spot of at least ∼250 nm in size, as described by the point‐spread function (PSF) [1, 2]. In conventional fluorescence microscopy, all the illuminated molecules emit their signal at the same time in a diffraction‐limited volume, limiting the spatial resolution of the final acquired image. Moreover, optical aberrations and out‐of‐focus blur also affect the signal‐to‐noise ratio and decrease the effectively achievable resolution, especially in the case of complex biological samples. Recently, super‐resolution microscopy techniques overcame this barrier using different approaches to distinguish fluorescent molecules that reside within the same diffraction‐limited volume [3, 4]. One of them, called STochastic Optical Reconstruction Microscopy (STORM), is based on single molecule localization by stochastically turning on photoswitchable fluorescent molecules within the diffraction limited volume at different time points so their signal does not substantially overlap [5, 6]. When isolated in space, the position of each individual fluorophore can be determined by localizing the centre positions of their signal. A super‐resolution image is then generated from numerous molecular localizations accumulated over time, offering a spatial resolution < 50 nm.
By improving the resolution towards the nanometre scale, STORM allows the visualization of nanoscopic structures while retaining the advantages of optical microscopy such as sample preparation, molecular identification through immunostaining and multicolour fluorescence imaging [4]. In the field of neuroscience, this new technique led to the characterization of the periodic structure of axonal cytoskeleton in neurons [7] and the spatial organization of synaptic proteins [8]. However, to date, STORM has mainly been used to image cultured cells and rodent brain samples [9].
In this study, we combined cell imaging protocols with neuropathology techniques to perform 2D‐, 3D‐ and two‐colour STORM on human brain samples from control subjects and patients with common age‐related neurodegenerative diseases.
Material and methods
Tissue samples
All post mortem brain samples used for STORM imaging were obtained from the Neurodegenerative Diseases Brain Bank of Angers University Hospital. Six patients with neurodegenerative disorders and three age‐matched control subjects were selected among the archived histological sections. Samples from prefrontal cortex, parietal cortex, temporal cortex, peri‐ventricular white matter and substantia nigra were considered. Clinical characteristics of the patients and controls are reported in Table S1.
Immunofluorescence staining
Cryopreserved brain samples were used for STORM imaging (samples bathed 30 s into iso‐pentane immersed in liquid nitrogen during brain autopsy and stored dry at −80°C). Tissue samples were cryo‐sectioned at −20°C into 5‐µm‐thick sections using a Leica CM3050 S cryostat (Leica Biosystems, Wetzlar, Germany) (Figure S1a). Each frozen section was retrieved onto 22 × 22 mm2 N°1 cover glass (Paul Marienfeld, Lauda‐Königshofen, Germany) and air‐dried for 45 min. After three washes in phosphate‐buffered saline (PBS) (PAN‐Biotech, Aidenbach, Germany), the sections were blocked and permeabilized in 0.1% triton X‐100 and 5 % bovine serum albumin (BSA) (Sigma‐Aldrich, Saint‐Louis, MO, USA) in PBS for 1 h at room temperature (RT). No antigen retrieval was used. The sections were then washed and incubated over night at 4°C with primary antibodies diluted in blocking solution. The next day, the sections were washed and incubated with appropriate AF‐conjugated secondary antibodies (Thermo Fisher Scientific, Waltham, MA, USA) diluted in blocking solution for 2 h at RT. TrueBlack® (Biotium, Fremont, CA, USA) was used to quench lipofuscin autofluorescence following manufacturer’s instructions. References and dilutions of antibodies and reagents are reported in Table S2.
STORM Acquisition and image reconstruction
For super resolution imaging, the cavity of a clean single depression slide (Paul Marienfeld, Lauda‐Königshofen, Germany) was filled with 50 μl of switching buffer (Abbelight, Paris, France), and covered by a coverslip, the sample side facing downward (Figure S1b). Excess buffer was carefully wiped away, and the coverslip was sealed with a two‐component silicone‐glue (Twinsil®, Picodent, Wipperfürth, Germany) (Figure S1c). After 10 min drying time, the device was placed on the stage of an inverted motorized microscope NIKON ECLIPSE Ti‐E (Nikon Instruments Europe, Amsterdam, The Netherlands) equipped with a CFI SR APO TIRF 100X ON1.49 objective, a Perfect Focus System and a total internal reflection fluorescence ILas2 module (Roper Scientific, Martinsried, Germany) (Figure S2). Image sequences were acquired with a single‐photon sensitive camera Evolve 128TM EMCCD 512 × 512 imaging array (Photometrics, AZ, USA). The signal was expanded with a 1.5× lens and focused on the EMCCD with pixel size of 16 μm. The pixel size on the image plane was 107 nm. Acquisitions were performed at fixed 25°C in a dark heating chamber (Okolab NA, Pozzuoli, Italy). Phase contrast was first used for orientation and focus adjustment. The TIRF angle was then adjusted for each channel to reduce the background excitation. The region of interest was defined using the 647 and 532 nm laser line, and the 405 nm laser line was used to assess the autofluorescence signal. Prior to STORM imaging, a multichannel conventional fluorescence microscopy image was acquired for subsequent comparison with STORM image (snapshot). The excitation power of either 647 or 532 nm laser line was then strongly increased (~50 to 100 mW before the objective lens) to induce fluorophore blinking and preform STORM imaging. The em‐gain of the camera was set to high amplification (300) to optimize the signal‐to‐noise ratio. Images were acquired with an integration time of 30 ms per frame.
Wavertracer software (Molecular devices, CA, USA) was used for real time localization and reconstruction [13, 14]. Spatial coordinates of each localized molecule were retrieved in real‐time in two or three dimensions. The blinking spot was detected using 6x6 pixels region in a 180‐250 intensity threshold range. In order to ensure optimal molecule density all along the acquisition process, an automatic feedback control on the 405 laser power was used. WaveTracer module uses a wavelet‐based segmentation algorithm filter, improving Gaussian fitting. The xy sigma of the PSF was fixed to 1. The localization uncertainty of the system, experimentally measured by imaging a point sample illuminated so that it emits a signal similar to the single molecules (on average), achieved 10.8 nm (R 2 0.96). The PSF Gaussian fitting of the image reconstruction was used to measure the standard deviation of the distribution. The total acquisition time points for each sequence were adapted to the observed structure and to the labelling density (5,000 to 20 000 frames) (Video S1). For high‐density STORM data sets (Video S3), we used a super‐resolution radial fluctuation (SRRF) analytical approach to prevent artefacts due to fluorophores overcrowding [15]. As in SMLM imaging, the input data were a sequential series of frames of the imaged structure. However, for SRRF analysis there was no requirement for the emitting fluorophores to be sparsely distributed. The following parameters were used for SRRF imaging: 0.3 Ring Radius, 10 Radiality Magnification and 8 Axes in Ring.
Imaris 8.0 software (Bitplane, Zurich, Switzerland) was used for image processing and visualization, and Metamorph 7.7 (Molecular Devices, CA, USA) was used for measurements. To estimate the diameter of filamentous structures (axons, branches of aggregated proteins), cross‐sectional widths were manually measured at multiple locations. To measure the area of rounded structures (axons, cores), the border was manually defined, and the area was automatically calculated. More than 300 STORM images have been acquired and analysed in total.
Immunohistochemistry
For immunohistochemistry, formalin‐fixed tissue samples embedded in paraffin from the same patients were sectioned at room temperature into 4 µm thick sections using a Leica RM125 microtome (Leica Biosystems, Wetzlar, Germany). Sections were collected on slides and immunostained using a fully automated IHC stainer Leica‐BOND III (Leica Biosystems, Wetzlar, Germany). Washes, blocking, antigen retrieval, incubation with appropriate primary and enzyme‐conjugated secondary antibody, enzymatic detection and haematoxylin counterstain were automatically performed following manufacturer’s instructions. Finally, samples were mounted and analysed under a Zeiss Axioscop40 microscope equipped with an Axio‐cam MRC5 camera, using AxioVisio 4.6 software (Carl Zeiss, Oberkochen, Germany).
Electron microscopy
Brain cortical samples from the middle frontal gyrus of the right hemisphere were collected between 1974 and 1986 [16]. Biopsies were immediately fixed in Karnovsky’s solution, postfixed in osmium and embedded in Araldite. Samples from a 38‐year‐old male subject who suffered a brain haemorrhage were used as control (laboratory identification #927). Samples from a 58‐year‐old male patient affected with a familial form of AD (M146L mutation of the PSEN1 gene) were used to study neurofibrillary tangles and senile plaques (laboratory identification #1507). Seventy‐nm‐thick sections were collected on copper grids and contrasted by incubation with 5% uranyl acetate followed by lead citrate for 5 min each. Sections were analysed with a Hitachi HT 7700 (Elexience, Verrieres le Buisson, France) electron microscope. More than 80 TEM images have been analysed in total for each sample.
Statistical analysis
Statistical analyses were conducted using PRISM software version 5.0 for Windows (GraphPad, La Jolla, CA). Comparisons of means were performed using Student’s t‐test for two groups analysis, and one‐way analysis of variance (anova) for multiple groups analysis, after verifying the Normal distribution and the homoscedasticity of data using Shapiro–Wilk’s test. In the absence of Normal distribution, the nonparametric Mann–Whitney U‐test was used. Differences were considered to be significant at P < 0.01.
Ethical approval
Brain tissue samples were stored in the biological resource centre « Neurodegenerative disorders» (national identifier BB‐0033‐00038) after being authorized by the regional ethics committee West II (declaration number DC‐2011‐146). The study protocol has been declared to the French commission for information technology and civil liberties (declaration number ar19‐0012v0). An anonymous database and a list of correspondence have been generated and stored on a hard disk of the University Hospital of Angers with restricted access.
Results
Super‐resolution imaging of human brain samples with STORM
To assess STORM imaging on human brain tissue, we first aimed to resolve well‐defined histological structures such as neocortical axon tracts. Frozen samples of prefrontal cortex from control subjects were cut by standard cryostat methods (Figure S1) and immunostained with an anti‐neurofilament primary antibody and a secondary antibody conjugated with the photoswitchable fluorophore Alexa Fluor (AF) 647. Coverslips with immunostained brain sections were bathed in a switching buffer (Figure S1) and placed on the stage of an inverted motorized microscope equipped with a high‐power laser module, a total internal reflection fluorescence (TIRF) system and an electron multiplying charge coupled device (EMCCD) single‐photon sensitive camera (Figure 1A, Figure S2).
Figure 1.
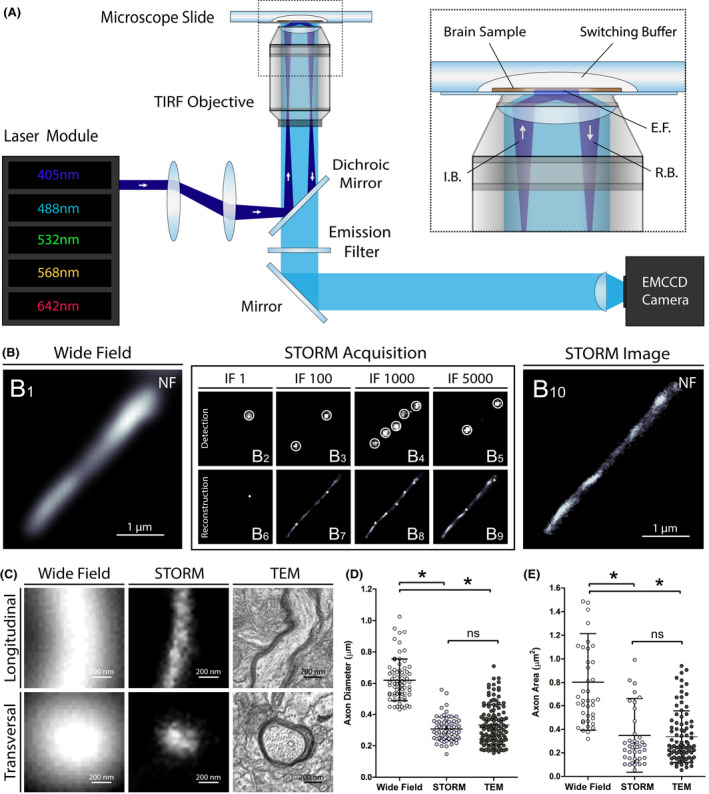
Super‐resolution imaging of human brain samples with STORM. (A) Schematic of the optical setup used for STORM imaging. I.B., incident beam; E.F, evanescent field; R.B., reflected beam. (B) STORM acquisition of a cortical axon in a human brain section immunostained for neurofilaments (NF): a conventional wide field fluorescence microscopy image was first acquired (B1), excitation power was then strongly increased to induce fluorophore blinking and thousands of frames were recorded (B2‐B5). The localization of the activated fluorescent molecules were detected on a per‐frame basis with sub‐pixel accuracy (B6‐B9). The accumulated localizations from all frames were then used to reconstruct a super‐resolution image (B10). IF, imaging frame. (C) Representative images of longitudinally and transversally sectioned prefrontal cortex axons acquired with conventional wide field fluorescence microscopy, STORM and transmission electron microscopy (TEM). (D and E) Axon diameters (longitudinal sections) and areas (transversal sections) measured in human brain using conventional fluorescence microscopy, STORM and TEM. Error bars indicate means with standard deviations. *P < .001
STORM is based on separating stochastically the emission of photoswitchable fluorescent molecules in time, so that each individual emitter can be distinguished from the others that reside within the same diffraction‐limited volume. For each acquisition, thousands of frames were recorded and the localizations of the activated fluorescent molecules were detected on a per‐frame basis with sub‐pixel accuracy. The accumulated localizations from all frames were then used to reconstruct a super‐resolution image (Figure 1B, Video S1). The total acquisition and reconstruction time for one super‐resolved image lasted from 5 to 10 min, depending on the number of acquired frames.
With the aim of assessing the final resolution of the technique, we imaged axon processes with conventional wide field fluorescence microscopy, STORM and transmission electron microscopy (TEM) in prefrontal cortex samples of age‐matched control subjects. Although neurofilament fibrils (~10 nm) could not be defined as well as when using TEM, the calibre of axons imaged with STORM was lower than when using conventional wide field fluorescence microscopy (Figure S3). For the same prefrontal axons, the apparent diameters and areas of longitudinally‐ and transversally‐sectioned axons were more than 50% smaller when measured with STORM compared to wide field fluorescence microscopy (P < 0.001 for the two parameters) (Figure 1C‐E, Tables S3 and S4). In contrast, there was no significant difference when comparing apparent diameters (P = 0.441) and areas (P = 0.596) measured with STORM and TEM (Figure 1D,E, Tables S3 and S4). STORM is thus a reliable technique allowing to image and analyse histological brain structures with a resolution <50 nm.
3D‐STORM and two‐colour STORM imaging of human brain samples
Three‐dimensional (3D) and multichannel imaging provide fundamental insights into the architectural organization of nanoscale structures. To perform 3D‐STORM in human brain sections we used an astigmatism‐based method [17]. After setting a cylindrical lens in the detection path of the microscope, the z coordinate of each fluorescent molecule was determined from the ellipticity of its image. Prefrontal cortical samples of control subjects were immunostained for neurofilaments (NF), and after reconstruction, axons were visualized in 3D with high resolution and an imaging depth of ~0.5 µm (Figure 2A, Video S2). The cylindrical shape of axon tracts was discernible and both longitudinal and transversal sections of the 3D modelled axons were similar in size to those obtained with 2D‐STORM.
Figure 2.
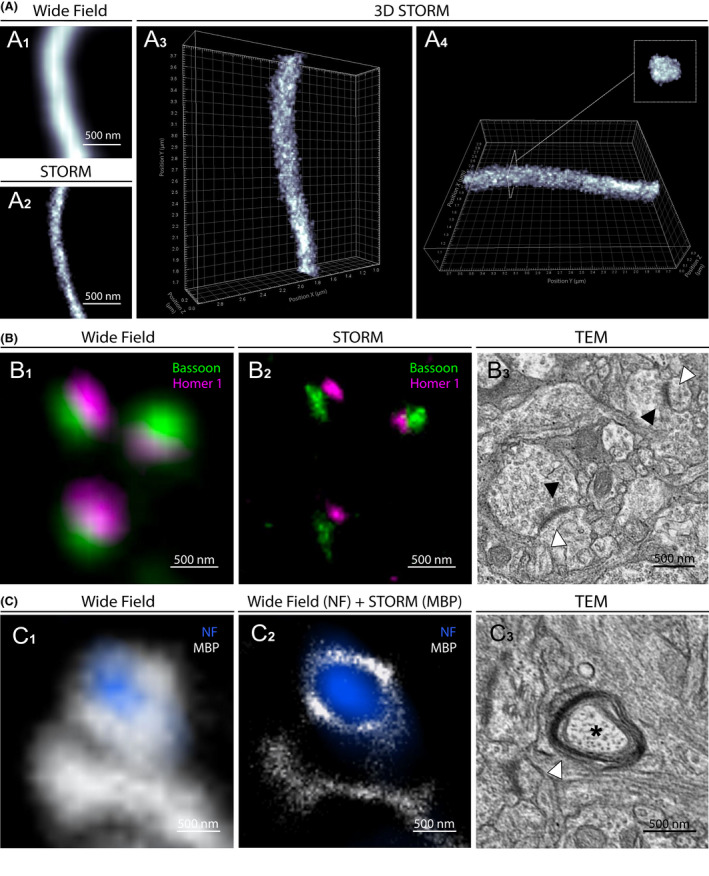
3D‐STORM and two‐colour STORM images of physiological structures in human brain. (A1) Conventional fluorescence microscopy image of a longitudinally sectioned axon immunostained for neurofilament (NF) in the prefrontal cortex. (A2) STORM image of the same area. (A3 and A4) 3D‐STORM reconstruction of the acquired axon. (B1) Conventional fluorescence microscopy image of pre and postsynaptic proteins Bassoon and Homer 1 in prefrontal cortex. (B2) Two‐colour STORM image of the same area resolving distinct synaptic clusters. (B3) Comparative TEM image of two synapses with pre (black arrowheads) and post (white arrowheads) synaptic compartments. (C1) Conventional fluorescence microscopy image of axonal NF and oligodendrocyte myelin basic protein (MBP) in peri‐ventricular white matter. (C2) Image of the same area combining conventional fluorescence microscopy (NF) and STORM (MBP). (C3) Comparative TEM image of a transversally sectioned axon (asterisk) surrounded by oligodendrocyte processes and myelin sheath (white arrowhead)
Then, to assess two‐colour STORM imaging on cortex samples, we immunostained human brain sections with two primary antibodies detecting nearby structures, the pre and postsynaptic Bassoon and Homer 1 proteins, and with secondary antibodies conjugated with the photoswitchable fluorophores AF 647 and AF 532 (Figure 2B, Figure S4). In conventional fluorescence microscopy, Bassoon and Homer 1 signals appeared diffuse and overlapping, and the synaptic clefts could not be precisely defined. In contrast, two‐colour STORM accurately distinguished between pre and postsynaptic protein clusters separated by the synaptic cleft, and defined the size, orientation and organization of the synapses. Interestingly, multichannel imaging combining conventional wide field fluorescence microscopy and STORM was also achievable, allowing super‐resolution imaging of brain structures using both photoswitchable and nonphotoswitchable fluorophores. Although less specific than two‐colour STORM, this technique provided valuable information about the layout of adjacent structures, as, for example the myelin sheath around axonal tracts (Figure 2C, Figure S4). The dimensions of the super‐resolved structures were comparable to those measured using TEM in the same regions (Figure 2B3,C3).
Together, these results demonstrate the possibility of performing 3D and two‐colour STORM imaging with a <50 nm resolution on human brain samples, providing valuable information to current topics in neuroscience, such as axonal organization, myelination and synaptic plasticity in the human brain. Understanding the pathophysiology of neurodegenerative disorders to identify novel therapeutic prospects is another major challenge in neuroscience. Since most of these diseases are defined by specific intra or extracellular protein aggregates in distinctive anatomical brain regions, novel insights must be disclosed on the precise characterization of the corresponding lesions. To this end, we performed STORM imaging of Amyloid‐β (Aβ), Tau, α‐synuclein and TDP‐43 aggregates in samples from patients affected with neurodegenerative disorders.
STORM imaging of Amyloid‐β and Tau proteinopathy
Alzheimer’s disease (AD) is the leading cause of dementia. The two main hallmarks of the disease are extracellular deposits of Aβ peptides, some of which constitute the core of senile plaques, and intraneuronal aggregates of hyperphosphorylated tau protein (p.Tau) called neurofibrillary tangles (NFT) [10]. Since aggregates can measure up to 100 µm, keeping an overall view of the whole lesions is critical to study Aβ and Tau pathology in the brain, whereas high‐resolution imaging is mandatory to characterize the nanoscale organization of the misfolded proteins.
Towards this goal, we imaged entire senile plaques and degenerating neurons with STORM. Tissue samples from the prefrontal, parietal and temporal cortex of AD patients were immunostained for Aβ and p.Tau (phospho Ser202, Thr205). Auto‐fluorescence quenchers were used to reduce the signal of lipofuscin, an autofluorescent pigment present in senescent neurons [18]. Nevertheless, lipofuscin signal was specifically detectable in neuron but did not preclude STORM acquisitions.
STORM images of ~30 μm diameter senile plaques and ~15 μm degenerating neurons with NFTs were acquired. While the Aβ fibrils and the paired helical filaments of Tau could not be identified as with TEM, STORM images provided highly resolved details of the nanoscale distribution and size of Aβ and p.Tau aggregates (Figure 3, Figure S5). Aggregated Aβ branches were reticulated and cross‐linked in the extracellular matrix, and their widths ranged from 60 to 240 nm (140.8 ± 39.6 nm, mean ± SD) (Figure S6). Intraneuronal p.Tau NFTs appeared denser, with a honeycombed structure in the soma and a filamentous organization in the axon. The presence of unstained spots within the aggregates suggested the inclusion of other components, such as proteins or organelles. These results emphasize that STORM can be used to image Aβ and p.Tau aggregates in brain samples from AD patients with high resolution.
Figure 3.
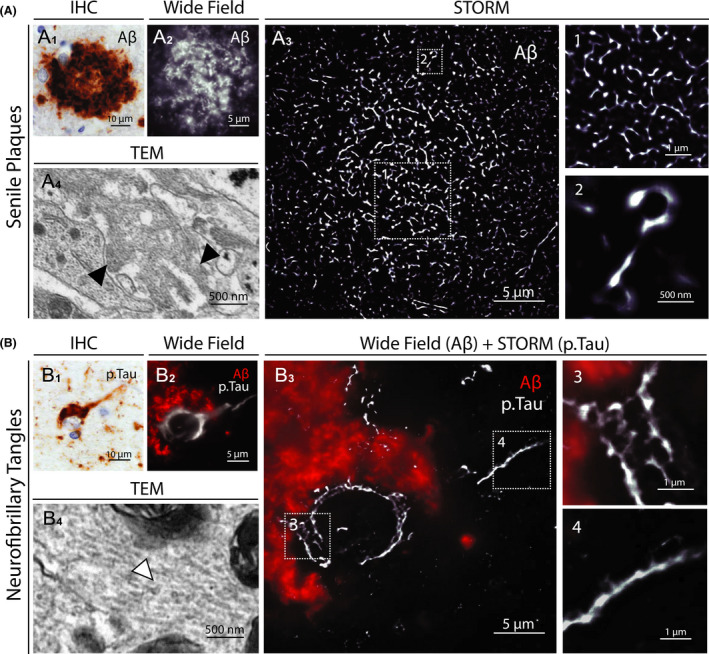
STORM images of senile plaques and neurofibrillary tangles in AD patient brain samples. (A1) Representative image of a senile plaque in the neocortex of an AD patient (immunohistochemical detection of Aβ). (A2) Conventional fluorescence microscopy image of a whole senile plaque in a neocortex section of the same patient immunostained for Aβ. (A3) STORM image of the same area. The insets (1 and 2) show close‐up details of the distribution and size of aggregated Aβ branches. (A4) Comparative TEM image of Aβ fibrils (black arrowheads) in a senile plaque. (B1) Representative image of neurofibrillary tangles in the neocortex of an AD patient (immunohistochemical detection of p.Tau). (B2) Conventional fluorescence microscopy image of neurofibrillary tangles within the soma of a whole degenerating neuron surrounded by Aβ deposition in a neocortex section of the same patient. (B3) Same neuron imaged by combining conventional fluorescence microscopy (Aβ) and STORM (p.Tau). The insets (3 and 4) show close‐up details of the honeycombed structure of p.Tau aggregates in the soma and the filamentous organization in the axon. (B4) Comparative TEM image of Tau filaments (white arrowhead) in neurofibrillary tangles
STORM Imaging Of Lewy pathology
Parkinson's disease (PD) and dementia with Lewy bodies (DLB) are two neurodegenerative diseases characterized by the presence of intraneuronal phosphorylated α‐synuclein (p.α‐syn) immuno‐reactive inclusions, called Lewy bodies (LB) [19]. The structure of LBs varies according to their localization within the central nervous system. Two main LB types can be observed by immunohistochemistry: typical Lewy bodies (TLB) with a pale core surrounded by a dense halo mainly found in the brainstem, and smaller cortical Lewy bodies (CLB), lacking the central core and mainly detected in the neocortex. Accumulation of p.α‐syn in dystrophic axons called Lewy neurites (LN) can also be observed. To date, the structure of LBs remains unclear, as the resolution of conventional fluorescence microscopy is too low to characterize their internal architecture and TEM does not provide sufficient information about their protein content and organization.
To characterize LB organization at the nanoscale level with a molecular staining approach, we performed STORM imaging on brain samples from PD and DLB patients. Substansia nigra and prefrontal cortex sections were immunostained with an anti‐p.α‐syn (phospho Ser129) antibody and images of TLBs, CLBs and LNs were acquired. The ring‐shaped appearance of TLBs were observed both with conventional and super‐resolution imaging, although only the STORM images defined precisely their architecture (Figure 4A). The pale core appeared unstained, whereas the peripheral dense halo was made of reticulated p.α‐syn. Likewise, STORM imaging of CLBs revealed dense honeycomb structures that could not be observed by conventional fluorescence microscopy (Figure 4B,C). As for p.Tau aggregates, the unstained cores and spots observed in LBs might correspond to protein partners or trapped organelles. Indeed, Lewy bodies are known to be multiprotein complexes composed of more than 100 proteins, including p.Tau [20]. We thus performed two‐colour STORM imaging of LBs using antibodies against p.Tau and p.α‐syn, to precisely define the internal architecture of the lesion and specifically distinguish one protein from the other (Figure 5A). STORM imaging accurately measured the width of aggregated p.α‐syn branches and the area of the unstained cores observed in CLBs, averaging 281.2 ± 95.8 nm and 0.133 ± 0.130 μm2 respectively (mean ± SD) (Figure 5B). Finally, two‐colour STORM imaging of LNs revealed the internal organization of the neurites with a core of aggregated p.α‐syn bound to neurofilaments (Figure 5C, Figure S7). These very first STORM images of p.α‐syn aggregates hold great promise for characterizing the composition and spatial organization of Lewy pathology in human brain.
Figure 4.
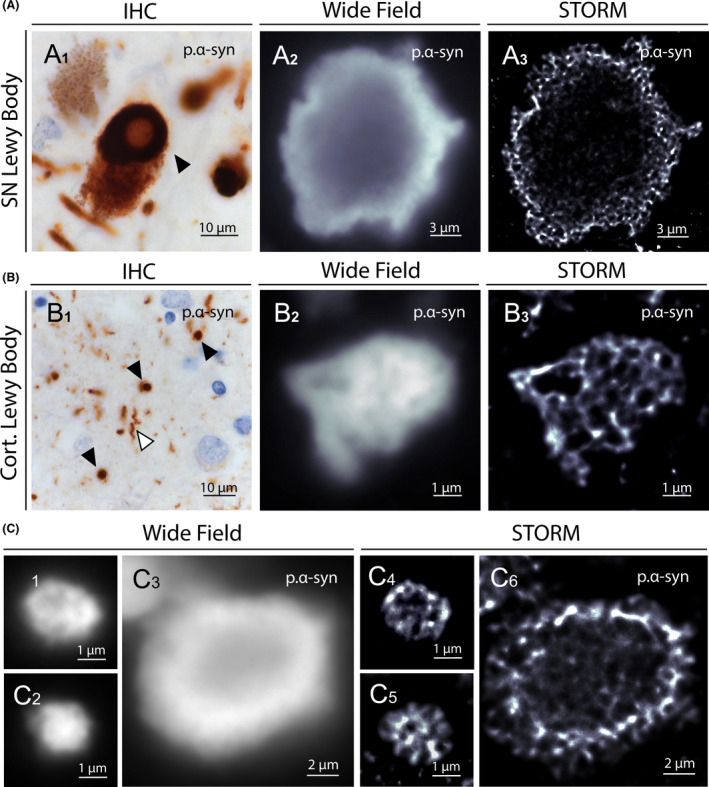
STORM images of Lewy bodies in PD and DLB patients brain samples. (A1) Representative image of a typical Lewy body (black arrowhead) in the substantia nigra of a PD patient (immunohistochemical detection of p.α‐syn). (A2) Conventional fluorescence microscopy image of a Lewy body in a substantia nigra section of the same patient immunostained for p.α‐syn. (A3) STORM image of the same LB resolving distinctly the α‐synuclein reticulated corona and the unstained core. (B1) Representative image of cortical Lewy bodies (black arrowheads) and Lewy neurite (white arrowhead) in the neocortex of an PD patient. (B2) Conventional fluorescence microscopy image of a cortical Lewy body in a neocortex section of the same patient immunostained for p.α‐syn. (B3) STORM image of the same area revealing the dense honeycombed organization of the Lewy body. (C) Conventional fluorescence microscopy representative images of cortical (C1 and C2) and SN (C3) Lewy bodies immunostained for p.α‐syn. STORM images of the same areas (C4, C5 and C6) highlight the distinct internal organization of the protein aggregates
Figure 5.
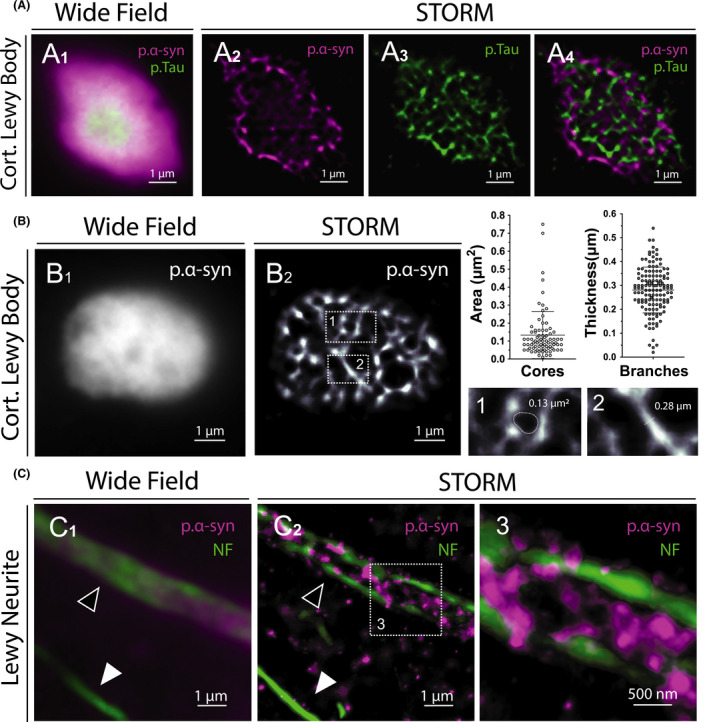
Colocalization and ultrastructural analysis of Lewy bodies and Lewy Neurites. (A1) Conventional fluorescence microscopy image of a cortical Lewy body immunostained for p.α‐syn and p.Tau. (A2‐A4) Two‐Colour STORM image of the same area revealing the internal architecture of the lesion and allowing to distinguish one protein from the other. (B) Ultrastructural analysis of a cortical Lewy body (B1) using STORM (B2). Areas of the unstained cores and widths of p.α‐syn branches were measured on super‐resolved images. Error bars indicate means with standard deviations. (C1) Conventional fluorescence microscopy image of a Lewy neurite (black arrowhead) immunostained for p.α‐syn and neurofilaments (NF). (C2) Two‐Colour STORM image of the same area showing p.α‐syn dense aggregates bounded by neurofilaments (enlarged in 3). Note the unaffected axon with normal calibre (white arrowhead)
STORM imaging of TDP‐43 neuronal inclusions
Transactive response DNA‐binding protein 43 (TDP‐43), encoded by TARDBP gene, is a DNA/RNA binding protein predominantly located in the nucleus of cells under physiological condition, whereas the accumulation of misfolded TDP‐43 in the cytosol and axons of degenerating neurons is a pathological hallmark of amyotrophic lateral sclerosis (ALS) and fronto‐temporal lobar degeneration (FTLD) [21, 22]. TDP‐43 aggregates are called neuronal cytoplasmic inclusions (NCI) when located in the cytosol, and dystrophic neurites (DN) when located in axons. The physiopathological mechanism leading to neuronal degeneration in both ALS and FTLD associates the cytoplasmic toxicity of aggregated TDP‐43 and the nuclear loss of function of the protein. In frontal cortical sections from a control subject, STORM imaging revealed the presence of dense nuclear TDP‐43 clusters, whereas other nuclear zones were unstained, whereas the TDP‐43 signal appeared diffuse and heterogeneous in conventional wide field fluorescence microscopy (Figure 6A, Figure S8). NCI and DN imaging with STORM in frontal and temporal cortex of patients affected with FTLD revealed compact and granular cytoplasmic structures, slightly denser and less reticulated than p.Tau and p.α‐syn aggregates (Figure 6B,C, Figure S8). Interestingly, empty vacuoles were also defined in the core of NCI and DN, suggesting again the presence of additional unstained components within these aggregates. Thus, STORM imaging of TDP‐43 allowed to resolve the physiological distribution of the protein in the nuclear compartment, and its spatial organization within pathological cytoplasmic aggregates.
Figure 6.
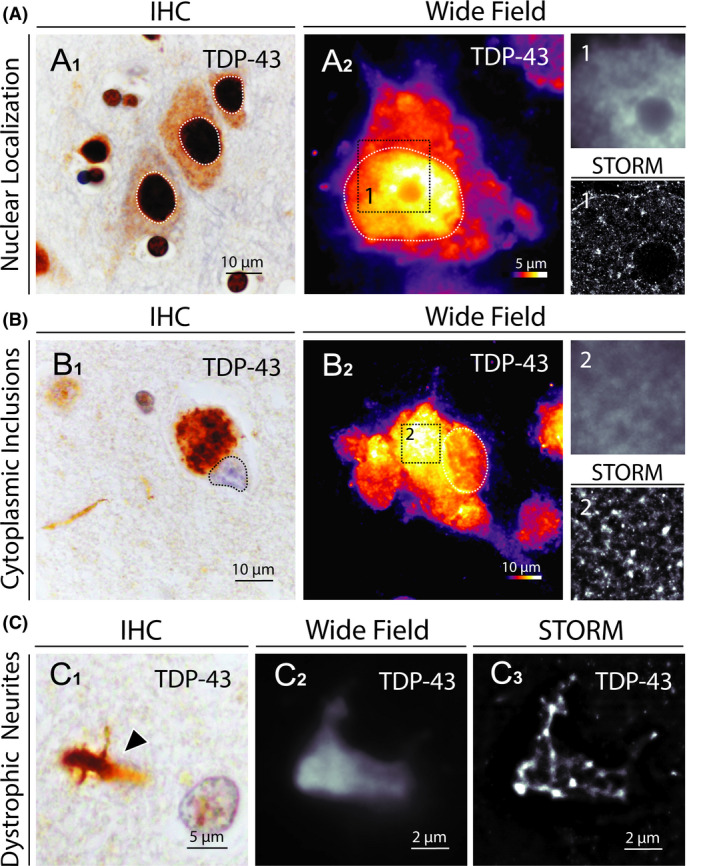
STORM images of TDP‐43 aggregates in FTLD brain samples. (A1) Representative image of normal TDP‐43 localization in cortical neurons (immunohistochemical detection of TDP‐43 in the prefrontal cortex of a control subject). (A2) Conventional fluorescence microscopy image of TDP‐43 distribution in a nondegenerating neuron in the prefrontal cortex of the same subject (signal intensity scale ranging from 1 to 254 UI). Nuclear compartments are defined with dotted lines. The insets (1) show close‐up details of TDP‐43 distribution within the nucleus as assessed by conventional wide field fluorescence microscopy (up) and STORM (down). (B1) Representative image of TDP‐43 cytoplasmic inclusions in a cortical neuron (immunohistochemical detection of TDP‐43 in the prefrontal cortex of a FTLD patient). (B2) Conventional fluorescence microscopy image of TDP‐43 distribution in a degenerating neuron in the prefrontal cortex of the same patient (signal intensity scale ranging from 1 to 254 UI). Nuclear compartments are defined with dotted lines. The insets (2) show close‐up details of TDP‐43 aggregate as assessed by conventional wide field fluorescence microscopy (up) and STORM (down). (C) Representative light microscopy, wide field fluorescence microscopy and STORM images of TDP‐43 aggregates in dystrophic neurites (black arrowhead in C1) in the prefrontal cortex of a FTLD patient
Discussion
In this work, we combined super resolution microscopy and neuropathological techniques to analyse human brain sections. This strategy characterized with a <50 nm resolution the architecture of physiological structures such as axons and synapses, and imaged with unprecedented details Aβ, Tau, α‐synuclein and TDP‐43 pathological aggregates in samples from patients affected with neurodegenerative disorders.
To date, the main approach to image nanoscopic structures in tissues relies on transmission electron microscopy, a time‐consuming technique which requires ultrathin tissue sections (50‐70 nm) with stringent sample preparation and limits immune‐targeting diversity and 3D acquisition. Conversely, STORM offers the advantages of optical fluorescence microscopy with respect to sample preparation, vast observation fields, multiple molecular labelling and 3D acquisition, with image acquisition and reconstruction taking only a few minutes. The sample preparation workflow developed in this work for human brain tissue observation is timesaving and easily reproducible, as it is inspired by protocols routinely used for conventional fluorescence microscopy. It can be used on samples stored in a brain bank, with reagents and antibodies commonly used in clinical neuropathology departments and neuroscience research laboratories.
Nevertheless, performing STORM on human brain samples requires fine adjustments to generate high resolution images. Two major issues when imaging tissue sections are tissue autofluorescence and sample‐induced aberrations that can distort and blur single‐molecule emission patterns. Here, to limit optical artefacts and reduce the background generated by fluorophores located outside the focal plane, we used 5 μm thin tissue sections and a TIRF illumination [23], where the incident laser beam is reflected at the coverslip and the sample is excited by the resulting evanescent wave. Imaging thicker brain sections could be considered but will require complementary techniques such as adaptive optics‐based correction, self‐interferences (SELFI) method or tissue clearing, to avoid excessive background and aberrations [24, 25, 26]. Sample preparation and immunostaining are also critical, the used fluorophores requiring high photoswitching kinetics and sufficient brightness to be detected properly. In this work, we used AF 647 and AF 532, as they exhibit appropriate photoswitching characteristics in antioxidant reagents. They also have long excitation and emission wavelengths, thus limiting background signal from tissue autofluorescence. Primary antibodies routinely used for immunostaining in clinical neuropathology departments have been used here for STORM, but the protocol can be adapted to smaller tags (fluorescent probes or primary antibodies directly conjugated with fluorophores) which would reduce linker length errors during the localization procedure.
To date, STORM has mainly been used to image nanoscale structures in cultured cells. However, the technique has recently been extended to rodent brain sections for histological analysis. As in our study, two‐colour STORM allowed visualization of the molecular architecture of synapses, accurately identifying pre and postsynaptic protein clusters and defining their size, morphology and orientation [8, 27, 28, 29]. STORM was also used to image pathological Aβ aggregates in mouse models of Alzheimer’s disease, leading to results comparable to ours in human, both for plaque morphologies and Aβ fibril widths, ranging from 50 to 300 nm [25, 30]. Finally, STORM imaging of α‐synuclein aggregates in a mouse model of Parkinson disease allowed to visualize α‐synuclein aggregation in dopaminergic neurons [31], although the inclusions remained scattered and unstructured and no Lewy bodies were observed. These last results underline the relevance of human brain analyses for studying the architecture and composition of pathological inclusions that cannot be induced in models.
To date, a single study reported STORM imaging on human brain tissue to visualize neurofilaments, myelin and astroglial processes within subcortical white matter [9]. This study found axonal diameters in the same range as our measurements and TEM values, highlighting the reliability and reproducibility of the technique. Nevertheless, the authors faced limitations in sample preparation, hindering multiple immunostaining and limiting STORM acquisition to the white matter. Conversely, the protocol developed in this study allowed us to observe physiological and pathological structures in multiple areas of the human brain with a nanometre‐scale precision.
The ultrastructural analysis of neuronal lesions is crucial to understand the pathogenesis and progression of neurodegenerative diseases. In this context and with the aim of improving neuropathological analyses, the development of novel imaging approaches is a current challenge in neuroscience. For instance the use of 3D multicolour STED microscopy correlative light and electron microscopy (CLEM) to analyse post mortem human brain tissue recently highlighted that Lewy bodies show an onion skin‐like architecture with a Ser129‐p aSyn external layer, and contain crowded membranous material from vesicles and fragmented organelles [32, 33]. Moreover, these techniques allowed to identify different pathological phenotypes of Lewy bodies within the CNS of the patients, which may reflect intracellular maturation stages of Lewy pathology [33]. Likewise, another new approach based on biochemical isolation of pathological TDP‐43 recently provided evidence that aggregates extracted from patients with different subtypes of FTLD exhibited distinct morphological features and composition [34]. All these results indicate that the clinical and pathological heterogeneity observed in neurodegenerative disorders could originate from alternate ultrastructure and composition of neuronal lesions.
Conclusions
By revealing novel structures and features that had not been previously visualized, STORM imaging of fixed human tissue samples opens further gates to a more comprehensive understanding of the human brain organization and revelations about the underlying mechanisms responsible for common neurological diseases. The convenience of this technique should open a straightforward extension of STORM applications for super‐resolution imaging of human brain samples, with promising new avenues to current challenges in neuroscience.
Ethical Approval
The use of human biological samples in this study has been given ethical approval by the regional ethics committee West II (biological resource centre national identifier BB‐0033‐00038, declaration number DC‐2011‐146). The study protocol has been declared to the French commission for information technology and civil liberties (declaration number ar19‐0012v0).
Author Contributions
P.C. initiated and led all aspects of the project, conducted the experiments, analysed data and wrote the manuscript. A.C. and F.L. supervised the project. A.C. optimized the imaging protocol. F.L., L.R., C.D. and J.P.J. helped in developing the tissue preparation method. S.M. conducted TEM acquisitions. P.C., F.L., S.M., L.R., M.D., C.V., G.L., C.D., J.‐P.J., J.C. and A.C. proofread the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare no Conflict of interest.
Supporting information
Figure S1. Sample preparation workflow for STORM acquisition. (a) Cryopreserved brain samples (black arrowhead) were cryo‐sectioned at −20°C into 5 µm thick sections and retrieved onto 22 × 22 mm2 N°1 cover glasses. (b) Once the sample immunostained, the cavity of a clean single depression slide was filled with 50 μl of switching buffer (black arrowhead) and covered by the coverslip. (c) Excess buffer was then carefully wiped away, and the coverslip was sealed on the slide with a two‐component silicone‐glue.
Figure S2. Detailed microscope setup used for STORM imaging. (a) Inverted motorized microscope NIKON ECLIPSE Ti‐E. (b) Central computer equipped with Metamorph 7.7 software. (c) Dark heating chamber. (d) Evolve 128TM EMCCD camera. (e) Andor NEO sCMOS camera. (f) Anti‐vibration table. (g) LED bench. (h) Piezoelectric driver system. (i) Total Internal Reflection Fluorescence (TIRF) module. (j) Laser bench.
Figure S3. Conventional wide field fluorescence and STORM imaging of the same axonal process. (A1) Wide field fluorescence image of a longitudinally sectioned axon immunostained for neurofilaments (NF) in prefrontal cortex. (A2) STORM image of the same area. (B) Representation of the cross‐sectional profiles (dotted lines in a) through axonal process. Grey values are normalized from 0 (minimum grey value) to 1 (maximum grey value). Error bars indicate mean with standard error, n = 8 measures per image.
Figure S4. Individual colour channel sequences of the composite images in Figure 2B,C.
Figure S5. Individual colour channel sequences of the composite images in Figure 3B.
Figure S6. (A) Conventional wide field fluorescence and STORM imaging of the same amyloid plaque. (B) Aβ fibrils width in senile plaques measured in STORM images.
Figure S7. Individual colour channel sequences of the composite images in Figure 5B.
Figure S8. Delimitation of the nuclear compartment in neurons (Figure 6A,B) using Lamin A/C signal.
Table S1. Controls and patients clinical and pathological characteristics.
Table S2. Antibodies and reagents.
Table S3. Apparent diameters of longitudinally sectioned prefrontal cortex axons acquired with conventional wide field fluorescence microscopy, stochastic optical reconstruction microscopy (STORM), and transmission electron microscopy (TEM).
Table S4. Areas of transversally sectioned prefrontal cortex axons acquired with conventional wide field fluorescence microscopy, stochastic optical reconstruction microscopy (STORM), and transmission electron microscopy (TEM).
Video S1. STORM imaging of a cortical axon in a human brain section immunostained for neurofilaments.
Video S2. 3DSTORM imaging of a cortical axon in a human brain section immunostained for neurofilaments.
Video S3. Representative STORM acquisition of a structure with high‐density emitting fluorophores (cortical Lewy body immunostained for α‐synuclein).
Acknowledgments
The authors are grateful to the donors and their families. We also thank Dr Khalid Hamid El Hachimi for providing biopsies for electron microscopy, and C. Dumez, I. Viau and L. Denechaud for technical assistance. We thank J.‐B. Sibarita, R. Galland and F. Cordelières (University of Bordeaux, France ) for technical assistance. This work was supported by the University Hospital of Angers (Grant No 2019‐264900_036), the French National Institute for Health and Medical Research (INSERM Research Fellow 2017–2019) and the European Regional Development Fund (ERDF).
Codron P., Letournel F., Marty S., Renaud L., Bodin A., Duchesne M., Verny C., Lenaers G., Duyckaerts C., Julien J.-P., Cassereau J. and Chevrollier A. (2021) Neuropathology and Applied Neurobiology 47, 127–142 STochastic Optical Reconstruction Microscopy (STORM) reveals the nanoscale organization of pathological aggregates in human brain
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Toomre D, Bewersdorf J. A new wave of cellular imaging. Annu Rev Cell Dev Biol. 2010; 26: 285–314 [DOI] [PubMed] [Google Scholar]
- 2. Baddeley D, Bewersdorf J. Biological insight from super‐resolution microscopy: What we can learn from localization‐based images. Annu Rev Biochem. 2018; 87: 965–989 [DOI] [PubMed] [Google Scholar]
- 3. Schermelleh L, Ferrand A, Huser T, Eggeling C, Sauer M, Biehlmaier O et al Super‐resolution microscopy demystified. Nat Cell Biol. 2019; 21: 72–84 [DOI] [PubMed] [Google Scholar]
- 4. Sigal YM, Zhou R, Zhuang X. Visualizing and discovering cellular structures with super‐resolution microscopy. Science 2018; 361: 880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rust MJ, Bates M, Zhuang X. Sub‐diffraction‐limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Methods. 2006; 3: 793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS et al Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006; 313: 1642–1645 [DOI] [PubMed] [Google Scholar]
- 7. Xu K, Zhong G, Zhuang X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 2013; 339: 452–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dani A, Huang B, Bergan J, Dulac C, Zhuang X. Superresolution imaging of chemical synapses in the brain. Neuron 2010; 68: 843–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hainsworth AH, Lee S, Foot P, Patel A, Poon WW, Knight AE. Super‐resolution imaging of subcortical white matter using stochastic optical reconstruction microscopy (STORM) and super‐resolution optical fluctuation imaging (SOFI). Neuropathol Appl Neurobiol. 2018; 44: 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al National Institute on Aging‐Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012; 123: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003; 24: 197–211 [DOI] [PubMed] [Google Scholar]
- 12. Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E et al A harmonized classification system for FTLD‐TDP pathology. Acta Neuropathol. 2011; 122: 111–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kechkar A, Nair D, Heilemann M, Choquet D, Sibarita JB. Real‐time analysis and visualization for single‐molecule based super‐resolution microscopy. PLoS One 2013; 8: e62918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Izeddin I, Boulanger J, Racine V, Specht CG, Kechkar A, Nair D et al Wavelet analysis for single molecule localization microscopy. Opt Express. 2012; 20: 2081–2095 [DOI] [PubMed] [Google Scholar]
- 15. Gustafsson N, Culley S, Ashdown G, Owen DM, Pereira PM, Henriques R. Fast live‐cell conventional fluorophore nanoscopy with ImageJ through super‐resolution radial fluctuations. Nat Commun. 2016; 7: 12471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Androuin A, Potier B, Nägerl UV, Cattaert D, Danglot L, Thierry M et al Evidence for altered dendritic spine compartmentalization in Alzheimer's disease and functional effects in a mouse model. Acta Neuropathol. 2018; 135: 839–854 [DOI] [PubMed] [Google Scholar]
- 17. Huang B, Wang W, Bates M, Zhuang X. Three‐dimensional super‐resolution imaging by stochastic optical reconstruction microscopy. Science 2008; 319: 810–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin‐like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999; 47: 719–730 [DOI] [PubMed] [Google Scholar]
- 19. Braak H, Del Tredici K. Invited article: Nervous system pathology in sporadic Parkinson disease. Neurology 2008; 70: 1916–1925 [DOI] [PubMed] [Google Scholar]
- 20. Arima K, Hirai S, Sunohara N, Aoto K, Izumiyama Y, Uéda K et al Cellular co‐localization of phosphorylated tau‐ and NACP/alpha‐synuclein‐epitopes in lewy bodies in sporadic Parkinson's disease and in dementia with Lewy bodies. Brain Res. 1999; 843: 53–61 [DOI] [PubMed] [Google Scholar]
- 21. Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H et al TDP‐43 is a component of ubiquitin‐positive tau‐negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006; 351: 602–611 [DOI] [PubMed] [Google Scholar]
- 22. Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT et al Ubiquitinated TDP‐43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006; 314: 130–133 [DOI] [PubMed] [Google Scholar]
- 23. Boulanger J, Gueudry C, Münch D, Cinquin B, Paul‐Gilloteaux P, Bardin S et al Fast high‐resolution 3D total internal reflection fluorescence microscopy by incidence angle scanning and azimuthal averaging. Proc Natl Acad Sci U S A. 2014; 111: 17164–17169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bon P, Linarès‐Loyez J, Feyeux M, Alessandri K, Lounis B, Nassoy P et al Self‐interference 3D super‐resolution microscopy for deep tissue investigations. Nat Methods. 2018; 15: 449–454 [DOI] [PubMed] [Google Scholar]
- 25. Mlodzianoski MJ, Cheng‐Hathaway PJ, Bemiller SM, McCray TJ, Liu S, Miller DA et al Active PSF shaping and adaptive optics enable volumetric localization microscopy through brain sections. Nat Methods. 2018; 15: 583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gustavsson AK, Petrov PN, Moerner WE. Light sheet approaches for improved precision in 3D localization‐based super‐resolution imaging in mammalian cells. Opt Express. 2018; 26: 13122–13147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schoen M, Reichel JM, Demestre M, Putz S, Deshpande D, Proepper C et al Super‐resolution microscopy reveals presynaptic localization of the ALS/FTD related protein FUS in hippocampal neurons. Front Cell Neurosci. 2016; 9: 496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sigal YM, Speer CM, Babcock HP, Zhuang X. Mapping synaptic input fields of neurons with super‐resolution imaging. Cell 2015; 163: 493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dudok B, Barna L, Ledri M, Szabó SI, Szabadits E, Pintér B et al Cell‐specific STORM super‐resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat Neurosci. 2015; 18: 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuan P, Condello C, Keene CD, Wang Y, Bird TD, Paul SM et al TREM2 haplodeficiency in mice and humans impairs the microglia barrier function leading to decreased amyloid compaction and severe axonal dystrophy. Neuron 2016; 90: 724–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wegrzynowicz M, Bar‐On D, Calo' L, Anichtchik O, Iovino M, Xia J et al Depopulation of dense α‐synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson's disease model. Acta Neuropathol. 2019; 138: 575–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shahmoradian SH, Lewis AJ, Genoud C, Hench J, Moors TE, Navarro PP et al Lewy pathology in Parkinson's disease consists of crowded organelles and lipid membranes. Nat Neurosci. 2019; 22: 1099–1109 [DOI] [PubMed] [Google Scholar]
- 33. Moors TE, Maat CA, Niedieker D, Mona D, Petersen D, Timmermans‐Huisman E et al Subcellular orchestration of alpha‐synuclein variants in Parkinson’s disease brains revealed by 3D multicolor STED microscopy. bioRxiv. 2019; 470476: https://www.biorxiv.org/content/10.1101/470476v3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laferrière F, Maniecka Z, Pérez‐Berlanga M, Hruska‐Plochan M, Gilhespy L, Hock EM et al TDP‐43 extracted from frontotemporal lobar degeneration subject brains displays distinct aggregate assemblies and neurotoxic effects reflecting disease progression rates. Nat Neurosci. 2019; 22: 65–77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sample preparation workflow for STORM acquisition. (a) Cryopreserved brain samples (black arrowhead) were cryo‐sectioned at −20°C into 5 µm thick sections and retrieved onto 22 × 22 mm2 N°1 cover glasses. (b) Once the sample immunostained, the cavity of a clean single depression slide was filled with 50 μl of switching buffer (black arrowhead) and covered by the coverslip. (c) Excess buffer was then carefully wiped away, and the coverslip was sealed on the slide with a two‐component silicone‐glue.
Figure S2. Detailed microscope setup used for STORM imaging. (a) Inverted motorized microscope NIKON ECLIPSE Ti‐E. (b) Central computer equipped with Metamorph 7.7 software. (c) Dark heating chamber. (d) Evolve 128TM EMCCD camera. (e) Andor NEO sCMOS camera. (f) Anti‐vibration table. (g) LED bench. (h) Piezoelectric driver system. (i) Total Internal Reflection Fluorescence (TIRF) module. (j) Laser bench.
Figure S3. Conventional wide field fluorescence and STORM imaging of the same axonal process. (A1) Wide field fluorescence image of a longitudinally sectioned axon immunostained for neurofilaments (NF) in prefrontal cortex. (A2) STORM image of the same area. (B) Representation of the cross‐sectional profiles (dotted lines in a) through axonal process. Grey values are normalized from 0 (minimum grey value) to 1 (maximum grey value). Error bars indicate mean with standard error, n = 8 measures per image.
Figure S4. Individual colour channel sequences of the composite images in Figure 2B,C.
Figure S5. Individual colour channel sequences of the composite images in Figure 3B.
Figure S6. (A) Conventional wide field fluorescence and STORM imaging of the same amyloid plaque. (B) Aβ fibrils width in senile plaques measured in STORM images.
Figure S7. Individual colour channel sequences of the composite images in Figure 5B.
Figure S8. Delimitation of the nuclear compartment in neurons (Figure 6A,B) using Lamin A/C signal.
Table S1. Controls and patients clinical and pathological characteristics.
Table S2. Antibodies and reagents.
Table S3. Apparent diameters of longitudinally sectioned prefrontal cortex axons acquired with conventional wide field fluorescence microscopy, stochastic optical reconstruction microscopy (STORM), and transmission electron microscopy (TEM).
Table S4. Areas of transversally sectioned prefrontal cortex axons acquired with conventional wide field fluorescence microscopy, stochastic optical reconstruction microscopy (STORM), and transmission electron microscopy (TEM).
Video S1. STORM imaging of a cortical axon in a human brain section immunostained for neurofilaments.
Video S2. 3DSTORM imaging of a cortical axon in a human brain section immunostained for neurofilaments.
Video S3. Representative STORM acquisition of a structure with high‐density emitting fluorophores (cortical Lewy body immunostained for α‐synuclein).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


