Abstract
The ability of ketogenic low‐carbohydrate (CHO) high‐fat (K‐LCHF) diets to enhance muscle fat oxidation has led to claims that it is the ‘future of elite endurance sport’. There is robust evidence that substantial increases in fat oxidation occur, even in elite athletes, within 3–4 weeks and possibly 5–10 days of adherence to a K‐LCHF diet. Retooling of the muscle can double exercise fat use to ∼1.5 g min−1, with the intensity of maximal rates of oxidation shifting from ∼45% to ∼70% of maximal aerobic capacity. Reciprocal reductions in CHO oxidation during exercise are clear, but current evidence to support the hypothesis of the normalization of muscle glycogen content with longer‐term adaptation is weak. Importantly, keto‐adaptation may impair the muscle's ability to use glycogen for oxidative fates, compromising the use of a more economical energy source when the oxygen supply becomes limiting and, thus, the performance of higher‐intensity exercise (>80% maximal aerobic capacity). Even with moderate intensity exercise, individual responsiveness to K‐LCHF is varied, with extremes at both ends of the performance spectrum. Periodisation of K‐LCHF with high CHO availability might offer opportunities to restore capacity for higher‐intensity exercise, but investigations of various models have failed to find a benefit over dietary approaches based on current sports nutrition guidelines. Endurance athletes who are contemplating the use of K‐LCHF should undertake an audit of event characteristics and personal experiences to balance the risk of impaired performance of higher‐intensity exercise with the likelihood of an unavoidable depletion of carbohydrate stores.
Keywords: athletic performance, exercise economy, substrate utilisation
Abstract figure legend CHO ox: rate of carbohydrate oxidation; CPT: carnitine palmitoyltransferase; Fat ox: rate of fat oxidation; FAT/CD36: Fatty Acid Translocase; GNG = gluconeogenesis; [Glycogen]: concentration of muscle glycogen; HSL: hormone sensitive lipase; [IMTG]: concentration of intramuscular triglyceride; Max: maximal; O2:oxygen; PDHa: active form of Pyruvate Dehydrogenase; ↔: remains the same; ↔: remains the same but with a variable response; ↑: is increased; ↓: is decreased.

Introduction
Endurance sports are classified as continuous events of >30 min duration, with activities lasting >4–5 h being considered ultra‐endurance (Saris et al. 2003). They rely on oxygen‐dependent resynthesis of adenosine triphosphate (ATP), which requires both adequate delivery of oxygen to the mitochondria and the availability of carbohydrate (CHO) and lipid fuels (Joyner & Coyle, 2008). Competitive success is awarded to athletes who sustain the highest power outputs/speeds for the duration of their event. Indeed, race pace in many endurance events (e.g. the marathon, cycling time trials, cross‐country skiing events) involves a very high percentage of an individual's maximal aerobic intensity (Joyner et al. 2011; Tucker, 2016; Burke et al. 2019). In longer events of lower ‘background’ intensity (e.g. Ironman triathlon, cycling road races and stage races), tactical, terrain and pacing characteristics require bursts of activity at or above critical velocity (Fernandez‐Garcia et al. 2000; Bentley et al. 2002; Tucker, 2016). Even when such pieces (e.g. breakaways, hill climbs, surges, sprint finishes) make a small contribution to overall energy costs, they are critical to the event outcome. Key characteristics of elite endurance athletes, accrued via genetics and training, involve the interaction of high peak aerobic capacity (), high muscle oxidative capacity and high exercise economy (Joyner & Coyle, 2008). Training and nutrition strategies aim to ensure adequate availability and capacity to integrate the use of the muscle's fuel stores to produce ATP according to the demands of the event; a concept that is becoming known as ‘metabolic flexibility’.
For the past 60 years, nutrition guidelines for endurance sports have focused on strategies to match the body's finite CHO stores to the event's fuel costs (Burke et al. 2018), using pre‐event CHO loading to optimise muscle glycogen content and/or CHO intake during the event to sustain high CHO availability for longer duration competitions. These approaches enhance endurance performance when they sustain high rates of CHO oxidation throughout exercise (Hawley et al. 1997; Stellingwerff & Cox, 2014) and support motor recruitment, pacing and perception of effort (Burke & Maughan, 2015). Clear benefits to the performance of elite athletes have been observed in laboratory and field situations (Hyman, 1970; Pfeiffer et al. 2012; Burke et al. 2017).
Low CHO, high fat (LCHF) diets upregulate the release, transport, uptake and utilisation of fat in the muscle, even in endurance athletes whose training enhances such adaptations (Spriet, 2014). Strategies explored over the past 40 years include exposure to non‐ketogenic (Lambert et al. 1994; Goedecke et al. 1999) and ketosis‐inducing (‘ketogenic’) models of LCHF diets (Phinney et al. 1983). Periodised high CHO availability following short‐term adaptation to a non‐ketogenic LCHF has also been studied (Burke et al. 2000; Carey et al. 2002, 2001; Havemann et al. 2006). Since 2012, both scientific and lay literature have scrutinised the putative benefits of the ketogenic low‐CHO high‐fat diet (K‐LCHF) on endurance performance (Noakes et al. 2014; Volek et al. 2015). Although a range of metabolic modifications have been attributed to this diet (Volek et al. 2015), proposed advantages for endurance performance are maximisation of rates of fat oxidation (>1.0 g min−1, with peak rates of fat oxidation shifting from ∼45% to ∼70% of aerobic capacity) and increased hepatic production of ketone bodies (‘ketones’) to provide an additional substrate for the muscle (Volek et al. 2015; Shaw et al. 2019) and central nervous system (CNS) (Volek et al. 2015). K‐LCHF diets are associated with sustained elevations (>0.5 mmol l−1) of plasma ketones (β‐hydroxybutyrate; β‐HB) (Volek et al. 2015; Shaw et al. 2019).
This narrative review addresses social media claims that the K‐LCHF is ‘the future of elite endurance sport’, with the focus on three issues: (1) Do maximal rates of fat oxidation achieved by K‐LCHF transfer to performance benefits in endurance sport?; (2) What is the apparent time course of ‘keto‐adaptation’? and (3) Could strategies that periodise K‐LCHF with high CHO availability provide alternative models for performance benefits? These themes extend previous summaries (Burke, 2015; McSwiney et al. 2019; Shaw et al. 2020) and address enthusiastic hypotheses and testimonials regarding K‐LCHF in sport, at a time when there is a spotlight on performance barriers such as the 2 h marathon (Burke et al. 2019; Hoogkamer et al. 2019) and interest in the benefits of training with low carbohydrate availability (Burke et al. 2018). This summary was assembled from a systematic review of publication databases, while cross‐checking reference lists and accessing newly published/in press studies from the author's own laboratory. Studies were included if they involved verifiable exposure to a K‐LCHF diet by participants undertaking regular endurance‐based training and/or sporting competition. The critique focuses on applications to the metabolism and performance of elite endurance athletes, and this author notes in discussing study ‘limitations’, that methodological imperfections are inevitable in resource‐intensive studies of this complexity and that many studies were focused on questions other than those of current interest to this review.
Definitions of diets involving LCHF and high CHO availability
A backdrop of uniform definitions and explanations of acute and chronic manipulations of fat and CHO in the athlete's diet is needed to avoid common misconceptions about the K‐LCHF diet (Burke et al. 2018). Table 1 summarises various dietary philosophies discussed in this review, with the spectrum ranging from achievement of high CHO availability (HCHO) around all sessions (to optimise training capacity or event performance) to chronic CHO restriction (to sustain reliance on muscle fat use). A hybrid approach to training nutrition, popularly known as periodised CHO availability, integrates sessions with HCHO with others exposed to low CHO availability according to the workout characteristics and the athlete's overall goals (Marquet et al. 2016; Burke et al. 2017). Such ‘train low’ strategies are acute and intermittently applied (i.e. 1–2 sessions at a time), via short‐term restriction of CHO rather than a high fat intake (Mirtschin et al. 2018). While sharing the goal of increased muscle oxidative capacity via enhanced mitochondrial biogenesis (Impey et al. 2018), the execution and other outcomes of this dietary approach differ from those of LCHF and should not be confused (Burke et al. 2018). While the K‐LCHF diet is proposed as a chronic training‐competition strategy that maximises fat as an exercise fuel, alternative models range from periodising a mesocycle (∼3–4 weeks) of K‐LCHF diet within a long‐term HCHO training plan to integrating occasional and specific sessions of HCHO within a chronic K‐LCHF diet (Table 1). Hybrid strategies for competition nutrition for the keto‐adapted athlete involve adding strategies that promote high CHO availability before and during an event (Table 1), with variable focus on restoring endogenous and/or exogenous CHO availability.
Table 1.
Summary of manipulations of dietary carbohydrate and fat to enhance endurance race performance identified in this review (Burke et al. 2018)
| Dietary principle | Overview of dietary strategy | Purported benefits for race performance |
|---|---|---|
| Training strategies | ||
|
High CHO availability (HCHO) |
|
|
| Periodised CHO availability (PCHO) |
|
|
| Non‐ketogenic low‐carb high‐fat (NK‐LCHF) |
|
|
| Ketogenic LCHF (K‐LCHF) diet |
|
|
| K‐LCHF diet with strategic training CHO |
|
|
| Race strategies | ||
| High CHO availability |
|
|
| K‐LCHF |
|
|
| Periodisation of NK‐LCHF or K‐LCHF + high CHO availability |
|
|
| K‐LCHF + strategic race CHO |
|
|
Effects of chronic adaptation to the K‐LCHF diet on endurance performance
Initial evidence (1983)
Interest in K‐LCHF and endurance sport emanated from a 1983 study by Phinney and colleagues, modelled on diets observed among Inuit tribes (Volek et al. 2015). Five well‐trained cyclists rode to exhaustion at ∼63% , after consuming two diets under metabolic ward conditions: 1 week of habitual CHO intake (∼57% energy) then 4 weeks adaptation to an energy‐matched, highly CHO‐restricted diet (<20 g day−1 CHO, 80% energy as fat). Despite a ∼50% decrease in muscle glycogen concentrations with K‐LCHF, exercise capacity did not decline according to prevailing beliefs around the importance of glycogen availability (Hawley et al. 1997), but was supported by a substantial increase in muscle fat oxidation (Table 2). The key finding of maintained endurance, however, masked a highly variable response to K‐LCHF treatment (Fig. 1), with the group average skewed by a substantial increase in exercise capacity in one individual (Phinney et al. 1983). No consistent relationship between changes in substrate utilization, as portrayed by respiratory exchange ratio (RER) values, and cycling endurance was apparent (Fig. 1).
Table 2.
Studies of adaptation to ketogenic low‐carbohydrate high‐fat diet (K‐LCHF) on endurance performance or exercise capacity of athletes
| Athletes and study design | K‐LCHF adaptation protocol (duration and daily intake) | Performance protocol | Nutritional support for performance | Performance advantage with K‐LCHF | Comments |
|---|---|---|---|---|---|
|
Trained athletes with verified ketogenic diet | |||||
|
Phinney et al. (1983) Well‐trained cyclists (n = 5 M) Cross‐over design with HCHO first |
28 days HCHO (7 d): CHO: 57% E; protein: 1.75 g kg−1 K‐LCHF: CHO < 20 g; protein: 1.75 g kg−1; fat = 85% E Energy‐matched and balanced. Controlled diets consumed with supervision; blood ketones measured to verify ketosis. |
Cycling TTE at ∼63% |
Both trials: Pre‐exercise meal = overnight‐fasted Intake during exercise = water |
No NS difference in TTE between trials (151 vs. 147 min for LCHF and HCHO). Group data skewed by 1 participant who increased time to fatigue by 156% on LCHF trial (see Fig. 1) |
Well controlled study but involved order effect and failure to provide optimal conditions for HCHO trial. K‐LCHF = fasting [β‐HB]: 1.3 mmol l−1; exercise rates of fat oxidation: 1.5 g min−1. |
|
Burke et al. (2017) Elite (international level) race walkers (n = 19 M: LCHF = 10; HCHO = 9) Parallel group design with non‐randomised treatments (allocation according to preference/belief) |
24 days HCHO: 231 kJ kg−1; CHO: 8.6 g kg−1 or 60% E; protein: 2.1 g kg−1 or 16% E K‐LCHF: 223 kJ kg−1; CHO: 0.5 g or 3.5% E; protein: 2.2 g kg−1 or 16% E Energy‐matched but allowing small energy deficit. Controlled diets consumed with supervision; blood ketones measured regularly to verify ketosis. Intensified and supervised training program (endurance + HIT + gym) |
Race walking 10,000 m real‐life track race |
Pre‐exercise meal at 2 h pre‐race: HCHO = 2 g kg1 CHO LCHF Race 2 = energy matched high‐fat meal Both races = water station on track; use of performance supplements as in real‐life (e.g. caffeine) as long as use was matched in both races |
No In fact, K‐LCHF failed to show the improvement seen in HCHO group HCHO: improved performance by 6.6% [4.1–9.9%, 90% CI] equivalent to 190 s faster K‐LCHF: NS change of −1.6% [−8.5%−5.3%] equivalent to 23 s slower |
No improvement in race performance with K‐LCHF, despite equal (∼3–7%) increase in aerobic capacity, greater loss of BM and substantial increase in fat oxidation rates (from ∼0.7 to 1.57 ± 0.32 g min−1) and fasting [β‐HB]: 1.8 mmol l−1. Note that race protocol was reliant on capacity for high‐intensity exercise rather than glycogen depletion. Reduced performance attributed to reduction in race walking economy due to additional oxygen demand of fat oxidation at high exercise intensities. |
|
McSwiney et al. (2018) Trained endurance athletes (runners, cyclists, triathletes) (n = 20: K‐LCHF = 9; HCHO = 11) Parallel group design with non‐randomised treatments (allocation according to preference/belief) |
12 weeks HCHO: 147 kJ kg−1; CHO: 5.2 g kg−1 or 61% E; protein: 1.2 g kg−1 or 14% E K‐LCHF: 158 kJ kg−1; CHO: 0.5 g kg−1 or 5% E; protein: 1.6 g kg−1 or 17% E; fat: 77% E Diets consumed in free‐living protocol with education and weekly contact + 3 day diary at 0 and 12 weeks to check compliance. Fasting plasma ketone concentrations checked on test day to verify ketosis. Intensified training programme (endurance + HIT + strength) |
Cycling Lab ergometer: 6 s sprint + 100 km TT + critical power test (CPT) undertaken on lab ergometer |
HCHO: Pre‐exercise meal at 2 h = CHO‐rich meal (52% E) During exercise: 30–60 g h−1 CHO K‐LCHF Post trial: 2 h post‐fat rich (64% E) Water electrolytes during trial |
Perhaps K‐LCHF significantly improved peak but not average power in 6 s and CPT; trend to faster 100 km (166 ± 12.4 vs. 161.5 ± 8.4 min; 2.5%). No differences in HCHO (169.6 ± 8.4 vs. 168.4 ± 9.1). However, 5 additional subjects were unable to adhere to K‐LCHF and 2 failed to complete post‐intervention testing. |
Both groups increased aerobic capacity by ∼7%. K‐LCHF group had higher body fat and BM at pre‐trial and although 3 day self‐reported food diaries suggested that energy intake was maintained over 12 weeks, K‐LCHF lost 5.9 kg including 4.6 kg body fat over the 12 weeks with minimal change in HCHO group. K‐LCHF = fasting [β‐HB]: 0.5 mmol l−1; exercise rates of fat oxidation: NA. Large degree of individual variability in response to K‐LCHF; negative experiences were not captured in the performance data. HCHO group may not have achieved optimal nutritional preparation in pre‐trial diet and within‐trial fuelling for 2.5 h protocol |
|
Shaw et al. (2019) Trained endurance runners, triathletes (n = 8 M) Cross‐over counterbalanced design (14–21 days washout) |
31 days ‘Habitual’ HCHO diet:178 kJ kg−1; CHO: 4.6 g kg−1; protein: 2.0 g kg−1 K‐LCHF = 191 kJ kg−1;; CHO: 0.5 g kg−1; protein: 2.0 g kg−1; fat: 78% E Energy‐matched. Diets consumed in free‐living protocol with education and regular monitoring of diet and ketosis (blood/urinary ketones) to check compliance |
Running TTE at ∼70% on treadmill |
HCHO: Pre‐exercise meal at 2 h = 2 g kg−1 CHO During exercise: 55 g h−1 CHO LCHF: Pre‐exercise meal at 2 h = energy matched fat‐rich foods During exercise = energy matched fat‐rich sources |
No NS difference in TTE (∼50 km) from pre‐ to post‐treatment with either diet: HCHO = 237 ± 44 vs. 231 ± 35 min, although post‐treatment with LCHF diet was associated increased variability in results (239 ± 27 vs. 219 ± 53 min, while HCHO treatment showed a reduction in range of post‐treatment results |
2 additional subjects failed to complete K‐LCHF treatment due to compliance issues. Other tests showed reduction in efficiency and increased oxygen cost of exercise at intensities >70% with K‐LCHF. K‐LCHF increased rates of max fat oxidation (0.57 ± 0.10 to 1.12 ± 0.10 g min−1 with Fatmax shifting from 43 to 70% . Fasting [β‐HB]: > 0.5 mmol l−1. HCHO may not have achieved optimal nutritional preparation for 4 h run. |
|
Prins et al. (2019) Recreational distance runners (n = 7 M) Cross‐over study (14 days washout) |
42 days HCHO diet: 173 kJ kg−1; CHO: 5.8 g kg−1 or 56% E; protein: 1.5 g kg−1 or 15% E K‐LCHF diet:179 kJ kg−1; CHO: 0.6 g kg−1; protein: 2.5 g kg−1 or 25% E; fat: 69% E Energy‐matched. Diets consumed in free‐living protocol with education and regular monitoring of diet and ketosis (blood ketones on race days) to check compliance |
Running 5 km treadmill TT (with constant collection of respiratory gases) Undertaken at 4, 14, 28 and 42 days |
All trials: Pre‐exercise = overnight fasted During exercise = nil |
No Impaired performance of D4 TT in K‐LCHF trial compared with HCHO trial (1231 s vs. 1182 s, p < 0.011), but NS difference between performance on other days. Mean intensity of TT pace = ∼82% |
K‐LCHF diet was higher in protein and lower in fat than typically observed but participants were in ketosis on TT days. Mean fasting [β‐HB] on TT days = 0.5 mmol l−1. Max rates of exercise fat oxidation significantly increased from 1.01 ± 0.21 to 1.26 ± 0.2 g min−1 over the 6‐w of K‐LCHF. Despite some counter‐balancing of treatment order, it is uncertain if 2 week washout was able to stabilise identical baseline metabolic and fitness conditions since mean fat oxidation peak at start of HCHO treatment was 0.67 ± 0.2 g min−1 and individuals showed substantial increases and decreases in peak fat oxidation over the treatment. |
|
Burke et al. (2020) Elite (international level) race walkers (LCHF = 9 M, 1 F; HCHO = 6 M, 2 F) Parallel group design with non‐randomised treatments (allocation according to preference/belief) |
25 days HCHO: 223 kJ kg1; CHO: 8.6 g kg−1 or 65% E; protein: 2.1 g kg−1 or 15% E K‐LCHF: 234 kJ kg−1; CHO: 0.5 g or 4% E; protein: 2.1 g kg−1 or 16% E Energy‐matched but allowing small energy deficit. Controlled diets consumed with supervision; blood ketones measured regularly to verify ketosis. Intensified and supervised training program (endurance + HIT + gym) |
Race walking 10,000 m real‐life track race |
Pre‐exercise meal at 2 h pre‐race: HCHO = 2 g kg1 CHO LCHF Race 2 = energy matched high‐fat meal Both races = water station on track; use of performance supplements as in real‐life (e.g. caffeine) as long as use was matched in both races |
No In fact K‐LCHF showed impairment of race performance while improvement seen in HCHO group HCHO: improved performance by 4.8% (134 s faster) but K‐LCHF: slower by 2.3% (86 s) (both P < 0.001) |
Study undertaken as replication of Burke et al. (2017) in new cohort. Previous findings were clearly repeated including margin of difference in race improvements between groups. Both groups had small increase in aerobic capacity. K‐LCHF group reduced BM by 2.6 kg, and increased maximal fat oxidation from 0.6 to 1.3 g min−1 with fasting [β‐HB]:0.8 mmol l−1. |
| Studies of interest but with major limitations in study design or application to young highly trained athletes | |||||
|
Zajak et al. (2014) Moderately trained off‐road cyclists (n = 8 M) Cross‐over design with 1 week of washout |
28 d HCHO: 202 kJ kg−1; CHO: 50% E; protein: 15% E K‐LCHF: 202 kJ kg−1; CHO: 15% E; protein: 15% E; fat: 70% E Energy matched to habitual diet (as assessed by 3 day food diaries). Unclear whether diets were controlled or self‐administered. High volume, moderate intensity training load |
Cycling 105 min with 90 min at 85% ‘LT’ and 15 min at 115% ‘LT’ |
Pre‐exercise meal at 3 h according to dietary treatment |
No Small increase in (56 vs. 59.2 ml kg−1 min−1 and at ‘LT’ for HCHO and K‐LCHF, p < 0.01) but reduction in maximum workload (350 vs. 362 W, P = 0.037) and workload at ‘LT’ |
Study was not truly ketogenic (fasting [β‐HB]: 0.15 mmol l−1) despite description in study title. No real measurement of exercise capacity or performance. Small favourable change in body composition with K‐LCHF (loss of ∼ 1.8 kg with body fat loss from 14.9% to 11.0% BM, p < 0.01). Increase in the oxygen cost of cycling at same workload |
|
Zinn et al. (2014) Case history of 5 moderately trained endurance runners and cyclists (4 F, 1 M) |
10 weeks Previous diet: CHO: > 45% E K‐LCHF; CHO: <50 g d−1, protein: 1.5 g kg−1, ad libitum fat Diets consumed in free‐living protocol with education and contact at week 5 and 10. Fasting plasma ketone concentrations checked on test day to verify ketosis Existing training continued ad lib |
Cycling on cycling ergometer |
NA |
No In fact, reduction in TTE, peak power and |
No control group or real measurement of exercise performance. Loss of BM (∼4 kg) and body fat achieved by all subjects. Ketosis with [β‐HB] >0.5 mmol l−1) maintained. Maximal rate of fat oxidation increased from 0.6 to 0.8 g min−1 with Fatmax shifting from 48 to 62% |
|
Heatherly et al. (2018) Cross‐over study of older recreationally competitive runners and triathletes (n = 8 M; 40 ± 10 years). Treatment order: all subjects undertook HCHO first |
3 weeks HCHO: 148 kJ kg−1; CHO: 3.9 g kg−1 or 43% E; protein: 1.4 g kg−1 or 17% E K‐LCHF: 99 kJ kg−1; CHO: 0.4 g kg−1 or 7% E; protein: 1.7 g kg−1 or 29% E; fat: 65% E Diets consumed in free‐living protocol with education and daily contact + 3 day diary on two occasions to check compliance. Ketone concentrations checked on test days to verify ketosis. |
Running 5 km TT on outside hilly course (and following 5 × 10 min treadmill running and various race speeds from 5 km to marathon pace) in heated environmental chamber |
Overnight fast and water only for both conditions |
No No significant difference (P = 0.25) in 5 km TT performance (K‐LCHF: 23.45 ± 2.25 min vs. HCHO: 23.92 ± 2.57 min). |
Order effect with subjects undertaking 3 days HCHO first, then 3 weeks K‐LCHF. K‐LCHF treatment associated with reduced energy intake and loss of ∼ 2.1 kg BM. Fasting [β‐HB] = 0.7 mmol l−1; Maximum observed rates of fat oxidation during exercise = 0.81 g min−1. Despite a lower BM, the oxygen cost of exercise at 10–42 km race pace was higher than with HCHO treatment. Differences in 5 km TT performance not significant. |
NA, not available; M, male; F, female; K‐LCHF, ketogenic low‐carbohydrate high‐fat diet; HCHO, high carbohydrate/high carbohydrate availability diet; CHO, carbohydrate; E, energy; , maximal oxygen capacity; W, watts; BM, body mass; ‘LT’, the so‐called lactate threshold; Fatmax, percentage of maximal aerobic capacity at which maximal rate of fat oxidation occurs; TT, time trial; TTE, time to exhaustion; [β‐HB], plasma concentrations of β‐hydroxybutyrate; NS, not significant.
Figure 1. Four‐week ketogenic LCHF diet and cycling endurance.
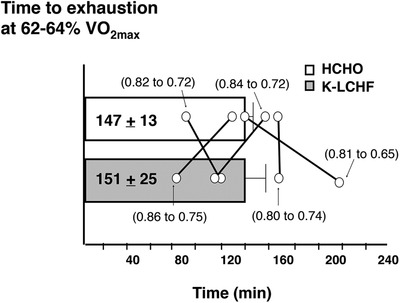
Time to exhaustion during cycling protocol at 1 week of habitual high‐carbohydrate diet (HCHO) followed by 4 weeks adaptation to ketogenic low‐carbohydrate, high‐fat (K‐LCHF) diet in 5 well‐trained cyclists (Phinney et al. 1983). Data are means ± SD with individual results identified (and mean changes from HCHO to K‐LCHF in respiratory exchange ratio provided in brackets).
Although this study provided novel and illuminating updates to concepts around exercise metabolism, its translation to elite endurance sport requires caution. Indeed, many attributes promoted the likelihood of a beneficial outcome following keto‐adaptation: (1) an order effect, with the K‐LCHF trial benefiting from an additional 4 week training plus protocol familiarisation; (2) a time to exhaustion protocol at modest exercise intensities which removed real‐life race characteristics such as pacing or higher exercise intensities; and (3) failure to provide optimal conditions for the HCHO trial (which involved an overnight fast and water during exercise). Furthermore, during tests on K‐LCHF, the authors noted that: ‘… the price paid for the conservation of CHO during exercise appears to be a limitation of the intensity of exercise that can be performed … there was a marked attenuation of respiratory quotient [RQ] value at suggesting a severe restriction on the ability of subjects to do anaerobic work.’ (Phinney et al. 1983). They postulated that ‘the controlling factor does not appear to be the presence or absence of substrate in the fibre. Rather it is more likely a restriction on substrate mobilization or fibre recruitment. The result, in any case, is a throttling of function near ’.
Re‐emergence of interest: supernova studies
In 2012, social and lay media commenced enthusiastic promotion of K‐LCHF for endurance performance, despite the absence of further scientific investigations of the claims and hypotheses (Burke, 2015). To address the lack of rigorous, sports‐focused evidence, this author's laboratory recruited internationally competitive race walkers to undertake 24 days of adaptation to K‐LCHF (3 weeks training + 3 days test protocol), using a battery of tests to interrogate metabolism and performance (Burke et al. 2017). Strict control of diet and training was maintained in a residential research camp setting (Mirtschin et al. 2018), with athletes following energy‐ and protein‐matched diets meeting the targets of HCHO, periodised CHO availability (PCHO) or an updated version of the Phinney diet, popularised in a lay book (Volek & Phinney, 2012).
Each group achieved an enhancement of aerobic capacity across the training camp, while the K‐LCHF group reported substantial increases in rates of fat oxidation during a graded economy test (∼60–90% ) and prolonged (2 h) training at a pace related to the 50 km race walking event (∼75–80% ). Despite this, K‐LCHF failed to achieve a faster time in a real‐life 10,000 m track race (−1.6%, NS), while the HCHO group improved significantly by 6.6% (Burke et al. 2017). This race protocol was criticised via social media as not being limited by glycogen depletion like longer athletic events (e.g. marathon/50 km race walk). In rebuttal, we chose an event which allowed peak performance to be repeated within a 3 week period and involved intensities of critical importance to the pacing strategies of international 20 and 50 km events (Huebsch, 2019). Furthermore, we note that 10 km personal best time has been reported as a good predictor of marathon performance in specialist distance runners (Noakes et al. 1990).
Because the increased fatigue and reduced quality of workouts associated with K‐LCHF abates after ∼2 weeks (Phinney et al. 1983; Burke et al. 2017, 2020), we did not feel it contributed to our race results. Instead, we attributed the failure of the K‐LCHF group to achieve expected performance improvements to reduced economy (i.e. increased oxygen utilisation) of race walking when reliant on fat oxidation (Fig. 2). An increased oxygen cost of producing ATP from the oxidation of fat relative to CHO has been known empirically for a century (Zuntz & Schumburg, 1901; Krogh & Lindhard, 1920), with shifts in substrate use being previously noted to change the economy of running (Kirwan et al. 1988) and cycling (Cole et al. 2014). This can be explained by the stoichiometry of oxidative reactions. Despite greater ATP yield per unit of substrate from fat, CHO metabolism produces a greater ratio of the reducing equivalent NADH (3 coupling sites in the electron transport chain) to FADH2 (2 coupling sides) than ß‐oxidation, thus achieving a greater ATP yield per unit of oxygen consumption via oxidative phosphorylation in the electron transport chain (Leverve et al. 2007). We propose that oxygen, rather than substrate, availability can become a limiting factor to performance of endurance events when dietary manipulations switch reliance from CHO to lipids. Although there may be adequate reserve for a greater, and perhaps imperceptible increase in, oxygen demand during moderate intensity exercise, this may be uncompensable at higher intensities. Our study provided a practical illustration of the effect of the substrate shift; following training, the HCHO group improved their relative economy because the oxygen cost of walking at race‐speeds accounted for a lower percentage of their enhanced aerobic capacity. Meanwhile, an increased absolute oxygen cost of exercise in K‐LCHF group negated the increase in , leaving no net change in relative exercise economy (see Fig. 2).
Figure 2. Changes in oxygen utilisation (in ml kg−1 min−1 .
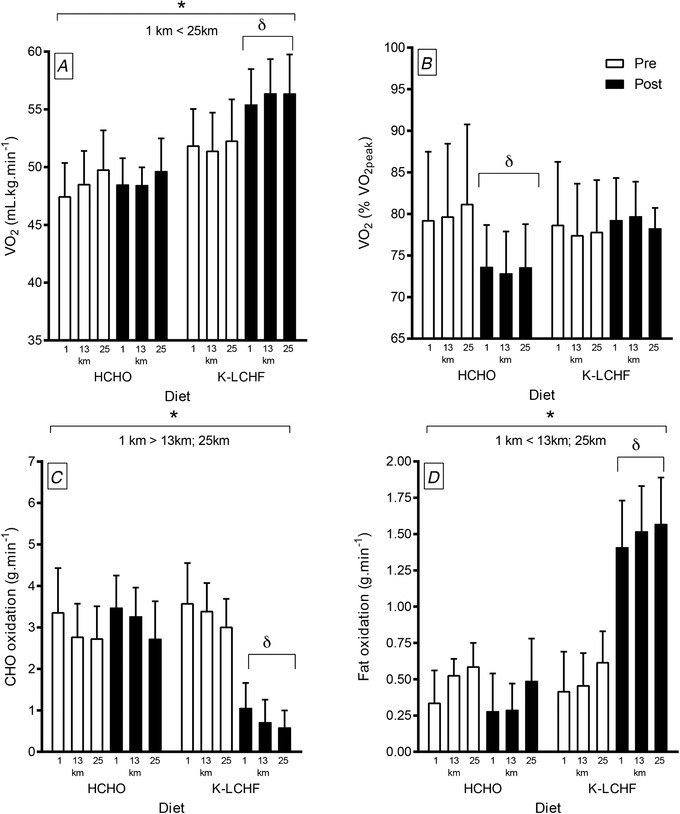
A, as a percentage of maximal aerobic capacity [], B), rates of carbohydrate oxidation (g min−1, C) and rates of fat oxidation (g min−1, D) in elite male race walkers during a 25 km (∼2 h) training session undertaken at a speed related to 50 km race pace. Data were collected before and after 3 weeks of adaptation to diets of either high carbohydrate availability (HCHO; n = 8) or ketogenic low‐carbohydrate high‐fat (K‐LCHF, n = 10). δSignificantly different to pre‐treatment (P < 0.01); *significant change over the 25 km walking session. Data are assembled from Burke et al. 2017.
Given the novelty of our observations and explanatory hypothesis, we tested their robustness via a replication study (Burke et al. 2020). This investigation (Table 2), including female participants within the elite race walker cohort, achieved almost identical outcomes to the earlier investigation in terms of exercise substrate use and economy changes. An equivalent margin between the pre‐ and post‐study 10,000 m track races (∼7% difference between the HCHO and K‐LCHF groups, with significant improvement (4.8%) and significant impairment (2.3%) of race times, respectively; Burke et al. 2020) provides additional confidence around our findings.
Other studies of K‐LCHF studies in endurance‐trained populations
Findings from further investigations of K‐LCHF in endurance athletes of 4–6 weeks duration (Table 2) both support and contradict the previously described work. In a study of free‐living runners and triathletes, Shaw and colleagues (2019) confirmed via self‐reported dietary intake data and occasional monitoring of blood and urine markers of ketosis, that core elements of the K‐LCHF diet can be implemented outside a controlled environment. Although the cross‐over ‘habitual diet’ treatment was lower in CHO than other HCHO interventions, before and during the performance protocol (treadmill run to exhaustion at 70% ), subjects received energy‐matched nutrition support appropriate to their dietary intervention. No differences in mean running time (∼4 h or ∼50 km), across the 31 days of adaptation in either treatments were found. However, in contrast to a reduction in the variability of results in the post‐treatment HCHO condition, there was a doubling of 90% confidence interval ranges with K‐LCHF. In addition, there was an impairment of higher‐intensity exercise, based on a ‘throttling of RER’, truncation of CHO oxidation, and a 1 km h−1 reduction in treadmill speed at (Shaw et al. 2019).
The oxygen cost of exercise, and comparisons between measured oxygen uptake versus RER‐based predictions of oxygen cost, were calculated during a graded aerobic capacity test, and the 1 and 2 h mark of the treadmill run to exhaustion (Shaw et al. 2019). Similar to Phinney et al. (1983), there were no differences in these indices at exercise intensities below 60% . However, above 70% , K‐LCHF demonstrated reduced economy, with oxygen uptakes being even higher than expected based on stoichiometry calculations. Specifically, RER‐based predictions accounted for only 14% of the increased oxygen uptake at 13.5 km h−1 (∼77% ), and 55% of the increase seen during the first 2 h of the treadmill run (Shaw et al. 2019). Potential explanations for these higher reductions in economy include observations, from a previous study of a 5 day non‐ketogenic LCHF, of an uncoupling of mitochondrial respiration specifically associated with high fat exposure (Leckey et al. 2018). The K‐LCHF diet has also been shown to alter the oral microbiome, reducing anaerobic bacteria involved in the conversion of dietary‐derived nitrate to nitrite within the enterosalivary pathway of nitric oxide (NO) production (Murtaza et al. 2019). Since other disturbances to the oral microbiome (e.g. via the use of antimicrobial mouthwashes) are associated with a reduction in functions attributed to NO production (Petersson et al. 2009), a background loss of NO activity might contribute to a baseline reduction in oxygen economy. Such speculations warrant targeted investigation.
A final element of the Shaw 2019 study involves the confirmation and a possible indicator of individual responsiveness to K‐LCHF. Indeed, in addition to increased variability in the endurance protocol outcomes, K‐LCHF included two subjects who failed to complete the treatment due to non‐compliance. The authors noted that athletes who maintained their running capacity with the high‐fat diet showed an RER of >1.0 at their , signalling a preservation of capacity for CHO oxidation at higher exercise intensities. Meanwhile, those with impaired endurance showed an RER <1.0 and an increase in plasma lactate concentrations, interpreted as reduced capacity for an oxidative fate of muscle glycogen stores. Further investigation is needed to confirm these findings and to interpret whether blunted CHO oxidation signals adaptation or mal‐adaptation to the K‐LCHF diet, and whether it represents a persistent difference between subjects, or a different time course of adaptation between individuals. Such information may discern whether it is a surrogate to predict overall suitability of the diet, or a potential marker of the adequacy of the time course of change.
Findings from a cross‐over‐designed project in recreational runners (Prins et al. 2019), involving a series of 5 km treadmill time trials (TTs) during 6 weeks of K‐LCHF or HCHO diets provide some contrasts to other studies (Table 2). A free‐living version of a K‐LCHF diet achieved sustained levels of ketosis (fasting [β‐HB]: ∼0.5 mmol l−1) and increases in maximal rates of fat oxidation (from 0.66 to 1.26 g min−1). Although the first TT (4 day adaptation) was slower than the corresponding HCHO trial, further trials undertaken after 14, 28 and 42 days, and a test of , failed to find significant differences in comparison to the HCHO treatment (Prins et al. 2019). The authors stated that their findings contradict the theory of impaired capacity for higher‐intensity endurance performance with K‐LCHF, noting that the TTs were undertaken at an oxygen cost of ∼80% . They attributed the continued contribution of CHO oxidation to fuel demands to a (speculated) preservation muscle glycogen stores. However, mean oxygen uptake during the TT decreased over the course of the HCHO treatment with the reverse occurring during the K‐LCHF treatment; this confirms the difference in exercise economy due to substrate choice. Furthermore, this does not preclude the impairment of glycogen utilisation and performance at even higher exercise intensities than achieved in the 5 km TT. Indeed, adaptation to a non‐ketogenic LCHF diet was able to maintain cycling intensities representing 80–85% of peak workload, but impaired workloads above 90% W peak (Peak Power Output). (Havemann et al. 2006). Whether some aspects of this model (recreational subjects, treadmill TT with the distraction of respiratory gases being collected throughout) are sufficiently valid and sensitive to detect alterations in the real‐life performance of elite athletes should also be considered.
The final study to merit discussion involves a 12 week adaptation to K‐LCHF in matched cohorts of free‐living endurance athletes (McSwiney et al. 2018). Twenty athletes completed the study, undertaking a specially designed 100 km cycling TT before and after structured training involving endurance, high‐intensity and resistance sessions. Both K‐LCHF and habitual ‘high’ CHO treatment groups achieved improvements in aerobic fitness without a significant change in total 100 km time (Table 2). However, K‐LCHF significant increased peak but not mean power (expressed as W kg−1 BM) in a 6 s sprint at the start of the TT and a 3 min critical power test at the end, designed to stimulate the finish of a race when glycogen depleted (McSwiney et al. 2018). Several caveats are noted in interpreting these results. First, the results represent nine athletes who completed the K‐LCHF diet and testing protocol; that a further seven subjects failed to comply with the diet or complete the post‐treatment testing reinforces the individual variability in response. Second, the K‐LCHF group had significantly higher body fat levels at the commencement of the treatment, and recorded a loss of ∼4 kg of body fat; a significant confounding effect on performance, especially for power reported relative to BM. Finally, the diet reported by the HCHO group failed to meet guidelines for a 2.5 h endurance event (Table 1) and may have led to sub‐optimal performance in the TT.
For the sake of completeness of this literature review, Table 2 recognises several studies of K‐LCHF diet and endurance performance which involve flaws in implementation (ketosis was not achieved; Zajac et al. 2014) or scientific control (lack of a control group: Zinn et al. 2017). A study involving middle‐aged recreational runners (Heatherley et al. 2018) has also been similarly included, although the application of its results to young and highly trained athletes must be treated with caution. However, a final comment is warranted regarding protocols used in all studies, including our own, which estimate muscle substrate utilisation during exercise from respiratory gas characteristics. This methodology assumes that and accurately reflect tissue O2 consumption and CO2 production, and that indirect calorimetry is valid for quantifying substrate oxidation in well‐trained subjects during exercise of up to ∼85% (Romijn et al. 1992). Two flaws are potentially present in all studies. First, all use conventional stoichiometric equations that fail to include the contribution of ketone oxidation to substrate use in the K‐LCHF trials. This represents a small (but potentially systematic) error in the calculations of fat and CHO oxidation from gas exchange information (Frayn, 1983). More importantly, at higher exercise intensities (e.g. above maximal lactate steady state), a non‐metabolic contribution to from the CO2 released as the bicarbonate pool buffers accumulating H+ ions means that stochiometric equations overestimate CHO and underestimate fat oxidation, respectively (Frayn, 1983; Jeukendrup & Wallis, 2005), particularly under non steady‐state conditions. Therefore, caveats apply to the data provided in some of the studies summarised in Table 2.
Summary from existing literature on K‐LCHF and endurance performance
Studies of K‐LCHF and endurance performance are sparse and heterogeneous in terms of athlete calibre, duration of adaptation and types of exercise protocol. Overall, they suggest that the performance of moderate‐intensity exercise may be preserved following keto‐adaptation, but individual results may be extreme in in both directions and include inability to tolerate or comply with the dietary restrictions. Importantly, there is no evidence of a consistent performance enhancement even at such intensities. Performance impairment of higher‐intensity exercise is likely, and potentially attributable to reduced exercise economy (higher oxygen cost) that prevents the athlete from sustaining workloads at high proportions of their maximal aerobic capacity. These findings support earlier suggestions that the K‐LCHF diet may unsuited to events in which higher intensity exercise is critical for success but may have utility in specific scenarios such as moderate‐intensity exercise that can be supported by fat oxidation, especially in the presence of an inability or unwillingness to use strategies to maintain high CHO availability (Burke, 2015). Benefits might also be seen when the K‐LCHF diet supports loss of body fat in athletes who are overweight.
In contemplating the potential use of K‐LCHF diets in terms of sports performance outcomes, endurance athletes should do an audit of their event or personal experiences to identify the relative importance of their capacity for high‐intensity aerobic exercise vs. the risk of depletion of CHO stores (Burke et al. 2020). Others have suggested an audit of the average or critical power production required in an event for the individual athlete, and whether fat oxidation can support this (Maunder et al. 2018). For example, analysis of an Ironman triathlon noted that elite athletes work at higher absolute rates of energy expenditure and a higher proportion of their individual energy expenditure ceiling than their slower counterparts. Modelling of the characteristics of top finishers (e.g. speeds, power outputs and exercise economy of running, cycling and swimming) suggests that enhanced fat oxidation typical of keto‐adaptation would fail to support the requirements of 8 h finish times, but might be sufficient for the energy needs of slower competitors with times >12 h (Maunders et al. 2018). Before examining an alternative concept, of rescuing keto‐adaptation with extra oxidative capacity from high CHO availability, the time course of adaptation and whether the studied protocols of K‐LCHF exposure have fully achieved the purported benefits to substrate use should be scrutinised.
Time course of adaptation to a K‐LCHF diet
Chronic exposure to a K‐LCHF diet is claimed to achieve a number of metabolic and functional outcomes (Volek et al. 2015). This review will focus only on issues that are of direct concern to sports performance: perception of effort or well‐being, and changes in substrate availability and utilisation. The time course of adaptation to these issues is contentious. The first study of the K‐LCHF diet and endurance athletes (Phinney et al. 1983) concluded that the 4 week exposure achieved a return to well‐being as well as remarkable shifts in fuel utilization during exercise. The next intervention study (Burke et al. 2017) was informed by this work, as well as statements within the popular books (Volek & Phinney, 2012) and published scientific reviews (Volek et al. 2015) responsible for the resurgence of interest in the K‐LCHF that keto‐adaptation occurred within ‘two or more weeks’ and ‘3–4 weeks’, respectively. Nevertheless, our study was firmly criticised as being too brief to allow important metabolic adaptations to occur, with suggestions that ‘several months’ are needed to achieve the full benefit; Burke et al. 2020). A systematic and well‐controlled investigation is clearly warranted of the time course of changes in perception of effort and well‐being, fuel utilisation and performance following exposure to K‐LCHF. However, Table 3 illustrates the lack of suitable information for undertaking such a review at the present time. Despite the shortcomings, this author will attempt to interpret the available data, extrapolating observations from short‐ to mid‐length (5 days to 12 weeks) intervention studies to the cross‐sectional studies of long‐term (>9 months) adapted endurance/ultra‐endurance athletes.
Table 3.
Ideal and actual models for investigating the time course of adaptation to ketogenic low‐carbohydrate, high‐fat (K‐LCHF) diet (well‐being, substrate utilisation, performance)
| Ideal scenario for investigating time course of K‐LCHF adaptation | Available data to investigate the time course of K‐LCHF adaptation |
|---|---|
|
|
Time course of changes in well‐being and perception of effort
Reductions in well‐being (e.g. fatigue, irritability, loss of concentration) and increases in perception of effort during exercise are clearly evident in the first few days of exposure to a K‐LCHF diet. These symptoms presumably reflect the lack of CHO substrate to the muscle and brain prior to enhancement of muscle fat utilisation and the establishment of sustained ketone support for the central nervous system (CNS). Lay literature also notes that ‘keto flu’ symptoms may reflect extensive electrolyte and fluid shifts resulting from the initial renal response to the ketogenic diet (Volek & Phinney, 2011). Advice around electrolyte supplementation (Volek & Phinney, 2011, 2012) has been incorporated into many of the K‐LCHF interventions summarised in this review (Phinney et al. 1983; Burke et al. 2017, 2020; McSwiney et al. 2018). McSwiney & colleagues (2018) reported that subjects suffered fatigue and performance impairment for the first 7–10 days of adaptation to the K‐LCHF diet, with a performance ‘lag’ persisting for 4–6 weeks. However, in the case of well‐trained athletes, training capacity is typically restored within 2–3 weeks (Phinney et al. 1983; Burke et al. 2017, 2020), although the consistent findings of individual responsiveness and difficulties with subject compliance in free‐living studies suggests that problems may persist for some athletes. Exercise at high intensities (>70% ) is associated with higher heart rates and perception of effort in athletes after ∼4 weeks of keto‐adaptation, even when training capacity has been restored (Burke et al. 2017; Shaw et al. 2019). Although this may simply reflect the increased oxygen cost and relative intensity of exercise required for fat oxidation to support the same power production, rather than ongoing impairment of exercise tolerance, a more systematic study of the time course of the changes in fatigue and well‐being associated with keto‐adaptation is warranted.
Time course of changes in ketone metabolism
Increased blood β‐HB concentrations occur quickly, reaching 0.8–2.0 mmol l−1 within 2–4 days of the K‐LCHF diet. Ketone bodies provide a substrate for peripheral tissues, including heart, CNS and skeletal muscle but their contribution as an exercise substrate is modified by factors including the protocol used to achieve ketonaemia (ketogenic diet, prolonged starvation/fasting, exogenous intake), training status, acute nutritional status and exercise characteristics (Evans et al. 2017; Pinckaers et al. 2017). The available studies of K‐LCHF diets in well‐trained/elite athletes have not specifically investigated the magnitude or importance of ketone utilisation during exercise on metabolism or performance. Therefore, no further commentary can be made.
Time course of changes in fat oxidation
Adaptation to the K‐LCHF diet is accompanied by a substantial increase in capacity for muscle fat oxidation during exercise, despite the increased muscle oxidative capacity and fat utilization achieved by training (Spriet, 2014). To date, no studies of K‐LCHF and endurance sport have directly investigated the mechanism(s) underpinning the enhancement of fat utilisation. However, previous studies involving NK‐LCHF diets have reported increases in intramuscular triglyceride (Yeo et al. 2008b ), hormone sensitive lipase (Stellingwerff et al. 2006), and the expression of fatty acid translocase FAT/CD36 protein (Cameron‐Smith et al. 2003) and carnitine palmitoyl transferase (Goedecke et al. 1999). Collectively, these changes suggest increases in fat availability, mobilisation and transport activities within the complex regulation of fat utilisation by the muscle.
Different methods have been used to measure and represent exercise fat oxidation within the current literature on K‐LCHF in endurance‐trained athletes. Some researchers (Volek et al. 2016) have undertaken standardised protocols for measuring maximal rates of fat oxidation (Fatmax) in which substrate utilisation is assessed from respiratory gas data during a graded economy test under fasted conditions, and modest increments in exercise intensity allow a prediction of the maximum rate of fat oxidation as well as the percentage of at which it occurs (Achten et al. 2002). Alternatively, rates of fat oxidation are simply measured during prolonged exercise, and may reflect substrate shifts over a longer period of exercise as well as intakes of high‐fat foods pre‐ and during the protocol (Burke et al. 2017, 2020). Although fat oxidation is typically reported in grams per minute, these rates vary according to the absolute power output (i.e. the athlete's body size and absolute aerobic capacity), the mode of exercise and acute nutritional characteristics. Despite these differences in methodologies, studies of 3–4 weeks duration involving rigorous control of the K‐LCHF intervention have reported mean values for maximal fat oxidation of ∼1.5 g min−1 representing 200–250% of values associated with a HCHO diet (Phinney et al. 1983; Burke et al. 2017, 2020; see Table 2). Whereas rates of up to 2 g min−1 can be observed in highly trained individuals (Burke et al. 2017; Webster et al. 2018), rates reported in other studies have been slightly lower (1.2–1.3 g min−1), perhaps reflecting lower training status/calibre or less strict dietary control under free‐living circumstances (Prins et al. 2019; Shaw et al. 2019). Fatmax protocols report a shift in the intensity at which maximal rates of fat oxidation occur from ∼45% to ∼70% (Shaw et al. 2019). Longer periods (6–12 weeks) of K‐LCHF diet exposure do not change these observations (McSwiney et al. 2018; Prins et al. 2019), suggesting that maximal enhancement of muscle fat oxidation occurs within 3–4 weeks of keto‐adaptation. Therefore, unless different information on processes and time course of substrate shifts during keto‐adaptation becomes available, 3–6 weeks should be considered a medium‐term period of adaptation, with long‐term exposure being >3–4 months.
In the absence of controlled intervention studies beyond 12 weeks in duration, insights on fat oxidation following longer‐term adaptation to K‐LCHF are best provided by cross‐sectional studies of well‐trained endurance athletes who have self‐selected such diets over prolonged periods. Two such projects have been published, involving cyclists (Webster et al. 2016) with 13 months adherence to a K‐LCHF diet (range: 8–22 months) or successful ultra‐endurance triathletes and runners (Volek et al. 2016) with 20 months exposure (9–26 months). These athletes were then compared to physical‐ and sports‐matched controls following habitual diets providing >50% E from CHO. Although self‐selection may have funnelled athletes to diets suiting their individual responsiveness, comparison of metabolic characteristics between groups could provide information about the optimal adaptive state for each approach. Cyclists habituated to a K‐LCHF diet (∼50 g day−1 or 7% energy CHO; ∼72% fat) sustained mean fat oxidation of 1.2 g min−1 during 2 h cycling at ∼70% while the comparison group following higher CHO diets achieved a gradual drift to 0.5 g min−1 (Webster et al. 2016), Meanwhile a graded protocol involving short (2 min) exercise periods in elite ultra‐distance athletes, showed a Fatmax of 1.54 ± 0.18 g min−1 at ∼70% ) in the K‐LCHF group (∼82 g day−1 or 10% CHO, ∼70% fat), compared to 0.67 ± 0.14 g min−1 at ∼55% in the control group (Volek et al. 2016). These same athletes had fat oxidation rates of 1.21 vs. 0.76 g min−1 over 3 h of submaximal treadmill running at 64% , following a pre‐exercise meal based on their habitual dietary intake (Volek et al. 2016). These findings suggest an absence of further changes in the capacity for fat oxidation beyond 3–4 weeks of K‐LCHF exposure.
A more interesting question is whether keto‐adaptation actually requires 3–4 weeks to achieve maximum rates of fat oxidation. The earliest studies of 3–4 days of CHO‐restriction (Ahlborg et al. 1967; Bergstrom et al. 1967) reported glycogen depletion and a lack of metabolic adaptation, and in agreement with Prins et al. (2019), an impairment of endurance capacity/performance. However, studies of a non‐ketogenic LCHF diet from our group (Burke et al. 2000, 2002; Carey et al. 2001) and from University of Cape Town (Goedecke et al. 1999; Havemann et al. 2006) reported robust cellular changes to fat mobilisation, transport, uptake and oxidation after 5–6 days exposure. Indeed, Goedecke and colleagues (1999) reported establishment of alterations in muscle characteristics and substrate utilisation in well‐trained cyclists by 5 days of NK‐LCHF that were not further enhanced after a 10 days exposure. We reported whole body rates of fat oxidation of∼60 and ∼70 µmol kg−1 min−1 in well‐trained cyclists during training sessions at 85% and 65% of , respectively with a 4–5 days adaptation period (Stepto et al. 2002). This approximates to absolute rates of fat oxidation of ∼1.3–1.5 g min−1, representing a doubling of this substrate use under the same conditions in a cross‐over HCHO treatment. Our more recent study of 5–6 days adaptation to a K‐LCHF diet showed mean rates of fat oxidation of ∼1.4 g min−1 during submaximal exercise in this time frame (Burke et al. unpublished data, Table 4). Therefore, the time course of adaptation to achieve high rates of fat oxidation in well‐trained athletes seems rapid (5–10 days) and robust, with similar outcomes achieved by both ketogenic and non‐ketogenic versions of the LCHF diet. On this basis, a definition of short‐term adaptation period as 5–10 days is proposed.
Time course of changes in CHO metabolism
The case for the effects of prolonged K‐LCHF on CHO oxidation at rest and during exercise is not as straightforward. Marked decreases in rates of CHO oxidation during exercise occur with LCHF diets, both in NK‐LCHF (Goedecke et al. 1999; Burke et al. 2000, 2002; Carey et al. 2001) and K‐LCHF forms Phinney et al. 1983; Burke et al. 2017, 2020; McSwiney et al. 2018; Shaw et al. 2019). Reduced availability of CHO substrate is an obvious explanation for this finding, although as discussed in the section below, impaired ability to oxidise available glycogen stores is also evident. Importantly, studies of 3–4 weeks K‐LCHF show the presence of endogenous CHO stores despite minimal CHO intake. For example, blood glucose concentrations are maintained at rest and during exercise, albeit at reduced levels, and increases in blood lactate concentrations during aerobic capacity tests are apparent (Phinney et al. 1983; Burke et al. 2017; Shaw et al. 2019). Even with the greatest dietary CHO restriction (<20 g days−1 over 4 weeks), Phinney and colleagues (1983) noted a 47% reduction rather than an absence of muscle glycogen stores. Because of the importance of glucose as a substrate for many tissues and a source of carbon for biosynthesis and anaplerosis, this confirms that humans can adapt to CHO deprivation by synthesising glucose from a variety of substrates (Soeters et al. 2012).
In comparing the findings of shorter‐term intervention studies and prolonged cross‐sectional studies of keto‐adaptation, respectively, Phinney et al. (1983) and Webster et al. (2016) used tracer technology and muscle biopsies to triangulate the changes in resting and exercise‐related CHO metabolism. Phinney and co‐workers (1983) estimated that glycogen utilisation was reduced 4‐fold, and blood glucose utilisation, 3‐fold, during moderate intensity (62–64% ) cycling after a 4 week K‐CHF diet, hypothesising that gluconeogenesis (from glycerol, lactate, pyruvate and gluconeogenic amino acids) was able to maintain blood glucose concentrations and permit glycogen restoration during recovery from exercise. Webster et al. (2016) measured glucose kinetics at rest and during 2 h of cycling at a slightly higher intensity (∼70% ), reporting lower endogenous glucose production (EGP) at rest and during exercise in long‐term keto‐adapted ultra‐endurance athletes compared to athletes consuming higher CHO diets. They hypothesised that the EGP decrease represented reduced liver glycogen breakdown without compensation by an absolute increase in gluconeogenesis. Although absolute rates of gluconeogenesis were similar between groups, they contributed a greater proportion of body CHO stores in the K‐LCHF group and came from different substrates; glycerol, in the case of the K‐LCHF group, and lactate in the case of the HCHO group (Webster et al. (2016). These two studies show a harmonised understanding of medium‐ and long‐term adaptation of CHO metabolism to K‐LCHF diets, both across the time course of changes and the various direct (biopsy and tracer‐enabled) and indirect (estimates from respiratory gas data) methodologies of assessing substrate use. However, observations of long‐term adaptation by Volek & colleagues (2016) provide a striking contradiction to the rest of the literature. Since these data have been adopted to justify criticisms of all studies of medium‐term K‐LCHF and to promote a unique theory of benefits of long‐term keto‐adaptation, closer scrutiny is required.
Ten chronically keto‐adapted high performance ultra‐runners/triathletes undertook a 3 h run at 65% after consuming a high‐fat snack (31 g fat, 80% E), then recovered for 2 h following repeated intake of this snack (Volek et al. 2016). The HCHO cohort completed the run with energy‐matched pre‐ and post‐exercise snacks providing 43 g CHO. High rates of fat oxidation (∼1.2 g min−1) and low rates of CHO oxidation (<0.5 g min−1) were seen in the K‐LCHF group, while CHO oxidation in the HCHO group declined from ∼1.7 to ∼1.2 g min−1 over the course of the water‐fed session (see Fig. 3 A). However, biopsy‐derived data showed a number of inconsistencies (Fig. 3 B): equal pre‐ and post‐exercise glycogen storage despite large differences in CHO intake, and equal glycogen degradation during exercise with a non‐oxidative fate for the K‐LCHF group. It was proposed that prolonged keto‐adaptation restores the muscle's ability to synthesise glycogen, which then has a non‐oxidative fate during exercise as a source of intermediates for the citric acid cycle and gluconeogenesis in the liver (Volek et al. 2016). The authors noted, in support of this hypothesis, that sled dogs (McKenzie et al. 2005) and horses (Hyyppa et al. 1999) are known to adapt to LCHF diets such that glycogen stores become normalised to levels associated with CHO‐rich diets.
Figure 3. Examination of issues around effects of long term adaptation to a ketogenic low‐carbohydrate high‐fat (K‐LCHF) on CHO metabolism from data reported by Volek et al. 2016 .
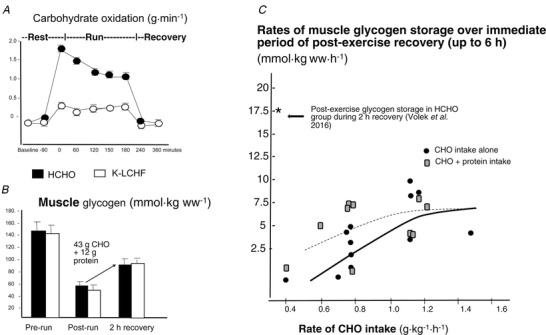
A, rates of CHO oxidation (g min−1) before, during and after 3 h treadmill running at ∼65% peak aerobic capacity in ultra‐endurance athletes habituated to K‐LCHF diet > 9 months. B, muscle glycogen content (mmol kg wet weight−1) in ultra‐endurance runners habitually consuming a K‐LCHF diet or a diet high in carbohydrate availability (HCHO) before and after a 3 h treadmill run, and after 2 h of post‐exercise recovery. Rates of muscle glycogen storage over 2 h recovery in HCHO adapted ultra‐endurance runners (Volek et al. 2016) are juxtaposed on literature summary of mean rates of glycogen storage in trained individuals over the 2–6 h period of post‐exercise recovery (redrawn from Betts & Williams, 2010).
While the concept proposed by Volek & colleagues (2016) is intriguing and merits further systematic evaluation, for the moment, there are strong reasons to reject the data on which it rests as atypical, rather than a basis for new operating beliefs about long‐term adaptation to K‐LCHF diets. First, these findings are contrary to observations from similar studies in long‐term keto‐adapted athletes and investigations of medium‐term adaptation. Second is the putative value of the non‐oxidative fate of large amounts of muscle glycogen, especially if the self‐selection aspect of the study indicates that these athletes have adapted optimally to the K‐LCHF diet. Indeed, Prins et al. (2019) and Shaw et al. (2019) noted that preservation of high rates of CHO oxidation in the face of medium‐term keto‐adaptation characterises the individual athletes who are able to preserve their endurance capacity/performance. Finally, the data can be evaluated at face value by comparing the results from the HCHO group within the study to a plentiful and non‐controversial literature, collected over nearly 6 decades, of muscle glycogen storage in endurance‐trained athletes. Figure 3 C, taken from Betts & Williams (2010) summarises nine biopsy‐based studies of glycogen storage during early post‐exercise recovery in relation to CHO intake over this time. When results from the HCHO group from Volek et al. (2016) are juxtaposed to this summary, they suggest the occurrence of an unusual event or issues with the measurement of glycogen, which probably extends to the results of glycogen storage in the K‐LCHF cohort. Taken together, there is a clear need for a re‐examination of the proposition that complete restoration of glycogen storage occurs under conditions of a chronic K‐LCHF diet as part of a dedicated investigation of long‐term effects of this diet. Although this theory may well be validated in the future, it is hard to justify on the presently available data.
Periodisation of K‐LCHF and HCHO strategies to optimise performance
A hybrid or periodised approach to strategies that separately optimise fat and CHO oxidation might achieve the ‘best of both worlds’ for metabolic flexibility for endurance athletes. A range of opportunities to include ketosis within periodised fueling strategies is possible, although adaptation to endogenous ketone sources requires 5+ days rather than hours involved with exogenous ketone supplements (Evans et al. 2017). Unfortunately, few studies of such periodisation are available (Table 4). The first investigation within the table emanated from a response to our 2017 publication on keto‐adaptation in elite race walkers (Burke et al. 2017). The modern phenomenon of social media allows study participants to describe their experiences to a wide audience, generating interest in their performances in sanctioned athletic events. Thus, a national record broken by a large margin by one study participant, 3 weeks after completing 24 days of keto‐adaptation within our research project, created social media speculation of similarities between K‐LCHF diet and altitude training, in creating a legacy of physiological changes that could integrate with the race taper and return to HCHO to enhance performance (Burke et al. 2020). To differentiate ‘the training camp effect’ from any special benefits of this periodisation model, we undertook a new investigation of controlled implementation of the K‐LCHF or CHO‐supported diets for 25 days, followed by a HCHO‐supported taper before the 20 km Australian Race Walking Road Championships. Different race analyses failed to detect a greater enhancement with K‐LCHF pre‐treatment than training with HCHO over the entire preparation (Burke et al. 2020). Therefore, we dismissed the proposal that the periodic adaptation to a K‐LCHF diet creates any legacy of importance to race performance in excess of normal training responses.
Table 4.
Effects of various strategies to periodise K‐LCHF diet with HCHO restoration or strategic CHO intake on performance
| Model of periodisation | Theoretical benefits | Available tests of model | Comment |
|---|---|---|---|
|
Periodisation of K‐LCHF + high CHO availability 1: (K‐LCHF adaptation periodised into base training phase with return to HCHO and taper prior to race) |
Model has been compared to altitude training; immediate response to exercise involving the additional metabolic stimulus of K LCHF creates performance decrement, but benefits of adaptation ‘carry over’ once returned to high CHO availability. Taper removes residual fatigue, allowing race performance to benefit from keto‐adaptation legacy plus immediate support from more economical CHO fuels |
Burke et al. (2020) Rigorously controlled trial of elite M + F race walkers with 6 weeks preparation for 20 km IAAF‐sanctioned road race; CHO+HCHO (n = 11): 6 weeks preparation with CHO support: K‐LCHF+HCHO (n = 8): 3.5 weeks K‐LCHF + 2.5 weeks HCHO 20 km race performance compared to baseline 10,000 m race |
No evidence of superior improvement compared with HCHO. No additional improvement in post‐study race performance due to periodisation of LCHF adaptation within preparation. In fact, statistical normalisation of the 20 km race time suggested that K‐LCHF‐HCHO group had no improvement in performance over 6 weeks training (197 ± 6%) while CHO‐HCHO had faster race time (191 ± 6%, P < 0.01). However, conversion of race times to IAAF points suggested that both groups showed improvement in 20 km race from baseline: 99 s (63–135 s) faster. |
|
Periodisation of K‐LCHF + high CHO availability 2: Chronic K‐LCHF adaptation plus acute CHO loading and HCHO for race day |
Acute CHO loading (24 h prior to race and pre‐race meal) prior to the race will restore muscle and liver glycogen content without time for de‐adaptation of enhanced rates of fat oxidation. Could return availability of glycogen to support higher intensity exercise |
Burke et al. unpublished data Rigorously controlled trial of elite M race walkers with 7 days HCHO (n = 6: (9.3 g kg−1, 2 g kg−1) or 6 days K‐LCHF (n (<50 g d−1, 2 g kg−1, fat = 80% E) + 1 days HCHO. All races undertaken with same 1 day CHO loading and light exercise and pre‐race meal (2 g kg−1) to achieve similar glycogen stores. 10,000 m races under IAAF conditions undertaken at baseline, followed by 7 days period of HCHO harmonization then intervention and post‐treatment race |
Impairment of performance compared with HCHO K‐LCHF increased rates of exercise fat oxidation (peak of 1.4 g min−1 during 4‐ stage economy test vs. 0.5 g min−1 at baseline). 1 day restoration of HCHO availability failed to completely restore rates of CHO oxidation, even at high exercise intensities (reduced by ∼41% and 27% at 50 km and 20 km race speeds, respectively, compared to baseline). Changes in performance from Race 1 to Race 2 were significantly different between groups (p = 0.009), with slower times in 6/7 K‐LCHF+ HCHO group (2.1% or 56 s slower [95% CI = 170 s slower to 58 s faster] while all HCHO group improved (5.7% or 163 s faster [40–286 s] |
| K‐LCHF diet + strategic exogenous CHO in training and race day | Occasional and targeted intake of exogenous CHO intake according to sports nutrition guidelines during training sessions involving high‐intensity or high‐quality performance against a background of chronic K‐LCHF may support better training and preserve CHO delivery (e.g intestinal absorption) and utilisation (e.g PDHa and oxidative pathway capability) without losing keto‐adaptation benefits, leading to better competition performance when also employing these fuel supporting strategies. |
Webster et al. (2018) Case study of elite (international level) triathlete (n = 1) who had followed K‐LCHF for 2 years. 2 × 3‐week standardised training with 3 day test block included at end. Block 1 = K‐LCHF (1 g kg−1 CHO = 8% E; 2.0 g kg−1 protein; fat = 75% E; water + electrolytes during sessions; Block 2 = K‐LCHF diet + 8 sessions of 60 g exogenous CHO during 1‐h session of high intensity swim/cycle/run training. Test block: D1 = fasted graded sub‐max Test + 2 × 30 s sprints, 4 min‐sprint (Block 2 = 3 × 10 g CHO in warm‐up to sprints); D2 = 20 km Cycle TT (Block 2: 30 g CHO during TT; D3 = 100‐km cycle TT (block 2 = 180 g CHO during TT). |
Some likely improvements in specific scenarios compared with LCHF Despite limitations of single case and order effect (CHO trials second), authors suggested based on real‐life significance in sport (Hopkins et al. 1999)
LCHF trials showed rates of fat oxidation of 1.5–1.7 g min−1 during both a graded Fatmax test and the100 km TT at 65% . CHO intake during 100 km TT produced small increase in rates of CHO oxidation (0.7 g min−1 to max of ∼1 g min−1) while fat oxidation was reduced by ∼0.3 g min−1 to min of ∼1.2 g min−1). |
M, male; F: female; K‐LCHF, ketogenic low‐carbohydrate high‐fat diet; HCHO, high carbohydrate/high carbohydrate availability diet; CHO, carbohydrate; E, energy; PDHa, activity of pyruvate dehydrogenase.
A more recent study from our group investigated potential benefits of acutely restoring liver and muscle glycogen after keto‐adaptation (Burke et al. unpublished data: Table 4) . Elite race‐walkers undertook two 10,000 m races separated by ∼2 weeks, each prepared with the same CHO intake (24 h of 8.5 g kg−1 plus pre‐race meal of 2 g kg−1) and light training to match pre‐race glycogen stores. Between races, athletes followed a HCHO diet for 7–8 days before being divided into intervention groups for continued HCHO diet or 5–6 days of K‐LCHF diet. Graded economy tests pre‐ and post‐intervention confirmed similar rates of fat and CHO utilisation in the HCHO group. However, even with the short adaptation period, the K‐LCHF group showed a substantial increase in maximal fat oxidation (from ∼0.4 to ∼1.4 g min−1). Additional testing during the race warm‐up showed that K‐LCHF was associated with higher rates of fat oxidation and only a partial restoration of CHO oxidation in Race 2, despite identical pre‐race dietary preparation. More importantly, race performance suggested a continued impairment of fuel utilisation with K‐LCHF. Whereas all participants in the HCHO group recorded faster times in Race 2 with a mean improvement of 5.7%, only one participant in the K‐LCHF improved in the second race (mean = 2.1% impairment); this replicated our familiar experience of ∼8% difference in performance between K‐LCHF and HCHO groups (Burke et al. 2017, 2020).
The consistent findings of this study series shows parallels with previous investigations of periodisation with a brief non‐ketogenic LCHF diet (Burke et al. 2000, 2002; Carey et al. 2001). The NK‐LCHF diet retooled the muscle to increase fat oxidation but pre‐exercise restoration of glycogen stores was thwarted by impaired oxidative CHO disposal due to a decrease in glycogenolysis and a reduction in the active form of pyruvate dehydrogenase (PDHa) (Stellingwerff et al. 2006). Havemann and colleagues (2006) demonstrated the practical implications of this: cyclists adapted to NK‐LCHF followed by restored CHO availability showed a small and non‐significant impairment of 100 km cycling TT, compared to a cross‐over trial undertaken with chronic HCHO. However, when required to undertake 1 km sprints at >90% of peak power output (∼95% of ) within the TT, there was a significant decrease in power output despite the maintenance of perceived effort and heart rate, and an increased attempt to recruit muscle fibres (Havemann et al. 2006). Thus, there is evidence of reduced metabolic flexibility (i.e. impaired oxidation of available glycogen) following adaptation to both ketogenic and non‐ketogenic versions of LCHF diets.
The final consideration is a case history of a competitive ultra‐endurance triathlete who investigated whether long‐term (∼2 y) keto‐adaptation was limiting his performance in higher‐intensity exercise domains (Webster et al. 2018). Three weeks of habitual training‐diet was compared with the same programme during which he consumed 60 g CHO in 8 × 1‐h higher‐intensity workouts, to potentially overturn a down‐regulation of absorption (Costa et al. 2017) and utilisation of CHO during exercise (Table 2). Although the study was unable to directly investigate mechanistic aspects of its working hypothesis, it provided some control and data collection around strategies being discussed in testimonial format in other media used by sports scientists, coaches and athletes. A series of exercise tests at the end of each period, fuelled by the prevailing dietary strategy, showed an apparent benefit to exercise reliant on CHO oxidation (higher intensity work of 30 s–20 min duration), but no effects on lower‐intensity (<60–65% ) and supra‐maximal (> maximal aerobic capacity) exercise when CHO was consumed acutely aroud each test. These benefits occurred without sacrificing the high rates of fat oxidation. The authors concede that their design cannot isolate muscle or CNS (Burke & Maughan, 2020) benefits of CHO intake during exercise. Neither can higher quality training at the targeted sessions be separated from adaptations to upregulate CHO absorption and utilisation. Although this case study encourages further exploration of this and other models of K‐LCHF/HCHO periodisation (e.g. acute glycogen restoration), it does not include comparisons to chronically high CHO availability.
In summary, various models integrating K‐LCHF and HCHO approaches to training and performance support are possible and are practiced in real‐life according to testimonials of successful and recreational athletes. Few have been systematically investigated. The available literature suggests that keto‐adaptation may impair muscle glycogen utilization, handicapping the ideal model of enhanced availability and capacity for both fat and CHO utilisation. However, there may be opportunities for strategic use of exogenous CHO intake during training sessions and events where higher intensity exercise is required.
Conclusion
The availability and capacity to use all muscle fuels to support the specific demands of exercise (‘metabolic flexibility’) is the Holy Grail for high‐performance endurance athletes, explaining continued fascination with strategies to better utilise the body's relatively unlimited fat stores. There is robust evidence that adaptation to a ketogenic LCHF creates substantial cellular changes to increase the mobilisation, transport, uptake and oxidation of fat during exercise, even in elite athletes who specifically train to optimise fat oxidative pathways. Furthermore, although the time course of all changes in body function with K‐LCHF requires systematic investigation, maximal changes to muscle fat metabolism occur within 3–4 weeks, and probably 5–10 days of adaptation. Changes in, and the significance of, muscle ketone use are, as yet, unknown. Although there is clear evidence that keto‐adaptation reduces muscle CHO oxidation, unresolved issues include the ability of long‐term ketoadaptation to restore muscle glycogen content to levels normally associated with a CHO‐rich diet and the impairment of muscle's ability to use glycogen for oxidative fates. This is important since CHO oxidation provides a more effective energy source when the oxygen supply becomes limiting. Inability to utilise available glycogen, albeit in reduced amounts with medium‐term adaptation to K‐LCHF or at normalised levels with strategies to restore/periodise CHO intake, limits performance of higher intensity endurance exercise. Furthermore, a wide variability in responsiveness to the K‐LCHF diet is well known, with extremes at both ends of the performance spectrum being possible. Athletes who are contemplating the use of ketogenic low‐carbohydrate high‐fat diets should undertake an audit of their event and their personal experiences to balance the risk of impaired performance of higher intensity exercise with the potential benefits of replacing an unavoidable depletion of carbohydrate stores with greater reliance on muscle fat use.
Additional information
Competing interests
The author of this paper has presented data included in this paper at professional conferences for which she has received travel support by conference organising committees. No other competing interests are declared.
Funding
Studies from this author's laboratory regarding the ketogenic low‐carbohydrate high‐fat diet have been funded by Program Grants from Australian Catholic University Research Fund and grants from the Australian Institute of Sport's High Performance Sport Research Fund.
Biography
Louise Burke is a sports dietitian with nearly 40 years of experience in the education and counselling of elite athletes. She was Head of Sports Nutrition at the Australian Institute of Sport during its existence from 1990–2018 and continues at the AIS as Chief of Nutrition Strategy. She was the team dietitian for the Australian Olympic Teams for the 1996–2012 Summer Olympic Games. Her publications include over 330 papers in peer‐reviewed journals and book chapters, and the authorship or editorship of several textbooks on sports nutrition. She is an editor of the International Journal of Sport Nutrition and Exercise Metabolism. She was awarded a Medal of the Order of Australia in 2009 for her contribution to sports nutrition. In 2014 she was appointed as Chair in Sports Nutrition in the Mary MacKillop Institute of Health Research at Australian Catholic University in Melbourne.

Edited by: Ian Forsythe & Scott Powers
This review was presented at the 2018 ACSM ‘Integrative Physiology of Exercise (IPE)’ conference, which took place at San Diego, California, 5–8 September 2018.
*The copyright line for this article was changed on 20 June 2020 after original online publication.
References
- Achten J, Gleeson M & Jeukendrup AE (2002). Determination of the exercise intensity that elicits maximal fat oxidation. Med Sci Sports Exerc 34, 92–97. [DOI] [PubMed] [Google Scholar]
- Ahlborg B, Bergstrom J, Brohult J, Ekelund LG, Hultman E & Maschio G (1967). Human muscle glycogen content and capacity for prolonged exercise after different diets. Forsvarsmedicin 3, 85–99. [Google Scholar]
- Bentley DJ, Millet GP, Vleck VE & McNaughton LR (2002). Specific aspects of contemporary triathlon: implications for physiological analysis and performance. Sports Med 32, 345–359. [DOI] [PubMed] [Google Scholar]
- Bergström J, Hermansen L, Hultman E & Saltin B (1967). Diet, muscle glycogen and physical performance. Acta Physiol Scand 71, 140–150. [DOI] [PubMed] [Google Scholar]
- Betts JA & Williams C (2010). Short‐term recovery from prolonged exercise exploring the potential for protein ingestion to accentuate the benefits of carbohydrate supplements. Sports Med 40, 941–959. [DOI] [PubMed] [Google Scholar]
- Burke LM (2015). Re‐examining high‐fat diets for sports performance: did we call the ‘nail in the coffin’ too soon? Sports Med 15 (Suppl. 1), S33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LM, Angus DJ, Cox GR, Cummings NK, Febbraio MA, Gawthorn K, Hawley JA, Minehan M, Martin DT & Hargreaves M (2000). Effect of fat adaptation and CHO restoration on metabolism and performance during prolonged cycling. J Appl Physiol 89,2413–2422. [DOI] [PubMed] [Google Scholar]
- Burke LM, Hawley JA, Angus DJ, Cox GR, Clark SA, Cummings NK, Desbrow B & Hargreaves M (2002). Adaptations to short‐term high‐fat diet persist during exercise despite high CHO availability. Med Sci Sports Exerc 34, 83–91. [DOI] [PubMed] [Google Scholar]
- Burke LM, Hawley JA, Jeukendrup A, Morton JP, Stellingwerff T & Maughan RJ (2018). Toward a common understanding of diet‐exercise strategies to manipulate fuel availability for training and competition preparation in endurance sport. Int J Sport Nutr Exerc Metab 28, 451–463. [DOI] [PubMed] [Google Scholar]
- Burke LM, Hawley JA, Wong SH & Jeukendrup AE. (2011). Carbohydrates for training and competition. J Sports Sci. 29 (Suppl 1), S17–27. [DOI] [PubMed] [Google Scholar]
- Burke LM, Jeukendrup AE, Jones AM & Mooses M (2019). Contemporary Nutrition Strategies to Optimize Performance in Distance Runners and Race Walkers. Int J Sport Nutr Exerc Metab 29, 117–129. [DOI] [PubMed] [Google Scholar]
- Burke LM & Maughan RJ (2015). The Governor has a sweet tooth ‐ mouth sensing of nutrients to enhance sports performance. Eur J Sport Sci 15, 29–40. [DOI] [PubMed] [Google Scholar]
- Burke LM, Ross ML, Garvican‐Lewis LA, Welvaert M, Heikura IA, Forbes SG, Mirtschin JG, Cato LE, Strobel N, Sharma AP & Hawley JA (2017). Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol 595, 2785–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LM, Sharma AP, Heikura IA, Forbes SF, Holloway M, McKay AKA, Bone JL, Leckey JJ, Welvaert M & Ross ML (2020). Crisis of confidence averted: Impairment of exercise economy and performance in elite race walkers by ketogenic Low Carbohydrate, High Fat (LCHF) diet is reproducible. PloS One (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron‐Smith D, Burke LM, Angus DJ, Tunstall RJ, Cox GR, Bonen A, Hawley JA & Hargreaves M (2003). A short‐term, high‐fat diet up‐regulates lipid metabolism and gene expression in human skeletal muscle. Am J Clin Nutr 77, 313–318. [DOI] [PubMed] [Google Scholar]
- Carey AL, Staudacher HM, Cummings NK, Stepto NK, Nikolopoulos V, Burke LM & Hawley JA (2001). Effects of fat adaptation and CHO restoration on prolonged endurance exercise. J Appl Physiol 91, 115–122. [DOI] [PubMed] [Google Scholar]
- Cole M, Coleman D, Hopker J & Wiles J (2014). Improved gross efficiency during long duration submaximal cycling following a short‐term high CHO diet. Int J Sports Med 35, 265–269. [DOI] [PubMed] [Google Scholar]
- Costa RJS, Miall A, Khoo A, Rauch C, Snipe R, Camoes‐Costa V & Gibson P (2017). Gut‐training: the impact of two weeks repetitive gut‐challenge during exercise on gastrointestinal status, glucose availability, fuel kinetics, and running performance. Appl Physiol Nutr Metab 42, 547–557. [DOI] [PubMed] [Google Scholar]
- Evans M, Cogan KE & Egan B (2017). Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol 595, 2857–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Garcia B, Perez‐Landaluce J, Rodriguez‐Alonso M & Terrados N (2000). Intensity of exercise during road race pro‐cycling competition. Med Sci Sports Exerc 32, 1002–1006. [DOI] [PubMed] [Google Scholar]
- Frayn KN (1983). Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol 55, 628–634. [DOI] [PubMed] [Google Scholar]
- Goedecke JH, Christie C, Wilson G, Dennis SC, Noakes TD, Hopkins WG & Lambert EV (1999). Metabolic adaptations to a high‐fat diet in endurance cyclists. Metabolism 48, 1509–1517. [DOI] [PubMed] [Google Scholar]
- Havemann L, West SJ, Goedecke JH, Macdonald IA, St Clair Gibson A, Noakes TD & Lambert EV (2006). Fat adaptation followed by CHO loading compromises high‐intensity sprint performance. J Appl Physiol 100, 194–202. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Schabort EJ, Noakes TD & Dennis SC (1997). Carbohydrate‐loading and exercise performance: An update. Sports Med 24, 73–81. [DOI] [PubMed] [Google Scholar]
- Heatherly AJ, Killen LG, Smith AF, Waldman HS, Seltmann CL, Hollingsworth A & O'Neal EK (2018). Effects of ad libitum low‐carbohydrate high‐fat dieting in middle‐age male runners. Med Sci Sports Exerc, 50, 570–579. [DOI] [PubMed] [Google Scholar]
- Hoogkamer W, Snyder KL & Arellano CJ (2019). Reflecting on Eliud Kipchoge's marathon world record: an update to our model of cooperative drafting and its potential for a sub‐2‐hour performance. Sports Med 49, 167–170. [DOI] [PubMed] [Google Scholar]
- Hopkins WG, Hawley JA & Burke LM (1999). Design and analysis of research on sport performance enhancement. Med Sci Sports Exerc, 31, 472–485. [DOI] [PubMed] [Google Scholar]
- Huebsch T (2019). The eye opening splits of the 50 km race walking world champions. https://runningmagazine.ca/50k-2017-iaaf-world-championships/. Cited July 1 2019
- Hyman M (1970). Diet and athletics. Br Med J 4, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyyppä S, Saastamoinen M & Reeta Pösö A (1999). Effect of a post exercise fat‐supplemented diet on muscle glycogen repletion. Equine Vet J. 30 (Suppl.), 493–498. [DOI] [PubMed] [Google Scholar]
- Impey SG, Hearris MA, Hammond KM, Bartlett JD, Louis J, Close GL & Morton JP (2018). Fuel for the work required: a theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med 48, 1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeukendrup AE (2017). Periodized nutrition for athletes. Sports Med 47, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeukendrup AE. & Wallis GA (2005). Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med, 26, 1–10. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Ruiz JR & Lucia A (2011). The two‐hour marathon: who and when? J Appl Physiol. 110, 275–277. [DOI] [PubMed] [Google Scholar]
- Joyner MJ & Coyle EF (2008). Endurance exercise performance: the physiology of champions. J Physiol. 586,35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan JP, Costill DL, Mitchell JB, Houmard JA, Flynn MG, Fink WJ & Beltz JD (1988). Carbohydrate balance in competitive runners during successive days of intense training. J Appl Physiol 65, 2601–2606. [DOI] [PubMed] [Google Scholar]
- Krogh A & Lindhard J (1920). The relative value of fat and CHO as sources of muscular energy: with appendices on the correlation between standard metabolism and the respiratory quotient during rest and work. Biochem J 14, 290–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert EV, Speechly DP, Dennis, SC & Noakes TD (1994). Enhanced endurance exercise in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur J Appl Physiol Occup Physiol 69, 287–293. [DOI] [PubMed] [Google Scholar]
- Leckey JJ, Hoffman NJ, Parr EB, Devlin BL, Trewin AJ, Stepto NK, Morton JP, Burke LM & Hawley JA (2018). High dietary fat intake increases fat oxidation and reduces skeletal muscle mitochondrial respiration in trained humans. FASEB J 32, 2979–2991. [DOI] [PubMed] [Google Scholar]
- Leverve X, Batandier C & Fontaine E (2007). Choosing the right substrate. Sepsis – new insights, new therapies. Novartis Found Symp 280, 108–121 [PubMed] [Google Scholar]
- Maunder E, Kilding AE & Plews DJ. (2018). Substrate metabolism during ironman Triathlon: different horses on the same courses. Sports Med 48, 2219–2226. [DOI] [PubMed] [Google Scholar]
- McKenzie E, Holbrook T, Williamson K, Royer C, Valberg S, Hinchcliff K, Jose‐Cunilleras E, Nelson S, Willard M & Davis M (2005). Recovery of muscle glycogen concentrations in sled dogs during prolonged exercise. Med Sci Sports Exerc 37, 1307–1312. [DOI] [PubMed] [Google Scholar]
- McSwiney FT, Wardrop B, Hyde PN, Lafountain RA, Volek JS & Doyle L (2018). Keto‐adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism 81, 25–34. [DOI] [PubMed] [Google Scholar]
- McSwiney FT, Doyle L, Plews DJ & Zinn C (2019). Impact of Ketogenic diet on athletes: current insights. Open Access J Sports Med 10, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquet LA, Brisswalter J, Louis J, Tiollier E, Burke LM, Hawley JA & Hausswirth C (2016). Enhanced endurance performance by periodization of CHO intake: “sleep low” strategy. Med Sci Sports Exerc 48, 663–672. [DOI] [PubMed] [Google Scholar]
- Mirtschin JG, Forbes SF, Cato LE, Heikura IA, Strobel N, Hall R & Burke LM (2018). Organisation of dietary control for nutrition‐training intervention involving periodized carbohydrate (CHO) availability and ketogenic low CHO high fat (LCHF) diet. Int J Sport Nutr Exerc Metab 28, 480–489. [DOI] [PubMed] [Google Scholar]
- Murtaza N, Burke LM, Vlahovich N, Charlesson B, O'Neill HM, Ross ML, Campbell KL, Krause L & Morrison M (2019). Analysis of the effects of dietary pattern on the oral microbiome of elite endurance athletes. Nutrients 11, pii: E614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes T, Volek JS & Phinney SD (2014). Low‐CHO diets for athletes: what evidence? Br J Sports Med 48(14), 1077–1078. [DOI] [PubMed] [Google Scholar]
- Noakes TD, Myburgh KH & Schall R (1990). Peak treadmill running velocity during the VO2 max test predicts running performance. J Sports Sci. 8, 35–45. [DOI] [PubMed] [Google Scholar]
- Petersson J, Carlström M, Schreiber O, Phillipson M, Christoffersson G, Jägare A, Roos S, Jansson EA, Persson AE, Lundberg JO & Holm L (2009). Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med. 46(8), 1068–1075 [DOI] [PubMed] [Google Scholar]
- Pfeiffer B, Stellingwerff T, Hodgson AB, Randell R, Pottgen K, Res P & Jeukendrup AE (2012). Nutritional intake and gastrointestinal problems during competitive endurance events. Med Sci Sports Exerc 44, 344–351. [DOI] [PubMed] [Google Scholar]
- Phinney SD, Bistrian BR, Evans WJ, Gervino E & Blackburn GL (1983). The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 32, 769–776. [DOI] [PubMed] [Google Scholar]
- Pinckaers PJ, Churchward‐Venne TA, Bailey D & van Loon LJ (2017). Ketone bodies and exercise performance: the next magic bullet or merely hype? Sports Med 47, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins PJ, Noakes TD, Welton GL, Haley SJ, Esbenshade NJ, Atwell AD, Scott KE, Abraham J, Raabe AS, Buxton JD, & Ault DL (2019) High rates of fat oxidation induced by a low‐carbohydrate, high‐fat diet, do not impair 5‐km running performance in competitive recreational athletes.J Sports Sci Med 18, 738–750. [PMC free article] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Hibbert J & Wolfe RR (1992). Comparison of indirect calorimetry and a new breath 13C/12C ratio, method during strenuous exercise. Am J Physiol Endocrinol Metab 263, E64–E71. [DOI] [PubMed] [Google Scholar]
- Saris WH, Antoine JM, Brouns F, Fogelholm M, Gleeson M, Hespel P, Jeukendrup AE, Maughan RJ, Pannemans D & Stich V (2003). PASSCLAIM ‐ Physical performance and fitness. Eur J Nutr 42 (Suppl. 1), I50–95. [DOI] [PubMed] [Google Scholar]
- Shaw DM, Merien F, Braakhuis A, Maunder E & Dulson DK (2019). Effect of a ketogenic diet on submaximal exercise capacity and efficiency in runners. Med Sci Sports Exerc 51, 2135–2146. [DOI] [PubMed] [Google Scholar]
- Shaw DM, Merien F, Braakhuis A, Maunder E & Dulson DK (2020). Exogenous ketone supplementation and keto‐adaptation for endurance performance: disentangling the effects of two distinct metabolic states. Sports Med 50, 641–656. [DOI] [PubMed] [Google Scholar]
- Soeters MR, Soeters PB, Schooneman MG, Houten SM & Romijn JA (2012). Adaptive reciprocity of lipid and glucose metabolism in human short‐term starvation. Am J Physiol Endocrinol Metab 303, E1397–1407. [DOI] [PubMed] [Google Scholar]
- Spriet LL (2014). New insights into the interaction of CHO and fat metabolism during exercise. Sports Med 44 (Suppl. 1), S87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellingwerff T & Cox GR (2014). Systematic review: Carbohydrate supplementation on exercise performance or capacity of varying durations . Appl Physiol Nutr Metabol 39, 998–1011. [DOI] [PubMed] [Google Scholar]
- Stellingwerff T, Spriet LL, Watt MJ, Kimber NE, Hargreaves M, Hawley JA & Burke LM (2006). Decreased PDH activation and glycogenolysis during exercise following fat adaptation with CHO restoration. Am J Physiol Endocrinol Metab 290, E380–E388 [DOI] [PubMed] [Google Scholar]
- Stepto NK, Carey AL, Staudacher HM, Cummings NK, Burke LM & Hawley JA (2002). Effect of short‐term fat adaptation on high‐intensity training. Med Sci Sports Exerc 34, 449–455. [DOI] [PubMed] [Google Scholar]
- Tucker R(2016). Science of sport: Marathon analysis. In: Marathon analysis. http://sportsscientists.com/thread/marathon-analysis. Accessed 8 August 2016.
- Volek J & Phinney S (2011). The art and science of low carbohydrate living: an expert guide to making the life‐saving benefits of carbohydrate restriction sustainable and enjoyable. Beyond Obesity Llc.
- Volek J & Phinney S (2012). The art and science of low carbohydrate performance. Beyond Obesity Llc.
- Volek JS, Noakes T & Phinney SD (2015). Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci 15, 13–20. [DOI] [PubMed] [Google Scholar]
- Volek JS, Freidenreich DJ, Saenz C, Kunces LJ, Creighton BC, Bartley JM, Davitt PM, Munoz CX, Anderson JM, Maresh CM, Lee EC, Schuenke MD, Aerni G, Kraemer WJ & Phinney SD (2016). Metabolic characteristics of keto‐adapted ultra‐endurance runners. Metabolism 65, 100–110. [DOI] [PubMed] [Google Scholar]
- Webster CC, Noakes TD, Chacko SK, Swart J, Kohn TA & Smith JA (2016). Gluconeogenesis during endurance exercise in cyclists habituated to a long‐term low CHO high‐fat diet. J Physiol 594, 4389–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster CC, Swart J, Noakes TD & Smith JA (2018). A carbohydrate ingestion intervention in an elite athlete who follows a LCH F diet. Int J Sports Physiol Perform 13, 957–960. [DOI] [PubMed] [Google Scholar]
- Yeo WK, Lessard SJ, Chen ZP, Garnham AP, Burke LM, Rivas DA, Kemp BE & Hawley JA (2008). Fat adaptation followed by CHO restoration increases AMPK activity in skeletal muscle from trained humans. J Appl Physiol 105, 1519–1526. [DOI] [PubMed] [Google Scholar]
- Zajac A, Poprzecki S, Maszczyk A, Czuba M, Michalczyk M & Zydek G (2014). The effects of a ketogenic diet on exercise metabolism and physical performance in off‐road cyclists. Nutrients 6, 2493–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn C, Wood M, Williden M, Chatterton S & Maunder E (2017). Ketogenic diet benefits body composition and well‐being but not performance in a pilot case study of New Zealand endurance athletes. J Int Soc Sports Nutr 14, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuntz N & Schumburg WK (1901). Studien zu einer Physiologie des Marsches. August Hirschwald, Berlin. [Google Scholar]


