Abstract
Aims
We aimed to examine temporal changes in left ventricular (LV) structures and their prognostic impacts in patients with heart failure (HF) and preserved ejection fraction (HFpEF).
Methods and results
In the Chronic Heart Failure Analysis and Registry in the Tohoku District‐2 (CHART‐2) study (n = 10 219), we divided 2698 consecutive HFpEF patients (68.9 ± 12.2 years, 32.1% female) into three groups by LV hypertrophy (LVH) and enlargement (LVE) at baseline: (−)LVH/(−)LVE (n = 989), (+)LVH/(−)LVE (n = 1448), and (+)LVH/(+)LVE (n = 261). We examined temporal changes in LV structures and their prognostic impacts during a median 8.7‐year follow‐up. From (−)LVH/(−)LVE, (+)LVH/(−)LVE to (+)LVH/(+)LVE at baseline, the incidence of the primary outcome, a composite of cardiovascular death or HF admission, significantly increased. Among 1808 patients who underwent echocardiography at both baseline and 1 year, we noted substantial group transitions from baseline to 1 year; the transition rates from (−)LVH/(−)LVE to (+)LVH/(−)LVE, from (+)LVH/(−)LVE to (−)LVH/(−)LVE, from (+)LVH/(−)LVE to (+)LVH/(+)LVE, and from (+)LVH/(+)LVE to (+)LVH/(−)LVE were 27% (182/671), 22% (213/967), 6% (59/967), and 26% (44/170), respectively. In the univariable Cox proportional hazard model, patients who transitioned from (+)LVH/(−)LVE to (+)LVH/(+)LVE or remained in (+)LVH/(+)LVE had the worst subsequent prognosis [hazard ratio (HR) 4.65, 95% confidence interval (CI) 3.09–6.99, P < 0.001; HR 4.01, 95% CI 2.85–5.65, P < 0.001, respectively], as compared with those who remained in (−)LVH/(−)LVE. These results were unchanged after adjustment for the covariates including baseline LV ejection fraction (LVEF) and 1‐year LVEF change.
Conclusion
In HFpEF patients, LV structures dynamically change over time with significant prognostic impacts, where patients who develop LVE with LVH have the worst prognosis.
Keywords: Heart failure with preserved ejection fraction, Cardiac structures, Prognosis
Introduction
Along with the aging of societies, the number of patients with heart failure (HF), especially those with HF and preserved ejection fraction (HFpEF), has been rapidly increasing worldwide. 1 , 2 However, the pathophysiology of HFpEF remains to be fully elucidated as the disorder may be heterogeneous, and no effective therapy is yet available. 3 , 4 , 5 , 6
Recently, the variability of left ventricular (LV) remodelling in HFpEF, at least in part, reflecting the heterogeneous pathophysiology, has been increasingly recognized. 7 Apart from LV ejection fraction (LVEF) deterioration, LV hypertrophy (LVH) and enlargement (LVE) are the important components of LV remodelling, which is known to be influenced by aging, cardiovascular (CV) risk factors, cardiac injury, neurohormonal activation, and sustained cardiac pressure and/or volume overload. 8 Indeed, it has been shown that LVH has negative prognostic impacts in the general population, hypertensive patients, and several populations with CV diseases such as atrial fibrillation (AF), prior myocardial infarction (MI), and HF including HFpEF. 9 , 10 , 11 , 12 , 13 , 14 Moreover, Zile et al. 15 recently reported that the assessment of LVE combined with LVH would help physicians further stratify the prognostic risk in older adults with predominantly preserved LVEF and without HF from the Cardiovascular Health Study (CHS). In HFpEF patients, however, it remains unexamined whether the classification of LV structures by LVH and LVE is useful to stratify the prognostic risk, and furthermore, whether temporal changes in LV structures influence the subsequent prognosis.
In the present study, we thus addressed these clinically important issues in the Chronic Heart Failure Analysis and Registry in the Tohoku District‐2 (CHART‐2) study, the largest multicentre, prospective observational study for chronic HF patients in Japan. 2 , 16 , 17
Methods
Study design
In the CHART‐2 study, we enrolled 10 219 consecutive patients with or at risk of HF between 2006 and 2010 according to the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines, 3 as previously described. 2 , 16 , 17 HF was diagnosed based on the Framingham Study criteria. 18 All information, including transthoracic two‐dimentional echocardiography data, was obtained at the time of enrolment and annually thereafter. The CHART‐2 study was approved by the ethics committee of each participating hospital and a written informed consent was obtained from all patients. Among HF patients in stage C/D (n = 4876) in the CHART‐2 study, we selected 2714 HFpEF patients (LVEF ≥50%) 3 , 4 after excluding those with valvular heart disease (VHD) as a primary aetiology of HF and those without echocardiography data at the time of enrolment (Figure 1 ). LVEF was calculated by the Teichholz formula or the Simpson method, as appropriate. We evaluated LVH and LVE in each patient based on the American Society of Echocardiography (ASE) guidelines. 19 LVH was defined as LV mass (LVM) indexed to body surface area (LVMI) >115 g/m2 in males and >95 g/m2 in females, while LVE was defined as LV diastolic dimension (LVDd) indexed to body surface area (LVDdI) >36 mm/m2 in males and >37 mm/m2 in women. LVM was calculated by LVDd, interventricular septal thickness at end‐diastole (IVSTD), and posterior wall thickness at end‐diastole (PWD) with the formula by Devereux et al. 20 as follows: LVM = 0.8 × (1.04 × [(IVSTD + LVDd + PWD)3 − LVDd3]) + 0.6. Based on these definitions, we divided 2714 patients into four LV structural groups: (−)LVH/(−)LVE (n = 989, 36.4%), (+)LVH/(−)LVE (n = 1448, 53.4%), (+)LVH/(+)LVE (n = 261, 9.6%), and (−)LVH/(+)LVE (n = 16, 0.6%). In the present study, we finally enrolled 2698 patients (68.9 ± 12.2 years, 32.1% female) from the former three groups after excluding those with (−)LVH/(+)LVE due to the small sample size (Figure 1 ). We followed them every year for a median of 8.7 years, including echocardiography evaluation. 2 , 16 , 17 The follow‐up rate was 98.6%. We examined the clinical characteristics and the prognostic impacts of baseline LV structural groups, and further explored temporal group transitions and their prognostic impacts. The primary outcome was a composite of CV death or HF admission. We also examined whether LV concentricity, defined as relative wall thickness (RWT) > 0.42, 19 affected long‐term prognosis in HFpEF patients. RWT was calculated by the following formula: RWT = 2 × PWD/LVDd. 19 Estimated glomerular filtration rate (eGFR) was calculated by the formula developed for the Japanese population. 21
Figure 1.
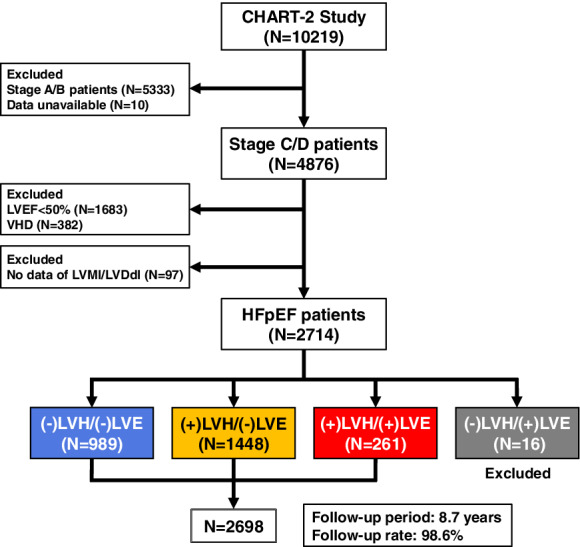
Study flowchart. HFpEF, heart failure with preserved ejection fraction; LVDdI, left ventricular diastolic dimension index; LVE, left ventricular enlargement; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; VHD, valvular heart disease.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median with interquartile range, as appropriate. Comparisons of these variables were performed by one‐way analysis of variance (ANOVA) or Kruskal–Wallis rank sum test. Categorical variables were expressed as numeral with percentage and were compared by Fisher's exact test.
To examine the prognostic impacts of baseline LV structural groups, Kaplan–Meier curves were utilized and compared with log‐rank test adjusted by the Holm's method for P‐values. The Cox proportional hazard models were also applied. In the multivariable Cox proportional hazard model, the following clinical covariates were adjusted: age, sex, ischaemic heart disease (IHD), hypertension, diabetes mellitus, AF, baseline LVEF, and medications, including beta‐blockers, renin–angiotensin system (RAS) inhibitors, calcium channel blockers (CCBs), diuretics, and statins. We examined the prognostic impacts of baseline values of LVEF, LVMI, and LVDdI as continuous variables in the Cox proportional hazard models. In Model 1, only the echocardiography variables were simultaneously incorporated, while in Model 2, the following clinical covariates were included for multivariable adjustment in addition to the echocardiography variables: age, sex, IHD, hypertension, diabetes mellitus, AF, and medications.
To examine the subsequent prognostic impacts of temporal group transitions from baseline to 1 year, we performed the landmark analyses. We compared the incidence rates per 1000 person‐years for the primary outcome with the mid‐P exact test adjusted by the Holm's method, utilizing the ‘epitools’ package of the R software. 22 We also applied the Cox proportional hazard models. In the multivariable Cox proportional hazard model, the following clinical covariates were adjusted: age, sex, IHD, hypertension, diabetes mellitus, AF, baseline LVEF, 1‐year LVEF change, and medications. We examined the prognostic impacts of baseline values and 1‐year changes in LVEF, LVMI, and LVDdI as continuous variables in the Cox proportional hazard models. In Model 1, only the echocardiography variables were simultaneously incorporated, while in Model 2, the following clinical covariates were included for multivariable adjustment in addition to the echocardiography variables: age, sex, IHD, hypertension, diabetes mellitus, AF, and medications. In these landmark analyses, only the primary outcome after 1 year was considered.
Furthermore, to examine the prognostic impacts of LVEF, LVMI, and LVDdI as time‐dependent variables, we constructed the Cox proportional hazard models with a time‐dependent approach (see details in online supplementary material). Moreover, we fit the joint modelling, 23 which is comprised of the Cox proportional hazard models for the primary outcome and multivariate linear mixed effects models of longitudinal measurements of LVEF, LVMI, and LVDdI in the overall HFpEF population, utilizing the ‘joineRML’ package of the R software. 24
For all steps, a two‐sided P < 0.05 was considered to be statistically significant. All statistical analyses were performed by the statistical computing software R version 3.6.1. 25
Results
Baseline patient characteristics
Baseline patient characteristics are summarized in Table 1 . From (−)LVH/(−)LVE, (+)LVH/(−)LVE to (+)LVH/(+)LVE, age, the proportion of females, the prevalence of prior history of HF admission and New York Heart Association (NYHA) functional class III–IV, and B‐type natriuretic peptide (BNP) levels increased, while heart rate, haemoglobin levels, and eGFR decreased. Body mass index (BMI), blood pressure (BP), and the prevalence of hypertensive heart disease (HHD), hypertrophic cardiomyopathy (HCM), hypertension, diabetes mellitus, and dyslipidaemia were highest in (+)LVH/(−)LVE, while the prevalence of IHD was highest in (−)LVH/(−)LVE. LVEF progressively decreased from (−)LVH/(−)LVE, (+)LVH/(−)LVE to (+)LVH/(+)LVE, while LVMI, LVDdI, and left atrial diameter (LAD) progressively increased. IVSTD, PWD, and RWT were highest in (+)LVH/(−)LVE, while RWT was lowest in (+)LVH/(+)LVE. Both E/A ratio and deceleration time (DT) were not significantly different among the three groups. From (−)LVH/(−)LVE, (+)LVH/(−)LVE to (+)LVH/(+)LVE, the prescription rates of RAS inhibitors and diuretics increased, while that of statins decreased. Beta‐blockers were most frequently prescribed in (+)LVH/(−)LVE.
Table 1.
Baseline patient characteristics
| (−)LVH/(−)LVE (n = 989) | (+)LVH/(−)LVE (n = 1448) | (+)LVH/(+)LVE (n = 261) | P‐value | |
|---|---|---|---|---|
| Age (years) | 66.8 ± 13.2 | 69.4 ± 11.4 | 73.7 ± 10.8 | <0.001 |
| Female sex, n (%) | 215 (21.7) | 524 (36.2) | 126 (48.3) | <0.001 |
| BMI (kg/m2) | 23.7 ± 3.6 | 24.8 ± 3.9 | 22.5 ± 3.4 | <0.001 |
| HF aetiology, n (%) | ||||
| IHD | 573 (57.9) | 763 (52.7) | 130 (49.8) | 0.009 |
| HHD | 216 (21.8) | 419 (28.9) | 70 (26.8) | 0.002 |
| DCM | 80 (8.1) | 119 (8.2) | 31 (11.9) | 0.14 |
| HCM | 13 (1.3) | 91 (6.3) | 6 (2.3) | <0.001 |
| Clinical history, n (%) | ||||
| Hypertension | 854 (86.4) | 1363 (94.1) | 240 (92.0) | <0.001 |
| Diabetes mellitus | 395 (39.9) | 611 (42.2) | 92 (35.2) | 0.10 |
| Dyslipidaemia | 824 (83.3) | 1215 (83.9) | 195 (74.7) | 0.001 |
| AF | 375 (37.9) | 589 (40.7) | 119 (45.6) | 0.07 |
| Stroke | 184 (18.6) | 305 (21.1) | 60 (23.0) | 0.19 |
| Prior MI | 359 (36.3) | 468 (32.3) | 80 (30.7) | 0.07 |
| HF admission | 385 (39.0) | 665 (45.9) | 162 (62.1) | <0.001 |
| Cancer | 131 (13.2) | 194 (13.4) | 42 (16.1) | 0.47 |
| NYHA class III–IV, n (%) | 63 (6.4) | 117 (8.1) | 43 (16.5) | <0.001 |
| Previous treatments, n (%) | ||||
| PCI | 383 (38.7) | 471 (32.5) | 75 (28.7) | 0.001 |
| CABG | 125 (12.6) | 131 (9.1) | 21 (8.0) | 0.01 |
| PMI | 70 (7.1) | 98 (6.8) | 40 (15.3) | <0.001 |
| Haemodynamics | ||||
| Systolic BP (mmHg) | 126.0 ± 17.8 | 130.3 ± 18.7 | 127.3 ± 19.8 | <0.001 |
| Diastolic BP (mmHg) | 73.0 ± 11.9 | 74.0 ± 11.7 | 70.2 ± 12.0 | <0.001 |
| Heart rate (bpm) | 72.6 ± 14.1 | 71.1 ± 14.5 | 69.9 ± 15.4 | 0.006 |
| Laboratory data | ||||
| Haemoglobin (g/dL) | 13.6 ± 1.8 | 13.2 ± 1.9 | 12.2 ± 2.1 | <0.001 |
| BUN (mg/dL) | 18.0 ± 7.6 | 19.5 ± 9.5 | 21.9 ± 11.9 | <0.001 |
| Creatinine (mg/dL) | 0.96 ± 0.41 | 1.05 ± 0.86 | 1.08 ± 0.83 | 0.003 |
| eGFR (mL/min/1.73 m2) | 64.7 ± 20.1 | 60.7 ± 21.0 | 57.8 ± 23.4 | <0.001 |
| Albumin (g/dL) | 4.1 ± 0.5 | 4.1 ± 0.5 | 3.9 ± 0.5 | <0.001 |
| LDL‐C (mg/dL) | 103.1 ± 30.4 | 106.4 ± 30.1 | 101.6 ± 29.4 | 0.03 |
| BNP (pg/mL) | 54.8 (19.9–133.0) | 87.4 (36.7–190.0) | 172.8 (72.8–318.8) | <0.001 |
| Echocardiography | ||||
| LVEF (%) | 66.3 ± 8.9 | 64.9 ± 8.8 | 61.2 ± 8.6 | <0.001 |
| LVM (g) | 151.1 ± 33.5 | 239.0 ± 66.0 | 257.1 ± 89.5 | <0.001 |
| LVMI (g/m2) | 91.3 ± 15.6 | 144.8 ± 33.9 | 172.4 ± 51.7 | <0.001 |
| LVDd (mm) | 45.7 ± 6.0 | 49.5 ± 6.3 | 58.7 ± 6.9 | <0.001 |
| LVDdI (mm/m2) | 27.9 ± 3.7 | 30.3 ± 3.4 | 39.8 ± 3.2 | <0.001 |
| EDV (mL) | 98.2 ± 29.1 | 118.1 ± 34.2 | 174.2 ± 47.2 | <0.001 |
| EDVI (mL/m2) | 59.5 ± 16.2 | 71.5 ± 17.3 | 116.7 ± 23.5 | <0.001 |
| IVSTD (mm) | 9.8 ± 1.8 | 12.5 ± 2.8 | 10.4 ± 2.2 | <0.001 |
| PWD (mm) | 9.6 ± 1.7 | 12.0 ± 2.3 | 10.4 ± 2.3 | <0.001 |
| RWT | 0.43 ± 0.13 | 0.50 ± 0.14 | 0.36 ± 0.08 | <0.001 |
| LAD (mm) | 39.1 ± 8.0 | 42.9 ± 8.5 | 46.5 ± 11.4 | <0.001 |
| E/A | 0.95 ± 0.48 | 0.94 ± 0.62 | 1.05 ± 0.75 | 0.14 |
| DT (ms) | 219.3 ± 68.1 | 222.7 ± 72.0 | 213.4 ± 70.0 | 0.30 |
| Medications, n (%) | ||||
| Beta‐blockers | 391 (39.5) | 707 (48.8) | 118 (45.2) | <0.001 |
| RAS inhibitors | 641 (64.8) | 1063 (73.4) | 210 (80.5) | <0.001 |
| CCBs | 413 (41.8) | 720 (49.7) | 105 (40.2) | <0.001 |
| Diuretics | 408 (41.3) | 710 (49.0) | 177 (67.8) | <0.001 |
| Statins | 443 (44.8) | 537 (37.1) | 85 (32.6) | 0.001 |
AF, atrial fibrillation; BMI, body mass index; BNP, B‐type natriuretic peptide; BP, blood pressure; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; CCB, calcium channel blocker; DCM, dilated cardiomyopathy; DT, deceleration time; EDV, end‐diastolic volume; EDVI, end‐diastolic volume index; eGFR, estimated glomerular filtration rate; HCM, hypertrophic cardiomyopathy; HF, heart failure; HHD, hypertensive heart disease; IHD, ischaemic heart disease; IVSTD, interventricular septal thickness at end‐diastole; LAD, left atrial diameter; LDL‐C, low‐density lipoprotein cholesterol; LVDd, left ventricular diastolic dimension; LVDdI, left ventricular diastolic dimension index; LVE, left ventricular enlargement; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVM, left ventricular mass; LVMI, left ventricular mass index; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PMI, pacemaker implantation; PWD, posterior wall thickness at end‐diastole; RAS, renin–angiotensin system; RWT, relative wall thickness.
Prognostic impacts of baseline left ventricular structures and causes of death
During the follow‐up period, 999 patients (37.0%) died and 817 patients (30.2%) developed the primary outcome, a composite of CV death or HF admission (CV death in 180, HF admission in 637). From (−)LVH/(−)LVE, (+)LVH/(−)LVE to (+)LVH/(+)LVE at baseline, the incidence of the primary outcome significantly increased (Figure 2 ), which was also the case for all‐cause death, CV death, and HF admission, except non‐CV death (online supplementary Figure S1 ). In the multivariable Cox proportional hazard model adjusted for the clinical covariates including baseline LVEF, the risk for the primary outcome significantly increased from (−)LVH/(−)LVE (reference), (+)LVH/(−)LVE to (+)LVH/(+)LVE (Figure 2 ). In the Cox proportional hazard models simultaneously incorporating baseline values of LVEF, LVMI, and LVDdI as continuous variables, baseline LVMI and LVDdI were significantly associated with poor prognosis, while baseline LVEF did not affect long‐term prognosis, regardless of multivariable adjustment for the clinical covariates (online supplementary Table S1 ). The incidence of the primary outcome did not differ by LV concentricity (RWT >0.42) in patients with LVH regardless of the presence or absence of LVE (online supplementary Figure S2 ). With regard to causes of death, the proportion of CV death, particularly HF death, increased from (−)LVH/(−)LVE, (+)LVH/(−)LVE to (+)LVH/(+)LVE (online supplementary Figure S3 ).
Figure 2.
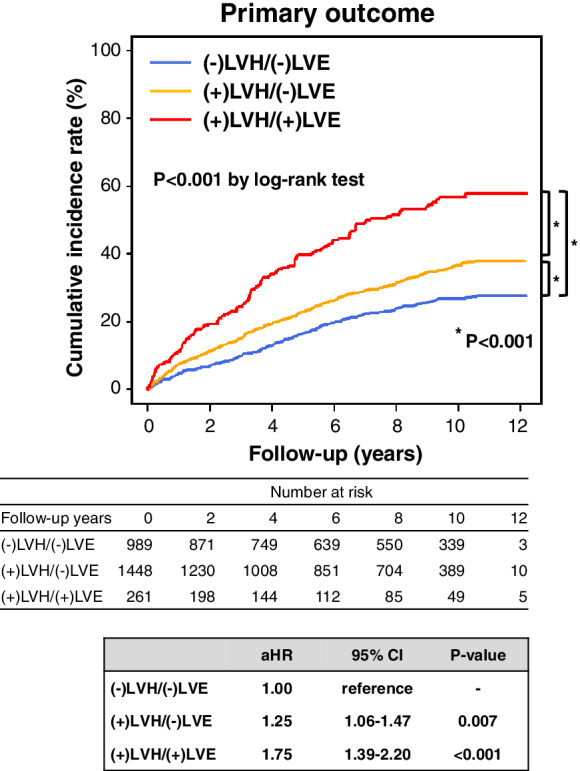
Prognostic impacts of baseline left ventricular structures in patients with heart failure and preserved ejection fraction. In the multivariable Cox hazard model, the following covariates were adjusted: age, sex, ischaemic heart disease, hypertension, diabetes mellitus, atrial fibrillation, baseline left ventricular ejection fraction, and medications. aHR, adjusted hazard ratio; CI, confidence interval; LVE, left ventricular enlargement; LVH, left ventricular hypertrophy.
Dynamic changes in left ventricular structures
Among 1808 patients who underwent echocardiography at both baseline and 1 year, substantial group transitions were noted from baseline to 1 year; the transition rates from (−)LVH/(−)LVE to (+)LVH/(−)LVE, from (+)LVH/(−)LVE to (−)LVH/(−)LVE, from (+)LVH/(−)LVE to (+)LVH/(+)LVE, and from (+)LVH/(+)LVE to (+)LVH/(−)LVE at 1 year were 27% (182/671), 22% (213/967), 6% (59/967), and 26% (44/170), respectively (Figure 3 ). The means and standard deviations of baseline values and changes from baseline in LVEF, LVMI, and LVDdI are shown in Figure 3 . Longitudinal trajectories of LVEF, LVMI, and LVDdI in the overall population are shown in online supplementary Figure S4 . In the overall population, the proportion of (−)LVH/(−)LVE gradually increased over time (Figure 3 ). The similar patterns of temporal group transitions were also noted in the analysis among 708 patients who survived through 5 years with complete data set of annual echocardiography follow‐up (online supplementary Figure S5 ).
Figure 3.
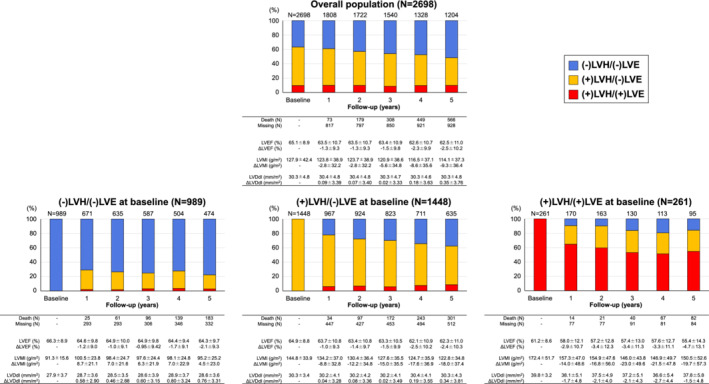
Dynamic changes in left ventricular structures in patients with heart failure and preserved ejection fraction. The stacked bars show the proportions of patients in each left ventricular structural group at every follow‐up year. The number of patients at every follow‐up year is shown above the bars. The means and standard deviations of baseline values and changes from baseline in left ventricular ejection fraction (LVEF), left ventricular mass index (LVMI), and left ventricular diastolic dimension index (LVDdI) are shown under the bars. LVE, left ventricular enlargement; LVH, left ventricular hypertrophy.
Prognostic impacts of temporal changes in left ventricular structures
To examine the subsequent prognostic impacts of temporal group transitions from baseline to 1 year, we performed the landmark analyses. The incidence rate per 1000 person‐years for the primary outcome after 1 year was high in patients who transitioned from (+)LVH/(−)LVE to (+)LVH/(+)LVE or remained in (+)LVH/(+)LVE at 1 year, while it was low in those who remained in (−)LVH/(−)LVE or transitioned from (+)LVH/(−)LVE to (−)LVH/(−)LVE (Figure 4 , middle panel). In the univariable Cox proportional hazard model, as compared with patients who remained in (−)LVH/(−)LVE (reference), those who transitioned from (−)LVH/(−)LVE to (+)LVH/(−)LVE at 1 year had worse prognosis [hazard ratio (HR) 1.93, 95% confidence interval (CI) 1.37–2.71, P < 0.001] (Figure 4 , lower panel). Furthermore, patients who transitioned from (+)LVH/(−)LVE to (+)LVH/(+)LVE or remained in (+)LVH/(+)LVE at 1 year had the worst prognosis (HR 4.65, 95% CI 3.09–6.99, P < 0.001; HR 4.01, 95% CI 2.85–5.65, P < 0.001, respectively) (Figure 4 , lower panel). In contrast, patients who transitioned from (+)LVH/(+)LVE to (+)LVH/(−)LVE at 1 year had comparable prognosis to those who remained in (+)LVH/(−)LVE. Similarly, patients who transitioned from (+)LVH/(−)LVE to (−)LVH/(−)LVE at 1 year had comparable prognosis to those who remained in (−)LVH/(−)LVE (Figure 4 , lower panel). These trends were also noted in the multivariable Cox proportional hazard model adjusted for the clinical covariates including baseline LVEF and 1‐year LVEF change (online supplementary Table S2 ). The Cox proportional hazard models, which simultaneously incorporated baseline values and 1‐year changes in LVEF, LVMI, and LVDdI as continuous variables, showed that baseline LVMI and LVDdI, and 1‐year increases in LVMI and LVDdI were significantly associated with poor prognosis in Model 1, although the impacts of 1‐year increase in LVDdI diminished in Model 2 (Table 2 ). These observations were evident in patients with LV concentricity but not in those without (online supplementary Table S3 ). Furthermore, the subgroup analyses by baseline LV structural groups showed that the negative prognostic impacts of 1‐year increases in LVMI and LVDdI were evident in patients with (+)LVH/(−)LVE (Table 3 ). Unlike LVMI or LVDdI, neither baseline LVEF nor 1‐year LVEF change affected long‐term prognosis in any sub‐populations, although 1‐year decrease in LVEF was significantly associated with poor prognosis only in Model 2 for the overall population (Tables 2 and 3 ). In the time‐dependent Cox proportional hazard model adjusted for the clinical covariates, LVEF was positively, while LVMI and LVDdI were negatively, associated with long‐term prognosis as time‐dependent variables (online supplementary Table S4 ). The joint modelling also demonstrated the negative prognostic impacts of longitudinal increases in LVMI and LVDdI (online supplementary Table S5 ).
Figure 4.
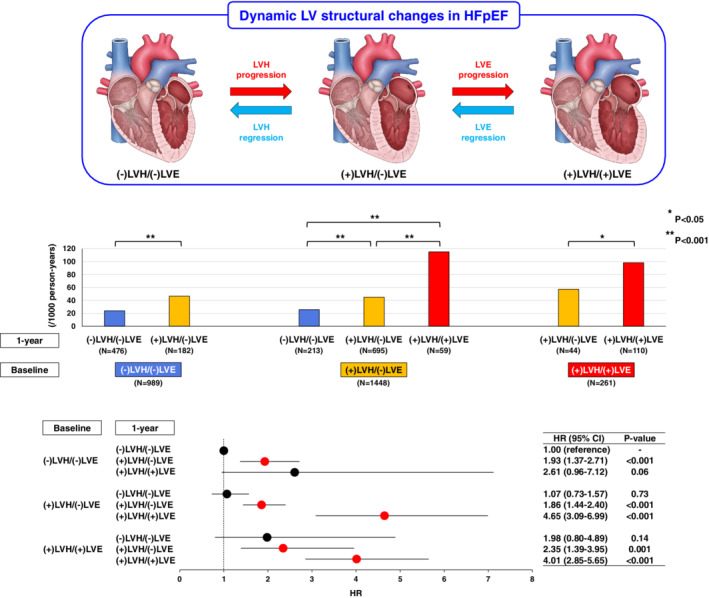
Prognostic impacts of temporal changes in left ventricular (LV) structures in patients with heart failure and preserved ejection fraction (HFpEF). The upper panel shows dynamic LV structural changes in HFpEF patients; temporal LV structural changes occur bidirectionally in terms of the progression/regression of LV hypertrophy (LVH) and enlargement (LVE). The middle panel shows the incidence rates per 1000 person‐years for the primary outcome after 1‐year changes in LV structures. The lower panel shows the subsequent risk for the primary outcome after 1‐year changes in LV structures in the univariable Cox proportional hazard model. CI, confidence interval; HR, hazard ratio.
Table 2.
Prognostic impacts of baseline values and 1‐year changes in left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index in the overall population
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | P‐value | aHR | 95% CI | P‐value | |
|
Baseline LVEF (per 10%) |
1.09 | 0.98–1.21 | 0.12 | 1.04 | 0.92–1.17 | 0.54 |
|
Increase in LVEF from baseline to 1 year (per 10%) |
0.93 | 0.84–1.03 | 0.14 | 0.88 | 0.79–0.97 | 0.01 |
|
Baseline LVMI (per 20 g/m2) |
1.11 | 1.07–1.16 | <0.001 | 1.10 | 1.04–1.15 | <0.001 |
|
Increase in LVMI from baseline to 1 year (per 20 g/m2) |
1.11 | 1.06–1.17 | <0.001 | 1.12 | 1.06–1.19 | <0.001 |
|
Baseline LVDdI (per 5 mm/m2) |
1.46 | 1.32–1.62 | <0.001 | 1.27 | 1.13–1.43 | <0.001 |
|
Increase in LVDdI from baseline to 1 year (per 5 mm/m2) |
1.28 | 1.11–1.48 | <0.001 | 1.13 | 0.98–1.31 | 0.0995 |
In Model 1, baseline values and 1‐year changes in LVEF, LVMI, and LVDdI were simultaneously incorporated.
In Model 2, the following clinical covariates were included for multivariable adjustment in addition to the echocardiography variables in Model 1: age, sex, ischaemic heart disease, hypertension, diabetes mellitus, atrial fibrillation, and medications.
aHR, adjusted hazard ratio; CI, confidence interval; LVDdI, left ventricular diastolic dimension index; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
Table 3.
Prognostic impacts of baseline values and 1‐year changes in left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index in left ventricular structural subgroups
| (−)LVH/(−)LVE | (+)LVH/(−)LVE | (+)LVH/(+)LVE | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |||||||||||||
| aHR | 95% CI | P‐value | aHR | 95% CI | P‐value | aHR | 95% CI | P‐value | aHR | 95% CI | P‐value | aHR | 95% CI | P‐value | aHR | 95% CI | P‐value | |
|
Baseline LVEF (per 10%) |
1.08 | 0.87–1.34 | 0.49 | 0.91 | 0.72–1.15 | 0.42 | 1.08 | 0.94–1.25 | 0.27 | 1.09 | 0.93–1.28 | 0.29 | 1.17 | 0.89–1.53 | 0.26 | 1.14 | 0.84–1.55 | 0.41 |
|
Increase in LVEF from baseline to 1 year (per 10%) |
0.91 | 0.75–1.11 | 0.36 | 0.83 | 0.67–1.03 | 0.09 | 0.93 | 0.81–1.06 | 0.28 | 0.90 | 0.78–1.04 | 0.15 | 0.94 | 0.75–1.18 | 0.60 | 0.87 | 0.68–1.12 | 0.27 |
|
Baseline LVMI (per 20 g/m2) |
1.01 | 0.81–1.27 | 0.90 | 1.00 | 0.76–1.30 | 0.99 | 1.14 | 1.07–1.21 | <0.001 | 1.12 | 1.04–1.20 | 0.003 | 1.13 | 1.01–1.27 | 0.03 | 1.17 | 1.01–1.34 | 0.03 |
|
Increase in LVMI from baseline to 1 year (per 20 g/m2) |
1.36 | 1.17–1.60 | <0.001 | 1.18 | 1.01–1.38 | 0.04 | 1.09 | 1.02–1.17 | 0.01 | 1.13 | 1.05–1.21 | <0.001 | 1.10 | 0.97–1.26 | 0.14 | 1.13 | 0.99–1.30 | 0.08 |
|
Baseline LVDdI (per 5 mm/m2) |
1.50 | 1.16–1.94 | 0.002 | 1.18 | 0.88–1.58 | 0.26 | 1.74 | 1.43–2.11 | <0.001 | 1.51 | 1.22–1.87 | <0.001 | 1.24 | 0.81–1.88 | 0.32 | 1.46 | 0.92–2.30 | 0.11 |
|
Increase in LVDdI from baseline to 1 year (per 5 mm/m2) |
1.16 | 0.83–1.63 | 0.37 | 1.06 | 0.73–1.53 | 0.76 | 1.40 | 1.14–1.72 | 0.001 | 1.28 | 1.04–1.58 | 0.02 | 1.17 | 0.87–1.56 | 0.29 | 1.23 | 0.89–1.70 | 0.22 |
In Model 1, baseline values and 1‐year changes in LVEF, LVMI, and LVDdI were simultaneously incorporated.
In Model 2, the following clinical covariates were included for multivariable adjustment in addition to the echocardiography variables in Model 1: age, sex, ischaemic heart disease, hypertension, diabetes mellitus, atrial fibrillation, and medications.
aHR, adjusted hazard ratio; CI, confidence interval; LVDdI, left ventricular diastolic dimension index; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
Discussion
The major findings of the present study are that LV structures, in terms of LVH and LVE, dynamically changed over time in HFpEF patients, and that these temporal changes, the progression/regression of LVH and LVE, had significant prognostic impacts (Figure 4 ). These findings demonstrate a novel aspect of HFpEF pathophysiology, which could be useful for better management of the disorder.
Prognostic impacts of baseline left ventricular hypertrophy and enlargement in HFpEF
The present study clearly showed that LV remodelling at baseline, in terms of LVH and LVE, was associated with poor prognosis in HFpEF patients, particularly when LVE was complicated with LVH. Although it has been well shown that LVE is associated with poor prognosis in patients with reduced LVEF, 26 , 27 , 28 it remained unclear whether LVE has prognostic impacts in patients with preserved LVEF, particularly in HFpEF patients. Thus, the present study has clinical significance as this is the first study to demonstrate the negative prognostic impacts of LVE in HFpEF patients.
As a conventional tool to define LV remodelling, LV concentricity (RWT >0.42) has been applied to divide LVH into two categories, namely, concentric and eccentric LVH. 19 However, despite its usefulness, this classification has a limitation, because it does not consider LVE, another important aspect of LV remodelling. 8 Indeed, Katz et al. 29 reported from the Northwestern HFpEF Registry that the conventional LV structural classification by LV concentricity was not necessarily useful for further risk stratification of HFpEF patients, particularly for those with LVH. In the present study, we thus examined LV structures in terms of LVH and LVE, and classified HFpEF patients into three groups: (−)LVH/(−)LVE, (+)LVH/(−)LVE, and (+)LVH/(+)LVE. With this classification, we found that the risk for the primary outcome progressively increased from (−)LVH/(−)LVE, (+)LVH(−)LVE to (+)LVH/(+)LVE, and confirmed that both LVH and LVE were substantially associated with worse prognosis in HFpEF patients. It was also indicated that baseline LVMI and LVDdI were prognostic predictors in HFpEF patients. These results demonstrate that LV structural classification by LVH and LVE is useful to stratify the prognostic risk in HFpEF patients.
Prognostic impacts of temporal changes in left ventricular hypertrophy and enlargement in HFpEF
The present study showed that LV structures, in terms of LVH and LVE, dynamically changed over time in HFpEF patients. Moreover, we clearly demonstrated that the progression of LV remodelling, in other words, LVH progression and LVE progression, had negative prognostic impacts; as compared with HFpEF patients who remained in (−)LVH/(−)LVE, those who transitioned from (−)LVH/(−)LVE to (+)LVH/(−)LVE had worse prognosis, and furthermore, those who transitioned from (+)LVH/(−)LVE to (+)LVH/(+)LVE or remained in (+)LVH/(+)LVE had the worst prognosis. The negative prognostic impacts of LVH progression and LVE progression were further confirmed by the observation that 1‐year increases in LVMI and LVDdI were associated with poor prognosis, particularly in patients with (+)LVH/(−)LVE. The present study also revealed that the transitions from (+)LVH/(+)LVE to (+)LVH/(−)LVE, namely LVE regression, and from (+)LVH/(−)LVE to (−)LVH/(−)LVE, namely LVH regression, were both associated with improved prognosis in HFpEF patients. These lines of observations that longitudinal progression and regression of LV remodelling in terms of LVH and LVE had negative and positive prognostic impacts, respectively, could be clinically significant, as no evidence‐based therapy has been established for HFpEF. 3 , 4
Prognostic impacts of temporal changes in left ventricular ejection fraction, left ventricular mass, and left ventricular volume in HFpEF
We and others previously reported that longitudinal LVEF deterioration is associated with poor prognosis in HFpEF patients. 17 , 30 , 31 In the present study, we demonstrated the negative prognostic impacts of 1‐year decrease in LVEF and 1‐year increases in LVMI and LVDdI in the overall HFpEF population. Moreover, we showed that the most recent echocardiography data had the most prognostic information, indicating that decrease in LVEF and increases in LVMI and LVDdI as time‐dependent variables had negative prognostic impacts. However, we further demonstrated that the prognostic impacts of 1‐year LVEF change were not so robust as compared with those of 1‐year change in LVMI or LVDdI. Indeed, unlike LVMI or LVDdI, 1‐year LVEF change had no prognostic impacts in any sub‐populations classified by LVH and LVE [i.e. (−)LVH/(−)LVE, (+)LVH/(−)LVE, and (+)LVH/(+)LVE]. Particularly, the negative prognostic impacts of 1‐year increases in LVMI and LVDdI were evident in patients with (+)LVH/(−)LVE, the most frequently observed LV structural phenotype in our HFpEF population. Thus, these results may indicate that we should pay more attention to temporal changes in LV mass and volume as compared with LVEF change in HFpEF patients at least for this LV structural phenotype.
Risk stratification by left ventricular remodelling in HFpEF
Recently, a series of studies reported that the four‐tiered classification based on LV concentricity and LVE was useful for further risk stratification of the general population with LVH from the Dallas Heart Study and hypertensive patients with LVH from the Losartan Intervention for Endpoint Reduction (LIFE) echocardiography sub‐study. 32 , 33 In contrast, Zile et al. 15 reported that the addition of RWT partition to the classification by LVH and LVE did not affect CV outcomes in older adults with predominantly preserved LVEF and without HF from the CHS. In the present study, the results showed that long‐term prognosis in patients with LVH did not differ by LV concentricity regardless of the presence or absence of LVE, while the prognostic impacts of LVMI and LVDdI themselves differed by LV concentricity. Thus, further studies are needed to examine whether the addition of LV concentricity information to the classification by LVH and LVE could improve the performance of risk stratification in HFpEF patients.
Study limitations
Several limitations should be mentioned for the present study. First, the CHART‐2 study enrolled only Japanese patients and caution should be taken when generalizing the present findings to other populations. Second, in the present study, the echocardiography data were obtained annually at each participating hospital, but not at the core laboratory. Thus, the echocardiography data could be subject to inter‐observer variability. However, the simulation study with random perturbation to the original echocardiography data confirmed the robustness of the prognostic impacts of baseline LV structural groups (online supplementary Table S6 ), suggesting that the influence of inter‐observer variability could be minimal. Third, given that we selected HFpEF patients according to the ACCF/AHA guidelines, 3 our HFpEF population was an amalgam of diseases including the predominant metabolic CV disease, cardiomyopathies, as well as restrictive/infiltrative diseases such as cardiac amyloidosis. 34 In addition, in relation to the aetiologies of HF, we have to mention that various risk factors, including age, obesity, and diabetes mellitus, were involved in the condition of HHD, and also that IHD coexisted with these risk factors. However, the sensitivity analysis suggested that the prognostic impacts of baseline LV structural groups and those of temporal group transitions did not differ regardless of these aetiologies (online supplementary Table S7 ). Fourth, the proportions of temporal group transitions may have been affected by the presence of missing echocardiography data and the survival bias. Indeed, the proportion of missing echocardiography data was approximately 30% at 1‐year follow‐up. In addition, given that patients with (−)LVH/(−)LVE had the most favourable prognosis among the three groups, the proportion of (−)LVH/(−)LVE group could have eventually increased over time. However, the similar patterns of temporal group transitions were also noted in the analysis among patients who survived through 5 years with complete data set of annual echocardiography follow‐up (online supplementary Figure S5 ). Furthermore, utilizing the ‘mice’ package of the R software, 35 we confirmed the robustness of the landmark analyses after multiply imputing missing echocardiography data at 1 year (online supplementary Table S8 ). Finally, since the CHART‐2 study is an observational study, there might be unmeasured confounding factors that could have influenced the present findings.
Conclusions
In HFpEF patients, LV structures dynamically change over time with significant prognostic impacts, where patients who develop LVE with LVH have the worst prognosis. Thus, for better management of HFpEF patients, more attention should be paid to temporal changes in LV mass and volume from the viewpoint of LV remodelling and reverse remodelling.
Supporting information
Figure S1. Incidences for all‐cause death, cardiovascular death, non‐cardiovascular death, and heart failure admission in HFpEF patients.
Figure S2. Incidence of the primary outcome by left ventricular concentricity in HFpEF patients with left ventricular hypertrophy.
Figure S3. Causes of death in HFpEF patients.
Figure S4. Longitudinal changes in left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index in the overall population.
Figure S5. Temporal changes in left ventricular structures in HFpEF patients who survived through 5 years with complete data set of annual echocardiography follow‐up.
Table S1. Prognostic impacts of baseline values of left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index.
Table S2. Prognostic impacts of temporal changes in left ventricular structures in the multivariable Cox proportional hazard model.
Table S3. Prognostic impacts of baseline values and 1‐year changes in left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index in patients with and without left ventricular concentricity.
Table S4. Prognostic impacts of left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index as time‐dependent variables.
Table S5. Prognostic impacts of left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index in the joint modelling.
Table S6. Simulation study to assess the robustness of the prognostic impacts of baseline left ventricular structural groups against random perturbation in left ventricular diastolic dimension and left ventricular wall thickness.
Table S7. Prognostic impacts of (A) baseline left ventricular structures and (B) temporal changes in left ventricular structures in the sensitivity analysis.
Table S8. Prognostic impacts of baseline values and 1‐year changes in left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index in the overall population after multiple imputation.
Acknowledgements
We thank all the members of the Tohoku Heart Failure Association and the staff of the Departments of Cardiovascular Medicine and Evidence‐based Cardiovascular Medicine, Tohoku University Graduate School of Medicine for their contributions.
Funding
This study was supported in part by the Grants‐in Aid from the Ministry of Health, Labour, and Welfare, the Ministry of Education, Culture, Sports, Science, and Technology, and the Agency for Medical Research and Development, Tokyo, Japan.
The Department of Evidence‐based Cardiovascular Medicine, Tohoku University Graduate School of Medicine, is supported in part by unrestricted research grants from Daiichi Sankyo (Tokyo, Japan), Bayer Yakuhin (Osaka, Japan), Kyowa Hakko Kirin (Tokyo, Japan), Novartis Pharma (Tokyo, Japan), Dainippon Sumitomo Pharma (Osaka, Japan), Astellas Pharma (Tokyo, Japan), AstraZeneca (Osaka, Japan), Chugai Pharmaceutical (Tokyo, Japan), GlaxoSmithKline (Tokyo, Japan), Kowa Pharmaceutical (Tokyo, Japan), Mitsubishi Tanabe Pharma (Osaka, Japan), Mochida Pharmaceutical (Tokyo, Japan), MSD (Tokyo, Japan), Nippon Boehringer Ingelheim (Tokyo, Japan), Otsuka Pharmaceutical (Tokyo, Japan), Shionogi (Osaka, Japan) and Takeda Pharmaceutical (Osaka, Japan).
Conflict of interest: H.S. has received lecture fees from Bayer Yakuhin (Osaka, Japan) and Daiichi Sankyo (Tokyo, Japan). All other authors have nothing to disclose.
[Correction added on 21 August 2020, after first online publication: the affiliations have been corrected in this current version.]
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail 2015;17:884–892. [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:e240–327. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 5. Parikh KS, Sharma K, Fiuzat M, Surks HK, George JT, Honarpour N, Depre C, Desvigne‐Nickens P, Nkulikiyinka R, Lewis GD, Gomberg‐Maitland M, O'Connor CM, Stockbridge N, Califf RM, Konstam MA, Januzzi JL Jr, Solomon SD, Borlaug BA, Shah SJ, Redfield MM, Felker GM. Heart failure with preserved ejection fraction expert panel report: current controversies and implications for clinical trials. JACC Heart Fail 2018;6:619–632. [DOI] [PubMed] [Google Scholar]
- 6. Komajda M, Lam CS. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J 2014;35:1022–1032. [DOI] [PubMed] [Google Scholar]
- 7. Shah AM. Ventricular remodeling in heart failure with preserved ejection fraction. Curr Heart Fail Rep 2013;10:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol 2000;35:569–582. [DOI] [PubMed] [Google Scholar]
- 9. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 10. Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000;35:580–586. [DOI] [PubMed] [Google Scholar]
- 11. Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Left ventricular geometry and outcomes in patients with atrial fibrillation: the AFFIRM trial. Int J Cardiol 2014;170:303–308. [DOI] [PubMed] [Google Scholar]
- 12. Verma A, Meris A, Skali H, Ghali JK, Arnold JM, Bourgoun M, Velazquez EJ, McMurray JJ, Kober L, Pfeffer MA, Califf RM, Solomon SD. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) echocardiographic study. JACC Cardiovasc Imaging 2008;1:582–591. [DOI] [PubMed] [Google Scholar]
- 13. Quiñones MA, Greenberg BH, Kopelen HA, Koilpillai C, Limacher MC, Shindler DM, Shelton BJ, Weiner DH. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. J Am Coll Cardiol 2000;35:1237–1244. [DOI] [PubMed] [Google Scholar]
- 14. Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE; I‐PRESERVE Investigators. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 2011;124:2491–2501. [DOI] [PubMed] [Google Scholar]
- 15. Zile MR, Gaasch WH, Patel K, Aban IB, Ahmed A. Adverse left ventricular remodeling in community‐dwelling older adults predicts incident heart failure and mortality. JACC Heart Fail 2014;2:512–522. [DOI] [PubMed] [Google Scholar]
- 16. Shiba N, Nochioka K, Miura M, Kohno H, Shimokawa H. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan – first report from the CHART‐2 study. Circ J 2011;75:823–833. [DOI] [PubMed] [Google Scholar]
- 17. Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, Shiroto T, Takahashi J, Miyata S, Shimokawa H. Characterization of heart failure patients with mid‐range left ventricular ejection fraction – a report from the CHART‐2 study. Eur J Heart Fail 2017;19:1258–1269. [DOI] [PubMed] [Google Scholar]
- 18. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham Study. N Engl J Med 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 20. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 21. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 22. Aragon TJ. epitools: Epidemiology tools. R package version 0.5‐10. 2017.
- 23. Hickey GL, Philipson P, Jorgensen A, Kolamunnage‐Dona R. Joint modelling of time‐to‐event and multivariate longitudinal outcomes: recent developments and issues. BMC Med Res Methodol 2016;16:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hickey GL, Philipson P, Kolamunnage‐Dona R, Gasparini A. joineRML: joint modelling of multivariate longitudinal data and time‐to‐event outcomes. R package version 0.4.4. 2020.
- 25. R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 26. Lee TH, Hamilton MA, Stevenson LW, Moriguchi JD, Fonarow GC, Child JS, Laks H, Walden JA. Impact of left ventricular cavity size on survival in advanced heart failure. Am J Cardiol 1993;72:672–676. [DOI] [PubMed] [Google Scholar]
- 27. St John Sutton M, Pfeffer MA, Plappert T, Rouleau JL, Moye LA, Dagenais GR, Lamas GA, Klein M, Sussex B, Goldman S, Menapace FJ Jr, Parker JO, Lewis S, Sestier F, Gordon DF, McEwan P, Bernstein V, Braunwald E; SAVE Investigators. Quantitative two‐dimensional echocardiographic measurements are major predictors of adverse cardiovascular events after acute myocardial infarction. The protective effects of captopril. Circulation 1994;89:68–75. [DOI] [PubMed] [Google Scholar]
- 28. Aleong RG, Mulvahill MJ, Halder I, Carlson NE, Singh M, Bloom HL, Dudley SC, Ellinor PT, Shalaby A, Weiss R, Gutmann R, Sauer WH, Narayanan K, Chugh SS, Saba S, London B. Left ventricular dilatation increases the risk of ventricular arrhythmias in patients with reduced systolic function. J Am Heart Assoc 2015;4:e001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katz DH, Beussink L, Sauer AJ, Freed BH, Burke MA, Shah SJ. Prevalence, clinical characteristics, and outcomes associated with eccentric versus concentric left ventricular hypertrophy in heart failure with preserved ejection fraction. Am J Cardiol 2013;112:1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail 2012;5:720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Savarese G, Vedin O, D'Amario D, Uijl A, Dahlstrom U, Rosano G, Lam CS, Lund LH. Prevalence and prognostic implications of longitudinal ejection fraction change in heart failure. JACC Heart Fail 2019;7:306–317. [DOI] [PubMed] [Google Scholar]
- 32. Bang CN, Gerdts E, Aurigemma GP, Boman K, de Simone G, Dahlof B, Kober L, Wachtell K, Devereux RB. Four‐group classification of left ventricular hypertrophy based on ventricular concentricity and dilatation identifies a low‐risk subset of eccentric hypertrophy in hypertensive patients. Circ Cardiovasc Imaging 2014;7:422–429. [DOI] [PubMed] [Google Scholar]
- 33. Garg S, de Lemos JA, Ayers C, Khouri MG, Pandey A, Berry JD, Peshock RM, Drazner MH. Association of a 4‐tiered classification of LV hypertrophy with adverse CV outcomes in the general population. JACC Cardiovasc Imaging 2015;8:1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, Dispenzieri A, Zeldenrust SR, Redfield MM. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2014;2:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Buuren S, Groothuis‐Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Incidences for all‐cause death, cardiovascular death, non‐cardiovascular death, and heart failure admission in HFpEF patients.
Figure S2. Incidence of the primary outcome by left ventricular concentricity in HFpEF patients with left ventricular hypertrophy.
Figure S3. Causes of death in HFpEF patients.
Figure S4. Longitudinal changes in left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index in the overall population.
Figure S5. Temporal changes in left ventricular structures in HFpEF patients who survived through 5 years with complete data set of annual echocardiography follow‐up.
Table S1. Prognostic impacts of baseline values of left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index.
Table S2. Prognostic impacts of temporal changes in left ventricular structures in the multivariable Cox proportional hazard model.
Table S3. Prognostic impacts of baseline values and 1‐year changes in left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index in patients with and without left ventricular concentricity.
Table S4. Prognostic impacts of left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index as time‐dependent variables.
Table S5. Prognostic impacts of left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index in the joint modelling.
Table S6. Simulation study to assess the robustness of the prognostic impacts of baseline left ventricular structural groups against random perturbation in left ventricular diastolic dimension and left ventricular wall thickness.
Table S7. Prognostic impacts of (A) baseline left ventricular structures and (B) temporal changes in left ventricular structures in the sensitivity analysis.
Table S8. Prognostic impacts of baseline values and 1‐year changes in left ventricular ejection fraction, left ventricular mass index, and left ventricular diastolic dimension index in the overall population after multiple imputation.


