Abstract
Background and Objectives
Laser‐pumped lasers enable driving a secondary wavelength through pumping with a primary device. Here we investigate the first 730 nm laser‐pumped laser for efficacy in tattoo removal.
Study Design/Materials and Methods
Fifteen subjects with 20 tattoos were enrolled to investigate the effect of a new 730 nm, titanium‐sapphire laser‐pumped laser at removing decorative tattoos. A total of four treatments were administered and photographic improvement of pre‐ and post‐treatment cross‐polarized digital images was evaluated by four blinded physician observers using an 11‐point scale.
Results
Blinded assessment of pre‐ and post‐treatment images found 70%, 77%, 83%, 83%, 26%, and 8% clearance from baseline images for black, green, blue, purple, red and yellow pigments, respectively. Side effects were limited to pinpoint bleeding and erythema immediately after treatment and some crusting and scale up to 1–2 weeks following treatment, and a localized allergic reaction in a single subject. There was no scarring or pigmentary alteration visible in any follow‐up images.
Conclusion
The new 730 nm, picosecond‐domain, titanium‐sapphire, laser‐pumped laser is safe and effective for removing multicolored tattoos. Green, blue, and purple pigments cleared the most as expected, but black ink cleared more completely than was predicted. Lasers Surg. Med. © 2020 The Authors. Lasers in Surgery and Medicine published by Wiley Periodicals LLC
Keywords: blue, green, ink, laser, picosecond, removal, tattoo, 730 nm
INTRODUCTION
For years the mainstay of laser tattoo removal were nanosecond‐domain lasers including the Q‐switched ruby laser and neodymium‐yttrium‐aluminum‐garnet lasers (Nd:YAG) [1, 2, 3, 4, 5, 6, 7]. The ruby laser is effective at removing black ink, as well as having some activity in removing blue and green inks but is not effective at removing red ink, or optimal for treating any color ink in very dark skin types due to the relatively strong melanin absorption at the 694 nm wavelength [1, 2, 3, 4, 5]. Q‐switched Nd:YAG lasers include a frequency‐doubling potassium‐titanyl‐phosphate (KTP) crystal to generate green light for treating red ink; in addition to delivering the long, 1,064 nm primary wavelength that is poorly absorbed by melanin pigment and thus ideal for use in all skin types for treating black inks [4, 5, 6, 7]. It has been theorized that shorter pulse durations in the sub‐nanosecond range would be ideal for treating particles the size of tattoo granules [8, 9, 10]. The actual size of particles that lasers target is the granules that have been aggregated by resident macrophages, which is where the ink resides in tattooed skin. Research lasers having shorter pulse durations in the nanosecond‐domain have been shown to be effective at removing tattoos [8, 9, 10]. These early picosecond‐domain lasers had delivered fairly low energies, requiring small beam diameters to get adequate fluences to elicit a tissue effect.
The newest additions to the armamentarium aimed at removing unwanted tattoos are the commercial picosecond‐domain lasers [11, 12, 13, 14, 15, 16, 17, 18, 19, 20]. The first of these lasers was an alexandrite laser having a pulse duration of approximately 0.5 nanoseconds [11, 12, 13]. Subsequently, Nd:YAG lasers have been developed that deliver picosecond‐domain pulses in the 0.3–0.5 nanoseconds range, and like their nanosecond counterparts incorporate a KTP frequency‐doubling crystal to enable treatment of red tattoos and pigmented lesions with the 532 nm wavelength [14]. Most of the commercially available picosecond‐domain lasers offer either laser‐pumped laser handpieces or dye cartridges that are either located inside the main laser casing or that connect as external handpieces to enable treatment of other colors of ink not covered by the primary laser wavelengths. In the case of the alexandrite laser, the additional wavelengths would include 1,064 and 532 nm for more specifically targeting black or red inks. In the case of a picosecond‐domain laser with the primary wavelength at 1,064 nm, the secondary wavelengths would be 532 nm which would be used to pump tertiary wavelengths in the 700 nm range to target green, blue, or purple inks. Such wavelengths include the 755 nm alexandrite laser and the 785 nm titanium‐sapphire (Ti:sapphire) laser [17]. The 785 nm laser‐pumped laser in a removable handpiece cartridge was shown to effectively remove blue, green, and purple inks, as well as showing efficacy for removing unwanted pigmentation. Here we investigate a new 730 nm, Ti:sapphire laser, by the same manufacturer as the above‐mentioned 785 nm laser, for safety and efficacy at removing multicolored decorative tattoos. This 730 nm laser‐pumped laser handpiece was developed as a potentially more effective wavelength for removing blue, green, and purple tattoos than the previous‐generation 785 nm laser, based on preliminary research. In addition, because shorter wavelengths in the visible range are more strongly absorbed by melanin pigment, it was postulated that the 730 nm Ti:sapphire laser would be more effective than the 785 nm wavelength at removing unwanted pigmentation. In this current study, we measure the safety and effectiveness of a new picosecond‐domain 730 nm, Ti:sapphire, laser‐pumped laser handpiece for removing multicolored tattoos.
MATERIALS AND METHODS
Subjects
The study was open to males or females ranging in age from 18 to 70 with unwanted decorative tattoos and any Fitzpatrick skin type. Approval for this study was received from an Institutional Review Board for the treatment of human subjects. A total of 15 subjects, 5 males and 10 females, with 20 tattoos were enrolled into this study and ranged in age from 30 to 63 years, averaging 45 years of age. Eleven subjects had a single tattoo, three had two tattoos and one had three tattoos. The study was open to all Fitzpatrick skin types. Subjects with Fitzpatrick skin types I‐III were enrolled with two subjects having skin type I, six skin type II, and seven had skin type III. Subjects could not have had any prior treatment to their tattoos, be pregnant, nursing or a female having unprotected sexual intercourse, within six months of a course of isotretinoin, or have a history of keloidal scarring. The most common tattoo color was black, being present in 18 tattoos, while nine contained green ink, three had blue ink, four contained purple ink, four had red ink, and five contained yellow ink. Yellow ink was often not visible on the initial presentation as it is frequently mixed with green ink to brighten the green color. Yellow ink is often revealed upon the removal of green ink to reveal the underlying yellow color.
Laser
The laser‐pumped laser used in the current study consists of a Ti:sapphire crystal placed in an accessory handpiece designed to fit on a commercial picosecond‐domain laser system (Picoway®; Candela Medical, Marlborough, MA). The Ti:sapphire laser crystal was pumped with the 532 nm wavelength generated from the Nd:YAG laser by a KTP crystal and delivered a maximum 100 mJ, with a 246 picoseconds pulse duration at a wavelength of 730 nm. The beam diameter can be selected from 2 to 6 mm in steps of 1 mm using lens cartridges that attach to the distal end of the laser‐pumped laser handpiece. The available fluences range from 0.6 to 4.0 J/cm2 with a selectable repetition rate ranging from 1 to 10 Hz.
Laser Treatment
The tattoo and surrounding skin were wiped with 70% isopropyl alcohol followed by 3% hydrogen peroxide and then dried with gauze. Intradermal 1% lidocaine with 1:100,000 epinephrine was administered in most subjects with some receiving 1% plain lidocaine, depending upon the subject's current medications. Treatments were administered through a clear hydrogel dressing to protect the epidermis and prevent tissue and blood splatter (Vigilon; CR Bard, Inc., Covington, CA). The dressing was left in place post‐treatment and covered with paper tape. Subsequent wound care consisted of gentle cleansing followed by application of an occlusive ointment (Aquaphor Healing Ointment; Beiersdorf AG, Hamburg, Germany).
Following local anesthesia and application of the hydrogel dressing, each tattoo was treated solely with the 730 nm wavelength. The 1,064 and 532 nm wavelengths were not used to treat colors typically considered optimally targeted with these wavelengths, such as black ink by the 1,064 nm wavelength or 532 nm for green and yellow pigments, to fully assess the efficacy of the 730 nm wavelength and short 246 picoseconds pulse duration at treating all tattoo colors included in this study. Since this wavelength and pulse duration have never been previously studied, their effects on multicolored or single‐colored, tattoos are unknown. Subjects were all scheduled to receive four treatments at 6–10‐week intervals, optimally at 8‐week intervals but varying as a function of holidays and other scheduling issues. The minimum fluences that resulted in immediate tissue whitening were selected to treat an individual tattoo. Fluences were increased to reach this endpoint and decreased if more than pinpoint bleeding was observed beneath the clear hydrogel dressing. With each subsequent treatment as the ink was removed, higher fluences were required to reach this endpoint, since less target pigment was present to absorb the administered laser energy (Table 1). To optimally visualize the tattoos through protective laser eyewear and through the hydrogel dressing, a cross‐polarizing headlamp was utilized for all treatments (v600; Syris Scientific, Gray, ME).
Table 1.
Laser Treatment Parameter
| Treatment parameter | Tx1 | Tx2 | Tx3 | Tx4 |
|---|---|---|---|---|
| Mean fluence (range) (J/cm2) | 0.96 (0.65–1.00) | 1.60 (0.70–1.80) | 1.72 (1.00–1.80) | 1.80 |
| Spot size (range) (mm) | 4 | (3–5) | (3–4) | 3 |
| No. of Txs | 19 | 19 | 19 | 14 |
| Median no. of pulses (range) | 214 (32–1,086) | 249 (46–1,063) | 145 (14–758) | 199 (82–892) |
Blinded Evaluation of Digital Images
Digital images were taken by the treating physician before the treatment and 8 weeks following the final treatment using a digital camera (D90; Nikon Corporation, Melville, NY) incorporating a cross‐polarizing flash system (Canfield Scientific, Fairfield, NJ). Cross‐polarized flashes eliminate any surface reflection, dramatically enhancing visualization of dermal tattoo pigment over even the unaided eye. Two fixed focal lengths were used depending upon the size of the tattoo to create reproducible, standardized images. Images were arranged in pairs, pre‐ and post‐treatment, and shown side‐by‐side, randomized as to which frame contained the before or after images. Four blinded independent physician reviewers rated clearance using an 11‐point clearance scale in 10% increments (0 = no improvement, 1 = 10%, 2 = 20% improvement, up to 10 = 100% or complete removal). If the baseline images were to be incorrectly selected by a reviewer, the score would be recorded as a negative score (i.e., a score of 2 would be recorded as a −2 or a 20% increase in tattoo pigment).
Side Effects
A 4‐point scale was used to record treatment effects immediately after treatment. Redness, swelling, bruising, crusting, pinpoint bleeding, and blistering were assessed by the treating physician. The scale used had a score of 0 representing none of the above effects and 1, 2, and 3 representing a mild, moderate, or severe effect, respectively. The treating physician evaluated treatment sites for pigmentary alterations or scarring 8 weeks following the final treatment and used the same 4‐point scoring scale.
RESULTS
Of the 15 subjects with 20 tattoos, one subject with a single black and green tattoo was withdrawn from the study due to a hypersensitivity reaction at the treatment site following the third treatment. All of the remaining subjects completed the study, with one subject having three tattoos missing a single treatment session due to scheduling issues receiving only three treatments instead of four. Example photos of tattoos taken at baseline and 2‐months after the fourth treatment are shown in Figure 1.
Figure 1.
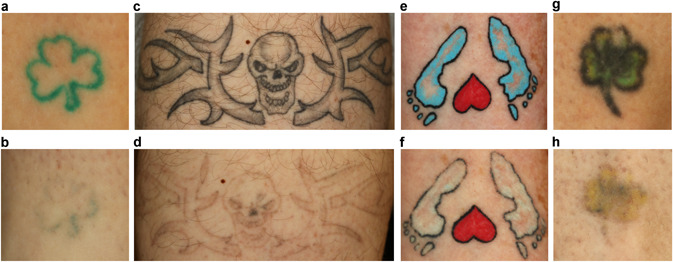
Cross‐polarized images at baseline (a, c, e, g) and follow‐up after four treatments (b, d, f, h). Cross‐polarized photography enhances the visibility of tattoos over conventional lighting or non‐polarized flash photography. The red heart in images (e) and (f) were not treated as per patient request and the blue was considered clear after only two treatments.
Blinded Evaluation of Digital Images
Blinded review by four physician reviewers performed on randomized side‐by‐side images before and 2 months following the final treatment were rated on an 11‐point scale revealing average clearances [mean ± SD (%)] of 8.3 ± 1.3 (83%) for purple, 8.3 ± 1.3 (83%) for blue, 7.7 ± 1.4 (77%) for green, 7.0 ± 1.9 (70%) for black, 2.6 ± 2.5 (26%) for red, and 0.8 ± 0.9 (8%) for yellow (Fig. 2). None of the blinded reviewers wrongly selected a pre‐treatment image as a post‐treatment image.
Figure 2.
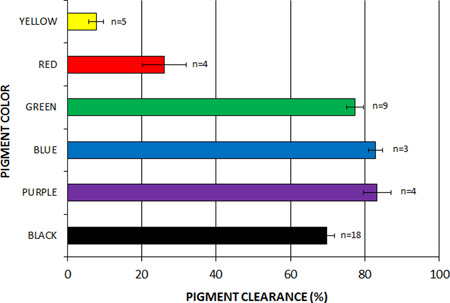
Average clearance for different tattoo ink colors using the 730 nm Ti: sapphire laser following four laser treatments. Error bars show standard error of the mean.
Side Effects
Out of a total of 71 administered treatments, one subject had mild pinpoint bleeding after a single treatment, and none was seen following any other treatments. Mild edema was noted immediately after 67 (94%) of treatments. There was clinical erythema after all but a single treatment, with mild erythema immediately after 64 (90%) treatments and moderate erythema after 6 (8%).
One subject reported diffuse swelling and intense itching around the treatment site following the 3rd treatment, beginning 3 days after the treatment and lasting approximately a week. On further questioning the subject believed a similar reaction occurred after the two previous treatments but considered it normal and did not report this finding to investigators. This subject was removed from the study and considered to have a localized allergic reaction following treatment. No purpura, crusting or blistering was seen immediately following any treatment, although some degree of crusting is common a few days after any tattoo laser treatment. There was no hyperpigmentation, hypopigmentation, or scarring seen at any 8‐week follow‐up period.
All subjects received intradermal 1% lidocaine injections with or without 1:100,000 epinephrine and reported pain scores for each of the four treatments of [median (range)] 0 (0–4), 2 (0–5), 2 (0–8), and 3 (0–6) for treatments 1, 2, 3 and 4, respectively.
DISCUSSION
The current study shows that the 730 nm, Ti‐sapphire, laser‐pumped‐laser is safe and effective for treating blue, purple, green, and black tattoo inks in Fitzpatrick skin types I‐III. The accessory handpiece was created to add an additional wavelength to a multi‐wavelength, picosecond‐domain laser platform. This wavelength was developed to treat unwanted epidermal pigmentation, such as freckles and lentigenes, in addition to targeting green, blue and purple tattoo pigments. The primary 1,064 nm wavelength is optimized for removing black ink and would be used for treating it by most laser operators; although, in the current study the 730 nm laser was effective at removing black ink as well, removing on average 70% of black pigment in four treatments as evaluated by blinded scoring of cross‐polarized images. Previously we reported on a Ti:sapphire 785 nm, 300 picoseconds pulse duration, laser‐pumped‐laser handpiece used with the same picosecond‐domain, 1,064 nm laser system used in the current study [17]. The current laser system emits at a significantly shorter wavelength, 730 nm, and would be expected to be more effective at removing brown spots such as freckles and lentigenes at equivalent fluences. In addition, the pulse duration of the 730 nm laser is shorter than the 785 nm laser at 246 vs. 300 picoseconds, respectively. Comparing the two studies, removal of purple, blue, and green pigments was quite similar, and both were poor at removing red and yellow inks as would be expected with a laser emitting in the red range. Truly comparing the efficacy of these two lasers requires a split‐tattoo study with each wavelength treating a different side of the same tattoo and using maximally tolerated fluences (MTFs). There was an interesting difference between the clearance of black ink with the 730 nm wavelength laser performing almost 10% better than the 785 nm laser. This isn't clinically important as the primary 1,064 nm, Nd:YAG laser would be used to remove black ink. Greater efficacy at removing epidermal pigmentation would favor the selection of the 730 nm laser over the 785 nm laser if one were to choose between the two, but the further use of both of these lasers in clinical practice should highlight relative benefits of each laser.
Traditionally, the Q‐switched, 755 nm alexandrite laser has been the treatment of choice for removing green, blue and purple tattoo inks [21, 22, 23, 24, 25, 26]. The 1,064 nm Nd:YAG laser has the advantage over the ruby laser of offering a 532 nm green wavelength, by incorporating a KTP crystal, for removing red ink as well. Q‐switched alexandrite lasers have the disadvantage of having a pulse duration in the 50–100 nanoseconds range, not optimal for small tattoo particles. The advent of the commercial picosecond‐domain lasers has had the greatest impact for wavelengths in the 755 nm range. Whereas 1,064 nm Nd:YAG lasers went from 5–10 nanoseconds pulse durations with Q‐switched lasers to approximately 0.5 nanoseconds with picosecond‐domain devices, a 10‐fold reduction in pulse duration; the alexandrite lasers went from pulse durations of 50–100 nanoseconds to 0.5 nanoseconds with picosecond‐domain devices which is over a 100‐fold shorter pulse duration. This results in higher peak powers and a pulse duration more appropriate for removing tattoo granules.
The shorter pulse durations of picosecond‐domain lasers theoretically convey an advantage for removing tattoo inks which are composed of small particles aggregated in dermal macrophages. Typically, Q‐switched, nanosecond‐domain Nd:YAG lasers have pulse durations in the 5–10 nanoseconds range, while Q‐switched alexandrite lasers emit pulses 5–10‐fold longer pulse durations at 50–100 nanoseconds. Thus, the pulse duration of Q‐switched Nd:YAG lasers are better optimized for removing small tattoo ink particles. Therefore, it is more critical to develop short, picosecond‐domain lasers in the wavelengths which are effective at removing blue, green, and purple inks, since the lasers which target these colors have relatively long pulse durations. The current 730 nm, 246 picoseconds Ti:sapphire laser emits the shortest picosecond‐domain pulse on the market to our knowledge, resulting in maximum peak power of 0.41 GW. A commercial picosecond‐domain alexandrite laser delivers a maximum peak power of 0.27 GW [11, 12], while a commercial nanosecond‐domain alexandrite laser delivers 0.02 GW [23]. Although a 752 nm Ti:sapphire laser was developed and tested by Herd et al[9] 20 years ago and was shown to be superior to an alexandrite laser, both lasers used in that study were far less advanced than the devices currently commercially available. Still, these investigators suggest far shorter pulse durations than are achievable today are optimal for tattoo removal and may remove all colors similarly and independent of the administered wavelength. Evidence for a qualitative difference in shorter pulse durations afforded by picosecond‐domain lasers for removing tattoos has been shown by the removal of yellow ink with picosecond‐domain, 532 nm KTP lasers as shown by Bernstein et al. and Geronemus’ group [13, 14], while yellow ink has been found to be quite resistant to removal by Q‐switched, 532 nm KTP lasers. It has been theorized that shorter pulse durations at least 10‐fold shorter than are currently achievable may result in even faster removal of all tattoo pigments and be independent of the wavelength, but so far this is not the case.
The crystal laser‐pumped‐laser handpiece produces single wavelengths of laser energy by using a simple handpiece that attaches to the distal end of the laser arm and is activated by the KTP laser contained in the main laser device. Laser‐pumped‐laser dye handpieces have been used for many years; however, they have been plagued by significant wavelength variation that can pose a danger to laser operators due to wavelengths emitted outside the wavelength range covered by laser eyewear, and by fairly rapid degradation. Newer laser‐pumped‐lasers contained inside the main laser casing may be solving some of those problems. Using laser crystals similar to those used in the primary laser device in external handpieces eliminates the issue of wavelength drift and may have advantages such as longevity and lower long‐term cost. The ability to create attachable handpieces of various wavelengths dramatically increases the versatility of a laser system without necessitating an entirely new laser device. The biggest advantage of the new picosecond‐domain devices is the versatility of many of these lasers which offer at least three wavelengths: 1,064, 532, and 755 nm or another wavelength in the 700‐range such as 785 or the 730 nm wavelength investigated here. Having the three main wavelengths used to remove all colors of tattoo ink effectively (except white or tan pigments) in a single device can save time, space and money that would be invested in having a second laser system. The future should bring even shorter pulse durations and never before used wavelengths to further optimize the laser treatment of tattoos.
ACKNOWLEGMENTS
The authors would like to acknowledge Joe Lowery, Liz Elliot, and Jessica Plugis for their help with IRB study documents, data entry, coordinating photos for blinded assessment, and for photography. Research funding for this project and loan of equipment was provided by Candela Medical.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: Dr. Bernstein is a consultant for Candela Medical and has equity in Candela Medical. Drs. Schomacker, Shang, and Paranjape were employees of Candela Medical at the time this study was completed.
REFERENCES
- 1. Taylor CR, Gange RW, Dover JS, et al. Treatment of tattoos by Q‐switched ruby laser. A dose‐response study. Arch Dermatol 1990;126:893–899. [PubMed] [Google Scholar]
- 2. Scheibner A, Kenny G, White W, Wheeland RG. A superior method of tattoo removal using the Q‐switched ruby laser. J Dermatol Surg Oncol 1990;16:1091–1098. [DOI] [PubMed] [Google Scholar]
- 3. Ashinoff R, Geronemus RG. Rapid response of traumatic and medical tattoos to treatment with the Q‐switched ruby laser. Plast Reconstr Surg 1993;91:841–845. [DOI] [PubMed] [Google Scholar]
- 4. Kilmer SL, Anderson RR. Clinical use of the Q‐switched ruby and the Q‐switched Nd:YAG (1064 nm and 532 nm) lasers for treatment of tattoos. J Dermatol Surg Oncol 1993;19:330–338. [DOI] [PubMed] [Google Scholar]
- 5. Levine VJ, Geronemus RG. Tattoo removal with the Q‐switched ruby laser and the Q‐switched Nd:YAG laser: A comparative study. Cutis 1995;55:291–296. [PubMed] [Google Scholar]
- 6. Kilmer SL, Lee MS, Grevelink JM, Flotte TJ, Anderson RR. The Q‐switched Nd:YAG laser effectively treats tattoos. A controlled, dose‐response study. Arch Dermatol 1993;129:971–978. [PubMed] [Google Scholar]
- 7. Jones A, Roddey P, Orengo I, Rosen T. The Q‐switched ND:YAG laser effectively treats tattoos in darkly pigmented skin. Dermatol Surg 1996;22:999–1001. [DOI] [PubMed] [Google Scholar]
- 8. Ross V, Naseef G, Lin G, et al. Comparison of responses of tattoos to picosecond and nanosecond Q‐switched neodymium: YAG lasers. Arch Dermatol 1998;134:167–171. [DOI] [PubMed] [Google Scholar]
- 9. Herd RM, Alora MB, Smoller B, Arndt KA, Dover JS. A clinical and histologic prospective controlled comparative study of the picosecond titanium:sapphire (795 nm) laser versus the Q‐switched alexandrite (752 nm) laser for removing tattoo pigment. J Am Acad Dermatol 1999;40:603–606. [DOI] [PubMed] [Google Scholar]
- 10. Izikson L, Farinelli W, Sakamoto F, Tannous Z, Anderson RR. Safety and effectiveness of black tattoo clearance in a pig model after a single treatment with a novel 758 nm 500 picosecond laser: A pilot study. Lasers Surg Med 2010;42:640–646. [DOI] [PubMed] [Google Scholar]
- 11. Brauer JA, Reddy KK, Anolik R, et al. Successful and rapid treatment of blue and green tattoo pigment with a novel picosecond laser. Arch Dermatol 2012;148:820–823. [DOI] [PubMed] [Google Scholar]
- 12. Saedi N, Metelitsa A, Petrell K, Arndt KA, Dover JS. Treatment of tattoos with a picosecond alexandrite laser: a prospective trial. Arch Dermatol 2012;148:1360–1363. [DOI] [PubMed] [Google Scholar]
- 13. Alabdulrazzaq H, Brauer JA, Bae YS, Geronemus RG. Clearance of yellow tattoo ink with a novel 532‐nm picosecond laser. Lasers Surg Med 2015;47:285–288. [DOI] [PubMed] [Google Scholar]
- 14. Bernstein EF, Schomacker KT, Basilavecchio LD, Plugis JM, Bhawalkar JD. A novel dual‐wavelength, Nd:YAG, picosecond‐domain laser safely and effectively removes multicolor tattoos. Lasers Surg Med 2015;47:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friedman DJ. Successful treatment of a red and black professional tattoo in skin type VI with a picosecond dual‐wavelength, neodymium‐doped yttrium aluminium garnet laser. Dermatol Surg 2016;42:1121–1123. [DOI] [PubMed] [Google Scholar]
- 16. Kauvar ANB, Keaney TC, Alster T. Laser treatment of professional tattoos with a 1064/532‐nm dual‐wavelength picosecond laser. Dermatol Surg 2017;43:1434–1440. [DOI] [PubMed] [Google Scholar]
- 17. Bernstein EF, Bhawalkar J, Schomacker KT. A novel titanium sapphire picosecond‐domain laser safely and effectively removes purple, blue, and green tattoo inks. Lasers Surg Med 2018;50:704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeon H, Geronemus RG. Successful treatment of a traumatic tattoo in a pediatric patient using a 755‐nm picosecond laser. Pediatr Dermatol 2018;35:e430–e431. [DOI] [PubMed] [Google Scholar]
- 19. Kato H, Doi K, Kanayama K, et al. Combination of dual wavelength picosecond and nanosecond pulse width neodymium‐doped yttrium‐aluminum‐garnet lasers for tattoo removal. Lasers Surg Med 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20. Feng H, Christman MP, Muzumdar S, Geronemus RG. Successful treatment of cosmetic oral mucosal tattoos using QS 694‐nm ruby laser and 755‐nm alexandrite picosecond laser. Lasers Surg Med 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Fitzpatrick RE, Goldman MP. Tattoo removal using the alexandrite laser. Arch Dermatol 1994;130:1508–1514. [PubMed] [Google Scholar]
- 22. Alster TS. Q‐switched alexandrite laser treatment (755 nm) of professional and amateur tattoos. J Am Acad Dermatol 1995;33:69–73. [DOI] [PubMed] [Google Scholar]
- 23. Bernstein EF, Bhawalkar J, Clifford J, Hsia J. Treatment of tattoos with a 755‐nm Q‐switched alexandrite laser and novel 1064 nm and 532 nm Nd:YAG laser handpieces pumped by the alexandrite treatment beam. J Drugs Dermatol 2010;9:1333–1339. [PubMed] [Google Scholar]
- 24. Stafford TJ, Lizek R, Tan OT. Role of the alexandrite laser for removal of tattoos. Lasers Surg Med 1995;17:32–38. [DOI] [PubMed] [Google Scholar]
- 25. Leuenberger ML, Mulas MW, Hata TR, Goldman MP, Fitzpatrick RE, Grevelink JM. Comparison of the Q‐switched alexandrite, Nd:YAG, and ruby lasers in treating blue‐black tattoos. Dermatol Surg 1999;25:10–14. [DOI] [PubMed] [Google Scholar]
- 26. Bukhari IA. Removal of amateur blue‐black tattoos in Arabic women of skin type (III‐IV) with Q‐switched alexandrite laser. J Cosmet Dermatol 2005;4:107–110. [DOI] [PubMed] [Google Scholar]


