Abstract
Introduction
Many recombinant and modified FIX products have been, and continue to be, developed with the aim of improving treatment for patients with haemophilia B. One such new product is dalcinonacog alfa, a recombinant FIX with modifications to provide improved features such as subcutaneous administration.
Aim
In view of previously observed assay discrepancies with modified FIX therapeutics, the aim of this study was to assess potential discrepancies in potency measurement of dalcinonacog alfa between and within different assay methods.
Methods
Potency of dalcinonacog alfa was measured against the 5th International Standard (IS) for FIX Concentrate and the 4th IS for FIX Plasma by One‐Stage Clotting Assay, using 9 different APTT reagents and 2 commercially available FIX chromogenic kits. Plasma‐derived concentrate and recombinant FIX samples were also included for comparison in every assay.
Results
Substantial discrepancies were observed when assaying dalcinonacog alfa using the one‐stage clotting assay against both standards. No statistically valid results were obtained when testing dalcinonacog alfa using either chromogenic kit. Increasing the incubation time with the activation reagent in both chromogenic kits resulted in valid assays and increased the potency to become more in line with potencies by one‐stage clotting assays. Increasing the incubation time in the chromogenic kits had no effect on the potencies of the plasma‐derived or recombinant samples. However, incubation time influenced in the one‐stage clotting assay using Dapttin.
Conclusions
Within and between assay method discrepancy was found when assaying dalcinonacog alfa. Methods for potency labelling and clinical monitoring should be given careful consideration.
Keywords: chromogenic assay, drug therapy, extended half‐life products, factor IX assay, haemophilia B, modified FIX, one‐stage clotting assay, standardization
1. INTRODUCTION
Haemophilia B is a genetic bleeding disorder caused by a deficiency in coagulation factor IX (FIX) and is commonly treated by FIX replacement therapy. Replacement factors can be derived from plasma or recombinant sources. Recently, novel recombinant FIX therapeutics has been developed with modifications mainly with the aim to improve half‐life and reduce infusion frequency.
There are two methods for measuring FIX activity: the one‐stage clotting assay (OSCA) and the functional chromogenic assay (ChA). Within these methods, there are multiple different reagents/kits. All licensed FIX therapeutics are potency labelled using the OSCA as described in the European Pharmacopeia (EP)1 against the current WHO International Standard (IS) for FIX, Concentrate. For clinical monitoring of postinfusion samples, OSCA against a plasma standard traceable to the current WHO IS for FIX, Plasma is routinely used.2 However, discrepancies between methods and reagents when assaying the extended half‐life and full‐length recombinant therapeutics have been reported and the compositional differences of the reagents/kits are believed to play a part in the observed discrepancies.3, 4, 5, 6, 7, 8, 9 It is also clear that these modified products have different characteristics than the IS, which are either plasma‐derived concentrate or normal plasma. Therefore, these potency evaluations deviate from the basic concept of biological standardization: ‘like versus like’.10, 11 Nevertheless, statistically valid assays and their estimates can be obtained when these modified products are assayed against the IS, which justify potency labelling in International Units (IU).3, 4, 5, 6, 8, 12, 13, 14
Catalyst Biosciences has developed a new rFIX therapeutic, dalcinonacog alfa (DA) (previously known as CB2679d/ISU304), which has been given orphan drug designation in Europe and the United States and has currently completed a Phase 1/2 proof of concept clinical trial.15 DA is a full‐length recombinant FIX molecule with amino acid substitutions in the protease domain that confer the product with increased potency, enhanced FVIIIa binding and reduced inhibition by antithrombin. These enhanced features allow for effective prophylaxis of patients by subcutaneous administration.16, 17
In view of the previously observed assay discrepancies with modified FIX therapeutics, the aim of this study was to assess any potential discrepancies in potency measurement of DA between and within different assay methods. To investigate this, one‐stage clotting and chromogenic assays were carried out using a range of reagents and kits. Plasma‐derived concentrate FIX (PD) and recombinant FIX (RB) samples were also included for comparison in every assay, and potencies were calculated against the 5th IS for FIX Concentrate (S1) and the 4th IS for FIX Plasma (S2).
2. MATERIALS AND METHODS
2.1. Samples
One vial of CB2679d (sample DA) (Catalyst Biosciences, lot F1601‐1) and 2 vials of Benefix (sample RB) (Pfizer, lot R36539) were reconstituted according to the manufacturers’ instructions and diluted to obtain a working stock of 10 IU/mL, based on the labelled potency, in Glyoxaline/HSA buffer, pH 7.3 (50 mmol/L Imidazole (Sigma Aldrich) + 100 mmol/L NaCl (VWR) with 1% human albumin (HSA) (BPL)). The working stocks of DA and RB were aliquoted, snap‐frozen and stored at −80°C. A new aliquot was thawed for 2 minutes at 37°C for every assay. A new ampoule of S1: 5th International Standard for FIX Concentrate, 14/148, 10.5 IU FIX/ampoule (NIBSC), S2: 4th International Standard for FIX Plasma, 09/172, 0.86 IU FIX/ampoule (NIBSC) and PD: a plasma‐derived FIX concentrate (6th British Working Standard for Coagulation Factors II, IX and X (07/326): 9.8 IU FIX/ampoule; NIBSC) were reconstituted according to the manufacturer's instructions for every assay.
2.2. One‐stage clotting assays
All test preparations, except S2, were prediluted to 1 IU/mL (for the initial assay, DA and RB were diluted based on assigned potency and subsequent assays based on the estimated potency from the first assay to ensure that the responses were in the same range as the standard) in FIX immune depleted plasma (Technoclone). All test preparations were further prediluted to approximately 0.1 IU/mL in Glyoxaline/HSA buffer. Assays were performed on an ACL TOP 550 automated coagulometer (Werfen) and each prediluted test preparation was tested at 3 working dilutions (neat, 1/3, 1/10) in Glyoxaline/HSA buffer in duplicate using a balanced assay design. Final concentrations of standards and samples were 0.1, 0.033 and 0.01 IU/mL. Dilutions were chosen so that the responses were in the linear portion of the dose‐response curve. APTT reagents used in OSCAs are listed in Table 1 and the test procedure; that is, proportions of reagents, calcium chloride concentration and incubation time were used as directed by the manufacturers’ instructions. Four independent assays were carried out for each APTT reagent and each assay included all test preparations and both standards.
Table 1.
List of APTT reagents used to perform One‐Stage Clotting Assays (OSCAs)
| Reagent | Phospholipid | Activator | Recommended incubation time (s) | Manufacturer |
|---|---|---|---|---|
| Actin‐FS | Soy | Ellagic acid | 180 | Siemens |
| APTT‐SP | Synthetic | Silica | 300 | Werfen |
| Cephascreen | Rabbit brain | Polyphenolic | 240 | Stago |
| CK Prest a | Rabbit brain | Kaolin | 180 | Stago |
| Dapttin a | Synthetic | Sulfatides & silica | 120‐300 b | Technoclone |
| Pathromtin SL | Vegetable | Silicon dioxide | 120 | Siemens |
| Siron LS | Synthetic | Ellagic acid | 240 | Technoclone |
| SynthAFax | Synthetic | Ellagic acid | 300 | Werfen |
| SynthasIL | Synthetic | Silica | 180 | Werfen |
Stir bar used with this reagent.
(120 s used for initial potency assays and 300 s used for incubation time investigations).
2.3. Chromogenic assays
Two commercial kits were investigated, Biophen Factor IX (Hyphen Biomed) and Rox Factor IX (Rossix). Both kits were automated on an ACL TOP 550 coagulometer and were used according to the kit manufacturers’ instructions except where the incubation time of reagent 2 (Hyphen kit) or reagent B (Rossix kit) were extended as described in the results section. Four independent assays were carried out with both kits and each assay included all test preparations and both standards. Each sample was assayed in duplicate within each assay using a balanced assay design. The dilutions for the first assay were based on DA and RB having an assumed potency of 10 IU/mL and for subsequent assays the dilutions were adjusted based on the estimated potency from the previous assay. This practice ensured that the test responses (mAbs/min) were in the same range as the standard. For the Rossix kit, test preparations were prediluted in kit assay buffer to approximately 0.02 IU/mL with further working dilutions of 1/2, 1/4, 1/8 (final concentrations: 0.01, 0.005 and 0.0025 IU/mL). For the Hyphen kit, in the initial potency assays, the test preparations were prediluted to approximately 0.1 IU/mL. Working dilutions performed were 1/10, 1/30, 1/100 (final concentrations: 0.01, 0.0033 and 0.001 IU/mL). When performing the Hyphen kit with the extended incubation time, samples were prediluted to 0.03 IU/mL to avoid substrate depletion (final concentrations: 0.003, 0.001 and 0.0003 IU/mL). Working dilutions performed were 1/10, 1/30, 1/100. Dilutions were chosen so that the responses were in the linear portion of the dose‐response curve.
2.4. Statistical analysis
Raw data were analysed using bioassay software Combistats (Version 5.0, EDQM), which calculates estimated potency by a parallel line model and assesses statistical validity under that model. This package determines linearity by an analysis of variance (ANOVA) F test and parallelism was assessed by equivalence testing. The latter is based on the calculation of the ratio of the slope of the test sample with the slope of the standard. The acceptance criteria for this ratio of 0.90‐1.11 for all test preparations were based on the ratio of the slope of the standard and test obtained for the plasma‐derived product as this was deemed to be most similar to the standard preparation. Overall potency estimates for each reagent were calculated as geometric means (GM) and variability was estimated using the geometric coefficient of variation (%GCV). One‐way ANOVA followed by Tukey's multiple comparisons test or an unpaired t test with the level of significance set at 5% (GraphPad Prism version 6.00 for Windows, GraphPad Software) were used to determine if there was a statistically significant difference between potency estimates from reagents or kits.
It is important to note that this study was not aimed at determining the accuracy of the labelled potency of DA and RB but focused on the agreement of values obtained by the different methods.
3. RESULTS
With the exception of DA vs S2 using the reagent APTT‐SP, one or more statistically valid assays were achieved for all samples with all APTT reagents (see Figure 1A‐F). Intra‐assay variability for OSCAs when assayed against S1 were low for all samples and all reagents, with the majority of GCVs < 10%. This indicated that assays were precise with good reproducibility (Table 2). Similar intra‐assay variability was observed when samples were assayed against S2 (data not shown). Table 3 shows the overall inter‐reagent variability for all preparations. Good agreement of potencies was obtained for PD with a GCV of ~3% against S1 and ~6% against S2. There was no discrepancy between OSCA reagents (P > .05). Higher variability was observed for RB with a GCV of ~15% against S1 and ~16% against S2, indicating that there was some disagreement in potency obtained with the different OSCA reagents. The highest OSCA potency estimate for RB was using Cephascreen (~11 IU/mL) and lowest using SynthaFax (~7 IU/mL) when assaying against S1 and S2 (Figure 1C and D). The variability for DA was at least 2‐fold higher than those obtained for RB with a GCV of 38% against S1 and 46% against S2. This indicates a poor agreement between reagents with CK Prest and Dapttin giving the highest potency estimates (~14 IU/mL vs S1) and Synthafax giving the lowest value (~5 IU/mL vs S1) (Table 2).
Figure 1.
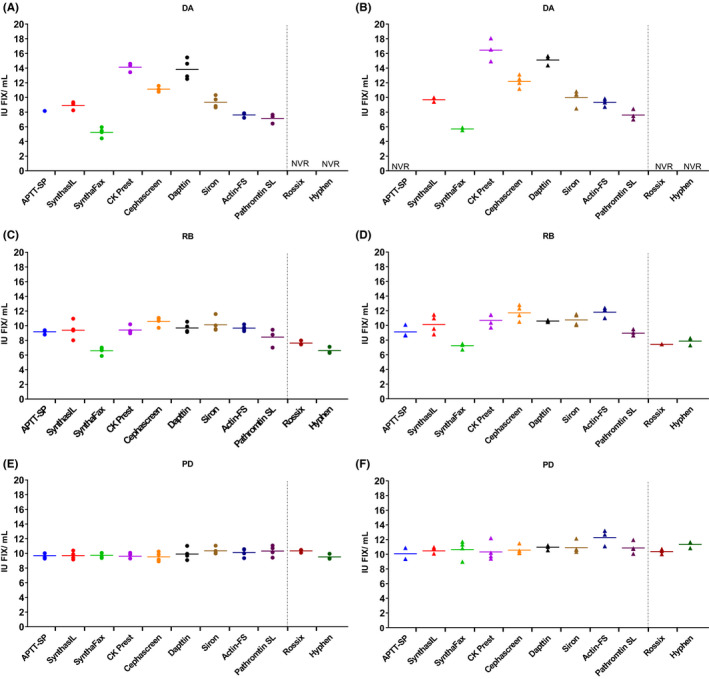
Potency estimates (IU/mL) from individual assays. Against S1 (FIX Concentrate International Standard): circles–panels A, C and E. Against S2 (FIX Plasma International Standard): triangles–panels B, D and F with geometric mean GM (lines) for dalcinonacog alfa (DA), recombinant FIX (RB) and plasma‐derived FIX (PD) when tested using all reagents/kits. NVR = no statistically valid results due to non‐parallelism
Table 2.
Geometric mean (GM) and intra‐assay variability expressed as geometric coefficient of variation (%GCV) for all reagents/kits when testing plasma‐derived FIX (PD), recombinant FIX (RB) and dalcinonacog alfa (DA) against the plasma‐derived concentrate international standard: S1, using different one‐stage clotting assays (OSCA) reagents and chromogenic assay (ChA) kits. No %GCV is given for APTT‐SP as only 1 valid assay was obtained using this reagent
| PD | RB | DA | ||||
|---|---|---|---|---|---|---|
| GM IU/mL | GCV (%) | GM IU/mL | GCV (%) | GM IU/mL | GCV (%) | |
| OSCA reagents | ||||||
| APTT‐SP | 9.7 | 3.9 | 9.2 | 2.9 | 8.2 | ‐ |
| SynthasIL | 9.7 | 5.7 | 9.4 | 13.6 | 8.9 | 6.8 |
| SynthaFax | 9.7 | 3.7 | 6.6 | 8.2 | 5.2 | 13.3 |
| CK Prest | 9.6 | 4.2 | 9.4 | 5.7 | 14.1 | 4.5 |
| Cephascreen | 9.5 | 6.6 | 10.6 | 6.2 | 11.1 | 3.0 |
| Dapttin | 9.9 | 8.4 | 9.7 | 6.8 | 13.8 | 10.5 |
| Siron | 10.3 | 4.8 | 10.1 | 9.9 | 9.4 | 8.7 |
| Actin‐FS | 10.1 | 5.9 | 9.7 | 4.1 | 7.6 | 4.5 |
| Pathromtin SL | 10.3 | 7.3 | 8.4 | 13.8 | 7.1 | 9.5 |
| ChA kits | ||||||
| Rossix | 10.3 | 2.1 | 7.6 | 4.0 | ‐ | ‐ |
| Hyphen | 9.5 | 4.0 | 6.6 | 6.9 | ‐ | ‐ |
Table 3.
Overall geometric mean (GM) and method GM (one‐stage clotting assays (OSCA) and chromogenic assays (ChA)) as well as inter‐assay variability (% geometric coefficient of variation (GCV) or range where only 2 kits tested) for plasma‐derived FIX (PD), recombinant FIX (RB) and dalcinonacog alfa (DA) against the plasma‐derived concentrate international standard: S1 and the plasma international standard: S2
| PD | RB | DA | |||||
|---|---|---|---|---|---|---|---|
| GM IU/mL | GCV/range | GM IU/mL | GCV/range | GM IU/mL | GCV/range | ||
| vs S1 | |||||||
| OSCA | 9.88 | 2.9% | 9.16 | 14.9% | 9.08 | 37.6% | |
| ChA | 9.92 | 9.5‐10.3 IU/mL | 7.10 | 6.6‐7.6 IU/mL | ‐ | ‐ | |
| Overall | 9.89 | 3.4% | 8.74 | 17.9% | 9.08 | 37.6% | |
| vs S2 | |||||||
| OSCA | 10.77 | 5.8% | 10.12 | 16.2% | 10.29 | 45.6% | |
| ChA | 10.84 | 10.4‐11.4 IU/mL | 7.77 | 7.7‐7.9 IU/mL | ‐ | ‐ | |
| Overall | 10.78 | 5.7% | 9.65 | 18.7% | 10.29 | 45.6% | |
When testing was carried out with ChAs, statistically valid results were obtained for both PD and RB with low intra‐assay variability (Figure 1C‐F and Table 3). No OSCA/ChA discrepancy was observed for PD while for RB there was a ~30% discrepancy between the OSCAs and ChAs with an overall GM for OSCAs of 9.2 IU/mL and ChAs of 7.1 IU/mL against S1 (Table 3). For DA, when ChAs were carried out according to the manufacturer's instructions, results for all assays against both standards were found to be statistically invalid due to non‐parallelism. Median slope ratios for DA against both S1 and S2, using both kits, were ~1.3 meaning that the slope of the dose‐response curve of the standards was steeper than the dose‐response curve of DA. Median slope ratios for PD and RB against both standards were within the 0.9‐1.11 limits set for non‐parallelism (all 1.0, except for RB against S2 using the Rossix kit, which had a slope ratio of 0.9). However, when the chromogenic assay kits were deconstructed and the incubation time of DA with the FXIa containing ‘activation reagent’ was extended, dose‐response curves with DA were parallel to both standards and statistically valid results were obtained (slope ratios close to 1.0). Figure 2 shows the potency obtained for DA and PD using the Hyphen kit manufacturers recommended incubation time of 3 minutes with reagent 2 along with results obtained when the incubation time was extended to 12 minutes. Statistically valid results were obtained for DA with 12 minutes incubation and the potency increased from ~3 to ~7 IU/mL. Increasing the incubation time to 12 minutes had no significant effect on the potency of PD. However, further increase in incubation time to 14 minutes resulted in a slight decrease in potency (data not shown) indicating issues with stability.
Figure 2.
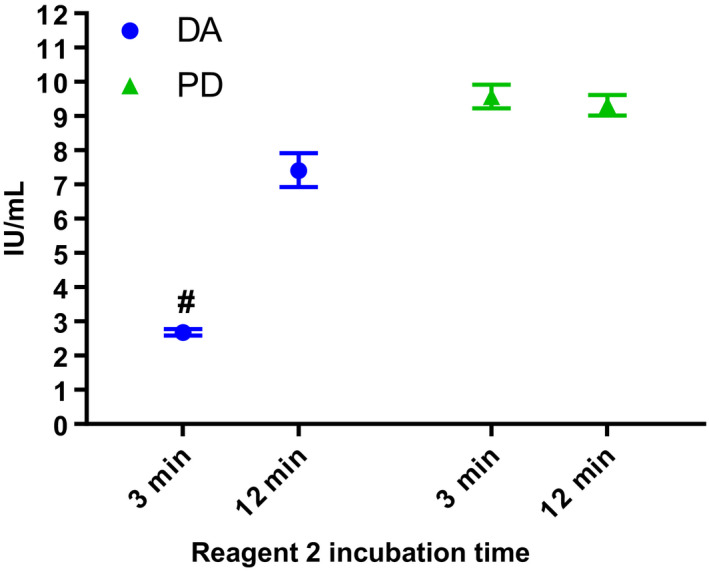
Estimated potency (IU/mL) with 95% confidence intervals for dalcinonacog alfa (DA) and plasma‐derived FIX (PD) when assayed using the Hyphen chromogenic assay (ChA) kit with the kit manufacturer's Reagent 2 recommended incubation time of 3 min and with the extended incubation time of 12 min. # = No assays were statistically valid for DA when tested with Reagent 2 incubation time of 3 min
A similar effect was observed when the incubation time of Reagent B of the Rossix kit was extended. Figure 3 shows the potency obtained for DA and PD with the kit manufacturers recommended incubation time of 8 minutes for reagent B, as well as with extended incubation times of 24‐ and 30‐minutes. Incubation time had no effect on potency of PD. Both 24‐ and 30‐minutes incubation generated statistically valid results for DA; potency increased from 1.2 IU/mL at 8 minutes incubation to 3.3 and 3.7 IU/mL for 24‐ and 30‐minutes incubation, respectively. The potency estimates at 24 and 30 minutes were significantly different from each other and to estimates obtained at 8 minutes incubation (t test, P < .05). However, at 30 minutes incubation increased assay variability was observed with both DA and PD. Incubation time was not extended further than 30 minutes due to limitations of the equipment and stability of the standards when carrying out long assays.
Figure 3.
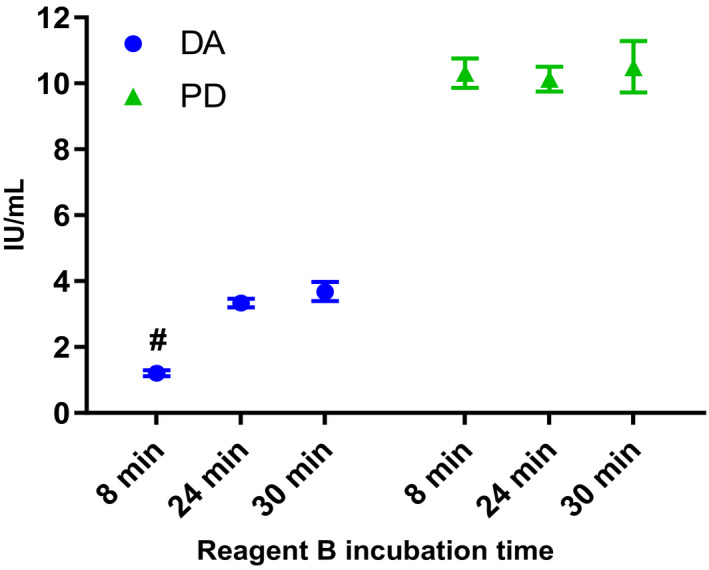
Estimated potency (IU/mL) with 95% confidence intervals for dalcinonacog alfa (DA) and plasma‐derived FIX (PD) when assayed using the Rossix chromogenic assay kit with the kit manufacturer's Reagent B recommended incubation time of 8 min and with the extended incubation times of 24‐ and 30‐ min. # = No assays were statistically valid for DA when tested with the manufacturers recommended incubation time (8 min) for reagent B
To determine if incubation time could explain the discrepancy seen for DA with the OSCA reagents, clotting times of the test preparations were compared over a range of incubation times from 1 to 8 minutes using 4 APTT reagents: APTT‐SP, SynthaFax, CK Prest and Dapttin. There was no divergence of clotting times of the test preparations over time for APTT‐SP, SynthaFax or CK Prest, indicating that incubation time of these 3 reagents does not influence potency. However, for Dapttin the difference in clotting times between the test preparations became greater with longer incubation time. Potencies of DA, RB and PD when assayed using Dapttin with an incubation time of 2 minutes compared to an incubation time of 5 minutes are shown in Figure 4A against S1 and 4B against S2. There was a reduction in potency for DA and RB against both standards and a reduction in potency of PD when assayed against S2. However, this decrease in potency was only significantly different against S2 (t test DA, P < .001; RB, P < .001; PD, P < .05).
Figure 4.
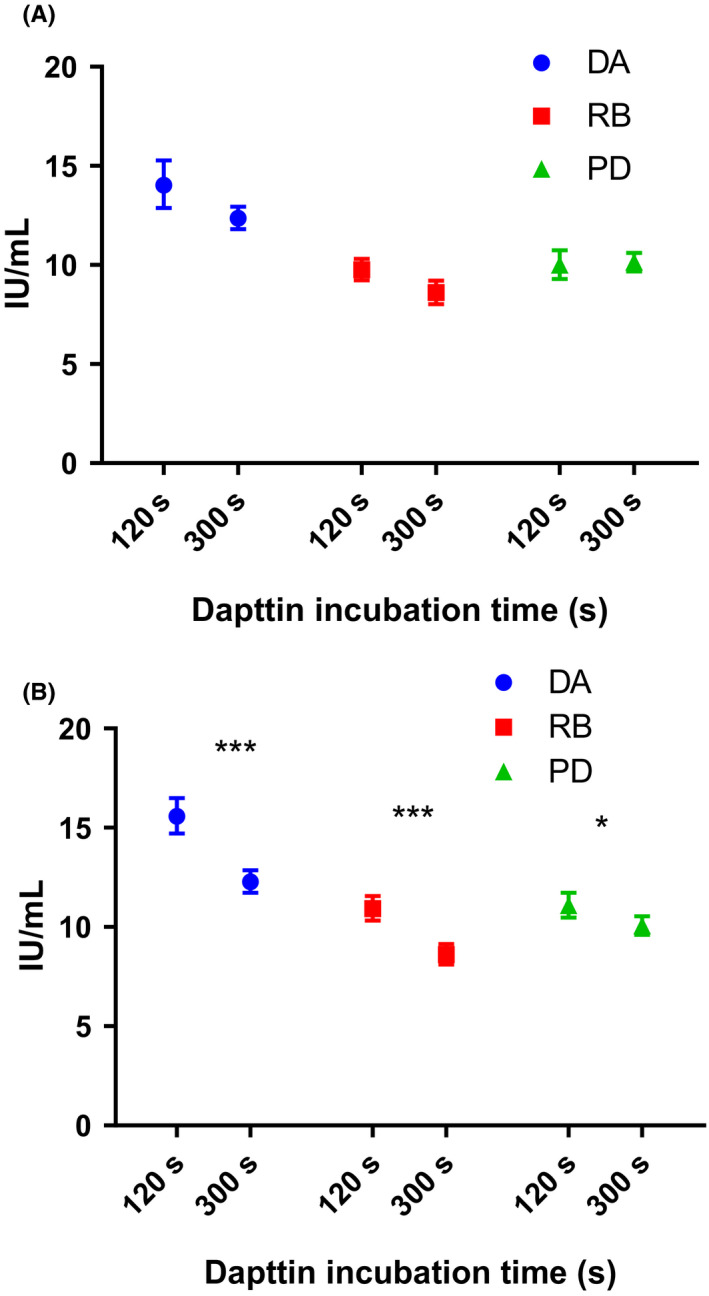
Estimated potency (IU/mL) with 95% confidence intervals for dalcinonacog alfa (DA), recombinant FIX (RB) and plasma‐derived FIX (PD) when assayed using one‐stage clotting assay with APTT reagent Dapttin using an incubation time of 120 and 300 s against S1: FIX Concentrate International Standard (panel A) and S2: FIX Plasma International Standard (panel B). Statistical differences between potency at 120 and 300 s were calculated using unpaired t test. When assayed against S1, P > .05 for all samples and against S2, DA; P < .001, RB; P < .001 and PD; P < .05)
4. DISCUSSION
It is widely known that assay discrepancies exist when measuring the potency of both full‐length and modified recombinant FIX products against plasma and plasma‐derived Concentrate FIX standards using different methods and reagents.4, 5, 6, 7, 8, 9, 12, 13, 14, 18, 19, 20, 21, 22 In this study, the degree of assay discrepancies of a novel modified rFIX therapeutic, dalcinonacog alfa (DA), was investigated and compared to those observed when assaying a plasma‐derived (PD) and full‐length rFIX (RB). No assay discrepancies were observed with PD against either standard. Both RB and DA showed discrepancies within OSCAs and between OSCA and ChAs, with DA showing greater differences than RB. Potency for RB by ChA was ~30% lower than by OSCA, the OSCA/ChA discrepancy with recombinant FIX has been described in a number of other studies.7, 8, 23, 24 For DA in the OSCAs, there was a roughly 3‐fold difference between the highest potency (CK Prest) and lowest potency (SynthFax) using both S1 and S2 standards. RB also showed lower potency when assayed using SynthaFax than the other APTT reagents, underestimation of Benefix with Synthfax has been previously reported.18 With the exception of Dapttin, CK Prest gave significantly higher potency for DA than all the other APTT reagents (one‐way ANOVA, P < .0001). However, when the assay was repeated using an incubation time of 300 seconds instead of 120 seconds, a lower potency was obtained, which was significantly different from the potency obtained by CK Prest (S1 ‐ Dapttin 300 seconds: 12.4 IU/mL, CK Prest: 14.2 IU/mL; t test P < .05, S2 ‐ Dapttin 300 seconds: 12.4 IU/mL, CK Prest: 15.9 IU/mL; t test P < .005). Unlike the other APTT reagents tested, the product insert for Dapttin does not specify an incubation time and instead provides a range between 120‐300 seconds. The effect on potency with different Dapttin incubation times has been described previously by our laboratory, where a 30% decrease in potency was observed for rFIX when assayed against the 4th IS for FIX concentrate when incubation time was increased from 120 to 300 seconds.22 Incubation time did not appear to influence potency estimates of DA by CK Prest, Synthafax or APTT‐SP. There was also no apparent relationship between potency and the activator/phospholipid composition of the APTT reagent. Studies into the causes of assay discrepancies with nonacog beta pegol (N9‐GP) by different APTT reagents suggest that different levels of overestimation were caused by varying levels of absorption of the PEG moiety attached to the FIX and by FXIa by silica activators 4 and underestimation likely to be caused by inhibition of FXIa by activators as well as steric hindrance.21 Differing levels of FXIa absorption/inhibition by activators in the APTT reagents could account for some of the deviating results observed for DA in the OSCAs.4
When DA was assayed by ChA according to the kit manufacturers’ instructions, there were no valid results against either standard due to non‐parallelism. In the ISTH/SSC published recommendations on potency labelling of FVIII and FIX concentrates, it states ‘Where only one method provides valid potency estimates relative to the WHO IS Concentrate (eg one‐stage clotting or chromogenic) this could be used for labelling’.20 In this study, only the OSCA method provided valid potency estimates which ties in well as it is the current EP method for potency labelling of FIX therapeutics. However, within the OSCA method not all APTT reagents generated statistically valid estimates: using APTT‐SP, no valid assays were attained against S2 (Plasma IS), and against S1 (Concentrate IS) only 1 out of the 4 assays were statistically valid. This indicates that APTT‐SP should be avoided for assaying this product. Furthermore, despite valid comparison against the IS for the other APTT reagents tested, assay discrepancies were still observed. This has been shown with other recombinant and modified FIX therapeutics.3, 4, 5, 6, 8, 13 In a situation where discrepancies are observed, the potency labelling method/reagent should correlate with the clinical outcome.20
Despite the lack of valid results when performing the ChAs as per the manufacturers’ instructions, modifications to the assay did result in valid results. When the incubation time of the ChA assay kit reagent containing FXIa, which activates the FIX in the sample, was extended parallelism was obtained and a concurrent increase in potency was observed with both kits. The increase in potency with increased incubation time using the Hyphen kit was closer to the expected potency based on the manufacturers labelled potency as well as the GM from the OSCAs. Whereas with the Rossix kit, the potency obtained after 30 minutes incubation was still low. Furthermore, modifying the ChAs to increase the incubation time would be difficult to implement in clinical haemostasis laboratories without extensive local validation and therefore would not be a viable solution for measurement of DA by ChA. Although both ChAs are based on a similar principle, there are differences in their composition, which may contribute to the discrepancy in estimated potency for DA by the two kits (Rossix < Hyphen). For example, the Hyphen kit contains thrombin to activate FVIII in the reaction, whereas the Rossix kit contains prothrombin from which the required thrombin is generated during the reaction. Also, Rossix contains factor V, whereas Hyphen does not, and the kits also have different FXa substrates. Differences in potency estimation by the 2 ChA kits have been reported elsewhere when testing other extended half‐life FIX therapeutics.9 One study showed that in a small data set of patients treated with FC fusion FIX (Alprolix), the Rossix kit recovered close to expected activity but the Hyphen kit was ~30% lower, while in patients treated with albumin fusion FIX (Idelvion) both kits recovered higher than expected median potencies.9 It is unclear how the differences in amino acid sequences between DA and wild‐type FIX could account for the differences in their activation by FXIa in the ChAs. However, DA binds FVIIIa ~8‐ to 10‐fold better than wild‐type FIX and is more resistant to inhibition by antithrombin,16, 17 which may play a different role in each of the assay types.
5. CONCLUSION
In conclusion, as seen for other modified FIX products, discrepancies were observed when assaying DA using different methods/reagents. Despite the OSCA discrepancies, with the exception of APTT‐SP, DA could be validly assayed against both the Concentrate FIX IS and the Plasma FIX IS using the APTT reagents investigated in this study. The OSCA is the EP method and thus the expected potency labelling method in Europe. Due to the assay discrepancies within the OSCA method, the reagent used for potency labelling and clinical monitoring needs to be carefully considered. Further optimization of the chromogenic assays would be required before DA could be reliably measured using these methods.
ACKNOWLEDGEMENTS
S. C. Williams designed and performed experiments, analysed and interpreted data and wrote the manuscript. E. Gray designed experiments, interpreted the data and reviewed the manuscript. The test material and funding for this research were provided by Catalyst Biosciences. The authors would like to thank Catalyst Biosciences reviewing the manuscript. They would also like to thank Dr Martin Lee for helpful discussions.
Williams SC, Gray E. Activity measurements of dalcinonacog alfa. Haemophilia. 2020;26:346–353. 10.1111/hae.13949
The copyright line for this article was changed on 12 February 2021 after original online publication.
REFERENCES
- 1. 2.7.11. Assay of human coagulation factor IX, in European Pharmacopoeia. Strasbourg, France: Council of Europe; 2008:20711. [Google Scholar]
- 2. Mackie I, Cooper P, Lawrie A, et al. Guidelines on the laboratory aspects of assays used in haemostasis and thrombosis. Int J Lab Hematol. 2013;35(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 3. Horn C, Negrier C, Kalina U, Seifert W, Friedman KD. Performance of a recombinant fusion protein linking coagulation factor IX with recombinant albumin in one‐stage clotting assays. J Thromb Haemost. 2019;17(1):138‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosen P, Rosen S, Ezban M, Persson E. Overestimation of N‐glycoPEGylated factor IX activity in a one‐stage factor IX clotting assay owing to silica‐mediated premature conversion to activated factor IX. J Thromb Haemost. 2016;14(7):1420‐1427. [DOI] [PubMed] [Google Scholar]
- 5. Sommer JM, Buyue Y, Bardan S, et al. Comparative field study: impact of laboratory assay variability on the assessment of recombinant factor IX Fc fusion protein (rFIXFc) activity. Thromb Haemost. 2014;112(5):932‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tiefenbacher S, Bohra R, Amiral J, et al. Qualification of a select one‐stage activated partial thromboplastin time‐based clotting assay and two chromogenic assays for the post‐administration monitoring of nonacog beta pegol. J Thromb Haemost. 2017;15(10):1901‐1912. [DOI] [PubMed] [Google Scholar]
- 7. Kitchen S, Gray E, Mertens K. Monitoring of modified factor VIII and IX products. Haemophilia. 2014;20(Suppl 4):36‐42. [DOI] [PubMed] [Google Scholar]
- 8. Wilmot HV, Hogwood J, Gray E. Recombinant factor IX: discrepancies between one‐stage clotting and chromogenic assays. Haemophilia. 2014;20(6):891‐897. [DOI] [PubMed] [Google Scholar]
- 9. Bowyer AE, Shepherd MF, Kitchen S, Maclean RM, Makris M. Measurement of extended half‐life recombinant factor IX products in clinical practice. Int J Lab Hematol. 2019;41(2):e46‐e49. [DOI] [PubMed] [Google Scholar]
- 10. Barrowcliffe TW, Hubbard AR, Kitchen S. Standards and monitoring treatment. Haemophilia. 2012;18(Suppl 4):61‐65. [DOI] [PubMed] [Google Scholar]
- 11. Campbell PJ. International biological standards and reference preparations. I. Preparation and presentation of materials to serve as standards and reference preparations. J Biol Stand. 1974;2(4):249‐258. [DOI] [PubMed] [Google Scholar]
- 12. Kitchen S, Kershaw G, Tiefenbacher S. Recombinant to modified factor VIII and factor IX ‐ chromogenic and one‐stage assays issues. Haemophilia. 2016;22(Suppl 5):72‐77. [DOI] [PubMed] [Google Scholar]
- 13. Kitchen S, Tiefenbacher S, Gosselin R. Factor activity assays for monitoring extended half‐life FVIII and factor IX replacement therapies. Semin Thromb Hemost. 2017;43(3):331‐337. [DOI] [PubMed] [Google Scholar]
- 14. Young G, Perry D. Laboratory assay measurement of modified clotting factor concentrates: a review of the literature and recommendations for practice. J Thromb Haemost. 2019;17(4):567‐573. [DOI] [PubMed] [Google Scholar]
- 15. Dose‐escalation Study to Investigate the Safety, PK, and PD of ISU304/CB2679d in Hemophilia B Patients. [cited 2019; Available from: https://ClinicalTrials.gov/show/NCT03186677
- 16. Hong S‐B, Levy H, Jung JY, et al. Pharmacokinetics of subcutaneously administered CB2679d/ISU304 in wild‐type and hemophilia B mice. Blood. 2016;128(22):1389‐1389. [Google Scholar]
- 17. You CW, Shin H‐J, Levy H, et al. Phase 1/2 trial of subcutaneously administered factor IX variant CB2679d/ISU304: pharmacokinetics and activity. Blood. 2017;130(Suppl 1):87‐87. [Google Scholar]
- 18. Bowyer AE, Hillarp A, Ezban M, Persson P, Kitchen S. Measuring factor IX activity of nonacog beta pegol with commercially available one‐stage clotting and chromogenic assay kits: a two‐center study. J Thromb Haemost. 2016;14(7):1428‐1435. [DOI] [PubMed] [Google Scholar]
- 19. Dodt J, Hubbard AR, Wicks SJ, et al. Potency determination of factor VIII and factor IX for new product labelling and postinfusion testing: challenges for caregivers and regulators. Haemophilia. 2015;21(4):543‐549. [DOI] [PubMed] [Google Scholar]
- 20. Hubbard AR, Dodt J, Lee T, et al. Recommendations on the potency labelling of factor VIII and factor IX concentrates. J Thromb Haemost. 2013;11(5):988‐989. [DOI] [PubMed] [Google Scholar]
- 21. Persson E, La Cour Christoffersen C. Underestimation of N‐glycoPEGylated factor IX one‐stage clotting activity owing to contact activator‐impaired activation. Res Pract Thromb Haemost. 2017;1(2):259‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilmot HV, Gray E. Potency estimates for recombinant factor IX in the one‐stage clotting assay are influenced by more than just the choice of activated partial thromboplastin time reagent. Haemophilia. 2018;24(5):e363‐e368. [DOI] [PubMed] [Google Scholar]
- 23. Barrowcliffe TW. Insights from factor IX activation studies with chromogenic assays: implications of disparate product results. Haemophilia. 2010;16(s6):9‐12. [DOI] [PubMed] [Google Scholar]
- 24. Kershaw GW, Dissanayake K, Chen VM, Khoo T‐L. Evaluation of chromogenic factor IX assays by automated protocols. Haemophilia. 2018;24(3):492‐501. [DOI] [PubMed] [Google Scholar]


