Abstract
Background
The recently introduced 2017 World Workshop on the classification of periodontitis, incorporating stages and grades of disease, aims to link disease classification with approaches to prevention and treatment, as it describes not only disease severity and extent but also the degree of complexity and an individual's risk. There is, therefore, a need for evidence‐based clinical guidelines providing recommendations to treat periodontitis.
Aim
The objective of the current project was to develop a S3 Level Clinical Practice Guideline (CPG) for the treatment of Stage I–III periodontitis.
Material and Methods
This S3 CPG was developed under the auspices of the European Federation of Periodontology (EFP), following the methodological guidance of the Association of Scientific Medical Societies in Germany and the Grading of Recommendations Assessment, Development and Evaluation (GRADE). The rigorous and transparent process included synthesis of relevant research in 15 specifically commissioned systematic reviews, evaluation of the quality and strength of evidence, the formulation of specific recommendations and consensus, on those recommendations, by leading experts and a broad base of stakeholders.
Results
The S3 CPG approaches the treatment of periodontitis (stages I, II and III) using a pre‐established stepwise approach to therapy that, depending on the disease stage, should be incremental, each including different interventions. Consensus was achieved on recommendations covering different interventions, aimed at (a) behavioural changes, supragingival biofilm, gingival inflammation and risk factor control; (b) supra‐ and sub‐gingival instrumentation, with and without adjunctive therapies; (c) different types of periodontal surgical interventions; and (d) the necessary supportive periodontal care to extend benefits over time.
Conclusion
This S3 guideline informs clinical practice, health systems, policymakers and, indirectly, the public on the available and most effective modalities to treat periodontitis and to maintain a healthy dentition for a lifetime, according to the available evidence at the time of publication.
Keywords: clinical guideline, grade, health policy, oral health, periodontal therapy, periodontitis, stage
1. INTRODUCTION
1.1. The health problem
1.1.1. Definition
Periodontitis is characterized by progressive destruction of the tooth‐supporting apparatus. Its primary features include the loss of periodontal tissue support manifest through clinical attachment loss (CAL) and radiographically assessed alveolar bone loss, presence of periodontal pocketing and gingival bleeding (Papapanou et al., 2018). If untreated, it may lead to tooth loss, although it is preventable and treatable in the majority of cases.
1.1.2. Importance
Periodontitis is a major public health problem due to its high prevalence, and since it may lead to tooth loss and disability, it negatively affects chewing function and aesthetics, is a source of social inequality, and significantly impairs quality of life. Periodontitis accounts for a substantial proportion of edentulism and masticatory dysfunction, has a negative impact on general health and results in significant dental care costs (Tonetti, Jepsen, Jin, & Otomo‐Corgel, 2017).
1.1.3. Pathophysiology
Periodontitis is a chronic multifactorial inflammatory disease associated with dysbiotic dental plaque biofilms.
1.1.4. Prevalence
Periodontitis is the most common chronic inflammatory non‐communicable disease of humans. According to the Global Burden of Disease 2010 study, the global age‐standardized prevalence (1990–2010) of severe periodontitis was 11.2%, representing the sixth‐most prevalent condition in the world (Kassebaum et al., 2014), while in the Global Burden of Disease 2015 study, the prevalence of severe periodontitis was estimated in 7.4% (Kassebaum et al., 2017). The prevalence of milder forms of periodontitis may be as high as 50% (Billings et al., 2018).
1.1.5. Consequences of failure to treat
Untreated or inadequately treated periodontitis leads to the loss of tooth‐supporting tissues and teeth. Severe periodontitis, along with dental caries, is responsible for more years lost to disability than any other human disease (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). Furthermore, periodontal infections are associated with a range of systemic diseases leading to premature death, including diabetes (Sanz et al., 2018), cardiovascular diseases (Sanz et al., 2019; Tonetti, Van Dyke, & Working Group 1 of the Joint EFP/AAP Workshop, 2013) or adverse pregnancy outcomes (Sanz, Kornman, & Working Group 3 of Joint EFP/AAP Workshop, 2013).
1.1.6. Economic importance
On a global scale, periodontitis is estimated to cost $54 billion in direct treatment costs and further $25 billion in indirect costs (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). Periodontitis contributes significantly to the cost of dental diseases due to the need to replace teeth lost to periodontitis. The total cost of dental diseases, in 2015, was estimated to be of $544.41 billion, being $356.80 billion direct costs, and $187.61 billion indirect costs (Righolt, Jevdjevic, Marcenes, & Listl, 2018).
2. AIM OF THE GUIDELINE
This guideline aims to highlight the importance and need for scientific evidence in clinical decision‐making in the treatment of patients with periodontitis stages I to III. Its main objective is therefore to support the evidence‐based recommendations for the different interventions used at the different steps of periodontal therapy, based on the best available evidence and/or expert consensus. In so doing, this guideline aims to improve the overall quality of periodontal treatment in Europe, reduce tooth loss associated with periodontitis and ultimately improve overall systemic health and quality of life. A separate guideline covering the treatment of Stage IV periodontitis will be published.
2.1. Target users of the guideline
Dental and medical professionals, together with all stakeholders related to health care, particularly oral health, including patients.
2.2. Targeted environments
Dental and medical academic/hospital environments, clinics and practices.
2.3. Targeted patient population
People with periodontitis stages I to III.
People with periodontitis stages I to III following successful treatment.
2.4. Exceptions from the guideline
This guideline did not consider the health economic cost–benefit ratio, since (a) it covers multiple different countries with disparate, not readily comparable health systems, and (b) there is a paucity of sound scientific evidence available addressing this question. This guideline did not consider the treatment of gingivitis (although management of gingivitis is considered as an indirect goal in some interventions evaluated), the treatment of Stage IV periodontitis, necrotising periodontitis, periodontitis as manifestation of systemic diseases and mucogingival conditions.
3. METHODOLOGY
3.1. General framework
This guideline was developed following methodological guidance published by the Standing Guideline Commission of the Association of Scientific Medical Societies in Germany (AWMF) (https://www.awmf.org/leitlinien/awmf‐regelwerk/awmf‐guidance.html) and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group (https://www.gradeworkinggroup.org/).
The guideline was developed under the auspices of the European Federation of Periodontology (EFP) and overseen by the EFP Workshop Committee. This guideline development process was steered by an Organizing Committee and a group of methodology consultants designated by the EFP. All members of the Organizing Committee were part of the EFP Workshop Committee.
To ensure adequate stakeholder involvement, the EFP established a guideline panel involving dental professionals representing 36 national periodontal societies within the EFP (Table 1a).
TABLE 1a.
Guideline panel
| Scientific society/organization | Delegate(s) |
|---|---|
| European Federation of Periodontology |
Organizing Committee, Working Group Chairs (in alphabetic order): Tord Berglundh, Iain Chapple, David Herrera, Søren Jepsen, Moritz Kebschull, Mariano Sanz, Anton Sculean, Maurizio Tonetti |
|
Methodologists: Ina Kopp, Paul Brocklehurst, Jan Wennström | |
|
Clinical Experts: Anne Merete Aass, Mario Aimetti, Bahar Eren Kuru, Georgios Belibasakis, Juan Blanco, Nagihan Bostanci, Darko Bozic, Philippe Bouchard, Nurcan Buduneli, Francesco Cairo, Elena Calciolari, Maria Clotilde Carra, Pierpaolo Cortellini, Jan Cosyn, Francesco D'Aiuto, Bettina Dannewitz, Monique Danser, Korkud Demirel, Jan Derks, Massimo de Sanctis, Thomas Dietrich, Christof Dörfer, Henrik Dommisch, Nikos Donos, Peter Eickholz, Elena Figuero, William Giannobile, Moshe Goldstein, Filippo Graziani, Thomas Kocher, Eija Kononen, Bahar Eren Kuru, France Lambert, Luca Landi, Nicklaus Lang, Bruno Loos, Rodrigo López, Pernilla Lundberg, Eli Machtei, Phoebus Madianos, Conchita Martín, Paula Matesanz, Jörg Meyle, Ana Molina, Eduardo Montero, José Nart, Ian Needleman, Luigi Nibali, Panos Papapanou, Andrea Pilloni, David Polak, Ioannis Polyzois, Philip Preshaw, Marc Quirynen, Christoph Ramseier, Stefan Renvert, Giovanni Salvi, Ignacio Sanz‐Sánchez, Lior Shapira, Dagmar Else Slot, Andreas Stavropoulos, Xavier Struillou, Jean Suvan, Wim Teughels, Cristiano Tomasi, Leonardo Trombelli, Fridus van der Weijden, Clemens Walter, Nicola West, Gernot Wimmer | |
| Scientific Societies | |
| European Society for Endodontology | Lise Lotte Kirkevang |
| European Prosthodontic Association | Phophi Kamposiora |
| European Association of Dental Public Health | Paula Vassallo |
| European Federation of Conservative Dentistry | Laura Ceballos |
| Other organisations | |
| Council of European Chief Dental Officers | Kenneth Eaton |
| Council of European Dentists | Paulo Melo |
| European Dental Hygienists' Federation | Ellen Bol‐van den Hil |
| European Dental Students' Association | Daniela Timus |
| Platform for Better Oral Health in Europe | Kenneth Eaton |
These delegates were nominated, participated in the guideline development process and had voting rights in the consensus conference. For the guideline development process, delegates were assigned to four Working Groups that were chaired by the members of the Organizing Committee and advised by the methodology consultants. This panel was supported by key stakeholders from European scientific societies with a strong professional interest in periodontal care and from European organizations representing key groups within the dental profession, and key experts from non‐EFP member countries, such as North America (Table 1b).
TABLE 1b.
Key stakeholders contacted and participants
| Institution | Acronym | Answer a | Representative |
|---|---|---|---|
| Association for Dental Education in Europe | ADEE | No answer | No representative |
| Council of European Chief Dental Officers | CECDO | Participant | Ken Eaton/Paula Vassallo |
| Council of European Dentists | CED | Participant | Paulo Melo |
| European Association of Dental Public Health | EADPH | Participant | Paula Vassallo |
| European Dental Hygienists Federation | EDHF | Participant | Ellen Bol‐van den Hil |
| European Dental Students' Association | EDSA | Participant | Daniella Timus |
| European Federation of Conservative Dentistry | EFCD | Participant | Laura Ceballos |
| European Orthodontic Society | EOS | No answer | No representative |
| European Prosthodontic Association | EPA | Participant | Phophi Kamposiora |
| European Society of Endodontology | ESE | Participant | Lise Lotte Kirkevang |
| Platform for Better Oral Health in Europe | PBOHE | Participant | Kenneth Eaton |
Messages sent 20 March 2019; reminder sent June 18.
In addition, EFP engaged an independent guideline methodologist to advise the panel and facilitate the consensus process (Prof. Dr. med. Ina Kopp). The guideline methodologist had no voting rights.
EFP and the guideline panel tried to involve patient organizations but were not able to identify any regarding periodontal diseases at European level. In a future update, efforts will be undertaken to include the perspective of citizens/patients (Brocklehurst et al., 2018).
3.2. Evidence synthesis
3.2.1. Systematic search and critical appraisal of guidelines
To assess and utilize existing guidelines during the development of the present guideline, well‐established guideline registers and the websites of large periodontal societies were electronically searched for potentially applicable guideline texts:
Guideline International Network (GIN)
The National Institute for Health and Clinical Excellence (NICE)
Canadian Health Technology Assessment (CADTH)
European Federation for Periodontology (EFP)
American Academy of Periodontology (AAP)
American Dental Association (ADA)
The last search was performed on 30 September 2019. Search terms used were “periodont*,” “Periodontal,” “Guidelines” and “Clinical Practice Guidelines.” In addition, content was screened by hand searches. See Table 2.
TABLE 2.
Results of the guideline search
| Database | Identified, potentially relevant guidelines | Critical appraisal |
|---|---|---|
| Guideline International Network (GIN) International Guidelines Library a | Comprehensive periodontal therapy: a statement by the American Academy of Periodontology. American Academy of Periodontology. NGC:008726 (2011) | 8 years old, recommendations not based on systematic evaluation of evidence, not applicable |
| DG PARO S3 guideline (Register Number 083‐029)— Adjuvant systemic administration of antibiotics for subgingival instrumentation in the context of systematic periodontitis treatment (2018) | Very recent, high methodological standard, very similar outcome measures ‐ relevant | |
| HealthPartners Dental Group and Clinics guidelines for the diagnosis and treatment of periodontal diseases. HealthPartners Dental Group. NGC:008848 (2011) | 8 years old, unclear methodology, not applicable | |
|
“Dentistry” category |
Health Partners Dental Group and Clinics Caries Guideline | Not applicable |
| The National Institute for Health and Clinical Excellence (NICE) b | No thematically relevant hits | Not applicable |
| National Guideline Clearinghouse (Agency for Healthcare Research and Quality) c | No thematically relevant hits | Not applicable |
| Canadian Health Technology Assessment (CADTH) d | Periodontal Regenerative Procedures for Patients with Periodontal Disease: A Review of Clinical Effectiveness (2010) | 9‐year‐old review article, not applicable |
| Treatment of Periodontal Disease: Guidelines and Impact (2010) | 9‐year‐old review article, not applicable | |
| Dental Scaling and Root Planing for Periodontal Health: A Review of the Clinical Effectiveness, Cost‐effectiveness, and Guidelines (2016) | Unclear methodology (follow‐up, outcome variables, recommendations, guideline group), not applicable | |
| Dental Cleaning and Polishing for Oral Health: A Review of the Clinical Effectiveness, Cost‐effectiveness and Guidelines (2013) | Unclear methodology (follow‐up, outcome variables, recommendations, guideline group), not applicable | |
| European Federation of Periodontology (EFP) e | No thematically relevant hits | Not applicable |
| American Academy of Periodontology (AAP) f | The American Journal of Cardiology and Journal of Periodontology Editors' Consensus: Periodontitis and Atherosclerotic Vascular Disease (2009) | Unclear methodology, 10 year‐old consensus‐based article, only limited clinically applicably recommendations, not applicable |
| Comprehensive Periodontal Therapy: A Statement by the American Academy of Periodontology (2011) | Unclear methodology (follow‐up, outcome variables, recommendations, guideline group), almost a decade old, not applicable | |
| Academy Statements on Gingival Curettage (2002), Local Delivery (2006), Risk Assessment (2008), Efficacy of Lasers (2011) | Unclear methodology, 10‐year‐old consensus‐based article, only limited clinically applicably recommendations, not applicable | |
| American Dental Association (ADA) g | Nonsurgical Treatment of Chronic Periodontitis Guideline (2015) | Outcome variable CAL (not PPD), no minimal follow‐up—not applicable |
Only guidelines published in English and with full texts available were included. The methodological quality of these guideline texts was critically appraised using the AGREE II framework (https://www.agreetrust.org/agree‐ii/).
Most of the identified guidelines/documents were considered not applicable due to (a) their age, (b) their methodological approach, or (c) their inclusion criteria. The recent German S3 guideline (Register Number 083‐029) was found to be potentially relevant, scored highest in the critical appraisal using AGREE II and was, therefore, used to inform the guideline development process.
3.2.2. Systematic search and critical appraisal of the literature
For this guideline, a total of 15 systematic reviews (SRs) were conducted to support the guideline development process (Carra et al., 2020; Dommisch, Walter, Dannewitz, & Eickholz, 2020; Donos et al., 2019; Figuero, Roldan, et al., 2019; Herrera et al., 2020; Jepsen et al., 2019; Nibali et al., 2019; Polak et al., 2020; Ramseier et al., 2020; Salvi et al., 2019; Sanz‐Sanchez et al., 2020; Slot, Valkenburg, & van der Weijden, 2020; Suvan et al., 2019; Teughels et al., 2020; Trombelli et al., 2020). The corresponding manuscripts are published within this special issue of the Journal of Clinical Periodontology.
All SRs were conducted following the “Preferred Reporting Items for Systematic Reviews and Meta‐Analyses” (PRISMA) framework (Moher, Liberati, Tetzlaff, & Altman, 2009).
3.2.3. Focused questions
In all 15 systematic reviews, focused questions in PICO(S) format (Guyatt et al., 2011) were proposed by the authors in January 2019 to a panel comprising the working group chairs and the methodological consultants, in order to review and approve them (Table 3). The panel took great care to avoid overlaps or significant gaps between the SRs, so they would truly cover all possible interventions currently undertaken in periodontal therapy.
TABLE 3.
PICOS questions addressed by each Systematic Review
| Reference | Systematic review title | Final PICOS (as written in manuscripts) |
|---|---|---|
| Suvan et al. (2019) | Subgingival Instrumentation for Treatment of Periodontitis. A Systematic Review. | #1. In patients with periodontitis, what is the efficacy of subgingival instrumentation performed with hand or sonic/ultrasonic instruments in comparison with supragingival instrumentation or prophylaxis in terms of clinical and patient reported outcomes? |
| #2. In patients with periodontitis, what is the efficacy of nonsurgical subgingival instrumentation performed with sonic/ultrasonic instruments compared to subgingival instrumentation performed with hand instruments or compared to the subgingival instrumentation performed with a combination of hand and sonic/ultrasonic instruments in terms of clinical and patient‐reported outcomes? | ||
| #3. In patients with periodontitis, what is the efficacy of full mouth delivery protocols (within 24 hr) in comparison with quadrant or sextant wise delivery of subgingival mechanical instrumentation in terms of clinical and patient‐reported outcomes? | ||
| Salvi et al. (2019) | Adjunctive laser or antimicrobial photodynamic therapy to non‐surgical mechanical instrumentation in patients with untreated periodontitis. A systematic review and meta‐analysis. | #1. In patients with untreated periodontitis, does laser application provide adjunctive effects to non‐surgical mechanical instrumentation alone? |
| #2. In patients with untreated periodontitis, does application of a PTD provide adjunctive effects to non‐surgical mechanical instrumentation alone? | ||
| Donos et al. (2019) | The adjunctive use of host modulators in non‐surgical periodontal therapy. A systematic review of randomized, placebo‐controlled clinical studies | In patients with periodontitis, what is the efficacy of adding host modulating agents instead of placebo to NSPT in terms of probing pocket depth (PPD) reduction? |
| Sanz‐Sanchez et al. (2020) | Efficacy of access flaps compared to subgingival debridement or to different access flap approaches in the treatment of periodontitis. A systematic review and meta‐analysis. | #1. In patients with periodontitis (population), how effective are access flaps (intervention) as compared to subgingival debridement (comparison) in attaining PD reduction (primary outcome)? |
| #2. In patients with periodontitis (population), does the type of access flaps (intervention and control) impact PD reduction (primary outcome)? | ||
| Polak et al. (2020) | The Efficacy of Pocket Elimination/Reduction Surgery Vs. Access Flap: A Systematic Review | In adult patients with periodontitis after initial non‐surgical cause‐related therapy and residual PPD of 5 mm or more, what is the efficacy of pocket elimination/reduction surgery in comparison with access flap surgery? |
| Teughels et al. (2020) | Adjunctive effect of systemic antimicrobials in periodontitis therapy. A systematic review and meta‐analysis. | In patients with periodontitis, which is the efficacy of adjunctive systemic antimicrobials, in comparison with subgingival debridement plus a placebo, in terms of probing pocket depth (PPD) reduction, in randomized clinical trials with at least 6 months of follow‐up. |
| Herrera et al. (2020) | Adjunctive effect of locally delivered antimicrobials in periodontitis therapy. A systematic review and meta‐analysis. | In adult patients with periodontitis, which is the efficacy of adjunctive locally delivered antimicrobials, in comparison with subgingival debridement alone or plus a placebo, in terms of probing pocket depth (PPD) reduction, in randomized clinical trials with at least 6 months of follow‐up. |
| Nibali et al. (2019) | Regenerative surgery versus access flap for the treatment of intra‐bony periodontal defects: A systematic review and meta‐analysis | #1. Does regenerative surgery of intraosseous defects provide additional clinical benefits measured as Probing Pocket Depth (PPD) reduction, Clinical Attachment Level (CAL) gain, Recession (Rec) and Bone Gain (BG) in periodontitis patients compared with access flap? |
| #2. Is there a difference among regenerative procedures in terms of clinical and radiographic gains in intrabony defects? | ||
| Jepsen et al. (2019) | Regenerative surgical treatment of furcation defects: A systematic review and Bayesian network meta‐analysis of randomized clinical trials | #1. What is the efficacy of regenerative periodontal surgery in terms of tooth loss, furcation conversion and closure, horizontal clinical attachment level (HCAL) and bone level (HBL) gain as well as other periodontal parameters in teeth affected by periodontitis‐related furcation defects, at least 12 months after surgery? |
| #2. NM: to establish a ranking in efficacy of the treatment options and to identify the best surgical technique. | ||
| Dommisch et al. (2020) | Resective surgery for the treatment of furcation involvement: A systematic review | What is the benefit of resective surgical periodontal therapy (i.e. root amputation or resection, root separation, tunnel preparation) in (I) subjects with periodontitis who have completed a cycle of non‐surgical periodontal therapy and exhibit Class II and III furcation involvement (P) compared to individuals suffering from periodontitis and exhibiting class II and III furcation involvement not being treated with resective surgical periodontal therapy but were not treated at all, treated exclusively by subgingival debridement or access flap surgery (C) with respect to 1) tooth survival (primary outcome), 2) vertical probing attachment (PAL‐V) gain and 3) reduction of probing pocket depth (PPD) (secondary outcomes) (O) evidenced by randomized controlled clinical trials, prospective and retrospective cohort studies and case series with at least 12 months of follow‐up (survival, PAL‐V, PPD) (S), respectively. |
| Slot et al. (2020) | Mechanical plaque removal of periodontal maintenance patients: A systematic review and network meta‐analysis | #1. In periodontal maintenance patients, what is the effect on plaque removal and parameters of periodontal health of the following: Power toothbrushes as compared to manual toothbrushes? |
| #2. In periodontal maintenance patients, what is the effect on plaque removal and parameters of periodontal health of the following: Interdental oral hygiene devices compared to no interdental cleaning as adjunct to toothbrushing? | ||
| #3. In periodontal maintenance patients, what is the effect on plaque removal and parameters of periodontal health of the following: Different interdental cleaning devices as adjuncts to toothbrushing | ||
| Carra et al. (2020) | Promoting behavioural changes to improve oral hygiene in patients with periodontal diseases: a systematic review of the literature. | What is the efficacy of behavioural interventions aimed to promote OH in patients with periodontal diseases (gingivitis/periodontitis), in improving clinical plaque and bleeding indices? |
| Ramseier et al. (2020) | Impact of risk factor control interventions for smoking cessation and promotion of healthy lifestyles in patients with periodontitis: a systematic review | What is the efficacy of health behaviour change interventions for smoking cessation, diabetes control, physical exercise (activity), change of diet, carbohydrate (dietary sugar) reduction and weight loss provided in patients with periodontitis?“. |
| Figuero, Roldan, et al. (2019) | Efficacy of adjunctive therapies in patients with gingival inflammation. A systematic review and meta‐analysis. | In systemically healthy humans with dental plaque‐induced gingival inflammation (with or without attachment loss, but excluding untreated periodontitis patients), what is the efficacy of agents used adjunctively to mechanical plaque control (either self‐performed or professionally delivered), as compared to mechanical plaque control combined with a negative control, in terms of changes in gingival inflammation (through gingivitis or bleeding indices)? |
| Trombelli et al. (2020) | Efficacy of alternative or additional methods to professional mechanical plaque removal during supportive periodontal therapy. A systematic review and meta‐analysis | #1. What is the efficacy of alternative methods to professional mechanical plaque removal (PMPR) on progression of attachment loss during supportive periodontal therapy (SPT) in periodontitis patients? |
| #2. What is the efficacy of additional methods to professional mechanical plaque removal (PMPR) on progression of attachment loss during supportive periodontal therapy (SPT) in periodontitis patients? |
3.2.4. Relevance of outcomes
A narrative review paper was commissioned for this guideline (Loos & Needleman, 2020) to evaluate the possible outcome measures utilized to evaluate the efficacy of periodontal therapy in relation to true patient‐centred outcomes like tooth retention/loss. The authors found that the commonly reported outcome variable with the best demonstrated predictive potential for tooth loss was the reduction in periodontal probing pocket depth (PPD). Therefore, for this guideline, PPD reduction was used as primary outcome for those systematic reviews not addressing periodontal regeneration, and where tooth survival data were not reported. When reviewing regenerative interventions, gains in clinical attachment were used as the primary outcome measure. To avoid introducing bias by including possibly spurious findings of studies with very short follow‐up, a minimal follow‐up period of six months was requested for all reviews.
3.2.5. Search strategy
All SRs utilized a comprehensive search strategy of at least two different databases, supplemented by a hand search of periodontal journals and the reference lists of included studies.
In all SRs, the electronic and manual search, as well as the data extraction, was done in parallel by two different investigators.
3.2.6. Quality assessment of included studies
In all SRs, the risk of bias of controlled clinical trials was assessed using the Cochrane risk of bias tool (https://methods.cochrane.org/bias/resources/rob‐2‐revised‐cochrane‐risk‐bias‐tool‐randomized‐trials). For observational studies, the Newcastle–Ottawa Scale was used http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
3.2.7. Data synthesis
Where applicable, the available evidence was summarized by means of meta‐analysis, or other tools aimed for pooling data (network meta‐analysis, Bayesian network meta‐analysis).
3.3. From evidence to recommendation: structured consensus process
The structured consensus development conference was held during the XVI European Workshop in Periodontology in La Granja de San Ildefonso Segovia, Spain, on 10–13 November 2019. Using the 15 SRs as background information, evidence‐based recommendations were formally debated by the guideline panel using the format of a structured consensus development conference, consisting of small group discussions and open plenary were the proposed recommendations were presented, voted and adopted by consensus and Murphy et al. (1998).
In the small group phase, delegates convened in four working groups addressing the following subtopics: (a) “periodontitis stages I and II”; (b) “periodontitis Stage III”; (c) “periodontitis Stage III with intraosseous defects and/or furcations”; and (d) “supportive periodontal care.” These working groups were directed by two chairpersons belonging to the EFP Workshop Committee. With the support of an expert in methodology in each working group, recommendations and draft background texts were generated and subsequently presented, debated and put to a vote in the plenary of all delegates. During these plenary sessions, the guideline development process and discussions and votes were overseen and facilitated by the independent guideline methodologist (I.K.). The plenary votes were recorded using an electronic voting system, checked for plausibility in then introduced into the guideline text.
The consensus process was conducted as follows:
3.3.1. Plenary 1
Introduction to guideline methodology (presentation, discussion) by the independent guideline methodologist (I.K.).
3.3.2. Working group Phase 1
Peer evaluation of declarations of interest and management of conflicts.
Presentation of the evidence (SR results) by group chairs and methodology consultants.
Invitation of all members of the working group to reflect critically on the quality of available evidence by group chairs, considering GRADE criteria.
-
Structured group discussion:
-
○
development of draft recommendation and their grading, considering GRADE‐criteria.
-
○
development of draft background texts, considering GRADE criteria.
-
○
invitation to comment draft recommendations and background text to suggest reasonable amendments by group chairs.
-
○
collection and merging of amendments by group chairs.
-
○
initial voting within the working group on recommendations and guideline text to be presented as group result in the plenary.
-
○
3.3.3. Plenary 2
Presentation of working group results (draft recommendations and background text) by working group chairs.
Invitation to formulate questions, statements and reasonable amendments of the plenary by the independent guideline methodologist/facilitator.
Answering of questions by working group chairpersons.
Collection and merging of amendments by independent moderator.
Preliminary vote on all suggestions provided by the working groups and all reasonable amendments.
Assessment of the strength of consensus.
Opening debate, where no consensus was reached or reasonable need for discussion was identified.
Formulation of tasks to be solved within the working groups.
3.3.4. Working group Phase 2
Discussion of tasks and potential amendments raised by the plenary.
Formulation of reasonable and justifiable amendments, considering the GRADE framework.
Initial voting within the working group on recommendations and guideline text for plenary.
3.3.5. Plenary 3
Presentation of working group results by working group chairpersons.
Invitation to formulate questions, statements and reasonable amendments of the plenary by the independent moderator.
Collection and merging of amendments by independent moderator.
Preliminary vote.
Assessment of the strength of consensus.
Opening debate, where no consensus was reached or reasonable need for discussion was identified.
Formulation of reasonable alternatives.
Final vote of each recommendation.
3.4. Definitions: rating the quality of evidence, grading the strength of recommendations and determining the strength of consensus
For all recommendations and statements, this guideline makes transparent.
the underlying quality of evidence, reflecting the degree of certainty/uncertainty of the evidence and robustness of study results
the grade of the recommendation, reflecting criteria of considered judgement the strength of consensus, indicating the degree of agreement within the guideline panel and thus reflecting the need of implementation
3.4.1. Quality of Evidence
The quality of evidence was assessed using a recommended rating scheme (Balshem et al., 2011; Schunemann, Zhang, Oxman, & Expert Evidence in Guidelines, 2019).
3.4.2. Strength of Recommendations
The grading of the recommendations used the grading scheme (Table 4) by the German Association of the Scientific Medical Societies (AWMF) and Standing Guidelines Commission (2012), taking into account not only the quality of evidence, but also considered judgement, guided by the following criteria:
relevance of outcomes and quality of evidence for each relevant outcome
consistency of study results
directness regarding applicability of the evidence to the target population/PICO specifics
precision of effect estimates regarding confidence intervals
magnitude of the effects
balance of benefit and harm
ethical, legal, economic considerations
patient preferences
TABLE 4.
Strength of recommendations: grading scheme (German Association of the Scientific Medical Societies (AWMF) and Standing Guidelines Commission, 2012)
| Grade of recommendation grade a | Description | Syntax |
|---|---|---|
| A | Strong recommendation |
We recommend (↑↑)/ We recommend not to (↓↓) |
| B | Recommendation |
We suggest to (↑)/ We suggest not to (↓) |
| 0 | Open recommendation | May be considered (↔) |
If the group felt that evidence was not clear enough to support a recommendation, Statements were formulated, including the need (or not) of additional research.
The grading of the quality of evidence and the strength of a recommendation may therefore differ in justified cases.
3.4.3. Strength of consensus
The consensus determination process followed the recommendations by the German Association of the Scientific Medical Societies (AWMF) and Standing Guidelines Commission (2012). In case, consensus could not be reached, different points of view were documented in the guideline text. See Table 5.
TABLE 5.
Strength of consensus: determination scheme (German Association of the Scientific Medical Societies (AWMF) and Standing Guidelines Commission, 2012)
| Unanimous consensus | Agreement of 100% of participants |
| Strong consensus | Agreement of > 95% of participants |
| Consensus | Agreement of 75%–95% of participants |
| Simple majority | Agreement of 50%–74% of participants |
| No consensus | Agreement of < 50% of participants |
3.5. Editorial independence
3.5.1. Funding of the guideline
The development of this guideline and its subsequent publication were financed entirely by internal funds of the European Federation of Periodontology, without any support from industry or other organizations.
3.5.2. Declaration of interests and management of potential conflicts
All members of the guideline panel declared secondary interests using the standardized form provided by the International Committee of Medical Journal Editors (ICMJE) (International Committee of Medical Editors).
Management of conflict of interests (CoIs) was discussed in the working groups, following the principles provided by the Guidelines International Network (Schunemann et al., 2015). According to these principles, panel members with relevant, potential CoI abstained from voting on guideline statements and recommendations within the consensus process.
3.6. Peer review
All 15 systematic reviews, and the position paper on outcome variables commissioned for this guideline, underwent a multistep peer review process. First, the draft documents were evaluated by members of the EFP Workshop Committee and the methodological consultants using a custom‐made appraisal tool to assess (a) the methodological quality of the SRs using the AMSTAR 2 checklist (Shea et al., 2017), and (b) whether all PICO(S) questions were addressed as planned. Detailed feedback was then provided for the SR authors. Subsequently, all 15 systematic reviews and the position paper underwent the regular editorial peer review process defined by the Journal of Clinical Periodontology.
The guideline text was drafted by the chairs of the working groups, in close cooperation with the methodological consultants, and circulated in the guideline group before the workshop. The methodological quality was formally assessed by an outside consultant using the AGREE framework. The guideline was subsequently peer‐reviewed for its publication in the Journal of Clinical Periodontology following the standard evaluation process of this scientific journal.
3.7. Implementation and dissemination plan
For this guideline, a multistage dissemination and implementation strategy will be actioned by the EFP, supported by a communication campaign.
This will include the following:
Publication of the guideline and the underlying systematic reviews and position paper as an Open Access special issue of the Journal of Clinical Periodontology
Local uptake from national societies, either by Commentary, Adoption, or Adaptation (Schunemann et al., 2017)
Generation of educational material for dental professionals and patients, dissemination via the EFP member societies
Dissemination via educational programmes on dental conferences
Dissemination via EFP through European stakeholders via National Societies, members of EFP
Long‐term evaluation of the successful implementation of the guideline by poll of EFP members.
The timeline of the guideline development process is detailed in Table 6.
TABLE 6.
Timeline of the guideline development process
| Time point | Action |
|---|---|
| April 2018 | Decision by European Federation of Periodontology (EFP) General Assembly to develop comprehensive treatment guidelines for periodontitis |
| May–September 2018 | EFP Workshop Committee assesses merits and disadvantages of various established methodologies and their applicability to the field |
| September 2018 | EFP Workshop Committee decides on/invites (a) topics covered by proposed guideline, (b) working groups and chairs, (c) systematic reviewers, and (d) outcomes measures |
| End of year 2018 |
Submission of PICO(S) questions by systematic reviewers to group chairs for internal alignment Decision on consensus group, invitation of stakeholders |
| 21 January 2019 | Organizing and Advisor Committee meeting. Decision on PICO(S) and information sent to reviewers |
| March–June 2019 | Submission of Systematic reviews by reviewers, initial assessment by workshop committee |
| June–October 2019 | Peer review and revision process, Journal of Clinical Periodontology |
| September 2019 | Submission of declarations of interest by all delegates |
| Before workshop | Electronic circulation of reviews and guideline draft |
| 10–13 November 2019 | Workshop in La Granja with moderated formalized consensus process |
| December 2019–January 2020 | Formal stakeholder consultation, finalization of guideline method report and background text |
| April 2020 | Publication of guideline and underlying Systematic Reviews in the Journal of Clinical Periodontology |
3.8. Validity and update process
The guideline is valid until 2025. However, the EFP, represented by the members of the Organizing Committee, will continuously assess current developments in the field. In case of major changes of circumstances, for example new relevant evidence, they will trigger an update of the guideline to potentially amend the recommendations. It is planned to update the current guideline regularly on demand in form of a living guideline.
4. PERIODONTAL DIAGNOSIS AND CLASSIFICATION
Periodontal diagnosis has been followed according to the classification scheme defined in the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions (Caton et al., 2018; Chapple et al., 2018; Jepsen et al., 2018; Papapanou et al., 2018).
According to this classification:
A case of clinical periodontal health is defined by the absence of inflammation [measured as presence of bleeding on probing (BOP) at less than 10% sites] and the absence of attachment and bone loss arising from previous periodontitis.
A gingivitis case is defined by the presence of gingival inflammation, as assessed by BOP at ≥10% sites and absence of detectable attachment loss due to previous periodontitis. Localized gingivitis is defined as 10%–30% bleeding sites, while generalized gingivitis is defined as >30% bleeding sites
A periodontitis case is defined by the loss of periodontal tissue support, which is commonly assessed by radiographic bone loss or interproximal loss of clinical attachment measured by probing. Other meaningful descriptions of periodontitis include the number and proportions of teeth with probing pocket depth over certain thresholds (commonly >4 mm with BOP and ≥6 mm), the number of teeth lost due to periodontitis, the number of teeth with intrabony lesions and the number of teeth with furcation lesions.
An individual case of periodontitis should be further characterized using a matrix that describes the stage and grade of the disease. Stage is largely dependent upon the severity of disease at presentation, as well as on the anticipated complexity of case management, and further includes a description of extent and distribution of the disease in the dentition. Grade provides supplemental information about biological features of the disease including a history‐based analysis of the rate of periodontitis progression; assessment of the risk for further progression; analysis of possible poor outcomes of treatment; and assessment of the risk that the disease or its treatment may negatively affect the general health of the patient. The staging, which is dependent on the severity of the disease and the anticipated complexity of case management, should be the basis for the patient's treatment plan based on the scientific evidence of the different therapeutic interventions. The grade, however, since it provides supplemental information on the patient's risk factors and rate of progression, should be the basis for individual planning of care (Tables 7 and 8) (Papapanou et al., 2018; Tonetti, Greenwell, & Kornman, 2018).
After the completion of periodontal therapy, a stable periodontitis patient has been defined by gingival health on a reduced periodontium (bleeding on probing in <10% of the sites; shallow probing depths of 4 mm or less and no 4 mm sites with bleeding on probing). When, after the completion of periodontal treatment, these criteria are met but bleeding on probing is present at >10% of sites, then the patient is diagnosed as a stable periodontitis patient with gingival inflammation. Sites with persistent probing depths ≥4 mm which exhibit BOP are likely to be unstable and require further treatment. It should be recognized that successfully treated and stable periodontitis patients will remain at increased risk of recurrent periodontitis, and hence if gingival inflammation is present adequate measures for inflammation control should be implemented to prevent recurrent periodontitis.
TABLE 7.
Periodontitis stage. Adapted from Tonetti et al. (2018)
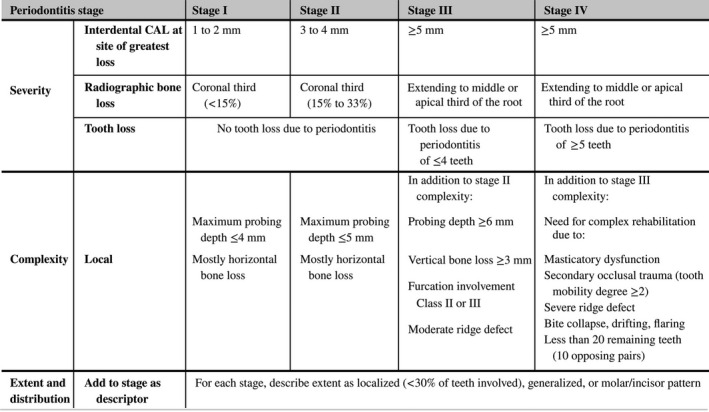
The initial Stage should be determined using CAL; if not available then RBL should be used. Information on tooth loss that can be attributed primarily to periodontitis – if available – may modify stage definition. This is the case even in the absence of complexity factors. Complexity factors may shift the Stage to a higher level, for example furcation II or III would shift to either Stage III or IV irrespective of the CAL. The distinction between Stage III and Stage IV is primarily based on complexity factors. For example, a high level of tooth mobility and/or posterior bite collapse would indicate a Stage IV diagnosis. For any given case only some, not all, complexity factors may be present, however, in general it only takes 1 complexity factor to shift the diagnosis to a higher Stage. It should be emphasized that these case definitions are guidelines that should be applied using sound clinical judgment to arrive at the most appropriate clinical diagnosis.
For post‐treatment patients CAL and RBL are still the primary stage determinants. If a stage shifting complexity factor(s) were eliminated by treatment, the stage should not retrogress to a lower stage since the original stage complexity factor should always be considered in maintenance phase management.
Abbreviations: CAL, clinical attachment loss; RBL, radiographic bone loss.
TABLE 8.
Periodontitis grade. Adapted from Papapanou et al. (2018)
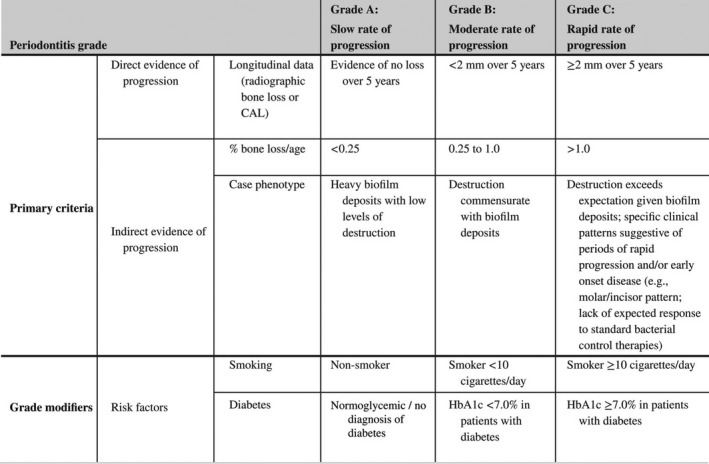
Grade should be used as an indicator of the rate of periodontitis progression. The primary criteria are either direct or indirect evidence of progression. Whenever available, direct evidence is used; in its absence indirect estimation is made using bone loss as a function of age at the most affected tooth or case presentation (radiographic bone loss expressed as percentage of root length divided by the age of the subject, RBL/age). Clinicians should initially assume Grade B disease and seek specific evidence to shift towards grade A or C, if available. Once grade is established based on evidence of progression, it can be modified based on the presence of risk factors. CAL, clinical attachment loss; HbA1c, glycated hemoglobin A1c; RBL, radiographic bone loss.
4.1. Clinical pathway for a diagnosis of periodontitis
A proposed algorithm has been used by the EFP to assist clinicians with this periodontal diagnosis process when examining a new patient (Tonetti & Sanz, 2019). It consists of four sequential steps:
Identifying a patient suspected of having periodontitis
Confirming the diagnosis of periodontitis
Staging the periodontitis case
Grading the periodontitis case
4.2. Differential Diagnosis
Periodontitis should be differentiated from the following clinical conditions (not an exhaustive list of conditions and diseases):
Gingivitis (Chapple et al., 2018)
Vertical root fracture (Jepsen et al., 2018)
Cervical decay (Jepsen et al., 2018)
Cemental tears (Jepsen et al., 2018)
External root resorption lesions (Jepsen et al., 2018)
Tumours or other systemic conditions extending to the periodontium (Jepsen et al., 2018)
Trauma‐induced local recession (Jepsen et al., 2018)
Endo‐periodontal lesions (Herrera, Retamal‐Valdes, Alonso, & Feres, 2018)
Periodontal abscess (Herrera et al., 2018)
Necrotizing periodontal diseases (Herrera et al., 2018)
4.3. Sequence for the treatment of periodontitis stages I, II and III
Patients, once diagnosed, should be treated according to a pre‐established stepwise approach to therapy that, depending on the disease stage, should be incremental, each including different interventions.
An essential prerequisite to therapy is to inform the patient of the diagnosis, including causes of the condition, risk factors, treatment alternatives and expected risks and benefits including the option of no treatment. This discussion should be followed by agreement on a personalized care plan. The plan might need to be modified during the treatment journey, depending on patient preferences, clinical findings and changes to overall health.
1. The first step in therapy is aimed at guiding behaviour change by motivating the patient to undertake successful removal of supragingival dental biofilm and risk factor control and may include the following interventions:
Supragingival dental biofilm control
Interventions to improve the effectiveness of oral hygiene [motivation, instructions (oral hygiene instructions, OHI)]
Adjunctive therapies for gingival inflammation
Professional mechanical plaque removal (PMPR), which includes the professional interventions aimed at removing supragingival plaque and calculus, as well as possible plaque‐retentive factors that impair oral hygiene practices.
Risk factor control, which includes all the health behavioural change interventions eliminating/mitigating the recognized risk factors for periodontitis onset and progression (smoking cessation, improved metabolic control of diabetes, and perhaps physical exercise, dietary counselling and weight loss).
This first step of therapy should be implemented in all periodontitis patients, irrespective of the stage of their disease, and should be re‐evaluated frequently in order to
Continue to build motivation and adherence, or explore other alternatives to overcome the barriers
Develop skills in dental biofilm removal and modify as required
Allow for the appropriate response of the ensuing steps of therapy
2. The second step of therapy (cause‐related therapy) is aimed at controlling (reducing/eliminating) the subgingival biofilm and calculus (subgingival instrumentation). In addition to this, the following interventions may be included:
Use of adjunctive physical or chemical agents
Use of adjunctive host‐modulating agents (local or systemic)
Use of adjunctive subgingival locally delivered antimicrobials
Use of adjunctive systemic antimicrobials
This second step of therapy should be used for all periodontitis patients, irrespective of their disease stage, only in teeth with loss of periodontal support and/or periodontal pocket formation*.
*In specific clinical situations, such as in the presence of deep probing depths, first and second steps of therapy could be delivered simultaneously (such as for preventing periodontal abscess development).
The individual response to the second step of therapy should be assessed once the periodontal tissues have healed (periodontal re‐evaluation). If the endpoints of therapy (no periodontal pockets >4 mm with bleeding on probing or no deep periodontal pockets [≥6 mm]) have not been achieved, the third step of therapy should be considered. If the treatment has been successful in achieving the endpoints of therapy, patients should be placed in a supportive periodontal care (SPC) programme.
3. The third step of therapy is aimed at treating those areas of the dentition non‐responding adequately to the second step of therapy (presence of pockets ≥4 mm with bleeding on probing or presence of deep periodontal pockets [≥6 mm]), with the purpose of gaining further access to subgingival instrumentation, or aiming at regenerating or resecting those lesions that add complexity in the management of periodontitis (intra‐bony and furcation lesions).
It may include the following interventions:
Repeated subgingival instrumentation with or without adjunctive therapies
Access flap periodontal surgery
Resective periodontal surgery
Regenerative periodontal surgery
When there is indication for surgical interventions, these should be subject to an additional patient consent and specific evaluation of risk factors or medical contra‐indications should be considered.
The individual response to the third step of therapy should be re‐assessed (periodontal re‐evaluation) and ideally the endpoints of therapy should be achieved, and patients should be placed in supportive periodontal care, although these endpoints of therapy may not be achievable in all teeth in severe Stage III periodontitis patients.
4. Supportive periodontal care is aimed at maintaining periodontal stability in all treated periodontitis patients combining preventive and therapeutic interventions defined in the first and second steps of therapy, depending on the gingival and periodontal status of the patient's dentition. This step should be rendered at regular intervals according to the patient's needs, and in any of these recall visits, any patient may need re‐treatment if recurrent disease is detected, and in these situations, a proper diagnosis and treatment plan should be reinstituted. In addition, compliance with the recommended oral hygiene regimens and healthy lifestyles are part of supportive periodontal care.
In any of the steps of therapy, tooth extraction may be considered if the affected teeth are considered with a hopeless prognosis.
The first part of this document was prepared by the steering group with the help of the methodology consultants, and it was carefully examined by the experts participating in the consensus and was voted upon in the initial plenary session to form the basis for the specific recommendations.
Strength of consensus strong consensus (0% of the group abstained due to potential CoI).
5. CLINICAL RECOMMENDATIONS: FIRST STEP OF THERAPY
The first step of therapy is aimed at providing the periodontitis patient with the adequate preventive and health promotion tools to facilitate his/her compliance with the prescribed therapy and the assurance of adequate outcomes. This step not only includes the implementation of patient's motivation and behavioural changes to achieve adequate self‐performed oral hygiene practices, but also the control of local and systemic modifiable risk factors that significantly influence this disease. Although this first step of therapy is insufficient to treat a periodontitis patient, it represents the foundation for optimal treatment response and long‐term stable outcomes.
This first step includes not only the educational and preventive interventions aimed to control gingival inflammation but also the professional mechanical removal of the supragingival plaque and calculus, together with the elimination local retentive factors.
5.1. Intervention: Supragingival dental biofilm control (by the patient)
R1.1 What are the adequate oral hygiene practices of periodontitis patients in the different steps of periodontitis therapy?
| Expert consensus‐based recommendation (1.1) |
|---|
| We recommend that the same guidance on oral hygiene practices to control gingival inflammation is enforced throughout all the steps of periodontal therapy including supportive periodontal care. |
| Supporting literature Van der Weijden and Slot (2015) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus [3.8% of the group abstained due to potential conflict of interest (CoI)] |
Background
Intervention
Supragingival dental biofilm control can be achieved by mechanical and chemical means. Mechanical plaque control is mainly performed by tooth brushing, either with manual or powered toothbrushes or with supplemental interdental cleaning using dental floss, interdental brushes, oral irrigators, wood sticks, etc. As adjuncts to mechanical plaque control, antiseptic agents, delivered in different formats, such as dentifrices and mouth rinses have been recommended. Furthermore, other agents aimed to reduce gingival inflammation have also been used adjunctively to mechanical biofilm control, such as probiotics, anti‐inflammatory agents and antioxidant micronutrients.
Available evidence
Even though oral hygiene interventions and other preventive measurements for gingivitis control were not specifically addressed in the systematic reviews prepared for this Workshop to Develop Guidelines for the treatment of periodontitis, evidence can be drawn from the XI European Workshop in Periodontology (2014) (Chapple et al., 2015) and the systematic review on oral hygiene practices for the prevention and treatment of gingivitis (Van der Weijden & Slot, 2015). This available evidence supports the following:
Professional oral hygiene instructions (OHI) should be provided to reduce plaque and gingivitis. Re‐enforcement of OHI may provide additional benefits.
Manual or power tooth brushing are recommended as a primary means of reducing plaque and gingivitis. The benefits of tooth brushing out‐weigh any potential risks.
When gingival inflammation is present, inter‐dental cleaning, preferably with interdental brushes (IDBs) should be professionally taught to patients. Clinicians may suggest other inter‐dental cleaning devices/methods when the use of IDBs is not appropriate.
R1.2 Are additional strategies in motivation useful?
| Expert consensus‐based recommendation (1.2) |
|---|
| We recommend emphasizing the importance of oral hygiene and engaging the periodontitis patient in behavioural change for oral hygiene improvement. |
| Supporting literature Carra et al. (2020) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus (1.3% of the group abstained due to potential CoI) |
Background
Intervention
Oral hygiene instructions (OHI) and patient motivation in oral hygiene practices should be an integral part of the patient management during all stages of periodontal treatment (Tonetti et al., 2015). Different behavioural interventions, as well as communication and educational methods, have been proposed to improve and maintain the patient's plaque control over time (Sanz & Meyle, 2010). See additional information in the next section on “Methods of motivation.”
R1.3 Are psychological methods for motivation effective to improve the patient's compliance in oral hygiene practices?
| Evidence‐based statement (1.3) |
|---|
| To improve patient's behaviour towards compliance with oral hygiene practices, psychological methods such as motivational interviewing or cognitive behavioural therapy have not shown a significant impact. |
| Supporting literature Carra et al. (2020) |
| Quality of evidence Five randomized clinical trials (RCTs) (1716 subjects) with duration ≥6 months in untreated periodontitis patients [(4 RCTs with high and 1 RCT with low risk of bias (RoB)] |
| Grade of recommendation Statement—unclear, additional research needed |
| Strength of consensus Strong consensus (1.3% of the group abstained due to potential CoI) |
Background
Intervention
Several different psychological interventions based on social cognitive theories, behavioural principles and motivational interviewing (MI) have been applied to improve OHI adherence in patients with periodontal diseases. The available evidence has not demonstrated that these psychological interventions based on cognitive constructs and motivational interviewing principles provided by oral health professionals have improved the patient's oral hygiene performance as measured by the reduction of plaque and bleeding scores over time.
Available evidence
The evidence includes two RCTs on MI (199 patients) and three RCTs on psychological interventions based on social cognitive theories and feedback (1,517 patients).
Risk of bias
The overall body of evidence was assessed at high risk of bias (four RCTs high and one RCT low).
Consistency
The majority of the studies found no significant additional benefit implementing psychological interventions in conjunction with OHI.
Clinical relevance and effect size
The reported effect size was not considered clinically relevant.
Balance of benefit and harm
Benefit and harm were not reported, and due to the fact that different health professionals were involved to provide interventions, no conclusion could be drawn.
Economic considerations
These studies did not assess a cost–benefit evaluation in spite of the expected additional cost related to the psychological intervention.
Patient preferences
No proper information was available to assess this issue.
Applicability
A psychological approach needs special training to be effectively performed.
5.2. Intervention: Adjunctive therapies for gingival inflammation
Adjunctive therapies for gingival inflammation have been considered within the adjunctive therapies to subgingival debridement, and therefore, they have been evaluated within the second step of therapy.
5.3. Intervention: Supragingival dental biofilm control (professional)
R1.4 What is the efficacy of supragingival professional mechanical plaque removal (PMPR) and control of retentive factors in periodontitis therapy?
| Expert consensus‐based recommendation (1.4) |
|---|
| We recommend supragingival professional mechanical plaque removal (PMPR) and control of retentive factors, as part of the first step of therapy. |
| Supporting literature Needleman, Nibali, and Di Iorio (2015); Trombelli, Franceschetti, and Farina (2015) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
The removal of the supragingival dental biofilm and calcified deposits (calculus) (here identified under the term “professional mechanical plaque removal” (PMPR)) is considered an essential component in the primary (Chapple et al., 2018) and secondary (Sanz et al., 2015) prevention of periodontitis as well as within the basic treatment of plaque‐induced periodontal diseases (van der Weijden & Slot, 2011). Since the presence of retentive factors, either associated with the tooth anatomy or more frequently, due to inadequate restorative margins, are often associated with gingival inflammation and/or periodontal attachment loss, they should be prevented/eliminated to reduce their impact on periodontal health.
Available evidence
Even though these interventions were not specifically addressed in the systematic reviews prepared for this Workshop to develop guidelines for the treatment of periodontitis, indirect evidence can be found in the 2014 European Workshop on Prevention, in which the role of PMPR was addressed both in primary prevention (Needleman et al., 2015) or in supportive periodontal care (SPC) (Trombelli et al., 2015). Some additional evidence can be found to support both procedures, as part of periodontitis therapy. A split‐mouth RCT, with a follow‐up of 450 days in 25 subjects, concluded that the performance of supragingival debridement, before subgingival debridement, decreased subgingival treatment needs and maintained the periodontal stability over time (Gomes, Romagna, Rossi, Corvello, & Angst, 2014). In addition, supragingival debridement may induce beneficial changes in the subgingival microbiota (Ximénez‐Fyvie et al., 2000). Moreover, it has been established that retentive factors may increase the risk of worsening the periodontal condition (Broadbent, Williams, Thomson, & Williams, 2006; Demarco et al., 2013; Lang, Kiel, & Anderhalden, 1983).
5.4. Intervention: Risk factor control
5.4.1. R1.5 What is the efficacy of risk factor control in periodontitis therapy?
| Evidence‐based recommendation (1.5) |
|---|
| We recommend risk factor control interventions in periodontitis patients, as part of the first step of therapy. |
| Supporting literature Ramseier et al. (2020) |
| Quality of evidence 25 clinical studies |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus (1.3% of the group abstained due to potential CoI) |
Background
Intervention
Smoking and diabetes are two proven risk factors in the etiopathogenesis of periodontitis (Papapanou et al., 2018), and therefore, their control should be an integral component in the treatment of these patients. Interventions for risk factor control have aimed to educate and advice patients for behavioural change aimed to reduce them and in specific cases to refer them for adequate medical therapy. Other relevant factors associated with healthy lifestyles (stress reduction, dietary counselling, weight loss or increased physical activities) may also be part of the overall strategy for reducing patient's risk factors.
Available evidence
In the systematic review (Ramseier et al., 2020), the authors have identified 13 relevant guidelines for interventions for tobacco smoking cessation, promotion of diabetes control, physical exercise (activity), change of diet, carbohydrate (dietary sugar reduction) and weight loss. In addition, 25 clinical studies were found that assess the impact of (some of) these interventions in gingivitis/periodontitis patients.
Risk of bias
It is explained specifically for each intervention.
Consistency
The heterogeneity in study design precludes more consistent findings, but adequate consistency may be found for studies on smoking cessation and diabetes control.
Clinical relevance and effect size
No meta‐analysis was performed; effect sizes can be found in the individual studies.
Balance of benefit and harm
In addition to periodontal benefits, all the tested interventions represent a relevant beneficial health impact.
Economic considerations
The various studies do not indicate a cost–benefit evaluation. However, it cannot be discarded an additional cost related to the psychological intervention. However, the systemic health benefits that can be obtained from these interventions, if they are successful, would represent reduced cost of healthcare services in different comorbidities.
Patient preferences
Interventions are heterogeneous, but the potential systemic health benefits may favour preference for them.
Applicability
Demonstrated with studies testing large groups from the general population; the practicality of routine use is still to be demonstrated.
R1.6 What is the efficacy of tobacco smoking cessation interventions in periodontitis therapy?
| Evidence‐based recommendation (1.6) |
|---|
| We recommend tobacco smoking cessation interventions to be implemented in patients undergoing periodontitis therapy. |
| Supporting literature Ramseier et al. (2020) |
| Quality of evidence Six prospective studies with, at least, 6‐month follow‐up |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (1.2% of the group abstained due to potential CoI) |
Background
Intervention
Periodontitis patients may benefit from smoking cessation interventions to improve periodontal treatment outcomes and the maintenance of periodontal stability. Interventions consist of brief counselling and may include patient referral for advanced counselling and pharmacotherapy.
Available evidence
In the systematic review (Ramseier et al., 2020), six prospective studies of 6‐ to 24‐month duration and performed at university setting were identified. Different interventions were tested (smoking cessation counselling, 5 A's [ask, advise, assess, assist, and arrange], cognitive behavioural therapy [CBT], motivational interview, brief interventions, nicotine replacement therapies). In three of the studies, the intervention was programmed in parallel with non‐surgical periodontal therapy (NSPT) and followed by SPC, in one study SPC patients were included and, in another, patients in NSPT and in SPC were compared; in one study, it was unclear. The success of smoking cessation was considered as moderate (4%–30% after 1–2 years), except in one study. Two studies demonstrated benefits in periodontal outcomes, when comparing former smokers to smokers and oscillators.
Additional factors have been discussed in the overall evaluation of risk factor control.
R1.7 What is the efficacy of promotion of diabetes control interventions in periodontitis therapy?
| Evidence‐based recommendation (1.7) |
|---|
| We recommend diabetes control interventions in patients undergoing periodontitis therapy. |
| Supporting literature Ramseier et al. (2020) |
| Quality of evidence Two 6‐month RCTs |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Periodontitis patients may benefit from diabetes control interventions to improve periodontal treatment outcomes and the maintenance of periodontal stability. These interventions consist of patient education as well as brief dietary counselling and, in situations of hyperglycaemia, the patient's referral for glycaemic control.
Available evidence
In the systematic review (Ramseier et al., 2020), two studies on the impact of diabetes control interventions in periodontitis patients were identified, two of them 6‐month RCTs, all of them performed at university settings. Periodontal interventions were not clearly defined. Different interventions were tested, including individual lifestyle counselling, dietary changes and oral health education. Some improvements were observed in the intervention groups, in terms of periodontal outcomes.
Additional factors have been discussed in the overall evaluation of risk factor control.
R1.8 What is the efficacy of increasing physical exercise (activity) in periodontitis therapy?
| Evidence‐based recommendation (1.8) |
|---|
| We do not know whether interventions aimed to increasing the physical exercise (activity) have a positive impact in periodontitis therapy. |
| Supporting literature Ramseier et al. (2020) |
| Quality of evidence One 12‐week RCT, one 12‐week prospective study |
| Grade of recommendation Grade 0—Statement: unclear, additional research needed |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Overall evidence from the medical literature suggests that the promotion of physical exercise (activity) interventions may improve both treatment and the long‐term management of chronic non‐communicable diseases. In periodontitis patients, the promotion may consist of patient education and counselling tailored to the patients' age and general health.
Available evidence
In the systematic review (Ramseier et al., 2020), two 12‐week studies on the impact of physical exercise (activity) interventions in periodontitis patients were identified, one RCT (testing education with comprehensive yogic interventions followed by yoga exercises) and one prospective study (with briefing followed by physical exercises; the control group was a dietary intervention), performed at university settings. Periodontal interventions were not clearly defined, although in the yoga study, standard therapy was delivered (by not described) in periodontitis patients, while no periodontal therapy was provided in the second study. Both studies reported improved periodontal parameters, including bleeding scores and probing depth changes, after 12 weeks (although in the yoga study also, the influence on psychological stress could not be discarded).
Additional factors have been discussed in the overall evaluation of risk factor control.
R1.9 What is the efficacy of dietary counselling in periodontitis therapy?
| Evidence‐based recommendation (1.9) |
|---|
| We do not know whether dietary counselling may have a positive impact in periodontitis therapy. |
| Supporting literature Ramseier et al. (2020) |
| Quality of evidence Three RCTs, four prospective studies |
| Grade of recommendation Grade 0—Statement: unclear, additional research needed |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Periodontitis patients may benefit from dietary counselling interventions to improve periodontal treatment outcomes and the maintenance of periodontal stability. These interventions may consist of patient education including brief dietary advices and in specific cases patient's referral to a nutrition specialist.
Available evidence
In the systematic review (Ramseier et al., 2020), seven studies on the impact of dietary counselling (mainly addressing lower fat intake, less free sugars and salt intake, increase in fruit and vegetable intake) in periodontitis (with or without other comorbidities) patients were identified: three RCTs (6 months, 8 weeks, 4 weeks) and four prospective studies (12 months, 24 weeks, 12 weeks, 4 weeks), performed at hospital and university settings. Periodontal interventions were not clearly defined, although in the 6‐month RCT, periodontal treatment was part of the protocol. Some studies showed significant improvements in periodontal parameters, but the RCT with the longest follow‐up was not able to identify significant benefits (Zare Javid, Seal, Heasman, & Moynihan, 2014).
In the systematic review (Ramseier et al., 2020), two studies specifically on the impact of dietary counselling aiming at carbohydrate (free sugars) reduction in gingivitis/periodontitis patients were identified, one 4‐week RCT (including also gingivitis patients) and one 24‐week prospective study. Periodontal interventions were not clearly defined. Both studies reported improved gingival indices.
Additional factors have been discussed in the overall evaluation of risk factor control.
5.4.2. What is the efficacy of lifestyle modifications aiming at weight loss in periodontitis therapy?
| Evidence‐based recommendation (1.10) |
|---|
| We do not know whether interventions aimed to weight loss through lifestyle modification may have a positive impact in periodontitis therapy. |
| Supporting literature Ramseier et al. (2020) |
| Quality of evidence Five prospective studies |
| Grade of recommendation Grade 0—Statement: unclear, additional research needed |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
5.4.3. Background
Intervention
Available evidence suggests that weight loss interventions may improve both the treatment and long‐term outcome of chronic non‐communicable diseases. In periodontitis patients, these interventions may consist of specific educational messages tailored to the patients' age and general health. These should be supported with positive behavioural change towards healthier diets and increase in physical activity (exercise).
Available evidence
In the systematic review (Ramseier et al., 2020), five prospective studies, in obese gingivitis/periodontitis patients, on the impact of weight loss interventions were identified, with different follow‐ups (18 months, 12 months, 24 weeks and two studies of 12 weeks). Periodontal interventions were not clearly defined. Intensity of lifestyle modifications aiming at weight loss interventions ranged from a briefing, followed by counselling in dietary change, to an 8‐week high‐fibre, low‐fat diet, or a weight reduction programme with diet and exercise‐related lifestyle modifications. Three studies reported beneficial periodontal outcomes and, the other two, no differences.
Additional factors have been discussed in the overall evaluation of risk factor control.
6. CLINICAL RECOMMENDATIONS: SECOND STEP OF THERAPY
The second step of therapy (also known as cause‐related therapy) is aimed at the elimination (reduction) of the subgingival biofilm and calculus and may be associated with removal of root surface (cementum). The procedures aimed at these objectives have received in the scientific literature different names: subgingival debridement, subgingival scaling, root planning, etc. (Kieser, 1994). In this guideline, we have agreed to use the term “subgingival instrumentation” to all non‐surgical procedures, either performed with hand (i.e. curettes) or power‐driven (i.e. sonic/ultrasonic devices) instruments specifically designed to gain access to the root surfaces in the subgingival environment and to remove subgingival biofilm and calculus. This second step of therapy requires the successful implementation of the measures described in the first step of therapy.
Furthermore, subgingival instrumentation may be supplemented with the following adjunctive interventions:
Use of adjunctive physical or chemical agents.
Use of adjunctive host‐modulating agents (local or systemic).
Use of adjunctive subgingival locally delivered antimicrobials.
Use of adjunctive systemic antimicrobials.
6.1. Intervention: Subgingival instrumentation
R2.1 Is subgingival instrumentation beneficial for the treatment of periodontitis?
| Evidence‐based recommendation (2.1) |
|---|
| We recommend that subgingival instrumentation be employed to treat periodontitis in order to reduce probing pocket depths, gingival inflammation and the number of diseased sites. |
| Supporting literature Suvan et al. (2019) |
| Quality of evidence One 3‐month RCT (n = 169 patients); 11 prospective studies (n = 258) ≥6 months |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (2.6% of the group abstained due to potential CoI) |
Background
Intervention
Subgingival instrumentation aims at reducing soft tissue inflammation by removing hard and soft deposits from the tooth surface. The endpoint of treatment is pocket closure, defined by probing pocket depth (PPD) ≤4 mm and absence of bleeding on probing (BOP).
Available evidence
One RCT on 169 patients with 3‐month outcomes addressed the PICOS question. Further 11 prospective studies (n = 258) with a follow‐up of ≥6 months which considered baseline measures and post‐treatment reductions in probing pocket depth (primary outcome) and bleeding on probing and percentage of closed pockets (secondary outcomes) were analysed.
Risk of bias
Study quality assessment identified a low risk of bias in all but one study, which had a high risk of bias.
Consistency
Evidence was consistent across all 11 studies that were included in the pre‐ and post‐treatment analysis and was therefore considered strong. Patient‐reported outcomes were inconsistently reported and adverse events, when reported, were rare. No indications of publication bias were observed but heterogeneity was high.
Clinical relevance and effect size
The evidence suggested a mean reduction of PPD of 1.7 mm at 6/8 months, a mean proportion of closed pockets of 74% and a mean reduction of BOP of 63%. Deeper sites (>6 mm) demonstrated a greater mean PPD reduction of 2.6 mm.
Balance of benefits and harm
An overall consideration of the benefit versus harm of subgingival instrumentation supports the strength of the recommendation.
Ethical considerations
Evaluation of the efficacy of subgingival debridement is ethically challenging as it would entail comparison with no subgingival intervention. Due to the lack of relevant RCTs, prospective studies were included and their data analysed.
Applicability
The majority of studies were conducted in well‐controlled research environments and included specifically selected populations, that is those with no systemic disease. While results from studies involving populations with systemic diseases were not included in the systematic review, there is a consensus that subgingival instrumentation is efficacious in these groups (Sanz et al., 2018, 2019), but the magnitude of the effect requires further study.
The evidence presented illustrates “efficacy” rather than “effectiveness”; therefore, generalizability to general dental practice settings is unclear.
6.1.1. R2.2 Are treatment outcomes of subgingival instrumentation better after use of hand, powered (sonic/ultrasonic) instruments or a combination thereof?
| Evidence‐based recommendation (2.2) |
|---|
| We recommend that subgingival periodontal instrumentation is performed with hand or powered (sonic/ultrasonic) instruments, either alone or in combination. |
| Supporting literature Suvan et al. (2019) |
| Quality of evidence Four RCTs (n = 132) with a follow‐up of ≥6 months. |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (6.2% of the group abstained due to potential CoI) |
Background
Intervention
Numerous types of instruments are available to perform subgingival instrumentation.
Available evidence
Four RCTs (n = 132) with a low overall risk of bias were included. Findings were evaluated at 6/8 months for PPD reduction (primary outcome) and clinical attachment level (CAL) gain (secondary outcome).
Risk of bias
Study quality assessment identified all four studies to be at low risk of bias.
Consistency
The evidence demonstrated that outcomes of treatment were not dependent on the type of instrument employed. The evidence was considered strong and consistent. No indications of publication bias were observed but heterogeneity was high.
Clinical relevance
No clinically or statistically significant differences were observed between the different types of instruments.
Balance of benefits and harm
The use of all types of instruments is technique‐sensitive and therefore requires specific training. Patient‐reported outcomes and adverse events were inconsistently reported. If present, no obvious differences between hand and powered instruments in terms of post‐operative sensitivity were noted.
Ethical considerations
There is a potential ethical dilemma in that patient preference may conflict with the clinician's preference in terms of type of instrument. Patient autonomy should be respected.
Economic considerations
Cost‐effectiveness has not been evaluated in these studies. Furthermore, there is no evidence that the use of one type of instrument is superior in terms of requisite treatment time.
Applicability
The majority of studies were conducted in well‐controlled research environments, in specifically selected populations and under local anaesthetic. Clinicians should be aware that new instrument choices (i.e. mini instruments) were not evaluated in the available studies. The choice of instrument should be based upon the experience/skills and preference of the operator together with patient preference.
R2.3 Are treatment outcomes of subgingival instrumentation better when delivered quadrant‐wise over multiple visits or as a full mouth procedure (within 24 hr)?
| Evidence‐based recommendation (2.3) |
|---|
| We suggest that subgingival periodontal instrumentation can be performed with either traditional quadrant‐wise or full mouth delivery within 24 hr. |
| Supporting literature Suvan et al. (2019) |
| Quality of evidence Eight RCTs (n = 212) with a follow‐up of ≥6 months. |
| Grade of recommendation Grade B—↑ |
| Strength of consensus Strong consensus (3.8% of the group abstained due to potential CoI) |
Background
Intervention
Subgingival instrumentation has traditionally been delivered during multiple sessions (e.g. quadrant‐wise). As an alternative, full‐mouth protocols have been suggested. Full‐mouth protocols included single stage and two‐stage therapy within 24 hr; however, protocols including antiseptics (full‐mouth disinfection) were not included in this analysis.
Available evidence
Eight RCTs (n = 212) with a follow‐up of ≥6 months were included demonstrating a low risk of bias. Outcome measures reported were PPD reduction (primary outcome), CAL gain, BOP reduction and pocket closure (secondary outcomes).
Risk of bias
Study quality assessment identified all eight studies at low risk of bias.
Consistency
The evidence suggested that outcomes of treatment were not dependent on the type of delivery (protocol) employed. The evidence was considered strong and consistent. No indications of publication bias were observed, and heterogeneity was low. The results confirm the findings of a recent Cochrane systematic review (Eberhard, Jepsen, Jervoe‐Storm, Needleman, & Worthington, 2015).
Clinical relevance
No substantial differences were observed between the two treatment modalities.
Balance of benefits and harm
Clinicians should be aware that there is evidence of systemic implications (e.g. acute systemic inflammatory response) with full‐mouth protocols. Thus, such an approach should always include careful consideration of the general health status of the patient.
Ethical considerations
There is a potential ethical dilemma in that patient preference may conflict with the clinician's recommendation in terms of mode of treatment delivery. Patient autonomy should be respected.
Legal considerations
Potential adverse systemic effects of full‐mouth treatment protocols in certain risk patients should be considered.
Economic considerations
Limited evidence on the cost‐effectiveness of different modes of delivery is available.
Patient preferences
Patient‐reported outcomes were inconsistently reported, and there is no evidence supporting one approach over the other. Reports of increased discomfort and side effects, evident in studies on full‐mouth disinfection, were not included in the present analysis.
Applicability
The majority of studies were conducted in well‐controlled environments, included specifically selected populations and were undertaken in a number of different continents.
6.2. Intervention: Use of adjunctive physical agents to subgingival instrumentation
R2.4 Are treatment outcomes with adjunctive application of laser superior to non‐surgical subgingival instrumentation alone?
| Evidence‐based recommendation (2.4) |
|---|
| We suggest not to use lasers as adjuncts to subgingival instrumentation. |
| Supporting literature Salvi et al. (2019) |
| Quality of evidence Two RCTs (n = 46, wavelengths 2,780 nm and 2,940 nm) and 3 RCTs (n = 101, wavelength range 810–980 nm) with single laser application reporting 6‐month outcomes. Two RCTs reported mean PPD changes. |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Simple majority (3.8% of the group abstained due to potential CoI) |
Background
Intervention
Lasers offer the potential to improve outcomes of subgingival root surface treatment protocols when used as adjuncts to traditional root surface instrumentation. Depending upon the wavelength and settings employed, some lasers can ablate subgingival calculus and exert antimicrobial effects. The evidence reported to inform the current guidelines has grouped lasers into two main wavelength categories: lasers with a wavelength range of 2,780–2,940 nm and lasers with a wavelength range of 810–980 nm.
Available evidence
Evidence was available from five RCTs (total n = 147) with a follow‐up of ≥6 months and a single laser application. Only RCTs reporting mean PPD changes were considered and this recommendation is made in the light of this approach to the systematic review.
Risk of bias
The majority of studies displayed unclear risk of bias.
Consistency
Studies differed in terms of laser type, tip diameter, wavelength, mode of periodontal treatment, number of treated sites, population and several possible combinations of these parameters.
Clinical relevance and effect size
There is insufficient evidence to recommend adjunctive application of lasers to subgingival instrumentation.
Balance of benefits and harm
The majority of the studies did not report on potential harm/adverse effects.
Economic considerations
Additional costs associated with adjunctive laser therapy may not be justified.
Patient preferences
Patient‐reported outcomes were rarely reported.
Applicability
The majority of studies were conducted in university settings, included specifically selected populations and were undertaken in a number of different countries.
R2.5 Are treatment outcomes with adjunctive antimicrobial photodynamic therapy (aPDT) superior to non‐surgical subgingival instrumentation alone?
| Evidence‐based recommendation (2.5) |
|---|
| We suggest not to use adjunctive a PDT at wavelength ranges of either 660–670 nm or 800–900 nm in patients with periodontitis. |
| Supporting literature Salvi et al. (2019) |
| Quality of evidence Five RCTs (n = 121, wavelength range 660–670 nm and wavelength range 800–900 nm) with single aPDT application reporting 6‐month outcomes. Three RCTs reported mean PPD changes. |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Consensus (1.3% of the group abstained due to potential CoI) |
Background
Intervention
Adjunctive antimicrobial photodynamic therapy (aPDT) is an approach used to improve the antimicrobial effects of traditional root surface decontamination methods. It functions by attaching a photosensitizing dye to the normally impermeable outer cell membrane of Gram‐negative bacteria and then uses laser light to generate reactive oxygen species through the membrane‐bound dye to locally destroy those bacteria.
Available evidence
Evidence was available from five RCTs (n = 121) with a follow‐up of ≥6 months and a single aPDT application. Only RCTs reporting mean PPD changes were included in the meta‐analysis, and this recommendation is made in the light of this approach to the systematic review.
Risk of bias
The majority of studies displayed unclear risk of bias.
Consistency
Substantial heterogeneity across the studies was identified, in terms of laser type, photosensitizer, wavelength, mode of periodontal treatment, number of treated sites, population and several possible combinations of these parameters.
Clinical relevance and effect size
No benefits were observed with the adjunctive application of aPDT.
Balance of benefits and harm
The majority of the studies reported on adverse events with no harm associated with the adjunctive application of aPDT.
Economic considerations
Additional costs associated with adjunctive laser therapy may not be justified.
Patient preferences
Patient‐reported outcomes were rarely reported, and there is no evidence supporting one approach over the other.
Applicability
All studies were conducted in well controlled university settings or specialist centres, included specifically selected populations and were undertaken in a number of different countries.
6.3. Intervention: Use of adjunctive host‐modulating agents (local or systemic) to subgingival instrumentation
R2.6 Does the adjunctive use of local statins improve the clinical outcome of subgingival instrumentation?
| Evidence‐based recommendation (2.6) |
|---|
| We recommend not to use local administration of statin gels (atorvastatin, simvastatin, rosuvastatin) as adjuncts to subgingival instrumentation. |
| Supporting literature Donos et al. (2019) |
| Quality of evidence Twelve placebo‐controlled RCTs (n = 753), for 1.2% atorvastatin (six RCTs, n = 180), 1.2% simvastatin gel (5 RCTs, n = 118) and 1.2% rosuvastatin gel (four RCTs, n = 122) |
| Grade of recommendation Grade A—↓↓ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Statins are known to have pleiotropic pharmacological effects in addition to their hypolipidemic properties. These include antioxidant and anti‐inflammatory effects, the stimulation of angiogenesis, improvements in endothelial function and the positive regulation of bone formation pathways (Adam & Laufs, 2008; Mennickent, Bravo, Calvo, & Avello, 2008; Petit et al., 2019). Recent evidence suggests that statins may also attenuate periodontal inflammation, as reflected by decreases in pro‐inflammatory and increases in anti‐inflammatory mediators within the gingival crevicular fluid (GCF) of patients with periodontitis (Cicek Ari et al., 2016).
Available evidence
Twelve placebo‐controlled RCTs (n = 753), all derived from the same research group, assessed the effect of local statin gels in adjunctive non‐surgical therapy for infrabony or furcation Class II defects. PPD reduction (primary outcome) was reported at 6 and 9 months for 1.2% atorvastatin (6 RCTs, n = 180), 1.2% simvastatin gel (five RCTs, n = 118) and 1.2% rosuvastatin gel (four RCTs, n = 122). Meta‐analysis was performed in nine RCTs (n = 607).
Risk of bias
There was a moderate overall risk of bias in the studies analysed. Three of 12 studies presented with a high risk of bias in at least one domain. One study was moderately underpowered. While pharmaceutical companies provided the statins in the included studies, the level of involvement of industry in the analysis and interpretation of the results is unclear.
Consistency
Meta‐analysis of nine RCTs where statins had been applied to a single site per patient demonstrated that adjunctive local application of 1.2% statin gels in infrabony defects led to a mean difference in PPD reduction of 1.83 mm (95% confidence interval (CI) [1.31; 2.36]) at 6 months and of 2.25 mm (95% CI [1.88; 2.61]) at 9 months. Only one study investigated locally delivered statins in Class II furcation defects.
Clinical relevance
Although the mean estimates suggested a clinically meaningful benefit from adding statin gels to subgingival instrumentation, there was a large prediction interval for PPD reduction at 6 months (−0.08 mm to 3.74 mm) and the I 2 (95.1%) indicating wide heterogeneity of data and therefore caution needs to be adopted when assessing the efficacy of statins. While the prediction interval at 9 months (1.16–3.34 mm) improved over 6‐month results, heterogeneity (I 2 statistic) of 65.4% still indicated moderate inconsistency in results. Since the outcomes of the different statin gels were considered as one group during the meta‐analysis, it is not possible to draw definitive conclusions on which statin offered higher efficacy.
Balance of benefits and harms
All studies included in the review reported that patients tolerated local statins well, without any complications, adverse reactions/side effects or allergic symptoms.
Economic considerations
There is an additional cost associated with the use of statins that is borne by the patient.
Ethical and legal considerations
The statin formulations included in the systematic review are “off‐label” and an approved formulation with appropriate good manufacturing practice quality control (Good Manufacturing Practice, GMP) and patient's safety validation is not available.
Applicability
The same research group published all data within the RCTs, thereby restricting the generalizability of the results, which need to be confirmed in future larger (multicentre) RCTs by independent groups, with multilevel analyses to account for potential confounding factors (e.g. medical history, smoking history). In addition, future studies will need to clarify which type of statin is more effective.
6.3.1. R2.7 Does the adjunctive use of probiotics improve the clinical outcome of subgingival instrumentation?
| Evidence‐based recommendation (2.7) |
|---|
| We suggest not to use probiotics as an adjunct to subgingival instrumentation |
| Supporting literature Donos et al. (2019) |
| Quality of evidence Five placebo controlled RCTs (n = 176) testing preparations containing L. ramnosus SP1, L. reuteri or the combination of S. oralis KJ3, S. uberis KJ2 and S. rattus JH145. |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” (FAO/WHO). It has been suggested that probiotics may alter the ecology of micro‐environmental niches such as periodontal pockets, and as such, they may disrupt an established dysbiosis. This may re‐establish a symbiotic flora and a beneficial interaction with the host via several mechanisms including modulation of the immune‐inflammatory response, regulation of antibacterial substances and exclusion of potential pathogens via nutritional and spatial competition (Gatej, Gully, Gibson, & Bartold, 2017). This guideline does not include evidence on the use of probiotics in supportive periodontal therapy.
Available evidence
Five placebo‐controlled RCTs (n = 176) assessed the adjunctive effect of probiotics to subgingival instrumentation. Two studies from the same group used a preparation containing L. ramnosus SP1 (2 × 107 colony forming units). Two other RCTs from another research group used a preparation containing L. reuteri. One study evaluated a combination of S. oralis KJ3, S. uberis KJ2 and S. rattus JH145. Meta‐analysis was performed on PPD reduction (primary outcome) at 6 months.
Risk of bias
All studies had an overall low risk of bias. Two out of the five studies declared industrial sponsorship, and three received the probiotics from industry.
Consistency
Meta‐analysis of five RCTs demonstrated that, compared with placebo, treatment with probiotics resulted in a mean difference in PPD reduction of 0.38 mm (95% CI [−0.14; 0.90]) at 6 months. The confidence interval and I 2 statistic (93.3%) suggested considerable heterogeneity for the effect of the treatment with the different formulations.
Clinical relevance
The mean estimated difference in PPD reduction between probiotics and placebo was not statistically significant and of limited clinical relevance (difference < 0.5 mm). Moreover, two groups published four out of the five RCTs included each of them using a different probiotic formulation. Preparations containing Lactobacillus reuteri were the only ones to demonstrate improved PPD reductions.
Given that probiotics embrace a broad range of micro‐organisms and types of preparations, combining such data within the same meta‐analysis poses an interpretational challenge.
Balance of benefits and harms
All formulations appeared to be safe and patients did not report adverse effects.
Economic considerations
There is an additional cost associated with the use of probiotics that is borne by the patient.
Applicability
All studies were conducted in two countries, and no conclusions can be drawn on the effectiveness of probiotics as adjuncts to subgingival instrumentation.
R2.8 Does the adjunctive use of systemic sub‐antimicrobial dose doxycycline (SDD) to subgingival instrumentation improve clinical outcomes?
| Evidence‐based recommendation (2.8) |
|---|
| We suggest not to use systemic sub‐antimicrobial dose doxycycline (SDD) as an adjunct to subgingival instrumentation. |
| Supporting literature Donos et al. (2019) |
| Quality of evidence Eight placebo‐controlled RCTs (14 publications, n = 610). Meta‐analysis on PPD reduction was performed in five RCTs (n = 484) |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Consensus (1.3% of the group abstained due to potential CoI) |
Background
Intervention
Sub‐antimicrobial dose doxycycline (up to 40 mg a day) is a systemic drug employed specifically for its anti‐inflammatory as opposed to its antimicrobial properties. The formulation offers anti‐collagenolytic activity, which may have utility in reducing connective tissue breakdown and augmenting healing responses following subgingival instrumentation in periodontitis patients.
Available evidence
Eight placebo‐controlled RCTs (14 publications, n = 610) reported on the systemic use of a sub‐antimicrobial dose doxycycline (SDD) (up to 40 mg a day) in combination with subgingival instrumentation. Meta‐analysis on PPD reduction (primary outcome) at 6 months post‐subgingival instrumentation was performed in five RCTs (n = 484).
Risk of bias
One study was considered to be at high risk of bias and the remaining studies presented some concerns in certain domains. Of the five studies included in meta‐analysis, three declared industrial sponsorship, one was sponsored by the academic institution, and the fifth did not declare funding.
Consistency
The systematic review included data from eight RCTs, but meta‐analysis was performed in five RCTs that stratified pockets into moderate (4–6 mm) versus deep (≥7 mm). The findings were consistent in all studies. The I 2 statistic was 0% (95% CI [0%; 64.1%]) for both moderate and deep pockets. Two out of five RCTs included did not report a power calculation. The strict experimental protocols employed by the five studies included in the meta‐analysis limits the generalizability of the outcomes.
Clinical relevance of outcomes and effect size
Additional PPD reductions reported following the use of SDD were 0.22 mm at 6 months and 0.3 mm at 9 months in moderate depth pockets. The mean prediction interval ranged from 0.06 mm to 0.38 mm at 6 months and from 0.15 mm to 0.45 mm at 9 months. At deep sites, the additional PPD reductions were more clinically relevant, with 0.68 mm mean additional PPD reductions at 6 months and 0.62 mm at 9 months. The mean prediction interval ranged from 0.34 mm to 1.02 mm at 6 months and from 0.28 mm to 0.96 mm at 9 months. Percentage of pocket closure was not reported.
Balance of benefits and harm
Most studies in the SDD category did not report any serious adverse events or patient dropouts that were directly attributed to the medication. However, it is known that doxycycline may lead to elevations in liver enzymes, which was evident for some patients in the results of one RCT included in the systematic review (Caton et al., 2000, 2001). The sustainability of the benefits or adverse events beyond the study period is unknown.
Ethical considerations
Current health policies on antibiotic stewardship and related public health concerns surrounding increasing antibiotic resistance need to be taken into account. The systemic effects of a drug taken over a 6‐ to 9‐month period during the initial phase of subgingival instrumentation require careful consideration when extrapolating outcomes from controlled research trials into general clinical practice.
Legal considerations
SDD is not approved or available in some European countries.
Economic considerations
There is a cost associated with the use of SDD that is borne by the patient.
Applicability
SSD is mainly effective in deep sites (≥7 mm), although SDD is used as a systemic rather than a site‐specific treatment. The clinical significance in deep sites (0.68 mm at 6 months and 0.62 mm at 9 months) is small, given that re‐treatment with non‐surgical root debridement might yield additional PPD reductions, and local drug delivery systems may yield similar effect sizes. Moreover, the five studies that did stratify results based upon pocket depth did not present an a priori statistical plan powered to stratify results in that manner.
R2.9 Does the adjunctive use of systemic/local bisphosphonates to subgingival instrumentation improve clinical outcomes?
| Evidence‐based recommendation (2.9) |
|---|
| We recommend not to use locally delivered bisphosphonate (BP) gels or systemic BPs as an adjunct to subgingival instrumentation. |
| Supporting literature Donos et al. (2019) |
| Quality of evidence Seven placebo‐controlled RCTs (n = 348), on local delivery of 1% alendronate gel (six studies) and 0.5% zolendronate gel (one study); two placebo‐controlled RCTs (n = 90) on systemic administration of BPs (alendronic acid and risedronate). |
| Grade of recommendation Grade A—↓↓ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Bisphosphonates (BPs) are a class of antiresorptive agents that act mainly by inhibiting osteoclast activity. BPs can also directly inhibit host degradative enzymes like matrix metalloproteinases released by osteoclasts and other cells of the periodontium. There is also evidence that BPs reduce osteoblast apoptosis, thus increasing bone density as an overall therapeutic outcome. It is therefore rational to speculate that BPs may benefit the management of inflammation‐mediated alveolar bone resorption in periodontitis patients (Badran, Kraehenmann, Guicheux, & Soueidan, 2009).
Available evidence
Seven placebo‐controlled RCTs (n = 348), all from the same research group, on local delivery of 1% alendronate gel (six studies) and 0.5% zolendronate gel (one study) in infrabony or furcation Class II defects were identified.
A meta‐analysis on PPD reduction at 6 months in five RCTs (n = 228) using either single or multiple sites per patient in infrabony defects was undertaken. Two placebo‐controlled RCTs (n = 90) evaluated systemic administration of BPs (alendronate and risedronate).
Risk of bias
Of the nine studies included, two were at high risk of bias and seven presented some concerns in at least one of the domains of the risk of bias assessment tool. One study was underpowered. All studies on local BPs were published by the same research group. While pharmaceutical companies provided bisphosphonates for local application in the included studies, the level of involvement of industry in the analysis and interpretation of the results is unclear.
Consistency
Nine RCTs were available, two involving systemic administration of BPs. No meta‐analysis was therefore undertaken for systemic BPs. Out of the seven RCTs involving local application of BPs, five were on infrabony defects (four employed 1% Alendronate gel and one study used 0.5% Zolendronate gel), while two were undertaken on furcation Class II defects (all using 1% Alendronate gel). A meta‐analysis of five studies using single or multiple sites per patient demonstrated a significant benefit in terms of PPD reduction of 2.15 mm (95% CI [1.75; 2.54]) after 6 months from non‐surgical periodontal therapy in infrabony defects, with a low level of heterogeneity (I 2 = 47.3%).
Clinical relevance
The results of the two studies on systemic BPs were poorly comparable as they were undertaken in different populations and involved different confounding factors (e.g. smoking).
Although the mean estimates suggested adjunctive benefits from adjunctive use of BP gels, the combined use of studies considering single and multiple sites per patient in the meta‐analysis should be taken into consideration.
Balance of benefits and harm
Both systemic and local BPs were well‐tolerated in the studies reported in the systematic review and were not associated with severe adverse reactions.
Economic considerations
There is an additional cost associated with the use of bisphosphonates that is borne by the patient.
Ethical and legal considerations
The balance of recognized potential severe risks (e.g. osteochemonecrosis of the jaws) versus benefits resulted in a consensus that systemic administration of BPs should not be recommended in the clinical management of periodontal bone loss. It is important to note that BP gel formulations are “off‐label” and an approved formulation with appropriate quality control (GMP) and patient safety validation is not available.
Applicability
The same research group/centre published all data on locally delivered BPs; therefore, the generalizability of the results requires substantiating in future larger (multicentre) RCTs, with multilevel analyses accounting for potential confounding factors (e.g. medical history, smoking history).
R2.9 Does adjunctive use of systemic/local non‐steroidal anti‐inflammatory drugs to subgingival instrumentation improve the clinical outcomes?
| Evidence‐based recommendation (2.10) |
|---|
| We recommend not to use systemic or local non‐steroidal anti‐inflammatory drugs (NSAIDs) as an adjunct to subgingival instrumentation |
| Supporting literature Donos et al. (2019) |
| Quality of evidence Two placebo‐controlled RCTs (n = 88) on local application (1% flurbiprofen toothpaste; irrigation with 200 ml buffered 0.3% acetylsalicylic acid); two placebo‐controlled RCTs (n = 133) on systemic applications (celecoxib, diclofenac potassium) |
| Grade of recommendation Grade A—↓↓ |
| Strength of consensus Strong consensus (1.3% of the group abstained due to potential CoI) |
Background
Intervention
Periodontitis is an inflammatory disease in which altered immune‐inflammatory responses to a dysbiotic biofilm drives connective tissue destruction and bone loss. It is reasonable therefore that non‐steroid anti‐inflammatory drugs (NSAIDs), may be effective as adjunctive periodontal therapies.
Available evidence
Two placebo‐controlled RCTs (n = 88) on local application, one using 1% flurbiprofen toothpaste twice daily for 12 months and a second using subgingival daily irrigation with 200 ml buffered 0.3% acetylsalicylic acid, were identified. Two placebo‐controlled RCTs (n = 133) on systemic applications, one RCT using systemic celecoxib (200 mg daily 6 months) and another using a cyclical regime of diclofenac potassium (50 mg 2‐months, then 2 months off, then 2 months on), were included. All studies reported on PPD reduction at 6 months. No meta‐analysis was performed due to the limited number of studies identified and their heterogeneity.
Risk of bias
Two out of four studies were considered at high risk of bias. All studies on NSAIDs either did not provide information on sample size calculation or were underpowered. All studies declared industry funding.
Consistency
It was not possible to undertake a meta‐analysis of local or systemic NSAID administration as an adjunct to subgingival instrumentation because the studies were heterogeneous (not comparable) in terms of the medication employed and the modality of administration.
Clinical relevance
Local NSAIDs did not enhance the clinical outcomes of subgingival instrumentation. Systemic NSAIDs exhibited limited clinical benefits, but their heterogeneity did not permit the drawing of clinically meaningful conclusions.
Balance of benefits and harm
No serious adverse events were reported.
Ethical considerations
Long‐term use of systemic NSAIDs carries a well‐known risk of unwanted side effects, which raises concerns over their use as adjuncts to subgingival instrumentation.
Economic considerations
There would be a cost to using NSAIDs which would ultimately transfer to the patient.
Applicability
We do not encourage everyday clinical use of systemic NSAIDs or to conduct future studies to test these medications in their current standard formulations or dosage regimes. No meaningful conclusions could be made regarding use of local NSAIDs. Based on the current limited evidence, local NSAIDs did not provide a clinical benefit.
R2.10 Does the adjunctive use of omega‐3 polyunsaturated fatty acids (PUFA) improve the clinical outcome of subgingival instrumentation?
| Evidence‐based recommendation (2.11) |
|---|
| We recommend not to use omega−3 PUFAs as an adjunct to subgingival instrumentation. |
| Supporting literature Donos et al. (2019) |
| Quality of evidence Three placebo‐controlled RCTs (n = 160) with 6‐month administration of omega‐3 PUFAs. |
| Grade of recommendation Grade A—↓↓ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
The recent discovery of pro‐resolving lipid mediators by Serhan and colleagues [reviewed by (Serhan, 2017)], some of which are produced by the metabolism of two major omega‐3 polyunsaturated fatty acids (PUFAs), namely eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA) to E‐ and D‐resolvins, respectively, raises the potential for essential dietary PUFAs as adjunctive host‐modulating therapeutics for non‐surgical periodontal treatment. However, few studies have investigated their efficacy in human trials.
Available evidence
Three placebo‐controlled RCTs (n = 160) with 6‐month administration of omega‐3 PUFAs. Heterogeneity in study designs precluded a meta‐analysis. One RCT investigated low dose omega‐3 PUFAs (6.25 mg eicosapentaenoic acid ‐EPA and 19.9mg docosahexaenoic acid ‐DHA) twice daily for 6 months; a second study employed high dose omega‐3 PUFAs (3 g) in combination with 81 mg aspirin daily for 6 months; and a third study used 1 g omega‐3 PUFAs twice daily for 6 months. All studies provided PPD reduction data at 6 months post‐subgingival instrumentation. No meta‐analysis was performed due to the limited number of studies identified and their heterogeneity.
Risk of bias
One out of three studies was considered to be at high risk of bias. One study reported industry support, one was supported by a University, and one did not disclose the funding source.
Consistency
No meta‐analysis could be performed due to the low number of available studies and study heterogeneity in terms of proposed regime and formulation.
Clinical relevance
Since the three RCTs used different doses and preparations of omega‐3 PUFAs and one out of three studies combined omega‐3 with 81 mg aspirin, it was not possible to draw clinically meaningful conclusions from the data.
Balance of benefits and harm
No adverse events were associated to the use of omega‐3 PUFAs, and they are essentially a relatively safe dietary supplement.
Economic considerations
There would be a cost to using omega‐3 PUFAs which would ultimately transfer to the patient.
Applicability
There is insufficient data to support or refute the use of omega‐3 PUFAs, either as a monotherapy or as a combined therapeutic adjunct to subgingival instrumentation. The combination of omega‐3 fatty acids and low‐dose aspirin also warrants further assessment of its use as an adjunct in the management of periodontitis.
R2.11 Does the adjunctive use of local metformin improve the clinical outcome of subgingival instrumentation?
| Evidence‐based recommendation (2.12) |
|---|
| We recommend not to use local administration of metformin gel as adjunct to subgingival instrumentation. |
| Supporting literature Donos et al. (2019) |
| Quality of evidence Six placebo‐controlled RCTs (n = 313) on locally delivered 1% metformin gel |
| Grade of recommendation Grade A—↓↓ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Metformin is a second‐generation biguanide used to manage type 2 diabetes mellitus. There is evidence suggesting that metformin decreases inflammation and oxidative stress and may also have an osteogenic effect by increasing the proliferation of osteoblasts and reducing osteoclast activity (Araujo et al., 2017). It is therefore plausible that this medication may be beneficial in treating a chronic inflammatory disease like periodontitis.
Available evidence
Six placebo‐controlled RCTs (n = 313) from the same research group investigated locally delivered 1% metformin gel as an adjunct to subgingival instrumentation. All studies reported on PPD reduction at 6 months post‐subgingival instrumentation, and a meta‐analysis was undertaken combining the six RCTs.
Risk of bias
Four out of six studies presented some concerns of risk of bias in most of the domains. All studies were published by the same research group. While pharmaceutical companies provided metformin, the level of involvement of industry in the analysis and interpretation of the results is unclear.
Consistency
Meta‐analysis of six studies (four considering single sites per patient and two considering multiple sites per patient) indicated that 1% metformin gel as adjunct to subgingival instrumentation led to an improved PPD reduction of 2.07 mm (95% CI [1.83; 2.31]) at 6 months. Heterogeneity between the studies was low (I 2 = 43%).
Clinical relevance
All studies reported a benefit in terms of PPD reduction when 1% metformin gel was used as an adjunct to subgingival instrumentation. However, studies using single and multiple sites per patients were combined.
Balance of benefits and harms
All studies included in the review reported that patients tolerated local metformin gel well, without any complications, adverse reactions/side‐effects, or symptoms of hypersensitivity.
Ethical and legal considerations
The metformin formulation included in the systematic review is “off‐label” and an approved formulation with appropriate quality control (GMP) and patient safety validation is not available.
Economic considerations
There is an additional cost associated with the use of metformin that is borne by the patient.
Applicability
The same research group published all data on local metformin; therefore, the generalizability of the results needs to be confirmed in future larger (multicentre) RCTs, with multi‐level analyses accounting for potential confounding factors (e.g. medical history, smoking history).
6.4. Intervention: Use of adjunctive chemical agents to subgingival instrumentation
R2.13 Does the adjunctive use of adjunctive chemotherapeutics (antiseptics) improve the clinical outcome of subgingival instrumentation?
| Expert consensus‐based recommendation (2.13) |
|---|
| Adjunctive antiseptics may be considered, specifically chlorhexidine mouth rinses for a limited period of time, in periodontitis therapy, as adjuncts to mechanical debridement, in specific cases. |
| Supporting literature da Costa, Amaral, Barbirato, Leao, and Fogacci (2017) |
| Grade of recommendation Grade 0—↔ |
| Strength of consensus Consensus (6.3% of the group abstained due to potential CoI) |
Background
Intervention
In order to control gingival inflammation during periodontal therapy, the adjunctive use of some agents has been proposed. Chlorhexidine mouth rinses have been frequently tested in this indication and frequently used in different clinical settings.
Available evidence
In the systematic reviews of the present European Workshop, the role of antiseptics in active periodontal therapy has not been directly addressed. However, some evidence is available based on studies on the role of chlorhexidine use after subgingival instrumentation (da Costa et al., 2017).
In addition, other factors should be considered:
It is unclear whether this should be a general recommendation for initial therapy.
It may be necessary to optimize mechanical plaque control before considering adjunctive chlorhexidine as an adjunct to subgingival instrumentation.
Specific considerations can be made when used in conjunction with full‐mouth disinfection approaches and/or with systemic antimicrobials.
The medical status of the patient.
Adverse effects (staining) and economical costs should be considered.
6.5. Intervention: Use of adjunctive locally administered antiseptics to subgingival instrumentation
R2.14 Do adjunctive locally administered antiseptics improve the clinical outcome of subgingival instrumentation?
| Evidence‐based recommendation (2.14) |
|---|
| Locally administered sustained‐release chlorhexidine as an adjunct to subgingival instrumentation in patients with periodontitis may be considered. |
| Supporting literature Herrera et al. (2020) |
| Quality of evidence Nine RCTs, 6–9 months. 718/719 patients. High risk of bias and heterogeneity among studies. |
| Grade of recommendation Grade 0—↔ |
| Strength of consensus Consensus (10.5% of the group abstained due to potential CoI) |
Background
Intervention
There is insufficient evidence on the benefits of locally administered sustained‐release antiseptics as an adjunct to subgingival debridement in patients with periodontitis.
Available evidence
The systematic review (Herrera et al., 2020) revealed results from studies on products containing chlorhexidine (Periochip n = 9, Chlosite n = 2). One product (Periochip) demonstrated statistically significantly greater PPD reduction following single or multiple applications as an adjunct to subgingival debridement on short‐term follow‐up (6–9 months) (weighted mean difference (WMD) = 0.23, 95% CI [0.12; 0.34], p < .001 and significant heterogeneity). There are no long‐term data available. No significant differences were found regarding CAL. Data on BOP were insufficient and no data on pocket closure or on number needed to treat (NNT) were provided.
Risk of bias
High risk of bias and heterogeneity among studies.
Clinical relevance and effect size
Effect size estimated for all PPD categories indicates an increased effect of about 10% in PPD reduction.
Balance of benefit and harm
No increase in adverse effects or differences in patient‐reported outcome measures (PROMs) were observed.
Economic considerations
The cost for the product and the limited availability of products in European countries need to be considered.
6.6. Intervention: Use of adjunctive locally administered antibiotics to subgingival instrumentation
R2.15 Do adjunctive locally administered antibiotics improve the clinical outcome of subgingival instrumentation?
| Evidence‐based recommendation (2.15) |
|---|
| Specific locally administered sustained‐release antibiotics as an adjunct to subgingival instrumentation in patients with periodontitis may be considered. |
| Supporting literature Herrera et al. (2020) |
| Quality of evidence PPD reduction (6–9 months): Atridox n = 2, 19/19 patients; Ligosan: n = 3, 232/236 patients; Arestin: n = 6, 564/567 patients. High risk of bias and heterogeneity in the majority of studies. |
| Grade of recommendation Grade 0—↔ |
| Strength of consensus Consensus (7.8% of the group abstained due to potential CoI) |
Background
Available evidence
Of the products available on the European market, the systematic review (Herrera et al., 2020) revealed statistically significantly improved PPD reduction of locally applied antibiotics as an adjunct to subgingival debridement on short‐term follow‐up (6–9 months) for Atridox (two studies, WMD = 0.80; 95% CI [0.08; 1.52]; p = .028), Ligosan (three studies, WMD = 0.52; 95% CI [0.28; 0.77]; p < .001) and Arestin (six studies, WMD = 0.28; 95% CI [0.20; 0.36]; p < .001). No significant adjunctive long‐term effect was evident. Statistically significantly improved CAL change for products used as an adjunct to subgingival debridement on short‐term follow‐up (6–9 months) was identified for Ligosan (n = 3, WMD = 0.41, 95% CI [0.06; 0.75]; p = .020) and Arestin (n = 4, WMD = 0.52; 95% CI [0.15; 0.88]; p = .019). Long‐term data did not show significant improvement of CAL for any product. Data on BOP and pocket closure were insufficient. No information on NNT was provided. Estimated effect size indicates an increased effect of 10%–30% in PPD reduction.
Risk of bias
High risk of bias and heterogeneity in the majority of studies.
Balance of benefit and harm
No increase in adverse effects or differences in PROMs were observed. Harm versus benefit considerations on the use of antibiotics need to be considered.
Economic considerations
High economic costs and limited availability of products in European countries need to be considered.
6.7. Intervention: Use of adjunctive systemically administered antibiotics to subgingival instrumentation
R2.16 Does adjunctive systemically administered antibiotics improve the clinical outcome of subgingival instrumentation?
| Evidence‐based recommendation (2.16) |
|---|
|
| Supporting literature Teughels et al. (2020) |
| Quality of evidence RCTs (n = 28) with a double‐blind, placebo‐controlled, parallel design. Risk of bias was low for 20 of the studies, while seven studies had a high risk. PPD reduction at 6 months; MET + AMOX: n = 8, 867 patients. PPD reduction at 12 months; MET + AMOX: n = 7, 764 patients, MET: n = 2, 259 patients. |
|
|
Background
Available evidence
While the results from the meta‐analysis (Teughels et al., 2020) revealed a statistically significantly improved outcome for systemically administrated antibiotics as an adjunct to subgingival debridement, the effect was confined to a limited group of antibiotics. A significantly improved PPD reduction at the 6‐month follow‐up was observed for metronidazole (MET) and amoxicillin (AMOX) (n = 8; WMD = 0.43, 95% CI [0.36; 0.51]). Analysis of 12‐month data revealed a significant adjunctive effect for MET + AMOX (n = 7; WMD = 0.54, 95% CI [0.33; 0.74]) and MET (n = 2; WMD = 0.26, 95% CI [0.13; 0.38]). The adjunctive use of MET + AMOX and MET resulted in a statistically significant additional percentage of pocket closure at 6 and 12 months. Statistically significantly greater CAL gain and BOP reduction for MET + AMOX at 6 and 12 months. The adjunctive effect of MET + AMOX on PPD reduction and CAL gain was more pronounced in initially deep than moderately deep pockets. There are no relevant data on the long‐term (>12 months) effect of using systemic antibiotics as an adjunct to subgingival debridement. NNT was not assessed.
Risk of bias
Low risk of bias and low heterogeneity among studies.
Consistency
High consistency of results.
Clinical relevance and effect size
Effect size estimation on PPD reduction as opposed to subgingival debridement alone indicates an increased effect of about 40%–50%.
Balance of benefit and harm
While the MET + AMOX combination had the most pronounced effects on the clinical outcomes among the different types of systemic antimicrobial therapy, the regimen was also associated with the highest frequency of side effects. Global concerns regarding the overuse of antibiotics and the development of antibiotic resistance must be considered. Benefit versus harm analysis includes considerations on the overall use of antibiotics for the individual patient and public health. Systemic antibiotic regimens have shown long lasting impact on the faecal microbiome, including an increase in genes associated with antimicrobial resistance.
Applicability
Due to concerns to patient's health and the impact of systemic antibiotic use to public health, its routine use as adjunct to subgingival debridement in patients with periodontitis is not recommended. Based on the available evidence, however, its adjunctive use may be considered for special patient categories (e.g. generalized periodontitis Stage III in young adults).
7. CLINICAL RECOMMENDATIONS: THIRD STEP OF THERAPY
The treatment of Stage III periodontitis should be carried out in an incremental manner, first by achieving adequate patient's oral hygiene practices and risk factor control during the first step of therapy and then, during the second step of therapy by professional elimination (reduction) of supra and subgingival biofilm and calculus, with or without adjunctive therapies. However, in periodontitis patients, the complete removal of subgingival biofilm and calculus at teeth with deep probing depths (≥6 mm) or complex anatomical surfaces (root concavities, furcations, infra bony pockets) may be difficult, and hence, the endpoints of therapy may not be achieved, and further treatment should be implemented.
The individual response to the second step of therapy should be assessed after an adequate healing period (periodontal re‐evaluation). If the endpoints of therapy (no periodontal pockets >4 mm with bleeding on probing or deep pockets [≥6 mm]) have not been achieved, the third step of therapy should be implemented. If the treatment has been successful in achieving these endpoints of therapy, patients should be placed in a SPC program.
The third step of therapy is, therefore, aimed at treating those sites non‐responding adequately to the second step of therapy with the purpose of getting access to deep pocket sites, or aiming at regenerating or resecting those lesions, that add complexity in the management of periodontitis (infrabony and furcation lesions). It may include the following interventions:
Repeated subgingival instrumentation with or without adjunctive therapies
Access flap periodontal surgery
Resective periodontal surgery
Regenerative periodontal surgery
Surgical approaches are subject to specific, additional patient consent and specific risk factors/presence of medical contra‐indications should be considered. The individual response to the third step of therapy should be assessed (periodontal evaluation), and ideally, the endpoints of therapy should be achieved, and patients should be placed in SPC. These endpoints of therapy may not be achievable in all teeth in severe Stage III periodontitis patients.
7.1. Intervention: access flap procedures
The first relevant question to evaluate the relative efficacy of the surgical interventions in the third step of therapy, for the treatment of Stage III periodontitis patients with residual pockets after the second step of periodontal therapy, is whether access flap procedures are more efficacious than subgingival re‐debridement for achieving the endpoints of therapy [probing depth (PD) ≤4 mm without BOP].
R3.1 How effective are access flaps as compared to repeated subgingival instrumentation?
| Evidence‐based recommendation (3.1) |
|---|
| In the presence of deep residual pockets (PPD ≥ 6 mm) in patients with Stage III periodontitis after the first and second steps of periodontal therapy, we suggest performing access flap surgery. In the presence of moderately deep residual pockets (4–5 mm), we suggest repeating subgingival instrumentation. |
| Supporting literature Sanz‐Sanchez et al. (2020) |
| Quality of evidence Thirteen RCTs (500 patients) with moderate‐to‐high risk of bias. Five studies were restricted to pockets associated with intrabony defects. Limited number of studies presented data for quantitative analyses. High consistency of results. |
| Grade of recommendation Grade B—↑ |
| Strength of consensus Consensus (1.4% of the group abstained due to potential CoI) |
Background
Available evidence
Statistically significantly greater PPD reduction was observed in access flaps (AF) than in subgingival debridement at 1 year. The difference was more pronounced at initially deep sites (PPD ≥ 6 mm) (four studies, WMD = 0.67, 95% CI [0.37; 0.97], at 1 year; WMD = 0.39; 95% CI [0.09; 0.70] at >1 year). The relative effect was 27.5%. These differences in PPD reduction also occurred in pockets associated with infrabony defects (four studies; WMD = 0.49, 95% CI [0.11; 0.86]). No statistically significant differences in CAL gain at initially deep pockets were observed between procedures. However, CAL gain was significantly greater in the subgingival debridement group at initially moderately deep pockets, and AF resulted in statistically significantly more attachment loss at sites with initial PPD ≤ 4 mm. Statistically significantly higher percentage of shallow pockets was achieved with AF than with subgingival debridement (three studies, WMD = 11.6%, 95% CI [6.76; 16.5]). The need of re‐treatment (four studies) was 8%–29% in the subgingival debridement group and 0%–14% in the AF. There were no statistically significant differences in PROMs between the interventions.
7.2. Intervention: different access flaps procedures
The second relevant question was whether there are specific surgical conservative surgical procedures that are more efficacious for achieving the endpoints of in the treatment of patients with Stage III periodontitis.
Conservative surgical procedures have been defined as those aiming to access the affected root surfaces without eliminating significant amounts of hard and soft tissues. These procedures have been classified depending on the amounts of marginal gingiva and interdental papillary tissue removal into:
open flap debridement with intra‐sulcular incisions (OFD);
flaps with para‐marginal incisions, such as modified Widman flap (MWF) and
papilla preservation flaps.
R3.2 How effective are the different access flap procedures?
| Evidence‐based recommendation (3.2) |
|---|
| In cases of deep (PPD ≥ 6 mm) residual pockets and intrabony defects in patients with Stage III periodontitis after adequate first and second steps of periodontal therapy, there is insufficient evidence for a recommendation on the choice of flap procedures. Access periodontal surgery can be carried out using different flap designs. |
| Supporting literature Sanz‐Sanchez et al. (2020) |
| Quality of evidence Three RCTs compared MWF with OFD. One RCT compared the efficacy of papilla preservation flaps (single flap approach versus OFD) in the presence of intrabony pockets. Two RCTs compared minimally invasive surgery with conventional surgery. Moderate to high risk of bias. Limited available data. |
| Grade of recommendation Grade 0—↔ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Available evidence
Out of three available studies comparing MWF with OFD, only one showed statistically significantly greater PPD reduction for MWF than OFD. There were no statistically significant differences in % PPD reduction in deep infrabony pockets between papilla preservation flap (single flap approach) and conventional flaps (one study). Two studies comparing minimally invasive surgery with conventional surgery did not demonstrate a significant added value in PPD reduction or CAL gain.
7.3. Intervention: resective flap procedures
The third relevant question was whether resective flap procedures (those that, in addition to gaining access for subgingival debridement, aim to change the architecture of the hard and/or the soft tissues to attain shallow probing depths) are more efficacious than conservative surgical procedures in achieving the endpoints of periodontal in the treatment of patients with Stage III periodontitis.
R3.3 What is the efficacy of pocket elimination/reduction surgery in comparison with access flap surgery?
| Evidence‐based recommendation (3.3) |
|---|
| In cases of deep (PPD ≥ 6 mm) residual pockets in patients with Stage III periodontitis after an adequate second step of periodontal therapy, we suggest using resective periodontal surgery, yet considering the potential increase of gingival recession. |
| Supporting literature Polak et al. (2020) |
| Quality of evidence Nine RCTs (four could be used for the quantitative analysis). High risk of bias. Limited available data. |
| Grade of recommendation Grade B—↑ |
| Strength of consensus Simple majority (2.6% of the group abstained due to potential CoI) |
Background
Available evidence
Resective periodontal surgery attained statistically significantly higher PPD reduction than access flaps at 6 months (WMD = 0.59 mm; 95% CI [0.06–1.12]) and one year (WMD = 0.47 mm; 95% CI [0.24; 0.7]). For pockets 4–6 mm, differences were statistically significant at 1 year (WMD = 0.34 mm; 95% CI [0.19; 0.48]), while pockets 7 mm or deeper showed greater difference between the groups (WMD = 0.76 mm; CI [0.35; 1.17]). The differences were lost with time (3‐ and 5‐year follow‐up). There were no differences in CAL gains between the surgical modalities in the long term (3–5 years). Post‐operative recession was statistically significantly greater following resective surgery than access flaps at 1‐year post‐op (two studies). No differences reported at 5‐year follow‐up (one study). No differences in recession over time in initially shallow pockets between the two modalities.
Risk of bias
High risk of bias, scarcity of quantitative data (only 4 RCTs).
Clinical relevance and effect size
The paucity of the data on percentage of shallow pockets or incidence of re‐treatment prevents assessments of the clinical relevance of the differences.
Balance of benefit and harm
Data on PROMs, the percentage of residual pockets or the need of re‐treatment were not reported in any of the studies.
7.4. General recommendations for periodontal surgical procedures
R3.4 What is the level of care required for management of deep residual pockets with or without presence of intrabony defects or furcation involvement after completion of steps 1 and 2 of periodontal therapy?
| Expert consensus‐based recommendation (3.4) |
|---|
| Surgical treatment is effective but frequently complex, and we recommend that it is provided by dentists with additional specific training or by specialists in referral centres. We recommend efforts to improve access to this level of care for these patients. |
| Supporting literature Expert opinion |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Advanced periodontal surgery (regenerative and furcation management) is beyond the scope and competence of education in general dental practice (Sanz & Meyle, 2010). Dental curricula include knowledge and familiarity with the approach but are not designed to provide competence to conduct such treatment: Additional specific training is required and is available through continuing professional development and periodontal learned societies in most countries. Post‐graduate periodontal education, on the other hand, is specifically designed to provide competence and proficiency towards the resolution of such complex problems (Sanz, van der Velden, van Steenberghe, & Baehni, 2006; Van der Velden & Sanz, 2010).
R3.5 If expertise is not available or referral is not an option, what is the minimum level of primary care required for management of residual pockets associated with or without intrabony defects or furcation involvement after completion of steps 1 and 2 of periodontal therapy?
| Expert consensus‐based recommendation (3.5) |
|---|
| As a minimum requirement, we recommend repeated scaling and root debridement with or without access flap of the area in the context of high‐quality step 1 and 2 treatment and a frequent program of supportive periodontal care including subgingival instrumentation. |
| Supporting literature Expert opinion [and systematic reviews for access flaps (Graziani et al., 2012, 2015)] |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Dental services are organized differently in various countries. Some are structured in both primary care and specialist care (usually delivered by referral to dental hospitals or specialist practices/centres); in other countries, dental services are based on a single level of care and interested general practitioners acquire broader periodontal skills through continuing professional development. Optimal management of Stage III and Stage IV periodontitis remains limited in most health systems with significant inequalities in availability and access to advanced/specialist periodontal care. There is an urgent need to improve patient access to the appropriate level of care given the high burden and costs associated with the sequelae of unmanaged severe (stages III and IV) periodontitis.
R3.6 What is the importance of adequate self‐performed oral hygiene in the context of surgical periodontal treatment?
| Expert consensus‐based recommendation (3.6) |
|---|
| We recommend not to perform periodontal (including implant) surgery in patients not achieving and maintaining adequate levels of self‐performed oral hygiene. |
| Supporting literature Expert opinion |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Proof‐of‐principle studies conducted in the 1970s have pointed to the negative effects (clinical attachment loss) of performing periodontal surgery in subjects with inadequate plaque control (Nyman, Lindhe, & Rosling, 1977; Rosling, Nyman, Lindhe, & Jern, 1976). Multiple RCTs on surgical periodontal intervention have shown a dose‐dependent effect of plaque control on healing outcomes. Similar data have been reported after implant surgery (van Steenberghe et al., 1990). The level of self‐performed oral hygiene is clinically assessed using a plaque control record [for an example, see (O'Leary, Drake, & Naylor, 1972)]. Plaque scores smaller than 20%–25% have been consistently associated with better surgical outcomes (see Step 1 and SPC clinical recommendations for detailed discussions on how to facilitate achieving stringent levels of self‐performed oral hygiene).
7.5. Intervention: Management of intrabony defects
R3.7 What is the adequate management of residual deep pockets associated with intrabony defects?
| Evidence‐based recommendation (3.7) |
|---|
| We recommend treating teeth with residual deep pockets associated with intrabony defects 3 mm or deeper with periodontal regenerative surgery. |
| Supporting literature Nibali et al. (2019) |
| Quality of evidence Twenty‐two RCTs (1,182 teeth in 1,000 patients)—four studies at low risk of bias—there is consistency of direction of benefit but high heterogeneity for superiority of regeneration over open flap debridement. |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Consensus (10% of the group abstained due to potential CoI) |
Background
Intervention
See previous sections. An algorithm for clinical decision‐making in the treatment by regenerative surgical therapy of intrabony defects and residual pockets is depicted in Figure 1.
FIGURE 1.
Regenerative surgical therapy of intrabony defects and residual pockets
Available evidence
The evidence base includes 22 RCTs with 1,000 patients. The quality of the evidence was rated as high.
Risk of bias
Study quality assessment identified four studies at low risk of bias and 15 studies at unclear risk of bias.
Consistency
Regenerative surgical therapy resulted in improved clinical outcomes (shallower pockets and higher CAL gain) compared with open flap debridement in the majority of studies. No indication of publication bias was observed. Moderate to substantial heterogeneity in the size of the adjunctive effect was observed. This could be partly explained by the use of specific biomaterials or flap designs.
Clinical relevance and effect size
The mean adjunctive benefit reported was 1.34 mm (95% CI [0.95; 1.73]) in CAL gain and 1.20 mm (95% CI [0.85; 1.55]) in pocket depth reduction. This represented an 80% (95% CI [60%; 100%]) improvement compared to the controls. A mean difference of this magnitude is deemed clinically relevant as it has the potential of decreasing risk of tooth loss. Observational and experimental studies reporting on tooth survival for a period of 3–20 years show improved tooth retention with periodontal regeneration in teeth under regular supportive periodontal therapy (28 RCTs summarized in unpublished data).
Balance of benefit and harm
No serious adverse event was reported in any of the studies included in the systematic review. The adverse events associated with regenerative therapy included local adverse events (wound failure) and post‐operative morbidity. No specific harm has been reported after regenerative surgery. Potential risk for disease transmission from well‐documented human‐derived or animal‐derived regenerative biomaterials is considered extremely low.
Ethical considerations
The perception that regenerative treatment of deep intrabony defects results in better outcomes than access flap is commonly held in the research and clinical community. Therefore, maximum tissue preservation flap with the application of documented regenerative biomaterials should be the standard of care. This perception is supported by the observation that only 22 of 79 RCTs included in the systematic review used access flap as the control and the majority of the body of evidence compared different regenerative techniques/biomaterials.
Regulatory consideration
It is important to emphasise that only few classes of regenerative materials are registered in Europe. In each class, only few materials satisfy the evidence base criteria set forth by these guidelines and the considerations should not be applied to materials that have not been adequately tested. Implementation of the new EU medical device regulations will prove useful.
Economic considerations
Regenerative surgery is more expensive than access flap surgery but cheaper than tooth replacement necessary as a consequence of tooth loss. In the absence of health‐economic data in RCTs included in the review, a pilot study has indicated that the initial increase in cost of regeneration is associated with lower cost of managing recurrence over a 20‐year period (Cortellini, Buti, Pini Prato, & Tonetti, 2017).
Patient preferences
No data are available about patient preference or acceptability. Religious issues may be present for segments of the population since some of the regenerative materials are of porcine or bovine origin. While the use for medical reasons is generally acceptable and has been approved by religious leaders, the sensitivity of individual subjects may pose a barrier.
R3.8 What is the adequate choice of regenerative biomaterials for promoting healing of residual deep pockets associated with a deep intrabony defect?
| Evidence‐based recommendation (3.8) |
|---|
| In regenerative therapy, we recommend the use of either barrier membranes or enamel matrix derivative with or without the addition of bone‐derived grafts* |
| Supporting literature Nibali et al. (2019) |
| Quality of evidence Twenty RCTs (972 patients)—four studies at low risk of bias—moderate‐to‐high heterogeneity for superiority of these biomaterials |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Consensus (18.1% of the group abstained due to potential CoI) |
*Clinicians should select a specific biomaterial to be used to promote regeneration at intrabony defects (or Class II furcation involvements) based on satisfaction of all of the following criteria (Proceedings of the, 1996 World Workshop in Periodontics, 1996): (a) availability of solid preclinical research identifying plausible mechanism(s) of action leading to periodontal regeneration; (b) human histological evidence of regeneration in the specific application; and (c) evidence of efficacy in applicable, high‐quality randomized controlled clinical trials. While there are biomaterials that satisfy all these criteria, it must be understood that many biomaterials do not meet them in spite of being CE (“Conformité Européene”) marked or Food and Drug Administration (FDA)‐approved/cleared.
Background
Intervention
See previous sections.
Available evidence
The evidence base includes 20 RCTs with 972 patients. The quality of the evidence was considered to be high.
Risk of bias
Study quality assessment identified four studies at low risk of bias and 15 studies at unclear risk of bias.
Consistency
Regenerative surgical therapy with a variety of biomaterials resulted in improved clinical outcomes compared with open flap debridement in the majority of studies. No indication of publication bias was observed. Moderate to substantial heterogeneity in the size of the adjunctive effect was observed.
Clinical relevance and effect size
The mean adjunctive benefit in term of CAL gain was 1.27 mm (95% CI [0.79; 1.74], equivalent to a 77% improvement) for EMD and 1.43 mm (95% CI [0.76; 2.22], equivalent to an 86% improvement) for guided tissue regeneration (GTR) compared with OFD. The combination of membrane with bone‐derived graft resulted in higher CAL gain of 1.5 mm (95% CI [0.66; 2.34], equivalent to a 90% improvement) compared with OFD. The comparison between EMD versus GTR resulted in no statistically significant difference in CAL gain. The choice of biomaterial or possible combinations should be based on defect configuration.
R3.9 What is the adequate choice of surgical flap design for the regenerative treatment of residual deep pockets associated with an intrabony defect?
| Evidence‐based recommendation (3.9) |
|---|
| We recommend the use of specific flap designs with maximum preservation of interdental soft tissue such as papilla preservation flaps. Under some specific circumstances, we also recommend limiting flap elevation to optimize wound stability and reduce morbidity. |
| Supporting literature Graziani et al. (2012); Nibali et al. (2019) |
| Quality of evidence Ancillary evidence arising from systematic reviews and expert opinion. |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Consensus (2.8% of the group abstained due to potential CoI) |
Background
Intervention
See previous sections.
Available evidence
The evidence base includes two systematic reviews.
Risk of bias
Study quality assessment identified five studies at low risk of bias and 15 studies at unclear risk of bias.
Consistency
No conclusion can be drawn.
Clinical relevance and effect size
Papilla preservation flaps have been shown to lead to increased CAL gain and PD reduction as well as reduced post‐surgical recession compared with OFD.
Balance of benefit and harm
No serious adverse event has been reported after application of papilla preservation flaps in regenerative periodontal surgery performed by adequately trained clinicians. The added complexity of the surgery requires additional training.
Applicability
Anatomical considerations related to the width of the interdental space advise on the choice of the preferred flap design to access the interdental area (Cortellini, Prato, & Tonetti, 1995, 1999). Location and configuration of the intrabony defect advise on the possibility to (a) minimize flap extension (Cortellini & Tonetti, 2007; Harrel, 1999), and (b) raise a single flap or the need to fully elevate the interdental papilla (Cortellini & Tonetti, 2009; Trombelli, Farina, Franceschetti, & Calura, 2009).
7.6. Intervention: Management of furcation lesions
R3.10 What is the adequate management of molars with Class II and III furcation involvement and residual pockets?
| Evidence‐based recommendation and statement (3.10) |
|---|
|
| Supporting literature Dommisch et al. (2020); Jepsen et al. (2019) |
|
Quality of evidence Regenerative treatment: 20 RCTs (575 patients) Resective treatment: Seven observational studies (665 patients) with low quality of evidence |
|
Grade of recommendation A. Grade A—↑↑ B. Statement |
|
A. Strength of consensus Strong consensus (1.5% of the group abstained due to potential CoI) B. Strength of consensus Consensus (1.5% of the group abstained due to potential CoI) |
Background
Intervention
See previous sections. An algorithm for clinical decision‐making in the treatment by periodontal surgery of molars with furcation involvement (Class I, Class II) and residual pockets is depicted in Figure 2.
FIGURE 2.
Periodontal surgery: molars with furcation involvement (Classes II and III) and residual pockets
Available evidence
The evidence base includes 20 RTCs with 575 patients (Class II buccal/lingual mandibular and maxillary buccal furcation involvement) and seven observational studies with 665 patients (Class II interproximal and Class III). Previous systematic reviews have addressed the clinical performance of periodontal therapy of teeth with furcation involvement (Huynh‐Ba et al., 2009; Nibali et al., 2016).
Risk of bias
High quality of evidence of RCTs. Low quality of evidence for observational studies.
Consistency
Following treatment, moderate to substantial heterogeneity in the size of the effect (wide ranges of tooth survival) was observed. The reasons cannot be determined from the existing data.
Clinical relevance and effect size
Following treatment, reasonable survival rates were observed over 4–30.8 years. Overall, the observed tooth survival rates were better in Class II furcation involvement than Class III.
Balance of benefit and harm
We did not identify data about harm directly related to procedures.
Economic considerations
Simulations based on the German health system have indicated that tooth retention after complex periodontal therapy of teeth with furcation involvement is more cost‐effective than their extraction and replacement with an implant supported fixed partial denture (Schwendicke, Graetz, Stolpe, & Dorfer, 2014). A study assessing the actual cost of retention of molars in the same health system showed that cost for retaining periodontally compromised molars were minimal (Schwendicke, Plaumann, Stolpe, Dorfer, & Graetz, 2016).
Patient preferences
There is a strong patient preference for tooth retention (IQWiG, 2016).
Applicability
The guideline can be applied since it is independent of availability of materials and a segment of the dental workforce has been trained or can be trained to deliver surgical furcation treatment in the different European health systems.
R3.11 What is the adequate management of residual deep pockets associated with mandibular Class II furcation involvement?
| Evidence‐based recommendation (3.11) |
|---|
| We recommend treating mandibular molars with residual pockets associated with Class II furcation involvement with periodontal regenerative surgery. |
| Supporting literature Jepsen et al. (2019) |
| Quality of evidence 17 RCTs ≥12 months (493 patients). |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Consensus (7.6% of the group abstained due to potential CoI) |
Background
Intervention
See previous sections.
Available evidence
The evidence base includes 17 RCTs with 493 patients. The quality of the evidence for the statement was assessed according to GRADE and considered to be high. In the systematic review underlying this recommendation (Jepsen et al., 2019), a standard meta‐analysis grouping all regenerative techniques versus OFD was performed altogether with ancillary analysis. Results indicated that regenerative therapies had a significant benefit over OFD in terms of both primary and surrogate outcomes.
Risk of bias
Study quality assessment identified an unclear risk of bias for the majority of the studies. Bearing in mind that six papers failed to disclose support and seven papers reported industry funding for the research.
Consistency
Regenerative treatment consistently demonstrated added benefits (in terms of furcation improvement, horizontal bone gain, horizontal and vertical attachment gain, pocket reduction) in comparison with OFD.
Clinical relevance and effect size
The mean adjunctive benefit of a regenerative treatment is clinically relevant (1.3 mm vertical CAL and greater PPD reduction), and the effect size is significant as furcation improvement showed an odds ratio (OR) of 21 (Bayesian credible interval 5.8–69.4) in favour of regenerative techniques.
Balance of benefit and harm
The benefit of regenerative therapies to promote tooth retention outweighs the adverse events which consist mainly of local wound failure.
Ethical considerations
The perception is that regenerative therapies to promote tooth retention are preferred over tooth extraction (and replacement) or open flap debridement.
Regulatory consideration
All the studies reported FDA‐ or CE‐approved devices.
Economic considerations
Regenerative surgery has additional costs, which appear to be justified by the added benefits (furcation improvements).
Patient preferences
Minimal data are available.
Applicability
Teeth presenting with favourable patient, tooth and defect‐related conditions.
R3.12 What is the adequate management of residual deep pockets associated with maxillary buccal Class II furcation involvement?
| Evidence‐based recommendation (3.12) |
|---|
| We suggest treating molars with residual pockets associated with maxillary buccal Class II furcation involvement with periodontal regenerative surgery. |
| Supporting literature Jepsen et al. (2019) |
| Quality of evidence Three RCTs ≥12 months (82 patients). |
| Grade of recommendation Grade B—↑ |
| Strength of consensus Consensus (8.5% of the group abstained due to potential CoI) |
Background
Intervention
See previous sections.
Available evidence
The evidence base includes three RCTs with 82 patients (Garrett et al., 1997; Hugoson et al., 1995; de Santana, Gusman, & Van Dyke, 1999). The quality of the evidence for the statement was assessed according to GRADE and considered to be moderate. Of these studies only one (de Santana et al., 1999) had a clear comparison towards OFD indicating an added benefit.
Risk of bias
Study quality assessment identified an unclear/high risk of bias.
Consistency
Regenerative treatment demonstrated added benefits.
Clinical relevance and effect size
It cannot be extrapolated.
Balance of benefit and harm
The benefit of regenerative therapies to promote tooth retention outweighs the adverse events which consist mainly of local wound failure.
Ethical considerations
The expert perception is that regenerative therapies to promote tooth retention are preferred over tooth extraction or open flap debridement.
Regulatory consideration
All the studies reported FDA‐ or CE‐approved devices.
Economic considerations
Regenerative surgery has additional costs which appear to be justified by the added benefits (furcation improvements).
Patient preferences
No data are reported.
Applicability
Teeth presenting with favourable patient, tooth and defect‐related conditions.
R3.13 What is the adequate choice of regenerative biomaterials for the regenerative treatment of residual deep pockets associated with Class II mandibular and maxillary buccal furcation involvement?
| Evidence‐based recommendation (3.13) |
|---|
| We recommend treating molars with residual pockets associated with mandibular and maxillary buccal Class II furcation involvement with periodontal regenerative therapy using enamel matrix derivative alone or bone‐derived graft with or without resorbable membranes* |
| Supporting literature Jepsen et al. (2019) |
| Quality of evidence Seventeen RCTs ≥12 months (493 patients) for mandibular class II, 3 RCTs ≥12 months (82 patients) for maxillary buccal Class II, and support from indirect evidence, expert opinion. |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Simple majority (12.7% of the group abstained due to potential CoI) |
*Clinicians should select a specific biomaterial to be used to promote regeneration at intrabony defects (or class II furcation involvements) based on satisfaction of all of the following criteria (Proceedings of the, 1996 World Workshop in Periodontics, 1996): (a) availability of solid preclinical research identifying plausible mechanism(s) of action leading to periodontal regeneration; (b) human histological evidence of regeneration in the specific application; and (c) evidence of efficacy in applicable, high‐quality randomized controlled clinical trials. While there are biomaterials that satisfy all these criteria, it must be understood that many biomaterials do not meet them in spite of being CE marked or FDA‐approved/cleared.
Background
Intervention
See previous sections.
Available evidence
The evidence base includes 17 RCTs with 493 patients for mandibular Class II and three RCTs with 82 patients for maxillary buccal Class II. The quality of the evidence for the statement was assessed according to GRADE and considered to be high/moderate. In the systematic review underlying this recommendation (Jepsen et al., 2019), a Bayesian network meta‐analysis was performed to assess which treatment modalities demonstrated the highest likelihood of success. For the outcome such as HBL, the highest‐ranked groups were bone replacement graft, GTR with a bone replacement graft or enamel matrix derivative.
Risk of bias
Study quality assessment identified an unclear risk of bias for the majority of the studies. There is a mix of researcher and industry‐initiated studies.
Consistency
The procedures with the highest ranking for horizontal bone gain are bone‐replacement graft, bone‐replacement graft with resorbable membranes or enamel matrix derivative.
Clinical relevance and effect size
It cannot be extrapolated among the therapies.
Balance of benefit and harm
The benefit of regenerative therapies to promote tooth retention outweighs the adverse events which consist mainly of local wound failure.
Ethical considerations
The perception is that regenerative therapies to promote tooth retention are preferred over tooth extraction and open flap debridement.
Regulatory consideration
All the studies reported FDA‐ or CE‐approved devices.
Economic considerations
Regenerative surgery has additional costs, which appear to be justified by the added benefits (furcation improvements).
Patient preferences
Enamel matrix derivative showed less postoperative swelling and pain than non‐resorbable membranes.
Applicability
Teeth presenting with favourable patient, tooth and defect‐related conditions.
R3.14 What is the adequate management of maxillary interdental Class II furcation involvement?
| Evidence‐based recommendation (3.14) |
|---|
| In maxillary interdental Class II furcation involvement non‐surgical instrumentation, OFD, periodontal regeneration, root separation or root resection may be considered. |
| Supporting literature Dommisch et al. (2020); Huynh‐Ba et al. (2009); Jepsen, Eberhard, Herrera, and Needleman, (2002) |
| Quality of evidence Six observational studies (633 patients) with low quality of evidence for non‐regenerative approaches and two systematic reviews with low quality of evidence for regenerative treatment. |
| Grade of recommendation Grade 0—↔ |
| Strength of consensus Consensus (4.3% of the group abstained due to potential CoI) |
Background
Intervention
See previous sections.
Available evidence
Six observational studies with 633 patients (Class II interproximal).
Risk of bias
Low quality of evidence for observational studies.
Consistency
Following non‐regenerative treatment of maxillary interproximal Class II furcation involvement, moderate to substantial heterogeneity in the size of the effect (wide ranges of tooth survival) was observed. The reasons cannot be determined from the existing data.
Clinical relevance and effect size
Following non‐regenerative treatment of maxillary interproximal Class II furcation involvement, reasonable survival rates were observed over 4–30.8 years.
Balance of benefit and harm
We did not identify data about harm directly related to procedures. Regarding tooth survival, a benefit of root amputation/resection, root separation or tunnelling compared to SRP or OFD cannot be currently stated. For the individual choice of procedure, however, the clinician should consider criteria beyond class of furcation involvement (e.g. bone loss, jaw).
Economic considerations
Simulations based on the German health system have indicated that tooth retention after complex periodontal therapy of teeth with furcation involvement is more cost‐effective than their extraction and replacement with an implant supported fixed partial denture (Schwendicke et al., 2014). A study assessing the actual cost of retention of molars in the same health system showed that cost for retaining periodontally compromised molars were minimal (Schwendicke et al., 2016).
Patient preferences
There is a strong patient preference for tooth retention (IQWiG, 2016).
Applicability
The guideline can be applied since it is independent of availability of materials and a segment of the dental workforce has been trained or can be trained to deliver surgical furcation treatment in the different European health systems.
R3.15 What is the adequate management of maxillary Class III furcation involvement?
| Evidence‐based recommendation (3.15) |
|---|
| In maxillary Class III and multiple Class II furcation involvement in the same tooth nonsurgical instrumentation, OFD, tunneling, root separation or root resection may be considered. |
| Supporting literature Dommisch et al. (2020) |
| Quality of evidence Six observational studies (633 patients) with low quality of evidence. |
| Grade of recommendation Grade 0—↔ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
See previous sections.
Available evidence
Six observational studies with 633 patients.
Risk of bias
Low quality of evidence for observational studies.
Consistency
Following treatment of maxillary Class III furcation involvement, moderate to substantial heterogeneity in the size of the effect (wide ranges of tooth survival) was observed. The reasons cannot be determined from the existing data.
Clinical relevance and effect size
Following treatment of maxillary Class III furcation involvement, reasonable survival rates were observed over 4–30.8 years.
Balance of benefit and harm
We did not identify data about harm directly related to procedures. Regarding tooth survival, a benefit of root amputation/resection, root separation or tunnelling compared to SRP or OFD cannot be currently stated. For the individual choice of procedure, however, the clinician should consider criteria beyond class of furcation involvement (e.g. bone loss, jaw).
Economic considerations
Simulations based on the German health system have indicated that tooth retention after complex periodontal therapy of teeth with furcation involvement is more cost‐effective than their extraction and replacement with an implant supported fixed partial denture (Schwendicke et al., 2014). A study assessing the actual cost of retention of molars in the same health system showed that cost for retaining periodontally compromised molars were minimal (Schwendicke et al., 2016).
Patient preferences
There is a strong patient preference for tooth retention (IQWiG, 2016).
Applicability
The guideline can be applied since it is independent of availability of materials and a segment of the dental workforce has been trained or can be trained to deliver resective treatment in the different European health systems.
R3.16 What is the adequate management of mandibular Class III furcation involvement?
| Evidence‐based recommendation (3.16) |
|---|
| In mandibular Class III and multiple Class II furcation involvement in the same tooth nonsurgical instrumentation, OFD, tunneling, root separation or root resection may be considered. |
| Supporting literature Dommisch et al. (2020) |
| Quality of evidence Seven observational studies (665 patients) with low quality of evidence. |
| Grade of recommendation Grade 0—↔ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
See previous sections.
Available evidence
Seven observational studies with 665 patients (with mandibular class III furcation).
Risk of bias
Low quality of evidence for observational studies.
Consistency
Following treatment mandibular Class III furcation involvement, moderate to substantial heterogeneity in the size of the effect (wide ranges of tooth survival) was observed. The reasons cannot be determined from the existing data.
Clinical relevance and effect size
Following treatment of mandibular Class III furcation involvement, reasonable survival rates were observed over 4–30.8 years.
Balance of benefit and harm
We did not identify data about harm directly related to procedures. Regarding tooth survival a benefit of root amputation/resection, root separation or tunnelling compared to SRP or OFD cannot be currently stated. For the individual choice of procedure, however, the clinician should consider criteria beyond class of furcation involvement (e.g. bone loss, jaw).
Economic considerations
Simulations based on the German health system have indicated that tooth retention after complex periodontal therapy of teeth with furcation involvement is more cost‐effective than their extraction and replacement with an implant supported fixed partial denture (Schwendicke et al., 2014). A study assessing the actual cost of retention of molars in the same health system showed that cost for retaining periodontally compromised molars were minimal (Schwendicke et al., 2016).
Patient preferences
There is a strong patient preference for tooth retention (IQWiG, 2016).
Applicability
The guideline can be applied since it is independent of availability of materials and a segment of the dental workforce has been trained or can be trained to deliver resective treatment in the different European health systems.
8. CLINICAL RECOMMENDATIONS: SUPPORTIVE PERIODONTAL CARE
Following completion of active periodontal therapy, successfully treated periodontitis patients may fall in one of two diagnostic categories: periodontitis patients with a reduced but healthy periodontium or periodontitis patients with gingival inflammation (Caton et al., 2018; Chapple et al., 2018). These subjects remain at high risk for periodontitis recurrence/progression and require specifically designed supportive periodontal care (SPC), consisting on a combination of preventive and therapeutic interventions rendered at different intervals which should including: appraisal and on monitoring of systemic and periodontal health, reinforcement of oral hygiene instructions, patient motivation towards continuous risk factor control, professional mechanical plaque removal (PMPR) and localized subgingival instrumentation at residual pockets. The professional interventions, also frequently referred as periodontal maintenance or supportive periodontal therapy, will require a structured recall system with visits customized to the patient needs, usually requiring 45‐ to 60‐min appointments. SPC also includes individual behaviours, since patients in SPC should be compliant with the recommended oral hygiene regimens and healthy lifestyles.
8.1. Supportive periodontal care: preliminary considerations
R4.1 At what intervals should supportive periodontal care visits be scheduled?
| Expert consensus‐based recommendation (4.1) |
|---|
| We recommend that supportive periodontal care visits should be scheduled at intervals of 3 to a maximum of 12 months and ought to be tailored according to patient's risk profile and periodontal conditions after active therapy. |
| Supporting literature Polak et al. (2020), Ramseier et al. (2019), Sanz et al. (2015), Trombelli et al. (2020), Trombelli et al. (2015) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Although not addressed directly in the systematic reviews underlying this guideline, different evidence supports the concept of defined intervals to perform SPC visit every 3–4 months are recommended in studies selected by Trombelli et al. (2020).
SPC every 3 months may be sufficient to control periodontitis progression after periodontal surgery (Polak et al., 2020).
In addition, the conclusions of the 2014 European Workshop on Prevention, based on the review by Trombelli et al. (2015), concluded that the recommended interval ranges 2–4 times per year and that it could be optimized if it is tailored according to patient's risk (Sanz et al., 2015).
A recent study (Ramseier et al., 2019), over 883 patients, reflected on the importance of SPC and the factors involved in its success.
R4.2 Is adherence to supportive periodontal care important?
| Expert consensus‐based recommendation (4.2) |
|---|
| We recommend that adherence to supportive periodontal care should be strongly promoted, since it is crucial for long‐term periodontal stability and potential further improvements in periodontal status. |
| Supporting literature Costa et al. (2014), Sanz et al. (2015), Trombelli et al. (2015) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Although not addressed directly in the systematic reviews underlying this guideline, different evidence supports the importance of complying with SPC visit, in which PMPR is performed:
Greater rates of tooth loss and disease progression in patients with irregular compliance, versus patients with regular compliance (Costa et al., 2014).
The conclusions of the 2014 European Workshop on Prevention, based on the review by Trombelli et al. (2015), concluded that compliance with the preventive professional intervention is crucial, based also on retrospective observational studies (Sanz et al., 2015).
8.2. Intervention: Supragingival dental biofilm control (by the patient)
R4.3 Are oral hygiene instructions important? How should they be performed?
| Expert consensus‐based recommendation (4.3) |
|---|
| We recommend repeated individually tailored instructions in mechanical oral hygiene, including interdental cleaning, in order to control inflammation and avoid potential damage for patients in periodontal SPC. |
| Supporting literature Slot et al. (2020) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
All surfaces exposed to the formation of intraoral biofilm have to be cleaned mechanically. Some of them will not be reached by toothbrushes even under optimized conditions. Interproximal cleaning therefore is essential in order to maintain interproximal gingival health, in particular for secondary prevention. It may be achieved using different devices, primarily inter‐dental brushes (IDB, which are not single‐tufted brushes), rubber/elastomeric cleaning sticks, wood sticks, oral irrigators and floss. However, all devices have the potential of side effects and their use has to be monitored not only with respect to efficacy but also with respect to early signs of trauma (e.g. onset of non‐carious cervical lesions).
Available evidence
Due to the scarcity of studies that met the inclusion criteria for each of the oral hygiene devices and the low certainty of the resultant evidence, no strong “evidence based” conclusion can be drawn concerning any specific oral hygiene device for patient self‐care in periodontal maintenance. The evidence that emerged from the search provided 16 papers reporting on 13 CCTs/RCTs, which included 17 comparisons. The differences of powered versus manual toothbrushes were evaluated in five comparisons, an interdental device was used as an adjunct to toothbrushing in five comparisons, and seven comparisons evaluated two different interdental devices. In total, the studies evaluated 607 patients.
Risk of bias
Study quality assessment identified one study at low risk of bias and 10 studies at high risk and two of an unclear risk of bias.
Consistency
The summary of findings table shows that the body of evidence is rather consistent.
Clinical relevance and effect size
Variable, depending on the comparisons established.
Balance of benefit and harm
The adverse events were not evaluated. There is a moderate risk of trauma due to the use of interdental cleaning devices, when not used properly. Therefore, individual instruction and adoption to the individual situation by professionals are crucial. In any case, the benefits overweigh the risks by far.
Economic considerations
A manual toothbrush is less expensive than a power toothbrush. Interdental brushes and oral irrigators are more expensive than dental floss, wood sticks and rubber and silicon interdental bristle cleaners.
Patient preferences
No data on patient preference arrive from the current review.
Applicability
The guideline can be applied to patients attending a periodontal maintenance program. There is an abundance of mechanical oral hygiene products available.
R4.4 How should we choose an appropriate design of manual, powered toothbrushes and interdental cleaning devices?
| Expert consensus‐based recommendation (4.4) |
|---|
| We recommend taking into account patients' needs and preferences when choosing a toothbrush design, and when choosing an interdental brush design. |
| Supporting literature Slot et al. (2020) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus (6.9% of the group abstained due to potential CoI) |
Background
Intervention
See previous section.
Available evidence
Scarcity or a lack of evidence does not necessarily imply that products may not be effective. Dental care professionals in clinical practice should tailor the best oral hygiene devices and methods according to patients' skill levels and preferences because patient acceptance is crucial for sustained long‐term use (Steenackers, Vijt, Leroy, De Vree, & De Boever, 2001). Clinical evidence indicates that the efficacy of interdental brushes depends on the relation between the size of the brush and the size and shape of the interdental space. Interdental spaces underlay a high variety regarding size and morphology, and interdental brushes have to be selected specific to the individual interdental space. The number of devices has to be limited to a certain number with respect to the ability of the patient to cope with this diversity. To reach this goal, compromises have to be found to achieve the individual optimum.
R4.5 Should we recommend a powered or a manual toothbrush?
| Evidence‐based recommendation (4.5) |
|---|
| The use of a powered toothbrush may be considered as an alternative to manual tooth brushing for periodontal maintenance patients. |
| Supporting literature Slot et al. (2020) |
| Quality of evidence Five RCTs (216 patients) with high risk of bias |
| Grade of recommendation Grade 0—↔ |
| Strength of consensus Strong consensus (22.5% of the group abstained due to potential CoI) |
Background
Intervention
See previous sections.
Available evidence
Based on the evidence from the systematic reviews underlying this guideline, toothbrushing is effective in reducing levels of dental plaque (Van der Weijden & Slot, 2015). Toothbrushes vary in size, design and the length, hardness and arrangement of the bristles. Some manufacturers have claimed superiority in modifications such as bristle placement, length and stiffness. Powered toothbrushes with various mechanical motions and features are available. The evidence that emerged from the search provided eight papers describing five CCT/RCT comparisons. In total, the studies evaluated 216 patients. The quality of the evidence for the statement was assessed according to GRADE.
Risk of bias
Study quality assessment showed that all studies at high risk of bias.
Consistency
The summary of findings table shows that the body of evidence is rather consistent.
Clinical relevance and effect size
No differences could be found. The statistically established clinical evidence was calculated for one study and showed no clinically relevant effect size.
Balance of benefit and harm
The adverse events were not evaluated.
Economic considerations
A manual toothbrush is less expensive than a power toothbrush.
Patient preferences
No data on patient preference arrive from the current review.
Applicability
The guideline can be applied to patients attending a periodontal maintenance program. There is an abundance of toothbrushes available.
R4.6 How should interdental cleaning be performed?
| Evidence‐based recommendation (4.6) |
|---|
| If anatomically possible, we recommend that tooth brushing should be supplemented by the use of interdental brushes. |
| Supporting literature Slot et al. (2020) |
| Quality of evidence Seven comparisons from four RCTs (290 patients) with low to unclear risk of bias |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Unanimous consensus (5.4% of the group abstained due to potential CoI) |
Background
Intervention
See previous sections.
Available evidence
The underlying systematic review (Slot et al., 2020) found evidence for a significantly better cleaning effect of interdental cleaning devices as adjuncts to tooth brushing alone, and a significantly better cleaning effect of interdental brushes than of flossing. Both the descriptive analysis and the NMA indicate that IDBs are the first choice for periodontal maintenance patients. Seven comparisons from four RCTs (290 patients) were identified.
Risk of bias
Low to unclear.
Consistency
High.
Clinical relevance and effect size
Considered as clinically relevant.
Balance of benefit and harm
There is a moderate risk of trauma due to the use of interdental brushes, when not used properly. Therefore, individual instruction and adaptation to the individual situation by professionals are crucial. In any case, the benefits overweigh the risks by far.
Economic considerations
Not considered.
Patient preferences
There is clinical evidence supporting that patients with open interdental spaces prefer the use of interdental brushes over the use of dental floss.
Applicability
The guideline can be applied since appropriate quantities and varieties of interdental brushes are available on the European market.
R4.7 What is the value of dental flossing for interdental cleaning in periodontal maintenance patients?
| Evidence‐based recommendation (4.7) |
|---|
| We do not suggest flossing as the first choice for interdental cleaning in periodontal maintenance patients. |
| Supporting literature Slot et al. (2020) |
| Quality of evidence Six comparisons from four RCTs (162 patients) with unclear to high risk of bias |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Consensus (5.6% of the group abstained due to potential CoI) |
Background
Intervention
See previous sections.
Available evidence
The underlying systematic review (Slot et al., 2020) found evidence for a significantly better cleaning effect of interdental brushes than of flossing. Both the descriptive analysis and the NMA indicate that IDBs are the first choice for periodontal maintenance patients. Six comparisons from four RCTs (162 patients) were identified.
Risk of bias
High to unclear.
Consistency
High.
Clinical relevance and effect size
Considered as clinically relevant.
Balance of benefit and harm
There is a moderate risk of trauma due to the use of interdental brushes or flossing, when not used properly. Therefore, individual instruction and adaptation to the individual situation by professionals are crucial.
Economic considerations
Not considered.
Patient preferences
There is clinical evidence supporting that patients with open interdental spaces prefer the use of interdental brushes over the use of dental floss.
Applicability
The guideline can be applied since appropriate quantities and varieties of interdental brushes are available on the European market.
R4.8 What is the value of other interdental devices for interdental cleaning in periodontal maintenance patients?
| Expert consensus‐based recommendation (4.8) |
|---|
| In interdental areas not reachable by toothbrushes, we suggest supplementing tooth brushing with the use of other interdental cleaning devices in periodontal maintenance patients. |
| Supporting literature Slot et al. (2020) |
| Grade of recommendation Grade B—↑ |
| Strength of consensus Consensus (4.1% of the group abstained due to potential CoI) |
Background
Intervention
Other interdental cleaning devices include rubber/elastomeric cleaning sticks, wood sticks, an oral irrigator or dental floss. Although there are very small and fine interdental brushes available on the market, it must be realized that not all interdental spaces are readily accessible with interdental brushes.
Available evidence
The underlying systematic review (Slot et al., 2020) identified three RCTs assessing the use of an adjunctive oral irrigator: two out of three studies demonstrated a significant effect of the irrigator on measures of gingival inflammation, but not on plaque scores. Rubber/elastomeric cleaning sticks are a relatively newly developed instruments with an increasing market share, and there only little evidence available on gingivitis patients that these devices are effective in reducing inflammation with no difference to interdental brushes (Abouassi et al., 2014; Hennequin‐Hoenderdos, van der Sluijs, van der Weijden, & Slot, 2018).
Risk of bias
High.
Consistency
Not evaluated.
Clinical relevance and effect size
Considered as moderate.
Balance of benefit and harm
Up to now no adverse effects have been reported.
Economic considerations
Not considered.
Patient preferences
Rubber/elastomeric cleaning sticks are highly accepted by patients as are oral irrigators.
Applicability
The guideline can be applied since appropriate quantities and varieties of interdental cleaning devices are available on the European market.
R4.9 What additional strategies in motivation are useful?
| Expert consensus‐based recommendation (4.9) |
|---|
| We recommend utilizing the “First Step of Therapy” section of this guideline. |
| Supporting literature Carra et al. (2020) |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Background information and the discussion of additional factors can be found in the section dealing with patients in active periodontal therapy (first step of therapy).
8.3. Intervention: Adjunctive therapies for gingival inflammation
R4.10 What is the value of adjunctive antiseptics/chemotherapeutic agents for the management of gingival inflammation?
| Expert consensus‐based recommendation (4.10) |
|---|
| The basis of the management of gingival inflammation is self‐performed mechanical removal of biofilm. Adjunctive measures, including antiseptic, may be considered in specific cases, as part of a personalized treatment approach. |
| Supporting literature Figuero, Roldan, et al. (2019) |
| Grade of recommendation Grade 0—↔ |
| Strength of consensus Consensus (11.8% of the group abstained due to potential CoI) |
Background
Intervention
In order to control gingival inflammation during periodontal maintenance, the adjunctive use of some agents has been proposed. These agents are mainly antiseptics agents, and can be delivered as dentifrices, as mouth rinses or both.
Available evidence
A systematic review (Figuero, Roldan, et al., 2019) was conducted, aiming to identify RCTs of, at least, 6 months of follow‐up, in treated periodontitis patients or in gingivitis patients, in which antiseptics, prebiotics, probiotics, anti‐inflammatory agents and antioxidant micronutrients were used as adjuncts to mechanical supragingival biofilm control. For antiseptic agents, the impact in the primary outcome, changes in gingival indices (analysed in 52 studies with 72 comparisons, including 5,376 test and 3,693 control patients), was statistically significant (p < .001) and the additional reduction, expressed as standardized weighted mean difference (S‐WMD), was −1.3 (95% CI [−1.489; −1.047]), with significant heterogeneity (p < .001). In treated periodontitis patients, analysed in 13 studies with 16 comparisons, including 1,125 test and 838 control patients, the impact was statistically significant (p < .001) and the additional reduction, expressed as S‐WMD, was −1.564 (95% CI [−2.197; −0.931]), with significant heterogeneity (p < .001). No conclusions could be made for other, non‐antiseptic, agents, since only one study was identified. Longer‐term studies in treated periodontitis patients are also relevant to assess periodontal stability. In the systematic review (Figuero, Roldan, et al., 2019), four long‐term studies (1.5–3 years) were identified, and no significant impact was observed for gingival indices. However, a 3‐year study demonstrated significant benefits in terms of frequency of deep periodontal pockets and in the number of sites that exhibited additional attachment and bone loss (Rosling et al., 1997).
Risk of bias
The great majority of these studies were industry‐funded, and there was a high risk of bias both within and across studies.
Consistency
Highly consistent across studies, 72 comparisons were included in the primary analysis.
Clinical relevance and effect size
Considered as clinically relevant.
Balance of benefit and harm
At least 31 studies assessed adverse events and PROMs and staining was the only relevant finding.
Economic considerations
The issue has not been addressed. For dentifrices, it may not be relevant, since a dentifrice has to be used combined with mechanical tooth brushing; for mouth rinse, the extra cost should be taken into consideration. It should also be noted that the evidence base contains studies using products that may no longer be available.
Patient preferences
Both dentifrices and mouth rinses are widely accepted by the population.
Applicability
Demonstrated with studies testing large groups from the general population. The adjunctive use of some agents has been proposed in those subjects who are not able to effectively remove supragingival biofilms by the sole use of mechanical procedures, but there is no direct evidence to support this statement.
R4.11 Should adjunctive chemotherapeutics be recommended for patients in supportive periodontal care?
| Evidence‐based recommendation/statement (4.11) |
|---|
|
A. The use of adjunctive antiseptics may be considered in periodontitis patients in supportive periodontal care in helping to control gingival inflammation, in specific cases. B. We do not know whether other adjunctive agents (such as probiotics, prebiotics, anti‐inflammatory agents, antioxidant micronutrients) are effective in controlling gingival inflammation in patients in supportive periodontal care. |
| Supporting literature Figuero, Roldan et al. (2019) |
| Quality of evidence 73 RCTs with, at least, 6‐month follow‐up |
|
A. Grade of recommendation Grade 0—↔ There is a need to define the term of use (e.g. 6 months?) Adverse effects should be taken into account. B. Grade of recommendation Grade 0—Statement: unclear, additional research needed |
| Strength of consensus Consensus (6.9% of the group abstained due to potential CoI) |
Background
Intervention
In order to control gingival inflammation during supportive periodontal care, the adjunctive use of some agents has been proposed. These agents are mainly antiseptics but some other agents, such as probiotics, prebiotics, anti‐inflammatory agents and antioxidant micronutrients, can be found in the literature. These products are mainly delivered as dentifrices or mouth rinses.
Available evidence
See also previous section. The adjunctive use of antiseptic agents has been proposed in those subjects who are not able to effectively remove supragingival biofilms by the sole use of mechanical procedures. Actually, the recommendations of the XI European Workshop in Periodontology (2014) highlighted that (Chapple et al., 2015) “For the treatment of gingivitis and where improvements in plaque control are required, adjunctive use of anti‐plaque chemical agents may be considered. In this scenario, mouth rinses may offer greater efficacy but require an additional action to the mechanical oral hygiene regime”. Recommending adjunctive antiseptics, to mechanical supragingival biofilm control, in a specific patient group, instead in the general population, is plausible, but there is no supporting evidence to defend it. Most studies assessing the adjunctive benefits of antiseptic formulations have been performed in general populations, with statistically significant benefits in plaque and gingival indices (Serrano, Escribano, Roldan, Martin, & Herrera, 2015). Therefore, different factors may be considered when deciding whether to recommend the use of an adjunctive agent to control gingival inflammation in patients in supportive periodontal care. It is noted that all patients need to use a toothbrush with a fluoride toothpaste. However, in those subjects who are not able to effectively control supragingival biofilms and/or gingival inflammation by the sole use of mechanical procedures, a decision is then made whether or not to utilise a toothpaste and/or a mouth rinse that contains a specific active agent (in addition to fluoride). This decision would follow a personalized approach to patient care and would need to consider two aspects:
Local factors consider levels of gingival inflammation related to plaque level, accessibility for cleaning, anatomical factors, etc.
General factors consider systemic factors, general health status, frailty, limited dexterity, etc., some of which may be more relevant in elderly patients.
The most frequent delivery format for antiseptic agents is dentifrices and mouth rinses, or even they can be delivered in both, simultaneously. The obvious benefit of dentifrice delivery is that no other delivery format is needed, and a dentifrice is going to be used anyway. Mouth rinse delivery offers a better distribution around the mouth (Serrano et al., 2015) and better pharmacokinetic properties (Cummins & Creeth, 1992). Some evidence suggests that the adjunctive use of mouth rinses may provide better outcomes than that of dentifrices. However, the evidence is conflictive and significant differences were only observed for the secondary outcome (Figuero, Roldan, et al., 2019). In addition, direct comparisons between similar agents/formulations, delivered either as dentifrice or mouth rinse, are not available.
The decision to select a specific toothpaste or a mouth rinse should be also based on a combination of factors:
Patient preferences including cost, taste, etc.
Unwanted effects including staining, burning sensation during use, etc.
Potential negative impacts on beneficial aspects of the oral microbiome highlighted in recent evidence (e.g. impact on nitric oxide pathway) (Bescos et al., 2020).
Potential negative impacts on blood pressure: one short‐term (7‐days) study suggested a non‐statistically significant “trend” for chlorhexidine mouth rinse to cause a small elevation in systolic blood pressure from 103 mmHg to 106 mHg (Bescos et al., 2020). The clinical significance of this is unknown.
Depending on the specific agent already selected, a decision must be made regarding their frequency and duration of use.
R4.12 Which antiseptic is the most effective in dentifrices?
| Evidence‐based recommendation (4.12) |
|---|
| If an antiseptic dentifrice formulation is going to be adjunctively used, we suggest products containing chlorhexidine, triclosan‐copolymer and stannous fluoride‐sodium hexametaphosphate for the control of gingival inflammation, in periodontitis patients in supportive periodontal care. |
| Supporting literature Escribano et al. (2016); Figuero, Herrera, et al. (2019); Figuero, Roldan, et al. (2019); Serrano et al. (2015) |
| Quality of evidence Twenty‐nine RCTs with, at least, 6‐month follow‐up |
| Grade of recommendation Grade B—↑ |
| Strength of consensus Consensus (17.4% of the group abstained due to potential CoI) |
Background
Intervention
In order to control gingival inflammation during supportive periodontal care, the adjunctive use of some agents has been proposed. These products can be delivered as dentifrices.
Available evidence
In the systematic review (Figuero, Roldan, et al., 2019), the adjunctive use of 14 different dentifrice formulations was evaluated for controlling gingival inflammation, with a clear heterogeneity in the number of available studies for each product. The magnitude of effect in gingival indices changes, in formulations with more than one study available, was headed by stannous fluoride with sodium hexametaphosphate (n = 2, S‐WMD = −1.503), followed by triclosan and copolymer (n = 18, S‐WMD = −1.313), and chlorhexidine (n = 2, S‐WMD = −1.278, not statistically significant), although comparing the formulations was not a specific objective of the review. Effects on plaque levels were best with chlorhexidine at high concentrations (n = 3, S‐WMD = −1.512) and triclosan and copolymer (n = 23, S‐WMD = −1.164). In a previously published network meta‐analyses, chlorhexidine and triclosan and copolymer were the most effective agents for plaque reduction, but no clear differences were observed for gingival index control (Escribano et al., 2016; Figuero, Herrera, et al., 2019).
Additional factors have been discussed in the overall evaluation of adjunctive agents.
R4.13 Which antiseptic is the most effective in mouth rinses?
| Evidence‐based recommendation (4.13) |
|---|
| If an antiseptic mouth rinse formulation is going to be adjunctively used, we suggest products containing chlorhexidine, essential oils and cetylpyridinium chloride for the control of gingival inflammation, in periodontitis patients in supportive periodontal care. |
| Supporting literature Escribano et al. (2016); Figuero, Herrera, et al. (2019); Figuero, Roldan, et al. (2019); Serrano et al. (2015) |
| Quality of evidence CoE Class I—24 RCTs with, at least, 6‐month follow‐up |
| Grade of recommendation Grade B—↑ |
| Strength of consensus Consensus (17.9% of the group abstained due to potential CoI) |
Background
Intervention
In order to control gingival inflammation during supportive periodontal care, the adjunctive use of some agents has been proposed. These products can be delivered as mouth rinses.
Available evidence
In the systematic review (Figuero, Roldan, et al., 2019), the adjunctive use of 11 different mouth rinse formulations were evaluated for controlling gingival inflammation, with a clear heterogeneity in the number of available studies for each product. The magnitude of effect in gingival indices changes, in formulations with more than one study available, ranged from S‐WMD = −2.248 (essential oils, n = 10), to S‐WMD = −1.499 (cetylpyridinium chloride, n = 5), and to S‐WMD = −1.144 (chlorhexidine at high concentrations, n = 5), although comparing the formulations was not a specific objective of the review. In a previously published network meta‐analyses (a statistical technique which allows the integration of data from direct and indirect comparisons, namely treatments compared among trials through a common comparator treatment), chlorhexidine and essential oil mouth rinses were ranked as the most efficacious agents in terms of changes in plaque and gingival indices (Escribano et al., 2016; Figuero, Herrera, et al., 2019).
Additional factors have been discussed in the overall evaluation of adjunctive agents.
8.4. Intervention: Supragingival dental biofilm control (professional)
R4.14 What is the value of professional mechanical plaque removal (PMPR) as part of SPC?
| Expert consensus‐based recommendation (4.14) |
|---|
| We suggest performing routine professional mechanical plaque removal (PMPR) to limit the rate of tooth loss and provide periodontal stability/improvement, as part of a supportive periodontal care program. |
| Supporting literature Trombelli et al. (2015) |
| Grade of recommendation Grade B—↑ |
| Strength of consensus Strong consensus (1.4% of the group abstained due to potential CoI) |
Background
Intervention
Professional mechanical plaque removal (PMPR) administered on a routine basis (i.e. at specific, predetermined intervals) as an integral part of supportive periodontal care has been shown to result in low rates of tooth loss and limited attachment level changes in both the short‐ and long‐term in patients treated for periodontitis (Heasman, McCracken, & Steen, 2002; Trombelli et al., 2015). In most of the studies, PMPR in SPC was often combined with other procedures (e.g. reinforcement of oral hygiene instruction, additional active treatment at sites showing disease recurrence), thus making it difficult to isolate information on the magnitude of the mere effect of PMPR on tooth survival and stability of periodontal parameters (Trombelli et al., 2015).
Available evidence
This issue has not been directly addressed in the systematic reviews prepared for this Workshop; however, ample evidence is available to support this statement. It has been demonstrated that professional mechanical plaque removal (PMPR), performed at defined intervals, together with the other interventions of supportive periodontal care may result in lower rates of tooth loss and attachment level changes. In a systematic review (Trombelli et al., 2015), presented at the 2014 European Workshop, a weighted mean yearly rate of tooth loss of 0.15 and 0.09 for follow‐up of 5 years or 12–14 years, respectively, was reported; the correspondent figures for mean clinical attachment loss lower than 1 mm at follow‐up ranging from 5 to 12 years. Information from this review, and also from other systematic reviews, collectively supports that patients with a history of treated periodontitis can maintain their dentition with limited variations in periodontal parameters when regularly complying with a SPC regimen based on routine PMPR (Sanz et al., 2015).
Risk of bias
The methodological quality was assessed with a specifically designed scale for the evaluation of non‐randomized observational studies, with a quality level ranging from 3 to 7, in a 9‐point scale, with 9 representing the highest quality (lowest risk of bias).
Consistency
Although no meta‐analysis was possible, the primary outcome (tooth loss) was reported in 12 studies, showing no or low incidence. Clinical attachment level (CAL) changes were reported in 10 studies, which consistently showed limited modifications in CAL, frequently as a slight CAL loss.
Clinical relevance and effect size
A weighted mean yearly rate of tooth loss of 0.15 for follow‐up of 5 years, and 0.09 for follow‐up of 12–14 years, can be considered as relevant.
Balance of benefit and harm
PROMs were not reported in the included studies.
Economic considerations
Ethics and legal aspects are not relevant for this intervention; economic aspects have not been frequently addressed. In a study in a private practice in Norway, it was demonstrated that regular maintenance was associated with less tooth loos than not regular maintenance, with follow‐ups of 16–26 years; the yearly cost of maintaining a tooth was estimated in 20.2 euro (Fardal & Grytten, 2014).
Patient preferences
Demonstrated with compliance in long‐term studies.
Applicability
Demonstrated with studies testing large groups from the general population.
R4.15 Should alternative methods be used for professional mechanical plaque removal (PMPR) in supportive periodontal care?
| Evidence‐based recommendation (4.15) |
|---|
| We suggest not to replace conventional professional mechanical plaque removal (PMPR) with the use of alternative methods (Er:YAG laser treatment) in supportive periodontal care. |
| Supporting literature Trombelli et al. (2020) |
| Quality of evidence One RCT |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Strong consensus (1.4% of the group abstained due to potential CoI) |
Background
Intervention
The systematic review (Trombelli et al., 2015) was retrieving available RCTs on any given alternative intervention to conventional PMPR (the latter including supragingival and/or subgingival removal of plaque, calculus and debris performed with manual and/or powered instruments) in the maintenance of periodontitis patients with a follow‐up of at least 1 year following the first administration of intervention/control treatment.
Available evidence
In the systematic review (Trombelli et al., 2020), only one RCT was identified, assessing Er:YAG laser as an alternative method to conventional PMPR. No statistically significant differences were found (Krohn‐Dale, Boe, Enersen, & Leknes, 2012).
Economic considerations
Cost‐benefit or cost‐effective analyses are missing and may be very relevant when considering this specific treatment option. The same is true for PROMs.
R4.16 Should adjunctive methods be used for professional mechanical plaque removal (PMPR) in supportive periodontal care?
| Evidence‐based recommendation (4.16) |
|---|
| We suggest not to use adjunctive methods (sub‐antimicrobial dose doxycycline, photodynamic therapy) to professional mechanical plaque removal (PMPR) in supportive periodontal care. |
| Supporting literature Trombelli et al. (2020) |
| Quality of evidence Two RCTs |
| Grade of recommendation Grade B—↓ |
| Strength of consensus Strong consensus (2.7% of the group abstained due to potential CoI) |
Background
Intervention
The systematic review (Trombelli et al., 2015) was retrieving available RCTs on any given additional intervention to conventional PMPR (the latter including supragingival and/or subgingival removal of plaque, calculus and debris performed with manual and/or powered instruments) in the maintenance of periodontitis patients with a follow‐up of at least 1 year following the first administration of intervention/control treatment.
Available evidence
In the systematic review (Trombelli et al., 2020), two RCTs were identified, one testing sub‐antimicrobial dose (20 mg b.i.d.) of doxycycline (Reinhardt et al., 2007), another evaluating photodynamic therapy (PDT) with a 0.01% methylene blue as photosensitizer and a diode laser (wavelength of 660 nm) (Carvalho et al., 2015). No statistically significant differences were observed in any study, although CAL gain was more relevant with adjunctive PDT (1.54 mm) in comparison with conventional PMPR alone (0.96 mm). The systematic review presented at this Workshop provided information, based on meta‐analysis, of the possible effects of the alternative/adjunctive methods mentioned, with no significant difference for the primary outcome (CAL changes), after 12‐month follow‐up, amounting −0.233 mm (95% CI [−1.065; 0.598; p = .351), favouring the control groups.
Economic considerations
For the adjunctive use of SDD, adverse effects and cost–benefit ratio have to be considered. For the adjunctive use of PDT, a previous systematic review (Xue et al., 2017), which included 11 RCTs, found better results for PDT, but only after 3 months, with 0.13 mm of additional impact in PPD reduction. No increase in adverse events were reported. Cost–benefit or cost‐effective analyses are missing and may be very relevant when considering this specific treatment option.
8.5. Intervention: Risk factor control
R4.17 What is the value of risk factor control in SPC?
| Expert consensus‐based recommendation (4.17) |
|---|
| We recommend risk factor control interventions in periodontitis patients in supportive periodontal care. |
| Supporting literature Ramseier et al. (2020) |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Periodontitis patients benefit from additional risk factor control interventions to improve the maintenance of periodontal stability. Interventions include patient education which be staged and adapted according to individual needs ranging from single brief advice to patient referral for advanced counselling and pharmacotherapy. Smoking and diabetes are two of the main risk factors for periodontitis, and they are currently included in the grading of periodontitis (Papapanou et al., 2018). Controlling these risk factors therefore would be critical for treatment response and for long‐term stability. In addition, other relevant factors, part a healthy life‐style counselling, are considered, including dietary counselling, physical exercise or weight loss. These interventions, together with those for tobacco cessation and diabetes control, are not direct responsibility of oral health professionals, and they may want to refer the patients to other health professionals. However, the direct/indirect role of oral health professionals in these interventions should be emphasized.
Available evidence
In the systematic review (Ramseier et al., 2020), the authors have identified 13 relevant guidelines for interventions for smoking cessation, diabetes control, physical exercise (activity), change of diet, carbohydrate (dietary sugar reduction) and weight loss. In addition, 25 clinical studies were found that assess the impact of (some of) these interventions in gingivitis/periodontitis patients. However, only some of them included patients in supportive periodontal care.
Additional factors have been discussed in the evaluation of risk factor control in patients in active periodontal therapy.
R4.18 What is the role of tobacco smoking cessation interventions in SPC?
| Evidence‐based recommendation (4.18) |
|---|
| We recommend tobacco smoking cessation interventions to be implemented in periodontitis patients in supportive periodontal care. |
| Supporting literature Ramseier et al. (2020) |
| Quality of evidence Six prospective studies with, at least, 6‐month follow‐up |
| Grade of recommendation Grade A—↑↑ |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Background information and the discussion of additional factors can be found in the section dealing with patients in active periodontal therapy.
R4.19 What is the role of promotion of diabetes control interventions in SPC?
| Expert consensus‐based recommendation (4.19) |
|---|
| We suggest promotion of diabetes control interventions in patients in maintenance therapy. |
| Supporting literature Ramseier et al. (2020) |
| Grade of recommendation Grade B—↑ |
| Strength of consensus Consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Periodontitis patients may benefit from the promotion of diabetes control interventions to improve the maintenance of periodontal stability. The promotion may consist of patient education including brief dietary counselling and possibly patient referral for glycaemic control.
Available evidence
In the systematic review (Ramseier et al., 2020), none of the identified studies was performed in patients in supportive periodontal care. Indirect evidence (see section on active periodontal therapy) suggests that diabetes control interventions ought to be implemented in supportive periodontal care patients.
Background information and the discussion of additional factors can be found in the section dealing with patients in active periodontal therapy.
R4.20 What is the role of physical exercise (activity), dietary counselling or lifestyle modifications aiming at weight loss in SPC?
| Evidence‐based recommendation/statement (4.20) |
|---|
| We do not know whether physical exercise (activity), dietary counselling or lifestyle modifications aiming at weight loss are relevant in supportive periodontal care. |
| Supporting literature Ramseier et al. (2020) |
| Grade of recommendation Grade 0—Statement: unclear, additional research needed |
| Strength of consensus Strong consensus (0% of the group abstained due to potential CoI) |
Background
Intervention
Overall evidence from the medical literature suggests that the promotion of physical exercise (activity) interventions may improve both treatment and long‐term management of non‐communicable diseases. In periodontitis patients, the promotion may consist of patient education specifically target to the patients' age and general health.
Available evidence
In the systematic review (Ramseier et al., 2020), none of the identified studies was performed in patients in supportive periodontal care.
Background information and the discussion of additional factors can be found in the section dealing with patients in active periodontal therapy (Billings et al., 2018).
CONFLICT OF INTEREST
Workshop participants filed detailed disclosure of potential conflict of interest relevant to the workshop topics, and these are kept on file. Declared potential dual commitments included having received research funding, consultant fees and speaker fee from the industries with economic interests in the interventions for prevention and therapy of Periodontitis. Those affected with potential conflict of interest abstained from vote in the specific recommendations following the required processes for S3 level clinical practice guideline. Individual potential conflict of interest forms were completed by all participants and are available on file at the European Federation of Periodontology and extracted in the Supporting Information, available online (Final Guideline‐Supporting Information_Potential conflict of interests). In addition, potential conflict of interest information of the chairs of the workshop is listed here.
Dr. Mariano Sanz (Chair) reports personal fees from Camlog implants, Colgate, Dentium Implants, Dentsply Sirona Implants, Geistlich, GSK, Klockner Implants, MIS Implants, Mozo Grau Implants, Nobel Biocare, Procter & Gamble, Straumann and Sunstar; grants from Camlog Implants, Dentaid, Dentium Implants, Dentsply Sirona Implants, Geistlich Pharma, Klockner Implants, MIS Implants, Mozo Grau Implants, Nobel Biocare, Sunstar, Straumann AG, Sweden and Martina Implants; and other support from Dentaid, outside the submitted work.
Dr. David Herrera (Chair) reports personal fees from Colgate, Dentaid, Dexcel Pharma, GSK, Johnson & Johnson, Klockner Implants, Procter & Gamble and Straumann and grants from Colgate, Dentaid, GSK, Kulzer and Zimmer‐Biomet, outside the submitted work.
Dr. Moritz Kebschull (Chair) reports personal fees from Colgate, Dexcel Pharma, Geistlich Pharma, Hu‐Friedy, NSK and Procter & Gamble and non‐financial support from Colgate, Dexcel Pharma, Geistlich Pharma, Hu‐Friedy, NSK and Procter & Gamble, outside the submitted work.
Dr. Iain Chapple (Chair) reports personal fees from Procter & Gamble and grants from GSK and Unilever, outside the submitted work. In addition, Dr. Chapple has eight patents on saliva diagnostics issued and his wife runs Oral Health Innovations that has the license for PreViser and DEPPA risk assessment software in the UK.
Dr. Sören Jepsen (Chair) reports personal fees from Colgate, Geistlich Pharma and Procter & Gamble, outside the submitted work.
Dr. Tord Berglundh (Chair) reports personal fees from Dentsply Sirona Implants and Straumann and grants from Dentsply Sirona Implants, outside the submitted work.
Dr. Anton Sculean (Chair) reports personal fees from Botiss Biomaterials, Geistlich Pharma, Oral Reconstruction Foundation, Osteology Foundation, Straumann AG, Regedent AG and Stoma and grants from Botiss Biomaterials, Geistlich Pharma, ITI Foundation, Oral Reconstruction Foundation, Osteology Foundation, Straumann AG and Regedent AG, outside the submitted work.
Dr. Maurizio Tonetti (Chair) reports personal fees from Geistlich Pharma AG, Procter & Gamble, Straumann AG, Sunstar SA anf Unilever; grants from Geistlich Pharma and Sunstar SA; and non‐financial support from Procter & Gamble, outside the submitted work.
Clinical Relevance.
Scientific rationale for the study: Implementation of the new classification of periodontitis should facilitate the use of the most appropriate preventive and therapeutic interventions, depending on the stage and grade of the disease. The choice of these interventions should be made following a rigorous evidence‐based decision‐making process.
Principal findings: This guideline has been developed using strict validated methodologies for assuring the best available evidence on the efficacy of the interventions considered and the most appropriate recommendations based on a structured consensus process, including a panel of experts and representatives from key stakeholders.
Practical implications: The application of this S3 Level Clinical Practice Guideline will allow a homogeneous and evidence‐based approach to the management of Stage I–III periodontitis.
ACKNOWLEDGEMENTS
The authors express their gratitude to all reviewers involved in the preparation of the systematic reviews. In addition, the organizations which accepted to participate in the guideline development process are also kindly and sincerely acknowledged: European Federation of Conservative Dentistry, European Association of Dental Public Health, European Society for Endodontology, European Prosthodontic Association, Council of European Dentists, European Dental Hygienists' Federation, European Dental Students' Association and Platform for Better Oral Health in Europe.
Appendix 1.
Workshop Participants
Anne Merete Aass, Mario Aimetti, Georgios Belibasakis, Juan Blanco, Ellen Bol‐van den Hil, Nagihan Bostanci, Darko Bozic, Philippe Bouchard, Nurcan Buduneli, Francesco Cairo, Elena Calciolari, Maria Clotilde Carra, Pierpaolo Cortellini, Jan Cosyn, Francesco D'Aiuto, Bettina Dannewitz, Monique Danser, Korkud Demirel, Jan Derks, Massimo de Sanctis, Thomas Dietrich, Christof Dörfer, Henrik Dommisch, Nikos Donos, Kenneth Eaton, Peter Eickholz, Elena Figuero, William Giannobile, Moshe Goldstein, Filippo Graziani, Phophi Kamposiora, Lise‐Lotte Kirkevang, Thomas Kocher, Eija Kononen, Bahar Eren Kuru, France Lambert, Luca Landi, Nicklaus Lang, Bruno Loos, Rodrigo Lopez, Pernilla Lundberg, Eli Machtei, Phoebus Madianos, Conchita Martín, Paula Matesanz, Paulo Melo, Jörg Meyle, Ana Molina, Eduardo Montero, Jose Nart, Ian Needleman, Luigi Nibali, Panos Papapanou, Andrea Pilloni, David Polak, Ioannis Polyzois, Philip Preshaw, Marc Quirynen, Christoph Ramseier, Stefan Renvert, Giovanni Salvi, Ignacio Sanz‐Sánchez, Lior Shapira, Dagmar Else Slot, Andreas Stavropoulos, Xavier Struillou, Jean Suvan, Wim Teughels, Daniela Timus, Cristiano Tomasi, Leonardo Trombelli, Fridus van der Weijden, Paula Vassallo, Clemens Walter, Nicola West, Gernot Wimmer
Methodological Consultants
Ina Kopp (chief consultant), Paul Brocklehurst, Jan Wennström
Workshop Organization
European Federation of Periodontology
Scientific societies involved in the guideline development process
European Federation of Conservative Dentistry
European Association of Dental Public Health
European Society for Endodontology
European Prosthodontic Association
Other organizations involved in the guideline development process
Council of European Dentists
European Dental Hygienists' Federation
European Dental Students' Association
Platform for Better Oral Health in Europe
Sanz M, Herrera D, Kebschull M, et al; On behalf of the EFP Workshop Participants and Methodological Consultants . Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J Clin Periodontol. 2020;47:4–60. 10.1111/jcpe.13290
EFP Workshop Participants and Methodological Consultants are presented in Appendix 1.
The copyright line for this article was changed on 12 February 2021 after original online publication.
Contributor Information
Mariano Sanz, Email: marsan@ucm.es.
EFP Workshop Participants and Methodological Consultants:
Anne Merete Aass, Mario Aimetti, Bahar Eren Kuru, Georgios Belibasakis, Juan Blanco, Ellen Bol‐van den Hil, Nagihan Bostanci, Darko Bozic, Philippe Bouchard, Nurcan Buduneli, Francesco Cairo, Elena Calciolari, Maria Clotilde Carra, Pierpaolo Cortellini, Jan Cosyn, Francesco D'Aiuto, Bettina Dannewitz, Monique Danser, Korkud Demirel, Jan Derks, Massimo de Sanctis, Thomas Dietrich, Christof Dörfer, Henrik Dommisch, Nikos Donos, Kenneth Eaton, Peter Eickholz, Elena Figuero, William Giannobile, Moshe Goldstein, Filippo Graziani, Phophi Kamposiora, Lise‐Lotte Kirkevang, Thomas Kocher, Eija Kononen, Nicklaus Lang, France Lambert, Luca Landi, Paulo Melo, Bruno Loos, Rodrigo Lopez, Pernilla Lundberg, Eli Machtei, Phoebus Madianos, Conchita Martín, Paula Matesanz, Jörg Meyle, Ana Molina, Eduardo Montero, Jose Nart, Ian Needleman, Luigi Nibali, Panos Papapanou, Andrea Pilloni, David Polak, Ioannis Polyzois, Philip Preshaw, Marc Quirynen, Christoph Ramseier, Stefan Renvert, Giovanni Salvi, Ignacio Sanz‐Sánchez, Lior Shapira, Dagmar Else Slot, Andreas Stavropoulos, Xavier Struillou, Jean Suvan, Wim Teughels, Daniela Timus, Cristiano Tomasi, Leonardo Trombelli, Fridus van der Weijden, Paula Vassallo, Clemens Walter, Nicola West, Gernot Wimmer, Ina Kopp, Paul Brocklehurst, and Jan Wennström
REFERENCES
- Abouassi, T. , Woelber, J. , Holst, K. , Stampf, S. , Doerfer, C. , Hellwig, E. , & Ratka‐Krüger, P. (2014). Clinical efficacy and patients' acceptance of a rubber interdental bristle. A randomized controlled trial. Clinical Oral Investigations, 18(7), 1873–1880. 10.1007/s00784-013-1164-3 [DOI] [PubMed] [Google Scholar]
- Adam, O. , & Laufs, U. (2008). Antioxidative effects of statins. Archives of Toxicology, 82(12), 885–892. 10.1007/s00204-008-0344-4 [DOI] [PubMed] [Google Scholar]
- Araujo, A. A. , Pereira, A. , Medeiros, C. , Brito, G. A. C. , Leitao, R. F. C. , Araujo, L. S. , … Araujo Junior, R. F. (2017). Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS ONE, 12(8), e0183506 10.1371/journal.pone.0183506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badran, Z. , Kraehenmann, M. A. , Guicheux, J. , & Soueidan, A. (2009). Bisphosphonates in periodontal treatment: A review. Oral Health and Preventive Dentistry, 7(1), 3–12. [PubMed] [Google Scholar]
- Balshem, H. , Helfand, M. , Schunemann, H. J. , Oxman, A. D. , Kunz, R. , Brozek, J. , … Guyatt, G. H. (2011). GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology, 64(4), 401–406. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- Bescos, R. , Ashworth, A. , Cutler, C. , Brookes, Z. L. , Belfield, L. , Rodiles, A. , & Hickson, M. (2020). Effects of Chlorhexidine mouthwash on the oral microbiome. Science Reports, 10(1), 5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings, M. , Holtfreter, B. , Papapanou, P. N. , Mitnik, G. L. , Kocher, T. , & Dye, B. A. (2018). Age‐dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP‐TREND 2008 to 2012. Journal of Clinical Periodontology, 45(Suppl 20), S130–S148. 10.1111/jcpe.12944 [DOI] [PubMed] [Google Scholar]
- Broadbent, J. M. , Williams, K. B. , Thomson, W. M. , & Williams, S. M. (2006). Dental restorations: A risk factor for periodontal attachment loss? Journal of Clinical Periodontology, 33, 803–810. 10.1111/j.1600-051X.2006.00988.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst, P. R. , McKenna, G. , Schimmel, M. , Kossioni, A. , Jerkovic‐Cosic, K. , Hayes, M. , … Muller, F. (2018). How do we incorporate patient views into the design of healthcare services for older people: A discussion paper. BMC Oral Health, 18(1), 61 10.1186/s12903-018-0513-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carra, M. C. , Detzen, L. , Kitzmann, J. , Woelber, J. P. , Ramseier, C. A. , & Bouchard, P. (2020). Promoting behavioural changes to improve oral hygiene in patients with periodontal diseases: A systematic review. Journal of Clinical Periodontology, 47(Suppl 22), 72–89. 10.1111/jcpe.13234 [DOI] [PubMed] [Google Scholar]
- Carvalho, V. F. , Andrade, P. V. , Rodrigues, M. F. , Hirata, M. H. , Hirata, R. D. , Pannuti, C. M. , … Conde, M. C. (2015). Antimicrobial photodynamic effect to treat residual pockets in periodontal patients: A randomized controlled clinical trial. Journal of Clinical Periodontology, 42(5), 440–447. 10.1111/jcpe.12393 [DOI] [PubMed] [Google Scholar]
- Caton, J. G. , Armitage, G. , Berglundh, T. , Chapple, I. L. C. , Jepsen, S. , Kornman, K. S. , … Tonetti, M. S. (2018). A new classification scheme for periodontal and peri‐implant diseases and conditions ‐ Introduction and key changes from the 1999 classification. Journal of Clinical Periodontology, 45(Suppl 20), S1–S8. 10.1111/jcpe.12935 [DOI] [PubMed] [Google Scholar]
- Caton, J. G. , Ciancio, S. G. , Blieden, T. M. , Bradshaw, M. , Crout, R. J. , Hefti, A. F. , … Walker, C. (2000). Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. Journal of Periodontology, 71(4), 521–532. 10.1902/jop.2000.71.4.521 [DOI] [PubMed] [Google Scholar]
- Caton, J. G. , Ciancio, S. G. , Blieden, T. M. , Bradshaw, M. , Crout, R. J. , Hefti, A. F. , … Walker, C. (2001). Subantimicrobial dose doxycycline as an adjunct to scaling and root planing: Post‐treatment effects. Journal of Clinical Periodontology, 28(8), 782–789. 10.1034/j.1600-051x.2001.280810.x [DOI] [PubMed] [Google Scholar]
- Chapple, I. L. C. , Mealey, B. L. , Van Dyke, T. E. , Bartold, P. M. , Dommisch, H. , Eickholz, P. , … Yoshie, H. (2018). Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Clinical Periodontology, 45(Suppl 20), S68–S77. 10.1111/jcpe.12940 [DOI] [PubMed] [Google Scholar]
- Chapple, I. L. C. , Van der Weijden, F. , Doerfer, C. , Herrera, D. , Shapira, L. , Polak, D. , … Graziani, F. (2015). Primary prevention of periodontitis: Managing gingivitis. Journal of Clinical Periodontology, 42(Suppl 16), S71–S76. 10.1111/jcpe.12366 [DOI] [PubMed] [Google Scholar]
- Cicek Ari, V. , Ilarslan, Y. D. , Erman, B. , Sarkarati, B. , Tezcan, I. , Karabulut, E. , … Berker, E. (2016). Statins and IL‐1beta, IL‐10, and MPO levels in gingival crevicular fluid: Preliminary results. Inflammation, 39(4), 1547–1557. 10.1007/s10753-016-0390-7 [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , Buti, J. , Pini Prato, G. , & Tonetti, M. S. (2017). Periodontal regeneration compared with access flap surgery in human intra‐bony defects 20‐year follow‐up of a randomized clinical trial: Tooth retention, periodontitis recurrence and costs. Journal of Clinical Periodontology, 44(1), 58–66. 10.1111/jcpe.12638 [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , Prato, G. P. , & Tonetti, M. S. (1995). The modified papilla preservation technique. A new surgical approach for interproximal regenerative procedures. Journal of Periodontology, 66(4), 261–266. 10.1902/jop.1995.66.4.261 [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , Prato, G. P. , & Tonetti, M. S. (1999). The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. International Journal of Periodontics and Restorative Dentistry, 19(6), 589–599. [PubMed] [Google Scholar]
- Cortellini, P. , & Tonetti, M. S. (2007). A minimally invasive surgical technique with an enamel matrix derivative in the regenerative treatment of intra‐bony defects: A novel approach to limit morbidity. Journal of Clinical Periodontology, 34(1), 87–93. 10.1111/j.1600-051X.2006.01020.x [DOI] [PubMed] [Google Scholar]
- Cortellini, P. , & Tonetti, M. S. (2009). Improved wound stability with a modified minimally invasive surgical technique in the regenerative treatment of isolated interdental intrabony defects. Journal of Clinical Periodontology, 36(2), 157–163. 10.1111/j.1600-051X.2008.01352.x [DOI] [PubMed] [Google Scholar]
- Costa, F. O. , Lages, E. J. , Cota, L. O. , Lorentz, T. C. , Soares, R. V. , & Cortelli, J. R. (2014). Tooth loss in individuals under periodontal maintenance therapy: 5‐year prospective study. Journal of Periodontal Research, 49(1), 121–128. 10.1111/jre.12087 [DOI] [PubMed] [Google Scholar]
- Cummins, D. , & Creeth, J. E. (1992). Delivery of antiplaque agents from dentifrices, gels, and mouthwashes. Journal of Dental Research, 71(7), 1439–1449. 10.1177/00220345920710071601 [DOI] [PubMed] [Google Scholar]
- da Costa, L. , Amaral, C. , Barbirato, D. D. S. , Leao, A. T. T. , & Fogacci, M. F. (2017). Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: A meta‐analysis. Journal of the American Dental Association, 148(5), 308–318. 10.1016/j.adaj.2017.01.021 [DOI] [PubMed] [Google Scholar]
- de Santana, R. B. , Gusman, H. C. , & Van Dyke, T. E. (1999). The response of human buccal maxillary furcation defects to combined regenerative techniques–two controlled clinical studies. Journal of the International Academy of Periodontology, 1(3), 69–77. [PubMed] [Google Scholar]
- Demarco, F. F. , Correa, M. B. , Horta, B. , Barros, A. J. , Peres, K. G. , & Peres, M. A. (2013). Multilevel analysis of the association between posterior restorations and gingival health in young adults: A population‐based birth cohort. Journal of Clinical Periodontology, 40(12), 1126–1131. 10.1111/jcpe.12168 [DOI] [PubMed] [Google Scholar]
- Dommisch, H. , Walter, C. , Dannewitz, B. , & Eickholz, P. (2020). Resective surgery for the treatment of furcation involvement: A systematic review. Journal of Clinical Periodontology, 47(Suppl 22), 292–391. 10.1111/jcpe.13241 [DOI] [PubMed] [Google Scholar]
- Donos, N. , Calciolari, E. , Brusselaers, N. , Goldoni, M. , Bostanci, N. , & Belibasakis, G. N. (2019). The adjunctive use of host modulators in non‐surgical periodontal therapy. A systematic review of randomized, placebo‐controlled clinical studies. Journal of Clinical Periodontology, 47(Suppl 22), 116–238. 10.1111/jcpe.13232 [DOI] [PubMed] [Google Scholar]
- Eberhard, J. , Jepsen, S. , Jervoe‐Storm, P. M. , Needleman, I. , & Worthington, H. V. (2015). Full‐mouth treatment modalities (within 24 hours) for chronic periodontitis in adults. Cochrane Database of Systematic Reviews, (4), CD004622 10.1002/14651858.CD004622.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano, M. , Figuero, E. , Martin, C. , Tobias, A. , Serrano, J. , Roldan, S. , & Herrera, D. (2016). Efficacy of adjunctive anti‐plaque chemical agents: A systematic review and network meta‐analyses of the Turesky modification of the Quigley and Hein plaque index. Journal of Clinical Periodontology, 43(12), 1059–1073. 10.1111/jcpe.12616 [DOI] [PubMed] [Google Scholar]
- Fardal, O. , & Grytten, J. (2014). Applying quality assurance in real time to compliant long‐term periodontal maintenance patients utilizing cost‐effectiveness and cost utility. Journal of Clinical Periodontology, 41(6), 604–611. 10.1111/jcpe.12252 [DOI] [PubMed] [Google Scholar]
- Figuero, E. , Herrera, D. , Tobias, A. , Serrano, J. , Roldan, S. , Escribano, M. , & Martin, C. (2019). Efficacy of adjunctive anti‐plaque chemical agents in managing gingivitis: A systematic review and network meta‐analyses. Journal of Clinical Periodontology, 46(7), 723–739. 10.1111/jcpe.13127 [DOI] [PubMed] [Google Scholar]
- Figuero, E. , Roldan, S. , Serrano, J. , Escribano, M. , Martin, C. , & Preshaw, P. M. (2020). Efficacy of adjunctive therapies in patients with gingival inflammation. A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(Suppl 22), 125–143. 10.1111/jcpe.13244 [DOI] [PubMed] [Google Scholar]
- Garrett, S. , Polson, A. M. , Stoller, N. H. , Drisko, C. L. , Caton, J. G. , Harrold, C. Q. , … DeRouen, T. A. (1997). Comparison of a bioabsorbable GTR barrier to a non‐absorbable barrier in treating human class II furcation defects. A multi‐center parallel design randomized single‐blind trial. Journal of Periodontology, 68(7), 667–675. 10.1902/jop.1997.68.7.667 [DOI] [PubMed] [Google Scholar]
- Gatej, S. , Gully, N. , Gibson, R. , & Bartold, P. M. (2017). Probiotics and periodontitis – A literature review. Journal of the International Academy of Periodontology, 19(2), 42–50. [PubMed] [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet, 392(10159), 1789–1858. 10.1016/s0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German Association of the Scientific Medical Societies (AWMF) , & Standing Guidelines Commission . (2012). AWMF Guidance manual and rules for guideline development. Retrieved from http://www.awmf.org/leitlinien/awmf‐regelwerk.html [Google Scholar]
- Gomes, S. C. , Romagna, R. , Rossi, V. , Corvello, P. C. , & Angst, P. D. (2014). Supragingival treatment as an aid to reduce subgingival needs: A 450‐day investigation. Brazilian Oral Research, 28, 10.1590/S1806-83242014.50000004 [DOI] [PubMed] [Google Scholar]
- Graziani, F. , Gennai, S. , Cei, S. , Cairo, F. , Baggiani, A. , Miccoli, M. , … Tonetti, M. (2012). Clinical performance of access flap surgery in the treatment of the intrabony defect. A systematic review and meta‐analysis of randomized clinical trials. Journal of Clinical Periodontology, 39(2), 145–156. 10.1111/j.1600-051X.2011.01815.x [DOI] [PubMed] [Google Scholar]
- Graziani, F. , Gennai, S. , Karapetsa, D. , Rosini, S. , Filice, N. , Gabriele, M. , & Tonetti, M. (2015). Clinical performance of access flap in the treatment of class II furcation defects. A systematic review and meta‐analysis of randomized clinical trials. Journal of Clinical Periodontology, 42(2), 169–181. 10.1111/jcpe.12327 [DOI] [PubMed] [Google Scholar]
- Guyatt, G. H. , Oxman, A. D. , Kunz, R. , Atkins, D. , Brozek, J. , Vist, G. , … Schunemann, H. J. (2011). GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology, 64(4), 395–400. 10.1016/j.jclinepi.2010.09.012 [DOI] [PubMed] [Google Scholar]
- Harrel, S. K. (1999). A minimally invasive surgical approach for periodontal regeneration: Surgical technique and observations. Journal of Periodontology, 70(12), 1547–1557. 10.1902/jop.1999.70.12.1547 [DOI] [PubMed] [Google Scholar]
- Heasman, P. A. , McCracken, G. I. , & Steen, N. (2002). Supportive periodontal care: The effect of periodic subgingival debridement compared with supragingival prophylaxis with respect to clinical outcomes. Journal of Clinical Periodontology, 29(Suppl 3), 163–172. 10.1034/j.1600-051x.29.s3.9.x [DOI] [PubMed] [Google Scholar]
- Hennequin‐Hoenderdos, N. L. , van der Sluijs, E. , van der Weijden, G. A. , & Slot, D. E. (2018). Efficacy of a rubber bristles interdental cleaner compared to an interdental brush on dental plaque, gingival bleeding and gingival abrasion: A randomized clinical trial. International Journal of Dental Hygiene, 16(3), 380–388. 10.1111/idh.12316 [DOI] [PubMed] [Google Scholar]
- Herrera, D. , Matesanz, P. , Martin, C. , Oud, V. , Feres, M. , & Teughels, W. (2020). Adjunctive effect of locally delivered antimicrobials in periodontitis therapy: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(Suppl 22), 239–256. 10.1111/jcpe.13230 [DOI] [PubMed] [Google Scholar]
- Herrera, D. , Retamal‐Valdes, B. , Alonso, B. , & Feres, M. (2018). Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo‐periodontal lesions. Journal of Clinical Periodontology, 45(Suppl 20), S78–S94. 10.1111/jcpe.12941 [DOI] [PubMed] [Google Scholar]
- Hugoson, A. , Ravald, N. , Fornell, J. , Johard, G. , Teiwik, A. , & Gottlow, J. (1995). Treatment of class II furcation involvements in humans with bioresorbable and nonresorbable guided tissue regeneration barriers. A randomized multi‐center study. Journal of Periodontology, 66(7), 624–634. 10.1902/jop.1995.66.7.624 [DOI] [PubMed] [Google Scholar]
- Huynh‐Ba, G. , Kuonen, P. , Hofer, D. , Schmid, J. , Lang, N. P. , & Salvi, G. E. (2009). The effect of periodontal therapy on the survival rate and incidence of complications of multirooted teeth with furcation involvement after an observation period of at least 5 years: A systematic review. Journal of Clinical Periodontology, 36(2), 164–176. 10.1111/j.1600-051X.2008.01358.x [DOI] [PubMed] [Google Scholar]
- International Committee of Medical Editors . ICMJE Form for disclosure of potential conflicts of interest. Retrieved from http://www.icmje.org/conflicts‐of‐interest/ [Google Scholar]
- IQWiG . (2016). Präferenzmessung bei Parodontopathien. Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen, 466. [Google Scholar]
- Jepsen, S. , Caton, J. G. , Albandar, J. M. , Bissada, N. F. , Bouchard, P. , Cortellini, P. , … Yamazaki, K. (2018). Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Clinical Periodontology, 45(Suppl 20), S219–S229. 10.1111/jcpe.12951 [DOI] [PubMed] [Google Scholar]
- Jepsen, S. , Eberhard, J. , Herrera, D. , & Needleman, I. (2002). A systematic review of guided tissue regeneration for periodontal furcation defects. What is the effect of guided tissue regeneration compared with surgical debridement in the treatment of furcation defects? Journal of Clinical Periodontology, 29(Suppl 3), 103–116, discussion 160–102. 10.1034/j.1600-051x.29.s3.6.x [DOI] [PubMed] [Google Scholar]
- Jepsen, S. , Gennai, S. , Hirschfeld, J. , Kalemaj, Z. , Buti, J. , & Graziani, F. (2019). Regenerative surgical treatment of furcation defects: A systematic review and Bayesian network meta‐analysis of randomized clinical trials. Journal of Clinical Periodontology, 47(Suppl 22)(Suppl 20), 269–374. 10.1111/jcpe.13238 [DOI] [PubMed] [Google Scholar]
- Kassebaum, N. J. , Bernabe, E. , Dahiya, M. , Bhandari, B. , Murray, C. J. , & Marcenes, W. (2014). Global burden of severe periodontitis in 1990–2010: A systematic review and meta‐regression. Journal of Dental Research, 93(11), 1045–1053. 10.1177/0022034514552491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassebaum, N. J. , Smith, A. G. C. , Bernabe, E. , Fleming, T. D. , Reynolds, A. E. , Vos, T. , …G. B. D. O. H. Collaborators . (2017). Global, regional, and national prevalence, incidence, and disability‐adjusted life years for oral conditions for 195 countries, 1990–2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. Journal of Dental Research, 96(4), 380–387. 10.1177/0022034517693566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser, J. B. (1994). Non‐surgical periodontal therapy In Lang N. P. & Karring T. (Eds.), Proceedings of the 1st European Workshop on Periodontology. (pp. 131.158). London, UK: Quintessence Publishing Co. [Google Scholar]
- Krohn‐Dale, I. , Boe, O. E. , Enersen, M. , & Leknes, K. N. (2012). Er:YAG laser in the treatment of periodontal sites with recurring chronic inflammation: A 12‐month randomized, controlled clinical trial. Journal of Clinical Periodontology, 39(8), 745–752. 10.1111/j.1600-051X.2012.01912.x [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , Kiel, R. A. , & Anderhalden, K. (1983). Clinical and microbiological effects of subgingival restorations with overhanging or clinically perfect margins. Journal of Clinical Periodontology, 10(6), 563–578. 10.1111/j.1600-051X.1983.tb01295.x [DOI] [PubMed] [Google Scholar]
- Loos, B. G. , & Needleman, I. (2020). Endpoints of active periodontal therapy. Journal of Clinical Periodontology, 47(Suppl 22), 61–71. 10.1111/jcpe.13253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennickent, C. S. , Bravo, D. M. , Calvo, M. C. , & Avello, L. M. (2008). Pleiotropic effects of statins. Revista Médica De Chile, 136(6), 775–782. 10.4067/S0034-98872008000600014 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Journal of Clinical Epidemiology, 62(10), 1006–1012. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Murphy, M. K. , Black, N. A. , Lamping, D. L. , McKee, C. M. , Sanderson, C. F. , Askham, J. , & Marteau, T. (1998). Consensus development methods, and their use in clinical guideline development. Health Technology Assessment, 2(3), i–iv, 1–88. 10.3310/hta2030 [DOI] [PubMed] [Google Scholar]
- Needleman, I. , Nibali, L. , & Di Iorio, A. (2015). Professional mechanical plaque removal for prevention of periodontal diseases in adults–systematic review update. Journal of Clinical Periodontology, 42(Suppl 16), S12–35. 10.1111/jcpe.12341 [DOI] [PubMed] [Google Scholar]
- Nibali, L. , Koidou, V. P. , Nieri, M. , Barbato, L. , Pagliaro, U. , & Cairo, F. (2020). Regenerative surgery versus access flap for the treatment of intra‐bony periodontal defects: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(Suppl 22), 320–351. 10.1111/jcpe.13237 [DOI] [PubMed] [Google Scholar]
- Nibali, L. , Zavattini, A. , Nagata, K. , Di Iorio, A. , Lin, G. H. , Needleman, I. , & Donos, N. (2016). Tooth loss in molars with and without furcation involvement – A systematic review and meta‐analysis. Journal of Clinical Periodontology, 43(2), 156–166. 10.1111/jcpe.12497 [DOI] [PubMed] [Google Scholar]
- Nyman, S. , Lindhe, J. , & Rosling, B. (1977). Periodontal surgery in plaque‐infected dentitions. Journal of Clinical Periodontology, 4(4), 240–249. 10.1111/j.1600-051x.1977.tb01896.x [DOI] [PubMed] [Google Scholar]
- O'Leary, T. J. , Drake, R. B. , & Naylor, J. E. (1972). The plaque control record. Journal of Periodontology, 43(1), 38 10.1902/jop.1972.43.1.38 [DOI] [PubMed] [Google Scholar]
- Papapanou, P. N. , Sanz, M. , Buduneli, N. , Dietrich, T. , Feres, M. , Fine, D. H. , … Tonetti, M. S. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Clinical Periodontology, 45(Suppl 20), S162–S170. 10.1111/jcpe.12946 [DOI] [PubMed] [Google Scholar]
- Petit, C. , Batool, F. , Bugueno, I. M. , Schwinte, P. , Benkirane‐Jessel, N. , & Huck, O. (2019). Contribution of statins towards periodontal treatment: A review. Mediators of Inflammation, 2019, 6367402 10.1155/2019/6367402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak, D. , Wilensky, A. , Antonoglou, G. N. , Shapira, L. , Goldstein, M. , & Martin, C. (2020). The efficacy of pocket elimination/reduction compared to access flap surgery: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(Suppl 22), 303–319. 10.1111/jcpe.13246 [DOI] [PubMed] [Google Scholar]
- Proceedings of the 1996 World Workshop in Periodontics . (1996). Consensus report. Periodontal regeneration around natural teeth. Annals of Periodontology, 1(1), 667–670. 10.1902/annals.1996.1.1.667 [DOI] [PubMed] [Google Scholar]
- Ramseier, C. A. , Nydegger, M. , Walter, C. , Fischer, G. , Sculean, A. , Lang, N. P. , & Salvi, G. E. (2019). Time between recall visits and residual probing depths predict long‐term stability in patients enrolled in supportive periodontal therapy. Journal of Clinical Periodontology, 46(2), 218–230. 10.1111/jcpe.13041 [DOI] [PubMed] [Google Scholar]
- Ramseier, C. A. , Woelber, J. P. , Kitzmann, J. , Detzen, L. , Carra, M. C. , & Bouchard, P. (2020). Impact of risk factor control interventions for smoking cessation and promotion of healthy lifestyles in patients with periodontitis: A systematic review. Journal of Clinical Periodontology, 47(Suppl 22), 90–106. 10.1111/jcpe.13240 [DOI] [PubMed] [Google Scholar]
- Reinhardt, R. A. , Stoner, J. A. , Golub, L. M. , Wolff, M. S. , Lee, H. M. , Meinberg, T. A. , … Payne, J. B. (2007). Efficacy of sub‐antimicrobial dose doxycycline in post‐menopausal women: Clinical outcomes. Journal of Clinical Periodontology, 34(9), 768–775. 10.1111/j.1600-051X.2007.01114.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righolt, A. J. , Jevdjevic, M. , Marcenes, W. , & Listl, S. (2018). Global‐, regional‐, and country‐level economic impacts of dental diseases in 2015. Journal of Dental Research, 97(5), 501–507. 10.1177/0022034517750572 [DOI] [PubMed] [Google Scholar]
- Rosling, B. , Nyman, S. , Lindhe, J. , & Jern, B. (1976). The healing potential of the periodontal tissues following different techniques of periodontal surgery in plaque‐free dentitions. A 2‐year clinical study. Journal of Clinical Periodontology, 3(4), 233–250. 10.1111/j.1600-051x.1976.tb00042.x [DOI] [PubMed] [Google Scholar]
- Rosling, B. , Wannfors, B. , Volpe, A. R. , Furuichi, Y. , Ramberg, P. , & Lindhe, J. (1997). The use of a triclosan/copolymer dentifrice may retard the progression of periodontitis. Journal of Clinical Periodontology, 24(12), 873–880. 10.1111/j.1600-051x.1997.tb01205.x [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , Stahli, A. , Schmidt, J. C. , Ramseier, C. A. , Sculean, A. , & Walter, C. (2020). Adjunctive laser or antimicrobial photodynamic therapy to non‐surgical mechanical instrumentation in patients with untreated periodontitis. A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(Suppl 22), 176–198. 10.1111/jcpe.13236 [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Baumer, A. , Buduneli, N. , Dommisch, H. , Farina, R. , Kononen, E. , … Winkel, E. (2015). Effect of professional mechanical plaque removal on secondary prevention of periodontitis and the complications of gingival and periodontal preventive measures: Consensus report of group 4 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri‐implant diseases. Journal of Clinical Periodontology, 42(Suppl 16), S214–220. 10.1111/jcpe.12367 [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Ceriello, A. , Buysschaert, M. , Chapple, I. , Demmer, R. T. , Graziani, F. , … Vegh, D. (2018). Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. Journal of Clinical Periodontology, 45(2), 138–149. 10.1111/jcpe.12808 [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Kornman, K. , & Working Group 3 of Joint EFP, AAP Workshop . (2013). Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Journal of Clinical Periodontology, 40(Suppl 14), S164–169. 10.1111/jcpe.12083 [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Marco del Castillo, A. , Jepsen, S. , Gonzalez‐Juanatey, J. R. , D'Aiuto, F. , Bouchard, P. , & Wimmer, G. (2019). Periodontitis and cardiovascular diseases: Consensus report. Journal of Clinical Periodontology, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz, M. , & Meyle, J. (2010). Scope, competences, learning outcomes and methods of periodontal education within the undergraduate dental curriculum: A consensus report of the 1st European Workshop on Periodontal Education–position paper 2 and consensus view 2. European Journal of Dental Education, 14(Suppl 1), 25–33. 10.1111/j.1600-0579.2010.00621.x [DOI] [PubMed] [Google Scholar]
- Sanz, M. , van der Velden, U. , van Steenberghe, D. , & Baehni, P. (2006). Periodontology as a recognized dental speciality in Europe. Journal of Clinical Periodontology, 33(6), 371–375. 10.1111/j.1600-051X.2006.00932.x [DOI] [PubMed] [Google Scholar]
- Sanz‐Sanchez, I. , Montero, E. , Citterio, F. , Romano, F. , Molina, A. , & Aimetti, M. (2020). Efficacy of access flap procedures compared to subgingival debridement in the treatment of periodontitis. A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(Suppl. 22), 282–302. 10.1111/jcpe.13259 [DOI] [PubMed] [Google Scholar]
- Schunemann, H. J. , Al‐Ansary, L. A. , Forland, F. , Kersten, S. , Komulainen, J. , Kopp, I. B. … Board of Trustees of the Guidelines International Network . (2015). Guidelines international network: Principles for disclosure of interests and management of conflicts in guidelines. Annals of Internal Medicine, 163(7), 548–553. 10.7326/M14-1885 [DOI] [PubMed] [Google Scholar]
- Schunemann, H. J. , Wiercioch, W. , Brozek, J. , Etxeandia‐Ikobaltzeta, I. , Mustafa, R. A. , Manja, V. , … Akl, E. A. (2017). GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE‐ADOLOPMENT. Journal of Clinical Epidemiology, 81, 101–110. 10.1016/j.jclinepi.2016.09.009 [DOI] [PubMed] [Google Scholar]
- Schunemann, H. J. , Zhang, Y. , Oxman, A. D. , & Expert Evidence in Guidelines Group . (2019). Distinguishing opinion from evidence in guidelines. British Medical Journal, 366, l4606 10.1136/bmj.l4606 [DOI] [PubMed] [Google Scholar]
- Schwendicke, F. , Graetz, C. , Stolpe, M. , & Dorfer, C. E. (2014). Retaining or replacing molars with furcation involvement: A cost‐effectiveness comparison of different strategies. Journal of Clinical Periodontology, 41(11), 1090–1097. 10.1111/jcpe.12315 [DOI] [PubMed] [Google Scholar]
- Schwendicke, F. , Plaumann, A. , Stolpe, M. , Dorfer, C. E. , & Graetz, C. (2016). Retention costs of periodontally compromised molars in a German population. Journal of Clinical Periodontology, 43(3), 261–270. 10.1111/jcpe.12509 [DOI] [PubMed] [Google Scholar]
- Serhan, C. N. (2017). Discovery of specialized pro‐resolving mediators marks the dawn of resolution physiology and pharmacology. Molecular Aspects of Medicine, 58, 1–11. 10.1016/j.mam.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano, J. , Escribano, M. , Roldan, S. , Martin, C. , & Herrera, D. (2015). Efficacy of adjunctive anti‐plaque chemical agents in managing gingivitis: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 42(Suppl 16), S106–138. 10.1111/jcpe.12331 [DOI] [PubMed] [Google Scholar]
- Shea, B. J. , Reeves, B. C. , Wells, G. , Thuku, M. , Hamel, C. , Moran, J. , … Henry, D. A. (2017). AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. British Medical Journal, 358, j4008 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot, D. E. , Valkenburg, C. , & van der Weijden, F. (2020). Mechanical plaque removal of periodontal maintenance patients: A systematic review and network meta‐analysis. Journal of Clinical Periodontology, 47(Suppl 22), 107–124. 10.1111/jcpe.13275 [DOI] [PubMed] [Google Scholar]
- Steenackers, K. , Vijt, J. , Leroy, R. , De Vree, H. , & De Boever, J. A. (2001). Short‐term clinical study comparing supragingival plaque removal and gingival bleeding reduction of the Philips Jordan HP735 to a manual toothbrush in periodontal patients in a maintenance program. Journal of Clinical Dentistry, 12(1), 17–20. [PubMed] [Google Scholar]
- Suvan, J. , Leira, Y. , Moreno, F. , Graziani, F. , Derks, J. , & Tomasi, C. (2020). Subgingival instrumentation for treatment of periodontitis. A systematic review. Journal of Clinical Periodontology, 47(Suppl 22), 155–175. 10.1111/jcpe.13245 [DOI] [PubMed] [Google Scholar]
- Teughels, W. , Feres, M. , Oud, V. , Martin, C. , Matesanz, P. , & Herrera, D. (2020). Adjunctive effect of systemic antimicrobials in periodontitis therapy. A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(Suppl 22), 212–281. 10.1111/jcpe.13264 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Eickholz, P. , Loos, B. G. , Papapanou, P. , van der Velden, U. , Armitage, G. , … Suvan, J. E. (2015). Principles in prevention of periodontal diseases: Consensus report of group 1 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri‐implant diseases. Journal of Clinical Periodontology, 42(Suppl 16), S5–S11. 10.1111/jcpe.12368 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Greenwell, H. , & Kornman, K. S. (2018). Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Clinical Periodontology, 45(Suppl 20), S149–S161. 10.1111/jcpe.12945 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Jepsen, S. , Jin, L. , & Otomo‐Corgel, J. (2017). Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. Journal of Clinical Periodontology, 44(5), 456–462. 10.1111/jcpe.12732 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , & Sanz, M. (2019). Implementation of the new classification of periodontal diseases: Decision‐making algorithms for clinical practice and education. Journal of Clinical Periodontology, 46(4), 398–405. 10.1111/jcpe.13104 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , & Van Dyke, T. E. , & Working Group 1 of the Joint EFP/AAP Workshop . (2013). Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Journal of Clinical Periodontology, 40(Suppl 14), S24–S29. 10.1111/jcpe.12089 [DOI] [PubMed] [Google Scholar]
- Trombelli, L. , Farina, R. , Franceschetti, G. , & Calura, G. (2009). Single‐flap approach with buccal access in periodontal reconstructive procedures. Journal of Periodontology, 80(2), 353–360. 10.1902/jop.2009.080420 [DOI] [PubMed] [Google Scholar]
- Trombelli, L. , Farina, R. , Pollard, A. , Claydon, N. , Franceschetti, G. , Khan, I. , & West, N. (2020). Efficacy of alternative or additional methods to professional mechanical plaque removal during supportive periodontal therapy. A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(Suppl 22), 144–154. 10.1111/jcpe.13269 [DOI] [PubMed] [Google Scholar]
- Trombelli, L. , Franceschetti, G. , & Farina, R. (2015). Effect of professional mechanical plaque removal performed on a long‐term, routine basis in the secondary prevention of periodontitis: A systematic review. Journal of Clinical Periodontology, 42(Suppl 16), S221–236. 10.1111/jcpe.12339 [DOI] [PubMed] [Google Scholar]
- Van der Velden, U. , & Sanz, M. (2010). Postgraduate periodontal education. Scope, competences, proficiencies and learning outcomes: Consensus report of the 1st European Workshop on Periodontal Education–position paper 3 and consensus view 3. European Journal of Dental Education, 14(Suppl 1), 34–40. 10.1111/j.1600-0579.2010.00622.x [DOI] [PubMed] [Google Scholar]
- van der Weijden, F. A. , & Slot, D. E. (2011). Oral hygiene in the prevention of periodontal diseases: The evidence. Periodontology 2000, 55(1), 104–123. 10.1111/j.1600-0757.2009.00337.x [DOI] [PubMed] [Google Scholar]
- Van der Weijden, F. A. , & Slot, D. E. (2015). Efficacy of homecare regimens for mechanical plaque removal in managing gingivitis: A metareview. Journal of Clinical Periodontology, 42(Suppl 16), S77–S91. 10.1111/jcpe.12359 [DOI] [PubMed] [Google Scholar]
- van Steenberghe, D. , Lekholm, U. , Bolender, C. , Folmer, T. , Henry, P. , Herrmann, I. , … Astrand, P. (1990). Applicability of osseointegrated oral implants in the rehabilitation of partial edentulism: A prospective multicenter study on 558 fixtures. International Journal of Oral and Maxillofacial Implants, 5(3), 272–281. [PubMed] [Google Scholar]
- Ximénez‐Fyvie, L. A. , Haffajee, A. D. , Som, S. , Thompson, M. , Torresyap, G. , & Socransky, S. S. (2000). The effect of repeated professional supragingival plaque removal on the composition of the supra‐ and subgingival microbiota. Journal of Clinical Periodontology, 27(9), 637–647. 10.1034/j.1600-051x.2000.027009637.x [DOI] [PubMed] [Google Scholar]
- Xue, D. , Tang, L. , Bai, Y. , Ding, Q. , Wang, P. , & Zhao, Y. (2017). Clinical efficacy of photodynamic therapy adjunctive to scaling and root planing in the treatment of chronic periodontitis: A systematic review and meta‐analysis. Photodiagnosis and Photodynamic Therapy, 18, 119–127. 10.1016/j.pdpdt.2017.01.183 [DOI] [PubMed] [Google Scholar]
- Zare Javid, A. , Seal, C. J. , Heasman, P. , & Moynihan, P. J. (2014). Impact of a customised dietary intervention on antioxidant status, dietary intakes and periodontal indices in patients with adult periodontitis. Journal of Human Nutrition and Dietetics, 27(6), 523–532. 10.1111/jhn.12184 [DOI] [PubMed] [Google Scholar]


