Abstract
Purpose
To determine change in visual acuity (VA) in the population of a previous amblyopia treatment study (Loudon 2006) and assess risk factors for VA decrease.
Methods
Subjects treated between 2001 and 2003 were contacted between December 2015 and July 2017. Orthoptic examination was conducted under controlled circumstances and included subjective refraction, best corrected VA, reading acuity, binocular vision, retinal fixation, cover‐uncover and alternating cover test. As a measure for degree of amblyopia, InterOcular VA Difference (IOD) at the end of occlusion therapy was compared with IOD at the follow‐up examination using Wilcoxon’s signed‐rank test. Regression analysis was conducted to determine the influence of clinical and socio‐economic factors on changes in IOD.
Results
Out of 303 subjects from the original study, 208 were contacted successfully, 59 refused and 15 were excluded because of non‐amblyopic cause of visual impairment. Mean IOD at end of therapy (mean age 6.4 years) was 0.11 ± 0.16 logMAR, and IOD at follow‐up examination (mean age 18.3 years) was 0.09 ± 0.21 logMAR; this difference was not significant (p = 0.054). Degree of anisometropia (p = 0.008; univariable analysis), increasing anisometropia (p = 0.009; multivariable), eccentric fixation (p < 0.001; univariable and multivariable); large IOD (p < 0.001; univariable and multivariable) and non‐compliance during therapy (p = 0.028; univariable) were associated with IOD increase.
Conclusion
Long‐term results of occlusion therapy were good. High or increasing anisometropia, eccentric fixation and non‐compliance during occlusion therapy were associated with long‐term VA decrease. Subjects with poor initial VA had a larger increase despite little patching, but often showed long‐term VA decrease.
Keywords: amblyopia, long‐term follow‐up, occlusion therapy, visual acuity
Introduction
Amblyopia is the most common cause of monocular visual acuity loss in children with a prevalence varying from 1.6% to 3.5% (Reeves 2002). It is mainly caused by strabismus and/or anisometropia or visual deprivation, which disrupts the equal input from both eyes to the visual cortex. Standard therapy is spectacle correction if necessary, and occlusion of the fellow eye several hours per day during the sensitive period (Stewart et al. 2004a, 2004b,2004a, 2004b).
Occlusion therapy is a very successful treatment; however, its success is hampered by non‐compliance (Loudon et al. 2003; Stewart et al. 2004a; Loudon et al. 2006; Stewart et al. 2007). Long‐term results of occlusion therapy vary widely (Leiba et al. 2001; Holmes et al. 2004; Rutstein & Corliss 2004; Repka et al. 2005; Scott et al. 2005; Bhola et al. 2006; Hertle et al. 2007; Simonsz‐Toth et al. 2007; Pediatric Eye Disease Investigator Group et al. 2008; Saxena et al. 2013). Visual acuity deteriorated in 7–75% of the cases, largely depending on duration of follow‐up and definition of outcome (Rutstein & Fuhr 1992; Hertle et al. 2007). Reported factors that negatively influenced the course of visual acuity after cessation of therapy included poor visual acuity at start of treatment, combined cause of amblyopia, eccentric fixation and age (Levartovsky et al. 1995; Bhola et al. 2006; Simonsz‐Toth et al. 2007). Almost all long‐term studies were done retrospectively. Persistent amblyopia causes a significant burden on society, financially as well as a reduced quality of life (van de Graaf et al. 2007). It also nearly doubles the time an individual spends with bilateral visual impairment due to loss of vision in the non‐amblyopic eye: this increases from 8 to 15.5 months, on average (van Leeuwen et al. 2007).
In our previous randomized controlled trial (RCT; 2001–2003, N = 303, NCT00131729), all newly diagnosed amblyopic children in four clinics in The Hague were registered. Included children received occlusion therapy, while compliance was measured electronically using the occlusion dose monitor (ODM). The purpose of the study was to determine whether compliance could be improved using an educational cartoon programme aimed at the child, and to identify risk factors for non‐compliance. We found predictors for non‐compliance to be a low initial visual acuity, poor parental fluency in the national language and low parental level of education. The educational programme significantly improved compliance throughout the study, limiting in particular the number of children who were not occluded at all. (Loudon et al. 2006).
The purpose of this study was to determine the long‐term course of the visual acuity after cessation of occlusion therapy for amblyopia and identify those at risk for visual acuity deterioration. At the time of this follow‐up measurement, all children were adolescents, most of them still living with their parents. They were contacted again for examination of their current visual acuity. Both the visual acuity measurements at the end of occlusion therapy and at the follow‐up examination were performed under the same strictly controlled conditions by the same research orthoptist (BST). Compliance during occlusion therapy had been measured electronically, and detailed clinical and socio‐demographic data were readily available of all subjects.
Materials and Methods
Study population and orthoptic examination in amblyopia treatment study 2001–2004
Subjects were derived from a previous RCT, in which all newly diagnosed amblyopic children had been recruited from the four clinics in The Hague from 2001 until 2003 (Loudon et al. 2006). The design for this prospective study has been reported in detail elsewhere (Loudon et al. 2006). Briefly, all amblyopic children were given standard orthoptic care with routine assessment every 3–4 months by the treating orthoptist. All measurements were conducted under controlled circumstances. Duration of occlusion (number of hours per day) for the first prescription was standardized according to the following formula: −6.63 × ratio acuity amblyopic eye/acuity better eye + 0.5 × age (years) + 4.97. Compliance was measured electronically using the ODM. Children were randomized to either the intervention group or the control group. The intervention group received an educational programme explaining to the child without words the reasons for patching. The control group received a picture to colour, without an educational message. The family’s socio‐economic status was ascertained using a 23‐item questionnaire.
Occlusion therapy was completed when the interocular difference in visual acuity was one logMAR line or less on two consecutive visits to the orthoptist. From 2004 the research orthoptist assessed best corrected visual acuity with the Landolt‐C chart 17.2’ in children whose occlusion treatment was either ‘completed’ by the orthoptists or ‘terminated’ by the parents (i.e. parents who failed to attend the appointments in clinic). The research orthoptist tested the best corrected visual acuity with the Landolt‐C chart 17.2’ minutes of distance between optotypes at 5m distance. At least 3 out of 5 optotypes had to be answered correctly per line. The luminance of the chart was measured during the tests. This ranged from 160 to 320 cd/m2, which is in accordance with the ISO‐8596 Standard.
Follow‐up examination 2015–2017
All orthoptic and demographic data from the original 303 files were readily available and analysed. From these files, last known contact information was obtained. The subjects were contacted from December 2015 until July 2017 (Fig. 1). Eighty‐nine (29%) could not be contacted using the available information from the original trial and six subjects had moved abroad. We were able to contact 208 subjects (69%), of whom 59 (19%) refused participation with a follow‐up examination. Reasons for refusing participation included time or interest issues (N = 33), not showing up for the appointment on multiple occasions (N = 20), no eye complaints (N = 3) or had recently visited the ophthalmology department or optician (N = 3). One subject could not be examined due to other disabilities. In total, 148 subjects were examined, of these 14 were excluded. Reasons for exclusion were diminished visual acuity due to other ocular diseases (e.g. optic neuritis, mild oculocutaneous albinism) or brain damage (e.g. haemorrhage). In two subjects, the visual acuity at the end of occlusion therapy was unknown and could not be obtained; in eight subjects, the diagnosis of amblyopia could not be confirmed, in hindsight.
Fig. 1.
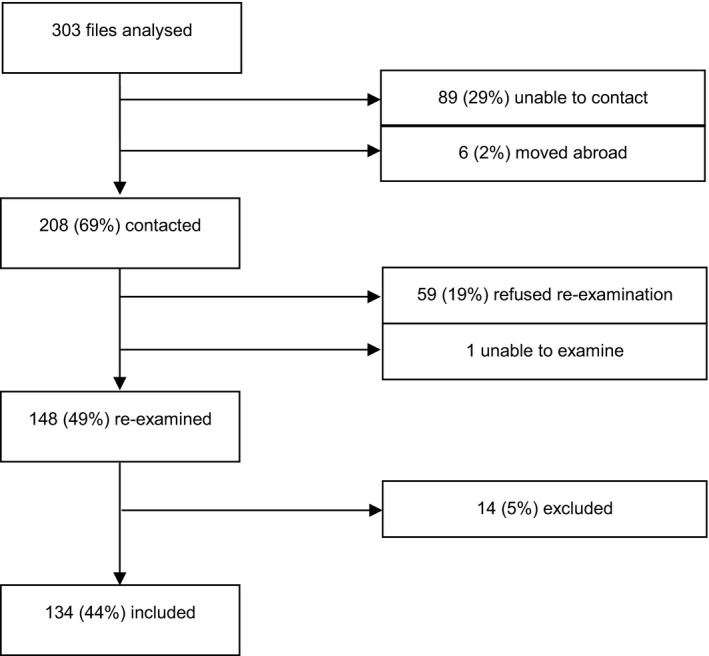
Recruitment procedure for re‐examination.
The Ethical Committee of Erasmus University Rotterdam and the boards of the participating clinics approved the protocol and informed consent forms. Written informed consent was obtained from each subject and/or from his or her parents or guardians. The research adhered to the tenets of the Declaration of Helsinki.
The follow‐up examinations were carried out at the outpatient clinic at Haaglanden Medical Center, Westeinde The Hague and conducted by the same research orthoptist (BST) who had examined the same subjects at the end of occlusion therapy, using the same protocol for measurement for visual acuity as in 2004. Examinations were performed through domiciliary visits if the subjects refused to visit the hospital. Binocular vision was assessed with Bagolini striated glasses, Titmus‐Fly and TNO‐test and expressed in five categories: (1) Bagolini negative; (2) Bagolini positive; (3) Bagolini and Titmus‐Fly test positive; (4) TNO plate 480”‐240”; (5) TNO plate 120”‐15”. Retinal fixation and ocular alignment were investigated with the cover‐uncover and alternating cover test at 30cm and 5m distance. Target for the cover test for fixation at near was a small object with detailed pictures to stimulate accommodation and to assess fixation. The target of the cover test at distance was a penlight. Reading acuity was tested using the Dutch version of the Radner Reading Chart (Maaijwee et al. 2008). Subjects were examined with their current spectacles. The best corrected visual acuity (BCVA) was determined using the Righton Retinomax handheld auto (kerato)‐refractor together with subjective refraction in all subjects. Cycloplegic refraction was part of the original protocol, but most of the subjects refused participation if this was obliged. Therefore, to ensure for a reliable calculation of the refractive error, we performed subjective refraction whereby the accommodation was eliminated with hypermetropic lenses. Degree of anisometropia was determined by calculating the difference in spherical equivalent between the two eyes based on the subjective refraction. Current degree of anisometropia was compared with the degree of anisometropia as measured with cycloplegic refraction at start of therapy: [Anisometropia follow‐up] – [Anisometropia start of therapy]. Loss of 2 logMAR lines or more in visual acuity was defined as ‘severe deterioration’.
Statistics
Differences in characteristics, that is clinical and socio‐economic data from the original study (N = 303), between subjects who completed the follow‐up examination (N = 134) and those who did not, were investigated to assess for potential bias. These differences were tested using T‐tests and Mann–Whitney tests for the following continuous variables: age at start of therapy, IOD at start, anisometropia at start, compliance during therapy, IOD at end of therapy. The chi‐square test was used to investigate the following categorical variables: eccentric fixation, gender, randomization group, fluency in the national language, level of education, number of working hours per week, country of origin and home‐ownership.
As a measure for the degree of amblyopia, we used the InterOcular VA Difference (IOD). The IOD at the end of occlusion therapy as measured by the research orthoptist (BST in 2004) was compared with the IOD as measured at the follow‐up examination (BST in 2016). The main outcome measure was this change in IOD after cessation of occlusion therapy, calculated with the following formula: [VAae – Vafe]follow‐up examination – [VAae – Vafe]end of occlusion treatment, with VAae the visual acuity of the amblyopic eye and VAfe the visual acuity of the fellow eye (logMAR). The Wilcoxon signed‐rank test was used to assess any significant changes in IOD and binocular vision between end of occlusion treatment and at the follow‐up examination. The Spearman correlation was used to determine the association between visual acuity and reading acuity. The influence of spectacle wearing on change in anisometropia was investigated with regression analysis.
In addition, we assessed risk factors for visual acuity deterioration. Univariable linear regression analysis was performed to investigate which clinical (i.e. age, gender, randomization, diagnosis, visual acuity, anisometropia, retinal fixation, compliance, and duration of occlusion therapy) and socio‐economic variables (i.e. parental fluency in the national language, parental level of education, number of working hours per week, country of origin and home‐ownership) influenced the change in IOD (dependent variable). Potential confounding was corrected for in a multivariable linear regression analysis. Variable selection using a stepwise backward approach with a p‐value cut‐off of 0.20 was performed. All statistical tests were two‐sided with a significance level of 0.05. Missing data were minimal, the variable ‘compliance’ had five missing data points, and therefore, complete case analyses were performed.
Results
Study population
Of the original cohort of 303 subjects, 208 were contacted successfully, 59 refused, 14 were excluded and we were unable to examine one subject. We included 134 (Fig. 1). Mean age at start of therapy was 4.7 (±2.0) years, 6.4 (±2.1) years at end of therapy and 18.3 (±2.1) years at follow‐up examination.
Subjects who completed the follow‐up examination and those who did not were comparable for the different baseline characteristics (p > 0.05), except for gender. The follow‐up group had significantly more females (p = 0.006; 44% in the original cohort versus 53% in the follow‐up group). Median compliance and interquartile range (IQR) in the original cohort (N = 303) was 71% (IQR 37–91) with 150 (50%) subjects in the intervention group. Median compliance in the follow‐up group (N = 134) was 73% (IQR 42–91) with 73 (55%) subjects from the intervention group. In 18 subjects, compliance was lower than 20%. Thirty‐three subjects had strabismus amblyopia, 75 anisometropic amblyopia, 21 had a combined cause of amblyopia and 5 had deprivation amblyopia. Retinal fixation was determined as central in 124 subjects and as eccentric in 10 subjects.
Interocular visual acuity difference
Mean IOD at the start of treatment was 0.27 (±0.25) logMAR and 0.11 (±0.16) logMAR at end of therapy. Mean IOD at follow‐up examination was 0.09 (±0.21) logMAR. There were 6 subjects who were prescribed occlusion treatment after their last examination, but failed to show up for their follow‐up appointments. Patching might have continued, but this could not be confirmed. There was no significant difference between mean IOD at end of therapy and at follow‐up examination (N = 134; p = 0.054). In 63 (47%) subjects, the IOD had decreased, that is less amblyopia; in 36 (27%) subjects it remained stable. The IOD had increased, that is more amblyopia, in 35 subjects: 14 of the 75 (19%) anisometropic, 8 of the 33 (24%) strabismic, 10 of the 21 (48%) combined subjects and 3 of the 5 (60%) subjects with deprivation amblyopia. Subjects were categorized based on the initial depth of amblyopia according to the PEDIG criteria (Holmes et al. 2003; Repka et al. 2003). Figure 2 shows the course of the IOD for each category. Subjects with severe amblyopia had improved most during occlusion therapy, but had deteriorated the most during follow‐up examination.
Fig. 2.
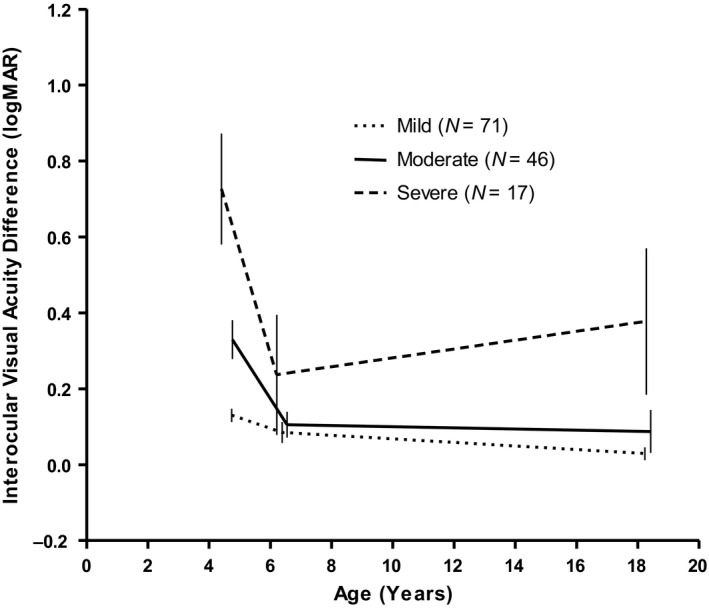
Mean Interocular Visual Acuity difference between the amblyopic eye and fellow eye at three points in time with 95% confidence intervals: at the start of occlusion therapy, at end of therapy and at the follow‐up examination 12–15 years later. Subjects are categorized, based on the visual acuity of the amblyopic eye at start of occlusion therapy: mild (≤0.2 logMAR), moderate (0.30–0.60 logMAR) and severe (0.70–1.3 logMAR).
Factors influencing the course of IOD
Univariable analysis showed that a large IOD and high anisometropia at start of occlusion therapy were both associated with an IOD increase after cessation of therapy. Eccentric fixation and non‐compliance during occlusion therapy were also significantly associated with IOD increase. Results of the univariable and multivariable analyses are listed in Table 1.
Table 1.
Results of the univariable and multivariable analyses: the influence of clinical and socio‐economic variables on the change in IOD between end of occlusion therapy and follow‐up examination.
| Independent variables | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| B | 95% CI | p‐Value | B | 95% CI | p‐Value | |
| Age at start of therapy (years) | −0.016 | −0.032, 0.000 | 0.055 | |||
| Gender** | ||||||
| Boys | 0.022 | −0.044, 0.088 | 0.515 | 0.058 | 0.007, 0.108 | 0.026 |
| Girls | Reference | |||||
| IOD at start of therapy (logMAR)** | 0.235 | 0.106, 0.363 | <0.001 | 0.332 | 0.205, 0.458 | <0.001 |
| IOD at end of therapy (logMAR)** | −0.357 | −0.557, −0.157 | 0.001 | −0.647 | −0.820, −0.473 | <0.001 |
| Anisometropia at start of therapy (D)* | 0.042 | 0.011, 0.073 | 0.008 | 0.020 | −0.009, 0.049 | 0.169 |
| Diagnosis | 0.052 | |||||
| Strabismus | −0.110 | −0.290, 0.070 | 0.227 | |||
| Anisometropia | −0.150 | −0.323, 0.023 | 0.090 | |||
| Combined | −0.037 | −0.223, 0.150 | 0.698 | |||
| Deprivation | Reference | |||||
| Eccentric fixation** | 0.279 | 0.163, 0.396 | <0.001 | 0.220 | 0.121, 0.319 | <0.001 |
| Compliance (%) | 0.000 | −0.001, 0.001 | 0.575 | |||
| Duration of occlusion therapy (years) | 0.011 | −0.028, 0.049 | 0.579 | |||
| Change in anisometropia (D)** | 0.025 | −0.009, 0.059 | 0.155 | 0.037 | 0.009, 0.064 | 0.009 |
| Intervention (educational programme) | 0.045 | −0.021, 0.111 | 0.177 | |||
| Parental fluency national language | 0.618 | |||||
| Excellent | 0.040 | −0.045, 0.126 | 0.353 | |||
| Good | −0.017 | −0.142, 0.107 | 0.782 | |||
| Moderate | −0.003 | −0.115, 0.110 | 0.964 | |||
| Poor | 0.076 | −0.060, 0.211 | 0.270 | |||
| None | Reference | |||||
| Highest level of education | 0.118 | 0.054 | ||||
| University | 0.069 | −0.096, 0.234 | 0.406 | 0.003 | −0.120, 0.127 | 0.957 |
| Higher education | 0.094 | −0.061, 0.250 | 0.233 | −0.033 | −0.152, 0.085 | 0.580 |
| Secondary education | 0.163 | 0.006, 0.320 | 0.041 | 0.051 | −0.068, 0.169 | 0.398 |
| Primary education | 0.061 | −0.098, 0.219 | 0.452 | −0.051 | −0.172, 0.069 | 0.401 |
| None | Reference | |||||
| Number of working hours per week | 0.000 | −0.003, 0.002 | 0.732 | |||
| Country of origin | 0.131 | |||||
| Natives | 0.048 | −0.039, 0.135 | 0.275 | |||
| Surinam | 0.028 | −0.102, 0.157 | 0.673 | |||
| Morocco | 0.063 | −0.045, 0.171 | 0.250 | |||
| Turkey | −0.074 | −0.186. 0.037 | 0.188 | |||
| Other | Reference | |||||
| Home‐ownership | ||||||
| Yes | −0.031 | −0.099, 0.038 | 0.379 | |||
| No | Reference | |||||
Variable significantly affecting change in IOD after univariable analysis (p < 0.05).
Variable significantly affecting change in IOD after multivariable analysis (p < 0.05).
Multivariable analysis showed that a large IOD at start of occlusion therapy, eccentric fixation at start of therapy and an increasing anisometropia were associated with IOD increase after cessation of treatment. Of the socio‐economic variables, only parental level of education was borderline significant (p = 0.054) in the multivariable analysis.
Increasing anisometropia and spectacle wearing
It has been suggested by Simonsz‐Tóth that amblyopia in children with increasing anisohypermetropia is more likely to deteriorate as new spectacles are needed frequently to keep up with the changing refractive error (Simonsz‐Toth et al. 2007). Overall, the degree of anisometropia was stable or had decreased (N = 91; 68%) with 0.09 (±1.0) dioptres (D): 0.90D (±1.0) at start of occlusion therapy and 0.80D (±1.2) at the follow‐up examination. Eleven (8%) of the 35 subjects in whom IOD had increased, also had an increase of their anisometropia. In the multivariable analysis (Table 1), an increase in anisometropia was significantly associated with an IOD increase after cessation of therapy (p = 0.009). To determine whether this association could be explained by spectacle wearing, we inquired about spectacle wearing in daily life in all 134 subjects and divided them into three categories. Sixty subjects (45%) wore their spectacles at least more than 50% of all waking hours; 20 (15%) wore spectacles <50% and 54 (40%) never wore spectacle correction or did not have any. Using univariable regression, a relationship could not be demonstrated (p = 0.064). Of the 54 subjects who never wore spectacles or did not have any, 17 showed an improvement with additional correction. Interestingly, of the 11 subjects who had an IOD increase as well as an increase in anisometropia, 6 seldom or never wore spectacles.
Compliance
Mean compliance with occlusion therapy as measured electronically was not significantly associated with a change in IOD (B = 0.000; p = 0.575). However, when comparing subjects who did not comply with therapy at all (i.e. compliance less than 20%) with subjects with compliance more than 20%, the non‐compliers were at risk for IOD deterioration after therapy (p = 0.028) in the univariable analysis. This cut‐off point of 20% was chosen as the lowest point in the bimodal distribution of compliance, which separated the children who had not been occluded regularly or not at all from the children who occluded routinely (Loudon et al. 2009). These non‐compliers had a mean IOD of 0.33 (±0.33) logMAR at start of therapy; 0.15 (±0.27) at end of their occlusion therapy and 0.23 (±0.32) at follow‐up examination. The VA in the amblyopic eye increased even with little patching, but deteriorated after therapy: 0.42 (±0.33) logMAR at start, 0.21 (±0.29) logMAR at end of occlusion therapy and 0.14 (±0.32) logMAR at follow‐up examination. Interestingly, of the 18 subjects with compliance less than 20%, 17 were in the control group; 1 in the intervention group who received the educational cartoon programme. The educational programme greatly reduced the number of non‐compliers in the original study and significantly improved the rate of VA increase (Tjiam et al. 2012).
Subjects with severe visual acuity deterioration after cessation of therapy
Of all 134 included subjects, five (4%) had visual acuity deterioration in the amblyopic eye of ≥0.2 logMAR lines (Fig. 3); visual acuity in the fellow eye was ≤0.0 logMAR. These five subjects all had a combination of microstrabismus, eccentric fixation and poor visual acuity of the amblyopic eye at start of occlusion therapy. In the first subject, the amblyopia was caused by anisometropia and strabismus; anisometropia was 3.50D and had not increased. Compliance with therapy at the time was 59%. In the second subject, the amblyopia was also caused by anisometropia and strabismus; anisometropia was 4.38 D and increased with 1.4 D, he ceased wearing his spectacles at age 12. Compliance was 0%. The third subject had a strabismus amblyopia; anisometropia was 0.25 D and increased with 0.50 D. She has never worn any refractive correction. Compliance was 99%. The fourth subject had strabismus and anisometropia amblyopia; anisometropia was 1.0 D and increased with 5 D, she did not wear adequate spectacle correction. Compliance was 98%. The fifth subject had strabismus and anisometropia amblyopia; anisometropia was 1 D and had not increased. Compliance was 0% at the time. He wore adequate spectacle correction.
Fig. 3.
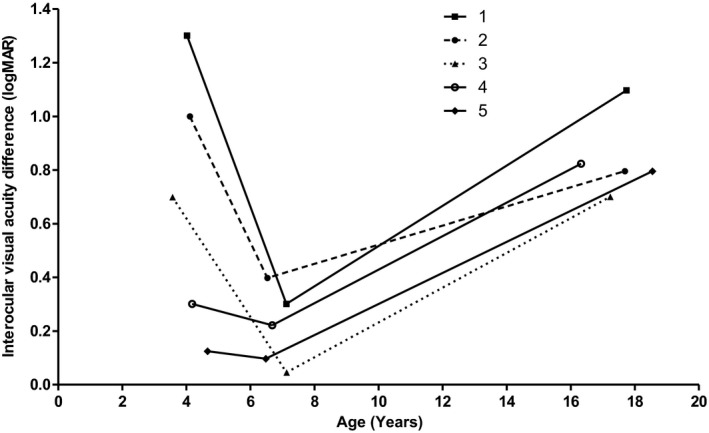
InterOcular VA Difference of the five subjects (number 1–5) with the largest VA deterioration measured at start of occlusion therapy, end of therapy and at follow‐up examination. Four had a combined cause of amblyopia.
Binocular vision
In 34 (25%) subjects, binocular vision at end of therapy was unknown and therefore, a comparison could not be made. Suppression as measured by Bagolini striated glasses was stable in the majority of the subjects (N = 95; 71%). Three subjects scored a positive Bagolini test at end of therapy, but showed suppression on the Bagolini test at follow‐up of whom two also showed profound deterioration in visual acuity of the amblyopic eye. Figure 4 shows the change in binocular vision of the 100 subjects at end of therapy and at the time of the follow‐up examination; this change was not statistically significant (p = 0.406).
Fig. 4.
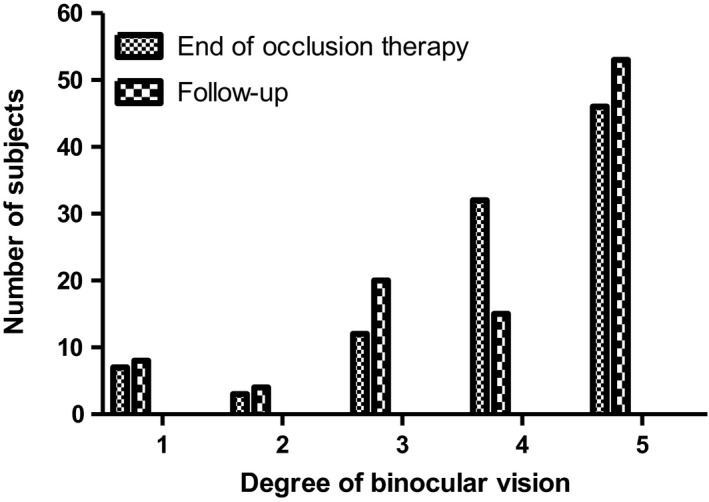
Binocular vision of the subjects at end of occlusion therapy and at the time of the follow‐up examination. The degree of binocular vision was arranged into five categories: 1. Bagolini negative, 2. Bagolini positive, 3. Bagolini and Titmus‐Fly positive, 4. TNO plate 480”‐240”, 5. TNO plate 120”‐15”.
Reading acuity
The mean reading acuity for the amblyopic eye was 0.19 ± 0.26 logMAR and 0.05 ± 0.14 logMAR for the fellow eye. Visual acuity at start of treatment was positively significantly correlated (Spearman correlation 0.377; p < 0.001) with reading acuity at follow‐up. Visual acuity at follow‐up and reading acuity at follow‐up were also significantly correlated (Spearman correlation 0.680; p < 0.001).
Discussion
This study evaluated the long‐term outcome of visual acuity in subjects who received occlusion therapy for amblyopia 12–15 years ago (Loudon et al. 2006). Visual acuity was measured under strictly controlled circumstances by the same orthoptist at end of therapy and at the long‐term follow‐up. Overall, we found good long‐term results of occlusion therapy: 74% had stable or improved IOD.
Risk factors for IOD increase included degree of anisometropia, increasing anisometropia, eccentric fixation and non‐compliance during occlusion therapy. A large IOD at the start of occlusion therapy was also significantly correlated with IOD increase after cessation of therapy. However, subjects with low initial VA also had worse compliance (Loudon et al. 2006) with patch wearing, but increased the most during therapy even with little patching. Subjects who increased the most during occlusion therapy were the most at risk for loss of logMAR lines after cessation of therapy explaining the found association between compliance and IOD increase after therapy.
Out of five subjects with severe deterioration (i.e. ≥2 logMAR lines) after cessation of occlusion therapy, four had a combined cause of amblyopia; three had increasing anisometropia and also had not worn their spectacles. One study comparable to ours was conducted by Simonsz‐Tóth (N = 137), who found 18 subjects (13%) with profound deterioration (>50% loss in visual acuity) in visual acuity 30 years after finishing occlusion treatment; 15 (11%) of these had an increase of anisometropia (Simonsz‐Toth et al. 2007). In that study, the orthoptist who had examined the subjects during occlusion therapy also took part in the follow‐up evaluation. In our study, we found a lower percentage with severe visual acuity deterioration. This difference could be attributed to differences in length of follow‐up time or improved spectacle wearing nowadays. We asked the subjects whether they had worn their spectacles, but on the basis of these data no statistically significant relationship was found.
Possible bias could be introduced to the analysis because of the 51% who either refused or could not be contacted. However, the statistical analysis showed that, except for gender, the subjects included for this study were a representative sample. We had more girls, maybe due to the fact that girls are more motivated to participate in a study. We also had missing data regarding the binocular vision, which could lead to a possible bias.
Another limitation in our study was that cycloplegic refraction was not performed. Our initial proposal did include cycloplegic refraction, but this was refused by most of the subjects. For calculation of the absolute refractive error, subjective refraction may not be ideal. However, for calculating the difference in refractive error between the eyes, that is the degree of anisometropia, it is expected that using subjective refraction is reliable. This is relevant as the degree of anisometropia and increasing anisometropia showed to be significantly correlated with IOD increase after cessation of occlusion therapy.
The Landolt‐C 17.2” chart does not fulfil all the criteria of a ‘crowded’ chart and therefore, visual acuity in the amblyopic eye may be over‐scored. As primary outcome measure in the study, we used visual acuity measured as much as possible according to ISO‐8596 Standard. The measurement of visual acuity with crowded optotypes is especially needed in the diagnosis and treatment of amblyopia, not so much in the final evaluation of the result of therapy. As we aimed to have comparable testing conditions for the follow‐up measurements, we preferred to use the same chart as in 2004 and measurements were performed by the same orthoptist (BST).
Finally, it cannot be ruled out that there are other unknown factors that could cause changes in visual acuity as children grow older. Therefore, as a measure for amblyopia, we used IOD as this would avoid part of the variability of the VA measurement.
We conclude that long‐term results of occlusion therapy as a treatment for amblyopia were successful. High or increasing anisometropia, eccentric fixation and non‐compliance during occlusion therapy were associated with increasing IOD and hence, with long‐term visual acuity decrease.
The corresponding author is a member of the Dutch Ophthalmological Societies.
References
- Bhola R, Keech RV, Kutschke P, Pfeifer W & Scott WE (2006): Recurrence of amblyopia after occlusion therapy. Ophthalmology 113: 2097–2100. [DOI] [PubMed] [Google Scholar]
- van de Graaf ES, van der Sterre GW, van Kempen‐du Saar H, Simonsz B, Looman CW & Simonsz HJ (2007): Amblyopia and Strabismus Questionnaire (A&SQ): clinical validation in a historic cohort. Graefes Arch Clin Exp Ophthalmol 245: 1589–1595. [DOI] [PubMed] [Google Scholar]
- Hertle RW, Scheiman MM, Beck RW et al. (2007): Stability of visual acuity improvement following discontinuation of amblyopia treatment in children aged 7 to 12 years. Arch Ophthalmol 125: 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Kraker RT, Beck RW et al. (2003): A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology 110: 2075–2087. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Beck RW, Kraker RT et al. (2004): Risk of amblyopia recurrence after cessation of treatment. J AAPOS 8: 420–428. [DOI] [PubMed] [Google Scholar]
- van Leeuwen R, Eijkemans MJ, Vingerling JR, Hofman A, de Jong PT & Simonsz HJ (2007): Risk of bilateral visual impairment in individuals with amblyopia: the Rotterdam study. Br J Ophthalmol 91: 1450–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiba H, Shimshoni M, Oliver M, Gottesman N & Levartovsky S (2001): Long‐term follow‐up of occlusion therapy in amblyopia. Ophthalmology 108: 1552–1555. [DOI] [PubMed] [Google Scholar]
- Levartovsky S, Oliver M, Gottesman N & Shimshoni M (1995): Factors affecting long term results of successfully treated amblyopia: initial visual acuity and type of amblyopia. Br J Ophthalmol 79: 225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon SE, Polling JR & Simonsz HJ (2003): Electronically measured compliance with occlusion therapy for amblyopia is related to visual acuity increase. Graefes Arch Clin Exp Ophthalmol 241: 176–180. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Fronius M, Looman CW, Awan M, Simonsz B, van der Maas PJ & Simonsz HJ (2006): Predictors and a remedy for noncompliance with amblyopia therapy in children measured with the occlusion dose monitor. Invest Ophthalmol Vis Sci 47: 4393–4400. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Passchier J, Chaker L et al. (2009): Psychological causes of non‐compliance with electronically monitored occlusion therapy for amblyopia. Br J Ophthalmol 93: 1499–1503. [DOI] [PubMed] [Google Scholar]
- Maaijwee K, Mulder P, Radner W & Van Meurs JC (2008): Reliability testing of the Dutch version of the Radner Reading Charts. Optom Vis Sci 85: 353–358. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group , Repka MX, Kraker RT et al.(2008): A randomized trial of atropine vs patching for treatment of moderate amblyopia: follow‐up at age 10 years. Arch Ophthalmol 126: 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves BC (2002): Taxonomy and epidemiology of amblyopia In: Mosely MJ & Fielder AR (eds). Amblyopia: a multidisciplinary approach. London: Butterworth‐Heinemann; 68–80. [Google Scholar]
- Repka MX, Beck RW, Holmes JM et al. (2003): A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol 121: 603–611. [DOI] [PubMed] [Google Scholar]
- Repka MX, Wallace DK, Beck RW et al. (2005): Two‐year follow‐up of a 6‐month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol 123: 149–157. [DOI] [PubMed] [Google Scholar]
- Rutstein RP & Corliss DA (2004): Long‐term changes in visual acuity and refractive error in amblyopes. Optom Vis Sci 81: 510–515. [DOI] [PubMed] [Google Scholar]
- Rutstein RP & Fuhr PS (1992): Efficacy and stability of amblyopia therapy. Optom Vis Sci 69: 747–754. [DOI] [PubMed] [Google Scholar]
- Saxena R, Puranik S, Singh D, Menon V, Sharma P & Phuljhele S (2013): Factors predicting recurrence in successfully treated cases of anisometropic amblyopia. Indian J Ophthalmol 61: 630–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott WE, Kutschke PJ, Keech RV, Pfeifer WL, Nichols B & Zhang L (2005): Amblyopia treatment outcomes. J AAPOS 9: 107–111. [DOI] [PubMed] [Google Scholar]
- Simonsz‐Toth B, Loudon SE, van Kempen‐du Saar H, van de Graaf ES, Groenewoud JH & Simonsz HJ (2007): [Evaluation of visual acuity in a historical cohort of 137 patients treated for amblyopia by occlusion 30–35 years ago]. Klin Monbl Augenheilkd 224: 40–46. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Fielder AR, Stephens DA & Cooperative M (2004a): Refractive adaptation in amblyopia: quantification of effect and implications for practice. Br J Ophthalmol 88: 1552–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Stephens DA & Fielder AR (2004b): Treatment dose‐response in amblyopia therapy: the Monitored Occlusion Treatment of Amblyopia Study (MOTAS). Invest Ophthalmol Vis Sci 45: 3048–3054. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Stephens DA, Fielder AR, Moseley MJ & Cooperative R (2007): Objectively monitored patching regimens for treatment of amblyopia: randomised trial. BMJ 335: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjiam AM, Holtslag G, Vukovic E, Asjes‐Tydeman WL, Loudon SE, Borsboom GJ, de Koning HJ & Simonsz HJ (2012): An educational cartoon accelerates amblyopia therapy and improves compliance, especially among children of immigrants. Ophthalmology 119: 2393–2401. [DOI] [PubMed] [Google Scholar]


