Abstract
Background
Warts are benign epithelial proliferations that result from human papillomavirus (HPV) infection occurring on the skin and mucosa. Patients express a significant reduction in quality of life due to this cosmetic nuisance, as well as functional problems and physical discomfort. Newer methods of wart removal include different energy‐based devices, mostly lasers. Nonablative lasers such as Nd:YAG have a higher success rate and are usually used with topical or infiltrative anesthesia. The procedure may be safer without anesthesia but still tolerable with an appropriate cooling and technique.
Aims
The purpose of this study is to report on our experience over 3 years since the approach without anesthesia has been utilized.
Patients/Methods
A retrospective chart review analysis of all 85 patients who underwent 1064 nm Nd:YAG wart removal without anesthesia between November 2016 and August 2019 was conducted. One of the main outcome measures was determining the number of sessions required in order to get full clearance.
Results
The mean number of sessions was 2.2 (range 1‐7). The mean VAS pain score during the procedure was 6 (range: 2‐10), and side effects were negligible.
Conclusion
Long‐pulse 1064‐nm Nd:YAG laser without any chemical anesthesia is safe and effective for the treatment of warts.
Keywords: 1064 nm, Nd:YAG, no anesthesia, warts
1. INTRODUCTION
Warts are benign epithelial proliferations, characteristically 1‐20 mm in diameter. The lesions result from more than 100 serotypes of human papillomavirus (HPV) infection occurring on the skin and mucosa 1 , 2 . Spontaneous regression can occur, but treatment is usually challenging and protracted. They are a common dermatological complaint, with an estimated incidence of 5%‐20% in children and adults, with peak incidence reported during teenage years 1 , 3 , 4 . Verrucas can be divided into two broad categories: cutaneous and extracutaneous. The cutaneous lesions include common warts, filiform warts, plane warts, plantar warts, anogenital warts, and bowenoid papulosis. Extracutaneous lesions occur on orificial mucous membranes and include oral common warts, oral condylomata acuminata, focal epithelial hyperplasia, oral florid papillomatosis, nasal papillomas, conjunctival papillomas, laryngeal papillomatosis, and cervical warts. 3 Patients often express a significant reduction in quality of life due to this cosmetic nuisance, as well as functional problems and physical discomfort when they occur on the palms of the hands and soles of the feet 5 . Therefore, cutaneous warts are one of the most common pathologies treated by the clinical dermatologist 6 .
Traditional therapeutic options for warts, such as topical therapy, cryotherapy, surgical excision, and electrocautery, have proven somewhat effective, but these approaches may offer incomplete and superficial results leading to high recurrence rates 4 . Topical management requires the application of drugs for long durations, and treatment success is therefore highly dependent on patient compliance. Newer methods include different energy‐based devices, mostly lasers. Ablative lasers, such as CO2 7 and Er:YAG 8 , 9 , have around a 70% success rate, similar to the “wait and see” approach. On the other hand, nonablative lasers have a higher success rate, 96% in the case of Nd:YAG 2 , 10 . One of the mechanisms of actions for this success is local hyperthermia, as shown by Huo 2 , 6 , 11 . Monochromatic light of a specific fluence and wavelength is absorbed by targeted tissue chromophores and converted to thermal energy, leading to selective tissue destruction. Depending on the pulse duration and energy density, this may result in either coagulation (photothermal effect) or blasting (photomechanical effect) of these structures 2 , 12 . Microscopic evaluation 7 days after the treatment showed separation of the dermo‐epidermal junction, epidermal necrosis, RBC extravasation, and destroyed blood vessels with dense inflammatory infiltrate in the dermis. This destruction may obliterate the nutrient supply to the wart 4 . Laser treatment was also shown to be effective in the destruction of HPV DNA, which is not achieved in cryotherapy of warts 6 .
The Nd:YAG laser is preferred over other laser treatment alternatives for its deeper penetrating 1064‐nm wavelength, which enables direct contact with warts while lowering the risk of pigmentation in dark skin types 5 , but is a relatively painful therapeutic option, so topical or infiltrative anesthesia is usually used 1 , 2 , 4 , 10 , 13 , 14 . However, direct, perilesional injections of anesthetic can lead to more local skin and tissue damage as reported by Smith 15 , and also seen first‐hand at our center.
Nd:YAG laser has been used at our clinic for many different indications, including wart removal, for over 15 years with great success. Since the procedure may be safer without anesthesia 15 but still tolerable with an appropriate cooling and technique, we have decided against anesthesia by default. This study presents our experience over 3 years since the approach without anesthesia has been utilized.
2. METHODS
2.1. Patients
This was a retrospective chart review study that was conducted at the Medilase Laser Center, Ljubljana, Slovenia. Ethics approval (No. 0120‐448/2019/9) was obtained from the National Medical Ethics Committee of the Ministry of Health of the Republic of Slovenia, and the study was conducted according to the Declaration of Helsinki.
All patients treated for wart removal at the Medilase Laser Center from November 2016 to August 2019 looking were included in this retrospective study. All the patients provided written informed consent before the treatment. Each wart was photographed before and a few weeks after the final treatment. Patients were asked about any previous treatments used for wart removal. Patients were also asked to evaluate the highest level of pain during the treatment on a 0‐10 scale. The highest reported score in all the sessions was recorded.
2.2. Procedure
At the initial visit, thick and hyperkeratotic warts were first thinned down using a scalpel or Er:YAG laser (SP Dynamis, Fotona, Slovenia). Patients were encouraged to remove the hyperkeratotic part of their warts at home as much as possible for the subsequent treatments. Laser treatment was administered using 1064‐nm long‐pulsed Nd:YAG laser (SP Dynamis, Fotona, Slovenia), as follows: handpiece, R33‐T; spot size, 2‐4 mm; pulse duration, 20‐25 ms; fluence, 130‐270 J/cm2. Lower fluence settings were used for thinner warts and patients with a lower pain threshold. All warts were administered multiple pulses as described by Bingol 2 , 14 . The pulse was not directed over the lesion; instead, we overlapped the circle of all pulses over the wart, with the aim of reaching the maximum energy level over the wart by centrally overlapping pulses, so as to protect the adjacent tissue from unintended damage. The treatment was stopped when a slight graying and contraction of the wart, and a 1‐ to 2‐mm margin of normal‐appearing tissue was observed. About 35‐40 shots were delivered (around 10 sequences of 3‐4 shots) for a wart with a diameter of around 1 cm (see Figure 1). Ice cubes were used in between sequences of 3‐4 laser pulses, and cold air (Cryo 6, Zimmer Germany) was used throughout the procedure, with the cooling level set at 5 to minimize pain and thermal damage to the surrounding tissue. All patients and the physician administering the treatment wore appropriate eye protection during application of the laser treatment. No topical or infiltrative chemical anesthetics were administered before laser treatment. No special aftercare ointment or dressing was used; patients were encouraged to use a regular moisturizer. The patients were instructed to come for a checkup in 4‐6 weeks.
FIGURE 1.
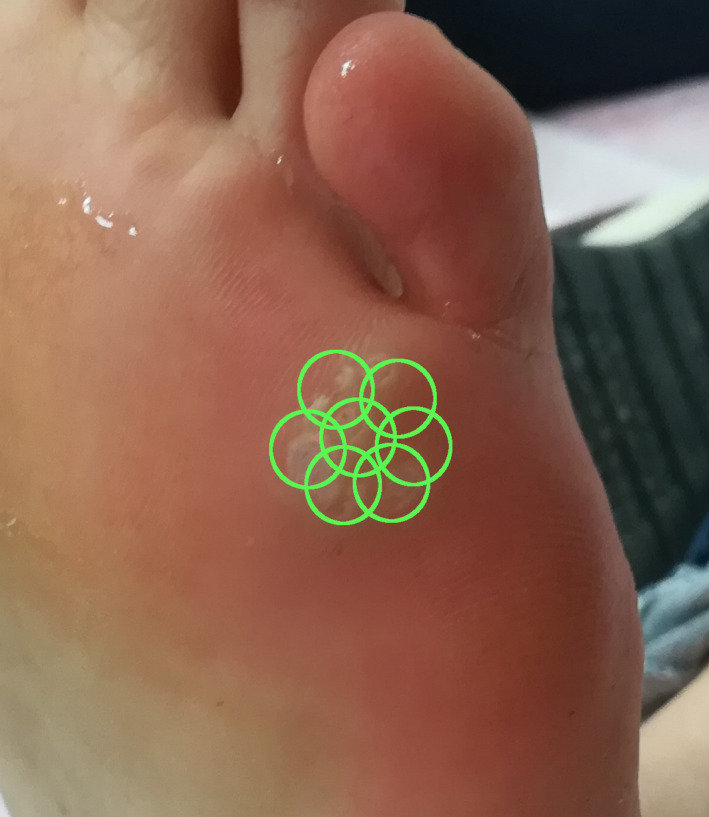
Pulse placement technique
3. RESULTS
85 patients, 45 women and 40 men, with a combined total of 174 warts were included. The mean patient age was 31 (range 7‐69 years) (Table 1). More than half of the lesions were plantar warts (n = 114, 66%); there were 22 warts on toes, 26 on fingers, 9 on palms, and 3 on knees. One third of the patients (n = 32, 37%) was treatment naive; the others had cryotherapy, topical treatments (mostly chloroacetic acid), or manual/mechanical removal; two patients had received all 3 procedures (Table 1).
TABLE 1.
Demographic characteristics of patients
| Age (mean (range)) | 31 (7‐69) |
| Duration of the disease (y) | 2 (0.2‐11) |
| Previous treatment (n, (%)) | |
| Cryotherapy | 25 (29) |
| Topical therapy | 31 (37) |
| Mechanical removal | 8 (9.4) |
| None | 32 (37) |
The minimal follow‐up period was 12 months for each patient, but in some patients, follow‐ups were as long as 3 years (see Figures 2, 3, 4, 5, 6, 7). Out of 85 patients included, a fifth of the patients (n = 17) did not finish with the treatments; 3 patients were lost to follow‐up, while 14 continued with other methods (surgical excision, cryotherapy, or OTC topical treatment). Four of these patients listed pain as the main reason; one said he was bothered by the smell during the procedure, while others did not provide a special reason for discontinuation of the laser therapy.
FIGURE 2.
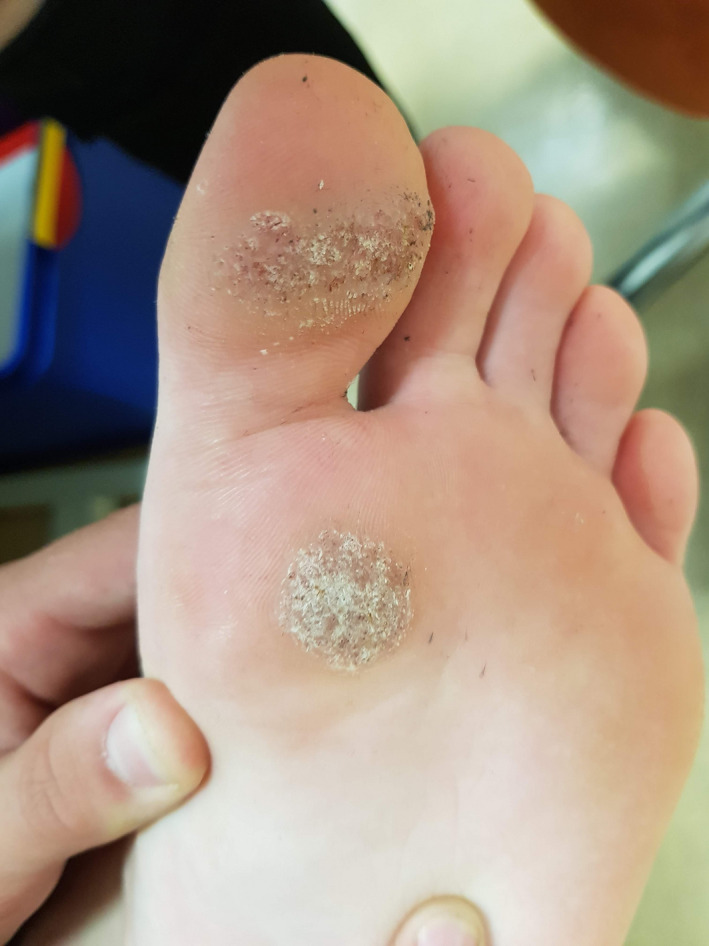
Patient 1—Before the treatment
FIGURE 3.
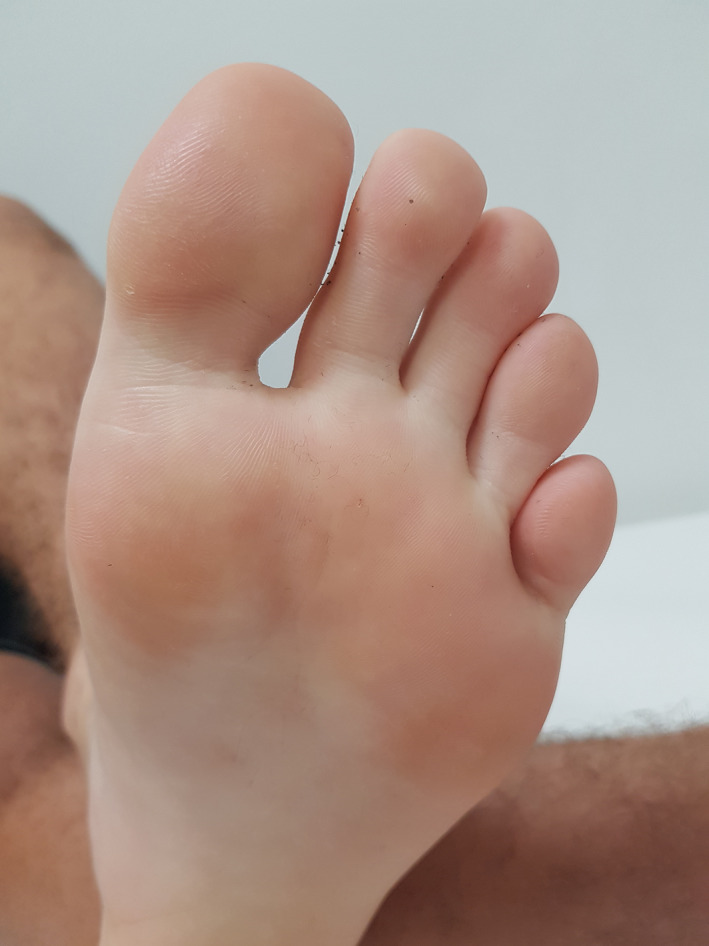
Patient 1—16 mo later (2 treatments)
FIGURE 4.
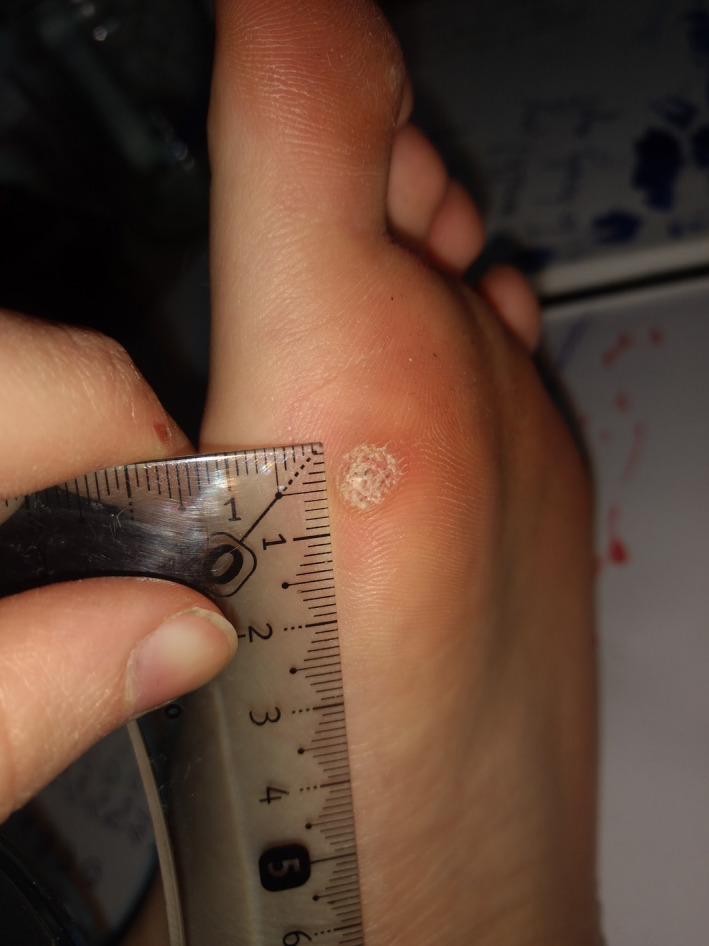
Patient 2—Before the treatment
FIGURE 5.
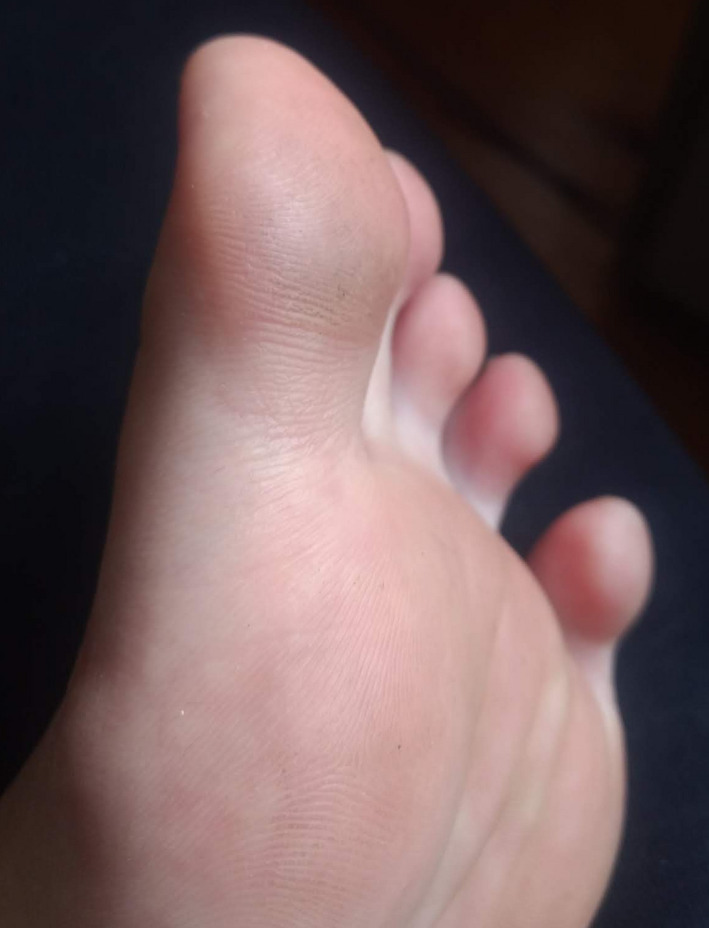
Patient 2—9 mo after a single treatment
FIGURE 6.
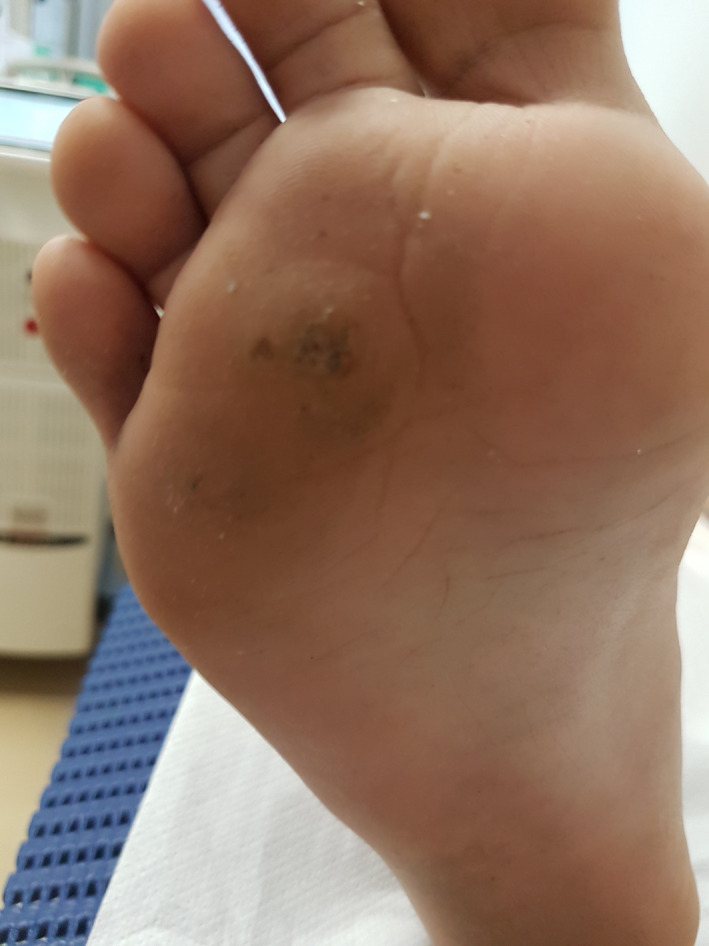
Patient 3—Before the treatment
FIGURE 7.

Patient 3—1 y after a single treatment
The mean number of sessions was 2.2 (range 1‐7); the number of treatments needed for complete clearance of warts in 68 patients is presented in Table 2, and the overall success rate is presented in Figure 8.
TABLE 2.
No. of treatments required to achieve complete clearance (n = 68). 17 patients discontinued their treatment
| No. of treatments | No. of patients | % of patients | Cumulative clearance rate (%) |
|---|---|---|---|
| 1 | 32 | 47 | 47 |
| 2 | 13 | 19 | 66 |
| 3 | 12 | 18 | 84 |
| 4 | 5 | 7.4 | 91 |
| 5 | 3 | 3.5 | 96 |
| 6 | 2 | 2.9 | 99 |
| 7 | 1 | 1.5 | 100 |
FIGURE 8.
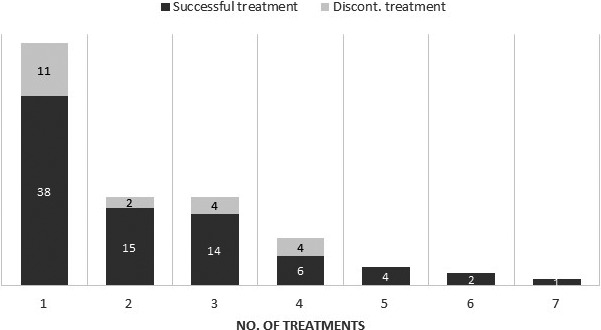
Treatment success rate. Results are presented as the percentage of patients who either successfully completed the treatment or discontinued the therapy after a specified number of treatment sessions (n = 85)
The intended treatment interval was 4‐6 weeks, but in fact it was a mean of 41 days between the 1st and 2nd session and 60 days between the 2nd and 3rd treatment, with a mean of 51 days for all sessions for the 68 patients who completed the treatment.
Associations between previous treatment, location of the wart, age of the patient, duration of the disease, and success of the treatment were tested using a chi‐square test of independence. No association tested was statistically significant.
The mean VAS pain score during the procedure was 6 (range: 2‐10). All treated lesions healed without major problems after the procedure; there were a few cases (4) of blisters and 14 patients reported slight pain in the next 2‐3 days, especially when exerting pressure on the wart, for example, walking. No hypo/hyperpigmentation or scarring was reported by any of the patients.
4. DISCUSSION
Warts are growths on the skin caused by HPV and can appear anywhere on the body. They are very common, usually asymptomatic and heal spontaneously. If lesions persist for a long time, increase in size or number, or cause pain or aesthetic problems, treatment is recommended. Topical, local, systemic, and intralesional medical treatments have been used to treat warts; however, none have been shown to be completely effective 16 . Most of these options require the application of drugs for long durations, and treatment success is therefore highly dependent on patient compliance. Surgical removal with a 1‐mm margin is also being used, but scarring and the potential for functional and cosmetic deformities is a major drawback 17 .
Recently, lasers have also been used to treat warts, including CO2 7 , Er:YAG 8 , 9 , 18 , PDL, and Nd:YAG laser 1 . Lasers can be used as a monotherapy or in conjunction with topical or intralesional agents 18 , 19 . The overall success rates vary substantially 16 . According to studies, the Nd:YAG laser has the highest success rate; the largest series of warts treated with Nd:YAG laser by Han 4 showed 96% in 4 sessions, Bingol 2 and Maletič 20 reported 100% clearance in 2 sessions 2 , and Alshami removed warts in 97% of his patients with 3 sessions 10 . Although the mechanism of action of Nd:YAG laser on warts remains unknown, the laser target is wart vessels, as damaging the vessels leads to necrosis of the wart. In addition, direct thermal injury to HPV may also play a role 6 .
The effectiveness of 1064 nm Nd:YAG laser is due to its deep penetration in the tissue; however, this can also lead to a relatively painful procedure, so topical or infiltrative anesthesia must be used in some cases. Our personal experience is that topical anesthesia (EMLA or similar) does not reduce the pain much more than placebo, so we usually do not use it. On the other hand, the use of infiltrative anesthesia was shown to lead to more skin and tissue destruction either through some direct effect as Smith theorized 15 or because of simply overtreating due to the lack of feedback from the patient. Therefore, we usually do not apply any chemical anesthesia and use only ice cubes and forced cold‐air cooling. Compared to previous studies reporting on the efficacy of wart laser therapy, the treatment success rate in this study is lower than in some studies 2 , 10 ; however, it is similar to the study by Han who treated 369 warts and had a 96% success rate in 4 sessions compared to our 91% when considering patients who received at least 4 sessions. When all patients (those that decided for a different method due to pain, smell, price, or were lost to FU) included in the study are considered, then the success rate after 4 treatments is 73%, which is similar to other studies where no anesthesia was used 15 .
The second reason for a lower success rate is that the interval between the treatments was longer than intended and reported in other studies, mainly due to patient compliance. Pain was the limiting factor; only a sequence of 3‐4 consecutive shots was tolerated by most of the patients, after which a short pause with contact ice cooling was needed. Patients who had experience with cryotherapy reported of similar discomfort during the procedure, but on the other hand reported of much less problems and pain over the following days after the laser therapy in comparison with cryotherapy. Overall side effects were negligible. Slight pain in the area, with or without serous or hemorrhagic bullae (see Figures 9, 10, 11, 12, 13, 14), over the next few days was considered normal and a part of the healing process 14 and only experienced by about 30% of the patients. Other, permanent side effects commonly reported by other authors using topical and/or infiltrative anesthesia were not observed (hypo/hyperpigmentation, ulceration, scarring) 2 , 4 , 10 , 13 , 15 . In our opinion, this is due to the fact that no chemical anesthesia was used, so the patients were able to give feedback information and the treatment could be adjusted or stopped.
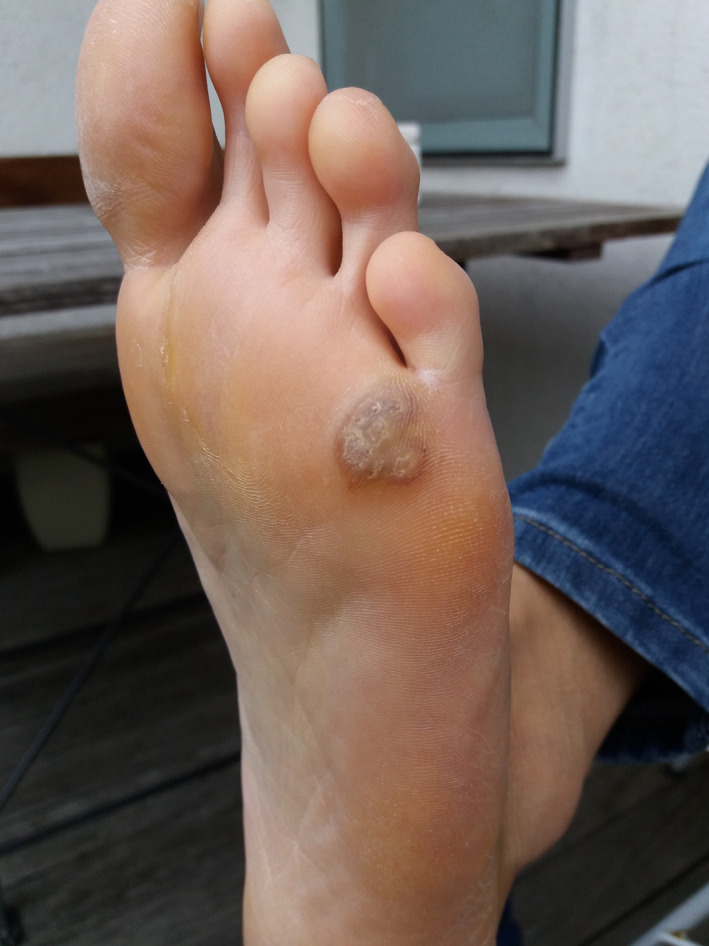
FIGURE 9.
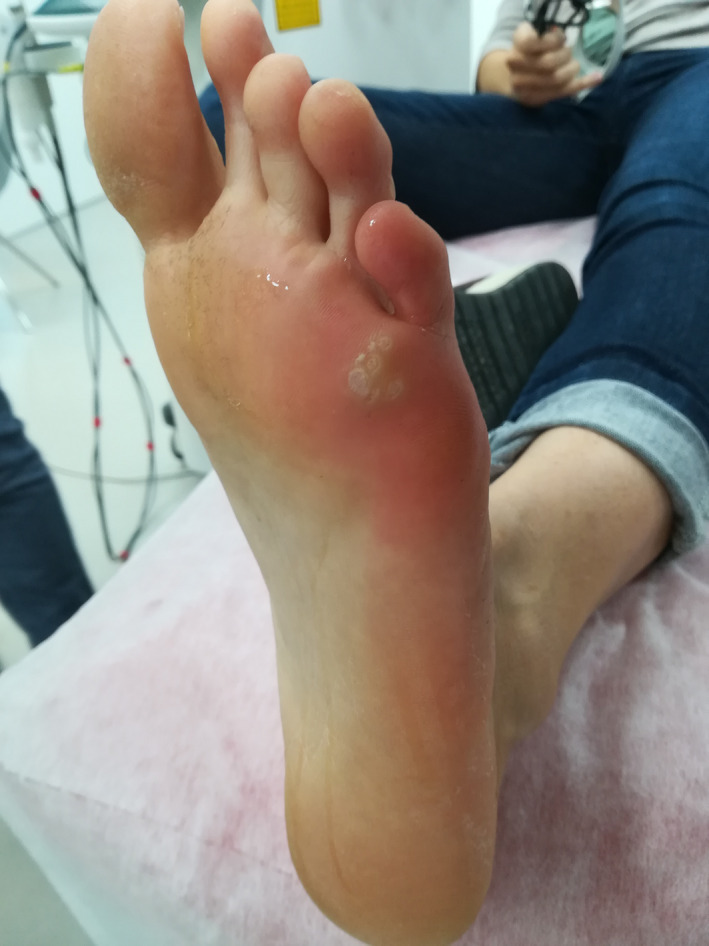
Patient 4—Before the treatment
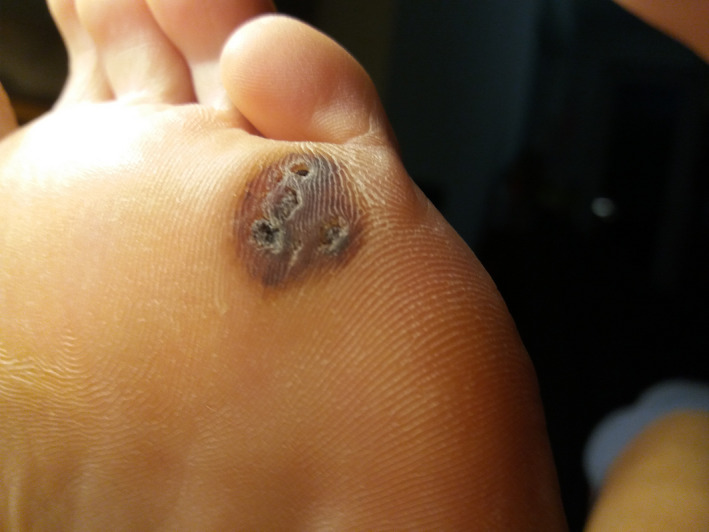
FIGURE 10.
Patient 4—5 d after the treatment
FIGURE 11.
Patient 4—20 d after the treatment
FIGURE 12.
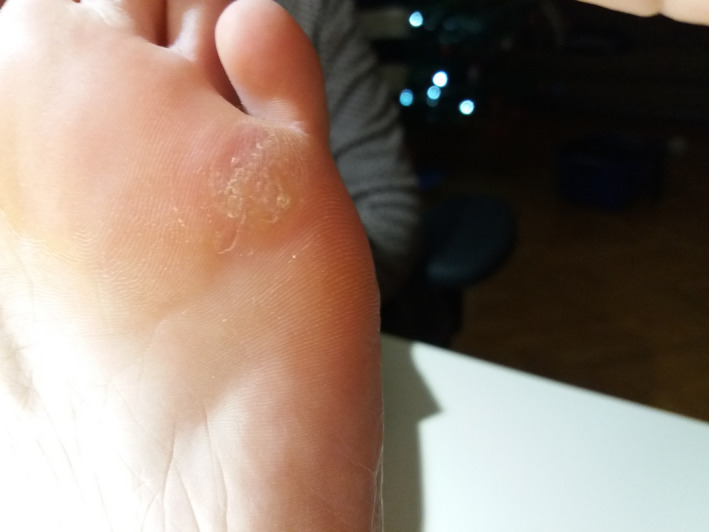
Patient 4—6 wk after the treatment
FIGURE 13.
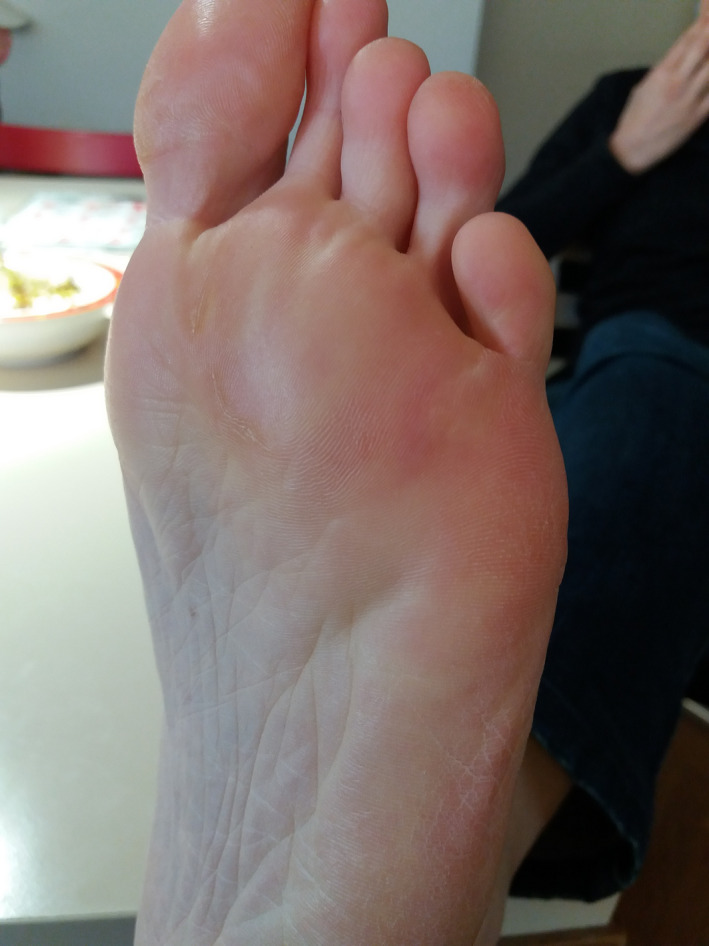
Patient 4—6 mo after the treatment
FIGURE 14.
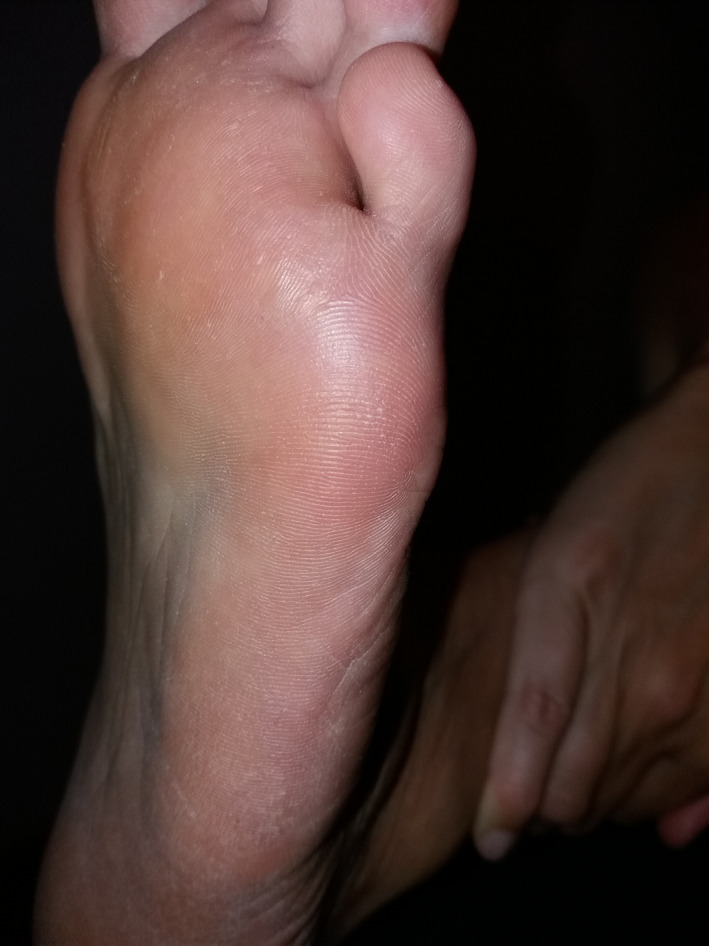
Patient 4—14 mo after a single treatment
We have not been able to show any statistically significant association between previous treatment, location of wart, age of the patient, duration of the disease, and success of the treatment when using a chi‐square test of independence, as was reported in some other studies 1 , 10 .
5. CONCLUSION
Long‐pulse 1064‐nm Nd:YAG laser without any chemical anesthesia is safe and effective for the treatment of warts, with response rates similar or higher than those obtained with conventional therapies, irrespective of wart location, age of the patient, and duration of the disease. Nevertheless, infiltrative anesthesia should be considered in specific cases—only 5% of our patients listed pain as the reason for discontinuation. More randomized control trials comparing Nd:YAG laser with standard treatments are needed in order for this method to be recommended as a first‐line treatment and therefore covered by insurance.
Zorman A, Koron N. Wart removal without anesthesia using long‐pulse 1064‐nm Nd:YAG laser. J Cosmet Dermatol.2021;20:506–512. 10.1111/jocd.13593
REFERENCES
- 1. El‐Mohamady AE‐S, Mearag I, El‐Khalawany M, Elshahed A, Shokeir H, Mahmoud A. Pulsed dye laser versus Nd:YAG laser in the treatment of plantar warts: a comparative study. Lasers Med Sci. 2014;29(3):1111‐1116. [DOI] [PubMed] [Google Scholar]
- 2. Bingol UA, Cömert A, Cinar C. The overlapped triple circle pulse technique with Nd:YAG laser for refractory hand warts. Photomed Laser Surg. 2015;33(6):338‐342. [DOI] [PubMed] [Google Scholar]
- 3. Cobb MW. Human papillomavirus infection. J Am Acad Dermatol. 1990;22(4):547‐566. [DOI] [PubMed] [Google Scholar]
- 4. Han TY, Lee JH, Lee CK, Ahn JY, Seo SJ, Hong CK. Long‐pulsed Nd:YAG laser treatment of warts: Report on a series of 369 cases. J Korean Med Sci. 2009;24(5):889‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsu VM, Aldahan AS, Tsatalis JP, Perper M, Nouri K. Efficacy of Nd:YAG laser therapy for the treatment of verrucae: a literature review. Lasers Med Sci. 2017;32(5):1207‐1211. [DOI] [PubMed] [Google Scholar]
- 6. El‐Tonsy MH, Anbar TED, El‐Domyati M, Barakat M. Density of viral particles in pre and post Nd:YAG laser hyperthermia therapy and cryotherapy in plantar warts. Int J Dermatol. 1999;38(5):393‐398. [DOI] [PubMed] [Google Scholar]
- 7. Boroujeni NH, Handjani F. Cryotherapy versus CO2 laser in the treatment of plantar warts: a randomized controlled trial. Dermatol Pract Concept. 2018;8(3):168‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wollina U, Konrad H, Karamfilov T. Treatment of common warts and actinic keratoses by Er:YAG laser. J Cutan Laser Ther. 2001;3(2):63‐66. [DOI] [PubMed] [Google Scholar]
- 9. Balevi A, Ustuner P, Ozdemir M. Use of Er:YAG for the treatment of recalcitrant facial verruca plana. J Dermatolog Treat. 2017;28(4):368‐371. [DOI] [PubMed] [Google Scholar]
- 10. Alshami MA, Mohana MJ. Novel treatment approach for deep palmoplantar warts using long‐pulsed 1064‐nm Nd:YAG laser and a moisturizing cream without prior paring of the wart surface. Photomed Laser Surg. 2016;34(10):448‐455. [DOI] [PubMed] [Google Scholar]
- 11. Huo W, Gao X, Sun X et al Local hyperthermia at 44°C for the treatment of plantar warts: a randomized, patient‐blinded, placebo‐controlled trial. J Infect Dis. 2010;201(8):1169‐1172. [DOI] [PubMed] [Google Scholar]
- 12. Kopera D. Verrucae vulgares: flashlamp‐pumped pulsed dye laser treatment in 134 patients. Int J Dermatol. 2003;42(11):905‐908. [DOI] [PubMed] [Google Scholar]
- 13. Shin YS, Cho EB, Park EJ, Kim KH, Kim KJ. A comparative study of pulsed dye laser versus long pulsed Nd:YAG laser treatment in recalcitrant viral warts. J Dermatolog Treat. 2017;28(5):411‐416. [DOI] [PubMed] [Google Scholar]
- 14. Kimura U, Takeuchi K, Kinoshita A, Takamori K, Suga Y. Long‐pulsed 1064‐nm neodymium:yttrium‐aluminum‐garnet laser treatment for refractory warts on hands and feet. J Dermatol. 2014;41(3):252‐257. [DOI] [PubMed] [Google Scholar]
- 15. Smith EA, Patel SB, Whiteley MS. Evaluating the success of Nd: YAG laser ablation in the treatment of recalcitrant verruca plantaris and a cautionary note about local anaesthesia on the plantar aspect of the foot. J Eur Acad Dermatology Venereol. 2015;29(3):463‐467. [DOI] [PubMed] [Google Scholar]
- 16. Jordan Witchey D, Brianne Witchey N, Roth‐Kauffman MM, Kauffman MK. Plantar warts: epidemiology, pathophysiology, and clinical management. J Am Osteopath Assoc. 2018;118(2):92‐105. [DOI] [PubMed] [Google Scholar]
- 17. Mulhem E. Treatment of nongenital warts. Am Fam Physician. 2011;84(3):288‐293. [PubMed] [Google Scholar]
- 18. Wollina U. Er:YAG laser followed by topical podophyllotoxin for hard‐to‐treat palmoplantar warts. J Cosmet Laser Ther. 2003;5(1):35‐37. [PubMed] [Google Scholar]
- 19. Baczako A, Krautheim V, Biedermann T, Volz T. Combination of surgery and Nd:YAG laser therapy for recalcitrant viral warts: a successful therapeutic approach for immunosuppressed patients. Acta Derm Venereol. 2019;99(3):349‐350. [DOI] [PubMed] [Google Scholar]
- 20. Maletic A, Maletic I, Maletic D. CASE REPORT: combination of Er:YAG and Nd:YAG laser for treatment of warts. J Laser Heal Acad. 2015;2015(1):1‐4. [Google Scholar]


