Abstract
Background
Delayed gastric emptying is the leading cause of enteral feeding intolerance (EFI) in critical illness. This phase 2a study compared TAK‐954, a selective agonist of 5‐hydroxytryptamine type 4 receptors, with metoclopramide in critically ill patients with EFI (NCT01953081).
Methods
A blinded, double‐dummy trial was conducted in mechanically ventilated patients with EFI (>200 mL gastric residual volume within 24 hours before randomization). Patients were randomized to receive either 0.5 mg intravenous TAK‐954 over 1 hour then 0.9% saline injection 4 times/d (sham metoclopramide) or the active comparator 10 mg intravenous metoclopramide 4 times/d and a 1‐hour 0.9% saline infusion. After initial dosing, participants received a radiolabeled meal of liquid nutrient (Ensure; 106 kcal), and gastric emptying was measured (scintigraphy). Adverse events (AEs) were recorded from the time of consent through to day 5; serious AEs were collected to day 30.
Results
Thirteen patients (TAK‐954, n = 7; metoclopramide, n = 6) participated. Five patients in the TAK‐954 group and 4 in the metoclopramide group experienced AEs (2 and 3, respectively, were serious). All AEs except 1 (diarrhea in the metoclopramide group) were considered unrelated to study drug. Following treatment, a greater proportion of patients receiving TAK‐954 had normal gastric retention (<13% retention at 180 minutes) than those receiving metoclopramide (6/7 vs 3/6 patients, respectively).
Conclusion
A single dose of 0.5 mg intravenous TAK‐954 appears to have at least similar efficacy in accelerating gastric emptying to multiple doses of 10 mg metoclopramide and was not associated with increased AEs.
Keywords: critically ill, enteral feeding intolerance, gastric emptying, metoclopramide, scintigraphy, TAK‐954 (td‐8954)
Introduction
Gastrointestinal motility is frequently decreased in critically ill patients receiving enteral nutrition. 1 , 2 Delayed gastric emptying is the leading cause of enteral feeding intolerance (EFI), which occurs in up to 30% of critically ill patients, compromises nutrition status, and is associated with increased morbidity and mortality. 3 , 4 EFI is usually defined by a large gastric residual volume (GRV). The treatment of EFI usually involves administration of a prokinetic drug, the most frequently prescribed agent being metoclopramide. 4 , 5 , 6 , 7 However, metoclopramide has an adverse event (AE) profile such that there is a need for alternative agents. 1 , 8
The nonselective serotonin (5‐hydroxytryptamine) type 4 (5‐HT4) receptor agonist cisapride was previously used as an effective gastrointestinal prokinetic drug to accelerate gastric emptying and reduce EFI in critically ill patients. 9 , 10 , 11 , 12 However, cisapride was withdrawn from the market because of the potential for serious cardiovascular events. 1 In contrast to nonselective agonists, highly selective 5‐HT4 receptor agonists, such as TAK‐954 (previously TD‐8954), appear to have an improved safety profile with respect to the risk of cardiac arrhythmias. 13 , 14 However, none of these agents have been tested in critically ill individuals.
The major objectives of this phase 2a study in critically ill patients with EFI included an evaluation of the safety and tolerability of a single dose of TAK‐954, gastric emptying after a single dose of TAK‐954 when compared with multiple doses of metoclopramide, and the pharmacokinetics of TAK‐954. An additional objective included assessment of the efficacy of a single dose of TAK‐954 on upper gastrointestinal motility as measured by GRV.
Methods
This was a single‐center, double‐blind, double‐dummy, phase 2a, randomized controlled trial. The study protocol was developed by collaboration between independent investigators (MJC, KLJ, and AMD) and the sponsor, Theravance Biopharma US, Inc. The study was conducted by the investigators, who had access to all data. The protocol was approved by the Human Research Ethics Committee of the Royal Adelaide Hospital, and prior written informed consent was obtained from each patient's surrogate decision maker.
The sponsor was responsible for the Safety Data Review Committee, which comprised the sponsor clinical study director, medical monitor, a scientist with expertise in pharmacokinetics, a professional biostatistician, and an independent external clinician experienced in the safety data review of critically ill patients. The sponsor was also responsible for registering the trial with ClinicalTrials.gov (NCT01953081).
Study Participants
Patients were eligible for inclusion in the study if they were aged ≥18 years and ≤85 years, invasively mechanically ventilated and anticipated to remain on mechanical ventilation for at least 2 days after enrollment, and had developed EFI (defined as a GRV ≥200 mL) during the delivery of enteral nutrition 3 within the 24 hours before randomization. Patients were selected for enrollment with the expectation that they would be alive for at least 4 days after randomization, be able to complete all study procedures, and, if receiving vasopressors at the time of randomization, be on stable or decreasing doses. 15 , 16
Patients were excluded from the study if they had undergone esophageal or gastric surgery or had experienced traumatic injury to the gastrointestinal tract resulting in this admission; had a known history of diabetic or idiopathic gastroparesis 17 ; had a blood glucose level >15 mmol/L at screening 18 ; were admitted because of a drug overdose; had received any investigational agent or had used an investigational medical device within 30 days of screening; had a hypersensitivity or contraindication to metoclopramide; had received a serotonin‐specific reuptake inhibitor, anticholinergic, or acetylcholinesterase inhibitor drug within the previous 72 hours; had impaired renal function (estimated creatinine clearance rate <30 mL/min using the Cockcroft–Gault formula) unless receiving intermittent or continuous dialysis at the time of randomization; had a serum bilirubin or alkaline phosphatase concentration over twice the upper limit of normal or a serum alanine aminotransferase or aspartate aminotransferase level over three times the upper limit of normal; had received erythromycin or metoclopramide in the 24 hours before screening, domperidone in the previous 48 hours, or azithromycin in the previous 2 weeks; had a heart rate of at least 150 beats per minute or >5 beats of ventricular tachycardia within 24 hours, a prolonged corrected QT interval (>450 ms in men or >470 ms in women), or a history of congenital long QT syndrome, acute myocardial ischemia, or infarction on this admission; were pregnant; or had a gastric pacemaker.
Participants were randomized in a 1:1 ratio to receive either the intervention (TAK‐954) or an active comparator (metoclopramide, control). Randomization was based on a computer‐generated list, with details held by the central hospital pharmacy.
Study Drugs
Study drugs were prepared by the Department of Pharmacy within the Royal Adelaide Hospital. TAK‐954 (0.5 mg/5 mL) was diluted in 100 mL of 0.9% sodium chloride and presented in a VIAFLEX bag (Baxter, Sydney, Australia). Sham TAK‐954 was 5 mL of 0.9% sodium chloride for injection, which was added to 100 mL of 0.9% sodium chloride and presented in a VIAFLEX bag. Metoclopramide hydrochloride (Pfizer, Melbourne, Australia; 10 mg/2 mL) was diluted in 8 mL of 0.9% sodium chloride for injection (the usual delivery method) and presented in a 10‐mL syringe. Sham metoclopramide was 10 mL of 0.9% sodium chloride for injection, presented in a 10‐mL syringe. All study and clinical personnel other than those involved in preparation were “blinded” to the intervention because study drugs and sham drugs were identical in appearance.
Patients were randomized either to receive a single 1‐hour 0.5‐mg intravenous infusion of TAK‐954 (115 mL) and intravenous injections of 0.9% sodium chloride (10 mL) every 6 hours (sham metoclopramide; total of 4 injections) or to receive an active comparator; these patients received a 1‐hour intravenous infusion of 0.9% sodium chloride (sham TAK‐954) and intravenous injections of 10 mg metoclopramide every 6 hours (total of 4 injections).
TAK‐954 (0.5 mg) or sham TAK‐954 was administered intravenously over 1 hour. Fifteen minutes before the end of the infusion, 10 mL metoclopramide or sham metoclopramide was also administered via slow intravenous injection. Participants subsequently received 10 mg metoclopramide or sham metoclopramide at regular intervals after the first injection (schedule in Figure 1).
Figure 1.
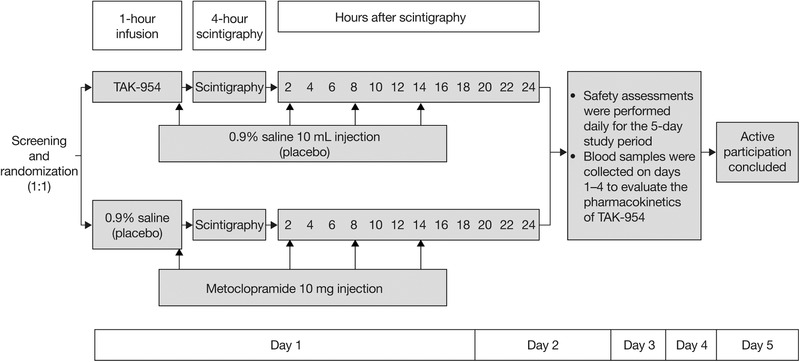
Study design.
TAK‐954 has previously been evaluated in a multiple‐dose IV study in healthy adult participants (18 to 45 years old) at doses ranging from 0.1 to 0.5 mg once/d for 5 consecutive days. 19 TAK‐954 was shown to be generally well tolerated upon repeated IV doses, with no serious adverse events reported or significant safety issues identified. 19 The IV dose of 0.5 mg TAK‐954 was selected for this study based on prior human data 19 and was anticipated to be well tolerated in the intended study population and to demonstrate an effect of increased gastrointestinal motility.
Test Meal
The test meal comprised 100 mL of Ensure (Abbott, Melbourne, Australia), a nutrient liquid representative of standard formulae (64% carbohydrate, 21% lipid, 13% protein; 1.06 kcal/mL), 20 radiolabeled with 20 MBq technetium‐99m calcium phytate (Radpharm Scientific, Belconnen, ACT, Australia). 21
Protocol
The study protocol is summarized in Figure 1. Enteral feeding was ended following randomization, before study drug infusion. When the intravenous infusion of TAK‐954 or sham TAK‐954 was completed (ie, at t = 1 hour), GRV was aspirated, and the test meal of 100 mL of radiolabeled Ensure was administered. Gastric emptying was then assessed over the subsequent 4 hours. At the end of this scintigraphic measurement period, enteral feeding was recommenced at the goal rate (determined in accordance with the usual feeding protocol of that intensive care unit), and GRVs were measured at 6‐hour intervals. 22
A study participant's drug infusion may have been prematurely stopped at any time if this was considered necessary based on the clinical judgment of the investigator. Furthermore, infusion was paused if one of the following situations occurred during the infusion: sustained (≥1 minute) heart rate of 150 bpm or more or sustained increase of 30 bpm or more compared with the pre‐dose measurement; sustained mean arterial pressure of <60 mmHg or sustained decrease of >10 mmHg compared with the pre‐dose measurement; or an AE of at least moderate severity that was considered potentially related to the infusion. Following this pause, infusion was resumed or not resumed, depending on whether the participant's condition improved.
Outcome Measures
Safety and tolerability
Safety analyses included assessments of systolic and diastolic blood pressure, heart rate, ventilation status, and body temperature (observed values and changes from baseline). These were performed at 30 and 60 minutes after the start of the infusion and again at 2, 3, 4, 6, 8, 10, and 12 hours. Data for vital signs were subsequently collected twice daily for 5 days after the first study drug administration. Electrocardiograms (ECGs) were performed in triplicate at intervals of ≈1 minute at 1, 6, 12, 24, and 48 hours after the start of study drug infusion. ECGs were subsequently carried out daily (single, not triplicate). Blood samples were obtained for full blood count, serum biochemistry evaluation, and liver function tests at screening and on days 2, 3, 4, and 5.
Gastric emptying
The radiolabeled meal was administered via a nasogastric tube over 5 minutes. A mobile γ camera (Digirad, Poway, CA, USA) recorded images in dynamic mode at 1‐minute intervals with the camera positioned in the left anterior oblique position to correct for γ ray attenuation. 23 Scintigraphic data were analyzed by a qualified nuclear medicine technologist (KLJ) who was blinded to the study conditions. Radioisotopic data were corrected for subject movement and radionuclide decay. A region of interest that was drawn around the total stomach and gastric emptying curves, expressed as percentage retention, was generated over time. 24 Intragastric retention was derived at 15‐minute intervals from t = 0 to t = 60 minutes and at 30‐minute intervals thereafter until t = 240 minutes. Normal gastric retention was defined as <13% at 180 minutes based on previously published data, 25 and abnormal gastric retention was defined as any proportion ≥13% at 180 minutes.
Data on AEs were recorded as per the International Conference on Harmonization Guideline for Good Clinical Practice from the time of consent through to the final follow‐up assessment on day 5. AEs could be observed by study or intensive care unit personnel, spontaneously reported by the participant, or reported in response to a standard question from study personnel. The relationship of AEs to study drug therapy was assessed as being not related or possibly/probably related for all AEs. The relationship was deemed as “possibly/probably related” if there was a reasonable temporal sequence from administration of a study drug or for which possible involvement of the study drug could not be excluded—although factors such as underlying diseases or concomitant treatments may be responsible. The relationship was deemed not related if the AE did not follow a reasonable temporal sequence from administration of a drug and/or could be reasonably be explained by other factors. Data for serious AEs were collected through day 30.
Pharmacokinetics
Drug concentrations were measured in arterial blood, obtained within 30 minutes of dosing for baseline, at the time of dosing commencement (t = 0 minute), at t = 30 minutes, and at t = 60 minutes (immediately before the end of the infusion). Samples were also collected at t = 75 minutes, t = 90 minutes, t = 2, 3, 4, 6, 8, 10, and 12 hours after the start of the infusion, and daily thereafter, censored at day 5. Drug concentrations were determined using liquid chromatography–mass spectrometry (Quintiles BioSciences, New York, NY, USA). Plasma concentration–time data were analyzed using standard noncompartmental methods. Summary statistics were calculated for plasma concentrations for each time point and treatment group. As part of the major study objectives, pharmacokinetic parameters were calculated to evaluate pharmacokinetic and pharmacodynamic characteristics of TAK‐954 because the pharmacokinetic profile of TAK‐954 is less well known than that of metoclopramide. Actual sampling times were used for pharmacokinetic analyses.
Statistical Analyses
Data are presented as frequencies and proportions for categorical variables and as mean (standard deviation [SD]) and/or median (25% quartile, 75% quartile) for continuous variables. For the primary pharmacodynamics end point, summary statistics were calculated for gastric retention for each treatment group at t = 180 minutes. Owing to the early termination of the trial and the small sample size, inferential analyses were not conducted. 26
Sample size
The proposed sample size of this study was selected on the basis of clinical considerations for early development studies. A sample size of 60 participants was considered adequate to provide initial characterization of safety assessments within this setting. Owing to slower‐than‐expected enrollment, the study was terminated early with the intent to provide descriptive summaries of the outcomes.
Results
The trial was conducted between February 1, 2014, and August 23, 2014. Participant involvement is summarized in Figure 2. Thirteen individuals were enrolled; 7 received TAK‐954, and 6 received metoclopramide. Patient characteristics are summarized in Table 1.
Figure 2.
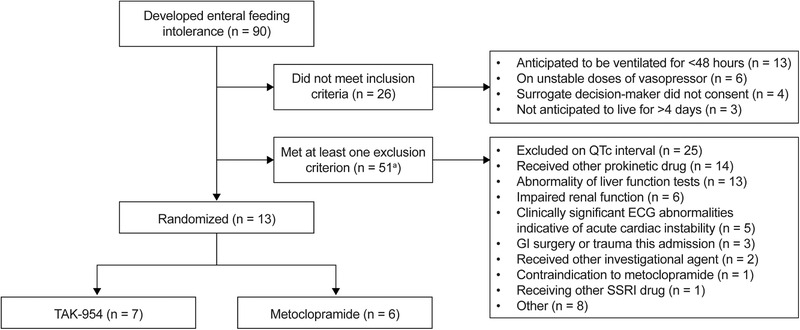
Consort‐style patient flow.a
aThere were 27 patients who met two exclusion criteria, meaning there were 78 exclusion episodes in 51 patients. ECG, electrocardiogram; GI, gastrointestinal; QTc interval, corrected QT interval; SSRI, selective serotonin reuptake inhibitor.
Table 1.
Baseline Patient Characteristics
| Characteristic | 0.5 mg TAK‐954 | 10 mg Metoclopramide |
|---|---|---|
| Number of participants | 7 | 6 |
| Admission diagnosis | ||
| Medical (nonoperative) | 2 | 3 |
| Surgical (postoperative) | 3 | 1 |
| Trauma (nonoperative) | 2 | 2 |
| Mean age, years (SD) | 54.0 (25.3) | 55.5 (13.4) |
| Sex, male, n (%) | 6 (86) | 4 (67) |
| Median BMI, kg/m2 (range) | 28 (23–39) | 24 (21–31) |
| Race, white, n (%) | 7 (100) | 6 (100) |
| Glasgow Coma Scale, mean (SD) | 11.0 (5.0) | 7.3 (6.3) |
| Acute renal failure/chronic health problems, n (%) | 0 (0) | 0 (0) |
| Median blood glucose level, mmol/L (range) | 7.7 (5.4–13.0) | 8.3 (7.1–8.8) |
| Median serum creatinine level, µmol/L (range) | 93 (47–199) | 51 (35–66) |
| Mean total time receiving enteral nutrition support, hours (SD)a | 124.0 (113.6) | 109.2 (105.9) |
| Median gastric residual volume at eligibility, mL (range) | 285 (210–1000) | 240 (210–350) |
| Median time from gastric residual volume eligibility to study drug, hours (range) | 16.3 (4.7–22.5) | 14.8 (12.3–20.8) |
| Mean gastric residual volume at baseline, mL (SD) | 97.9 (118.2) | 106.2 (99.2) |
| APACHE III score (median IQR) | 66 (30) | 57 (30) |
APACHE, Acute Physiologic Assessment and Chronic Health Evaluation; BMI, body mass index; SD, standard deviation.
Before day 1 of the study.
Safety and Tolerability
All participants received the complete dosing regimen of study drug and sham drug. Two participants (29%) in the TAK‐954 group and 3 (50%) in the metoclopramide group died before completion of the 30‐day follow‐up. Five participants (71%) in the TAK‐954 group and 4 (67%) in the metoclopramide group experienced an AE (Table 2). Of these events, 2 and 3 were defined as serious in the TAK‐954 and metoclopramide groups, respectively. Only 1 nonserious AE (diarrhea in 1 patient in the metoclopramide group) was considered to be related to study drug. No serious AEs (including deaths) were considered to be related to study drug in either group. The time (study day) at which each AE occurred is reported in Table 2.
Table 2.
Summary of AEs
| Category of AE | 0.5 mg TAK‐954 | 10 mg Metoclopramide | Day Reported (Relative to Baseline) |
|---|---|---|---|
| Total number of patients, n | 7 | 6 | – |
| Total number of AEs, n | 8 | 5 | – |
| Participants identified to have at least 1 AEa, n (%) | 5 (71) | 4 (67) | – |
| Participant AE reported as moderate or severe, n (%) | 3 (43) | 3 (50) | – |
| Cerebral hemorrhage | 1 (14) | 0 (0) | 3 |
| Intracranial hemorrhage | 0 (0) | 1 (17) | 3 |
| Subarachnoid hemorrhage | 0 (0) | 1 (17) | 7 |
| Disease progression | 0 (0) | 1 (17) | 6 |
| Respiratory failure | 1 (14) | 0 (0) | 11 |
| Decubitus ulcer | 1 (14) | 0 (0) | 7 |
| Hypertension | 0 (0) | 1 (17) | 3 |
| Treatment‐related moderate or severe AEs, n (%) | 0 (0) | 0 (0) | – |
| Serious AEs, n (%) | 2 (29) | 3 (50) | |
| Cerebral hemorrhage | 1 (14) | 0 (0) | 3 |
| Intracranial hemorrhage | 0 (0) | 1 (17) | 3 |
| Subarachnoid hemorrhage | 0 (0) | 1 (16) | 7 |
| Disease progression | 0 (0) | 1 (16) | 6 |
| Respiratory failure | 1 (14) | 0 (0) | 11 |
| Treatment‐related serious AEsb, n (%) | 0 (0) | 0 (0) | – |
| AEs leading to discontinuation of treatment, n (%) | 0 (0) | 0 (0) | – |
| Any AEs leading to treatment interruption, n (%) | 0 (0) | 0 (0) | – |
| Deathsb, n (%) | 2 (29) | 3 (50) | – |
AE, adverse event.
Only 1 AE (mild diarrhea in the metoclopramide group) was considered to be potentially treatment related.
No serious AEs (including deaths) were considered to be treatment related in either group.
No AEs led to treatment discontinuation or interruption in either group. In both groups, all vital sign changes from baseline were minimal (Supplementary Table S1), with no clinically relevant changes in ECG readings (Supplementary Table S2), full blood counts, or results of serum biochemistry and liver function tests (Supplementary Table S3).
Gastric Emptying
Individual data on scintigraphy are presented in Figure 3. With respect to gastric emptying (intragastric retention <13% at 180 minutes), a greater proportion of participants had normal gastric retention in the TAK‐954 group than those receiving metoclopramide (6 of 7 participants [86%] vs 3 of 6 participants [50%]). At t = 180 minutes, the median (interquartile range) percentages for gastric retention were 1.0 (0–10.0) and 15.0 (1.0–26.0) in the TAK‐954 and metoclopramide groups, respectively. Median GRVs are summarized in Table 3.
Figure 3.
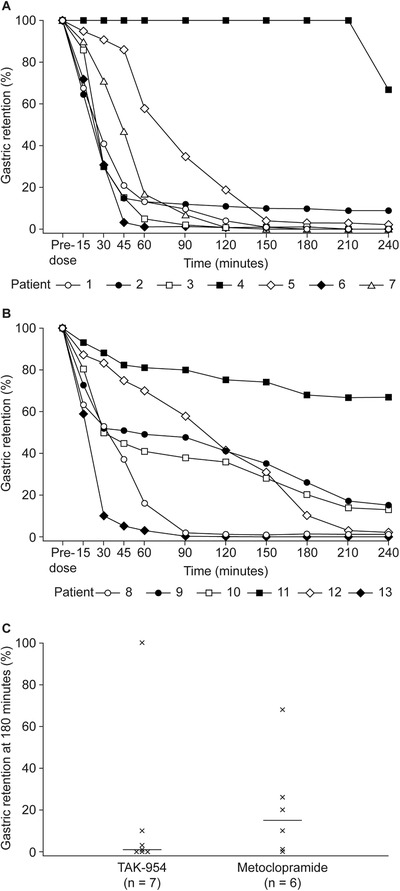
Gastric retention assessed by individual scintigraphy in participants treated with (A) TAK‐954 0.5 mg (n = 7) and (B) metoclopramide 10 mg (n = 6). (C) Median gastric emptying at 180 minutes.a
aEach cross in Figure 3C represents the gastric retention of a patient at 180 minutes. Each horizontal line is the median value of the patients' gastric retention in each group.
Table 3.
Median (Range) GRVs
| Time, Median (Min, Max) | 0.5 mg TAK‐954 | 10 mg Metoclopramide |
|---|---|---|
| 4 hours, n = 7 and 6a | 0 (0, 24) | 5.5 (0, 65) |
| 10 hours, n = 7 and 6a | 20 (0, 150) | 27.5 (0, 160) |
| 16 hours, n = 7 and 5a | 15 (0, 60) | 10 (0, 140) |
| 22 hours, n = 6 and 6a | 10 (0, 300) | 30 (0, 420) |
All volumes are in milliliters.
GRVs immediately post‐scintigraphy (ie, 4 hours after the infusion of the study drug) and 6 hours thereafter.
GRV, gastric residual volume.
Numbers of patients for the TAK‐954 and metoclopramide groups, respectively.
Pharmacokinetics
Samples were obtained in 6 of 7 participants receiving TAK‐954 for pharmacokinetics assessment. Data are summarized in Supplementary Figure S1. After the 1‐hour infusion of TAK‐954, the median time to maximum plasma drug concentration (T max) was observed at 30 minutes, with individual values ranging from 30 minutes to 1 hour. The mean (SD) half‐life (t 1⁄2) after a 1‐hour infusion of TAK‐954 was 15.9 (9.2) hours. After reaching the maximum serum concentration (C max; mean ± SD: 5040 ± 1780 pg/mL), plasma concentrations of TAK‐954 declined multi‐exponentially; there was moderate variability in C max across patients, with a coefficient of variation of 35%).
Discussion
EFI is a clinical condition that may result from delayed gastric emptying. In this phase 2a trial, a greater proportion of critically ill patients with EFI had “normal” gastric emptying after receiving a single dose of TAK‐954 than those receiving metoclopramide. Within the limitations of a small cohort, treatment with TAK‐954 was not associated with an increase in AEs compared with metoclopramide. Among the 13 study participants, only 1 nonserious AE was considered to be associated with treatment: diarrhea in a patient receiving metoclopramide. No serious AEs were considered to be treatment related.
Previous studies have found that patients with a large GRV are more likely to experience negative clinical outcomes (higher mortality, prolonged length of stay in intensive care, and fewer ventilator‐free days) than those with normal GRVs, accounting for underlying medical conditions. 4 , 27 Two nonselective 5‐HT4 receptor agonists, cisapride and tegaserod, have been reported to be effective therapies in decreasing GRV and improving tolerance to enteral feedings, but both have been removed from the market because of concerns regarding cardiovascular safety. 9 , 10 , 11 , 12 , 28 Metoclopramide, which is available and routinely used as a first‐line prokinetic drug, is a dopamine receptor antagonist with weak mixed serotonergic effects, including partial agonism of the 5‐HT4 receptor. 29 In critically ill patients, metoclopramide accelerates gastric emptying and is shown to be beneficial in patients with EFI. 30 , 31 , 32 , 33 However, the use of metoclopramide in clinical practice can be limited by the development of tachyphylaxis when used for prolonged periods (>7 days), 34 or earlier in the case of patients with traumatic brain injury, 35 and the possibility of adverse central nervous system effects such as extrapyramidal symptoms or mental status changes. 36 , 37 Therefore, there is a need for novel, efficacious drugs with an acceptable safety profile. 8 , 38 , 39
As aforementioned, the safety and tolerability of pharmacokinetics and pharmacodynamics of TAK‐954 have been studied in a phase 1 randomized study in 12 healthy adults. Participants were allocated to 2 consecutive cohorts each receiving a 1‐hour infusion of TAK‐954 daily over 5 days. 19 Overall, TAK‐954 was tolerated for daily doses up to 0.5 mg, with no severe or serious AEs reported. 19 The most common AEs were headache and postural dizziness, all of which were mild or moderate. 19 Two participants in the first cohort experienced moderate postural dizziness on day 1, which resolved but resulted in a protocol adaptation for the second cohort so that participants received 0.1 mg TAK‐954 on day 1 rather than 0.5 mg, with 0.5 mg TAK‐954 administered on days 2–4. 19 Following IV infusion, TAK‐954 concentrations declined in a biphasic manner, with variable mean half‐life values across cohorts and study days (range 18.0 to 18.9 hours on day 5 vs 15.9 hours in the current study). 19 Variability in C max, as measured by the coefficient of variation, ranged from 22% to 31% (vs 35% in the current study). 40 Excretion of TAK‐954 was predominantly via urine, with a smaller proportion via feces (data on file; data from the Clinical Study Report from the TAK‐954 Phase 1 ADME study).
Only 1 clinical trial has previously compared a 5‐HT4 receptor agonist with metoclopramide in critically ill patients. MacLaren and colleagues conducted a blinded parallel‐group trial of 14 critically ill patients with large GRVs (≥150 mL) receiving 10 mg enteral cisapride or 10 mg metoclopramide for a maximum of 7 days. 12 In this study, both drugs improved gastric motility, but metoclopramide reduced GRVs to a greater extent than cisapride. These differences, though statistically significant, were not clinically relevant, as maximum feeding rates were equally achieved with both cisapride and metoclopramide. 12 In contrast to these results with a nonselective 5‐HT4 receptor agonist, the results of the current study suggest that a selective 5‐HT4 receptor agonist (TAK‐954) may be a more potent prokinetic agent than metoclopramide, although this requires confirmation in a larger trial. This finding is supported by the results of studies in individuals with diabetic gastroparesis and preoperative patients receiving opiates, which suggest that, in both groups, 5‐HT4 receptor agonists accelerate gastric emptying to a greater extent than metoclopramide. 41 , 42
The strengths of the current trial include the use of the “gold‐standard” scintigraphic technique to quantify gastric emptying precisely. 43 In our study, TAK‐954 was also evaluated alongside an active comparator, metoclopramide, representing the current standard of care. The investigators remained blinded throughout to all study procedures and analyses. The limitations of the trial include its small sample size, its single‐center design, and the fact that only a short‐term, single‐dose effect was measured. Another limitation is that gastric emptying was not measured before application of the study drug, but a GRV of ≥200 mL was used to define slow gastric emptying in a binary (yes or no) manner. Consequently, it is unknown whether baseline gastric emptying was different between the 2 groups. Any difference may have affected the results observed after administration. Although larger GRVs are generally considered of more relevance clinically, a GRV of 200 mL identifies a population with slow gastric emptying, 25 specifically for recruitment into proof‐of‐concept trials to determine drug efficacy. Gastric emptying is a continuous variable that fluctuates over time; dichotomizing a continuous variable may lead to a loss of information or incorrect inferences. 44 In addition, it is unknown whether accelerating gastric emptying improves patient‐centered outcomes. Furthermore, the trial was terminated by the sponsor at an interim analysis, with the subsequent risk of inflating treatment effects. 26 Finally, this trial evaluated the effect of TAK‐954 on a physiological outcome (GRV as surrogate marker of gastric emptying). Accordingly, larger trials to evaluate the effect of repeated doses on clinically relevant outcomes are warranted. 45 , 46
In conclusion, in a small cohort of critically ill patients with pre‐existing slow gastric emptying indicated by a large GRV, a single dose of 0.5 mg intravenous TAK‐954 appeared to have at least similar efficacy in accelerating gastric emptying to multiple doses of 10 mg metoclopramide. Treatment with TAK‐954 was not associated with an increase in AEs compared with metoclopramide. These preliminary results support further evaluation of TAK‐954 in critically ill patients with EFI in larger trials of multiple doses.
Statement of Authorship
M. J. Chapman contributed to the conception of the research; M. J. Chapman, K. L. Jones, and A. M. Deane contributed equally to the design of the research; C. N. Barnes and D. Nguyen contributed to the design of the research; K. L. Jones contributed to the acquisition and analysis of the data; M. J. Chapman, K. L. Jones, C. Almansa, and A. M. Deane contributed equally to the interpretation of the data; A. M. Deane drafted the manuscript; and all authors critically revised the manuscript. All authors agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Financial disclosure: The study was supported by an unrestricted grant from Theravance Biopharma to the Royal Adelaide Hospital. K.L.J. received funding from Theravance Biopharma to perform the gastric emptying analyses. A.M.D. received a travel grant from Takeda to present these data at Digestive Diseases Week 2018. M.J.C. did not receive payment, stock, or travel grants from Theravance Biopharma and has no duality of interest. A.M.D. wrote the initial draft of the manuscript, which was edited by all authors. Takeda provided manuscript support via a third‐party medical writing service, Oxford PharmaGenesis Ltd, who updated the manuscript on behalf of the authors, collating the supplementary materials, developing figures, editing and quality‐checking the content, and supporting submission.
Conflicts of interest: M.J.C. has received support for her research from Baxter, Fresenius Kabi, and Nestlé. K.L.J. has received research funding from Sanofi Aventis, AstraZeneca and Theravance Biopharma, and trial medication from Merck, Sharp & Dohme. Her salary is supported by a University of Adelaide William T Southcott Research Fellowship in Nuclear Medicine. C.A. is an employee of Takeda. C.B. was an employee of Theravance Biopharma US, Inc. at the time of the study. D.N. is an employee of Theravance Biopharma US, Inc. A.M.D. or his institution have received honoraria, travel grants or project grant funding from Baxter, Cardinal Health, Fresenius Kabi, GSK, Medtronic and Nutricia. AMD has participated on advisory boards for Lyric Pharmaceuticals and Takeda.
References
- 1. Deane AM, Chapman MJ, Reintam Blaser A, McClave SA, Emmanuel A. Pathophysiology and treatment of gastrointestinal motility disorders in the acutely ill. Nutr Clin Pract. 2019;34(1):23‐36. [DOI] [PubMed] [Google Scholar]
- 2. Chapman MJ, Deane AM. Gastrointestinal dysfunction relating to the provision of nutrition in the critically ill. Curr Opin Clin Nutr Metab Care. 2015;18(2):207‐212. [DOI] [PubMed] [Google Scholar]
- 3. Reintam Blaser A, Starkopf L, Deane AM, Poeze M, Starkopf J. Comparison of different definitions of feeding intolerance: a retrospective observational study. Clin Nutr. 2015;34(5):956‐961. [DOI] [PubMed] [Google Scholar]
- 4. Gungabissoon U, Hacquoil K, Bains C, et al. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN J Parenter Enteral Nutr. 2015;39(4):441‐448. [DOI] [PubMed] [Google Scholar]
- 5. van Zanten AR, van der Meer YG, Venhuizen WA, Heyland DK. Still a place for metoclopramide as a prokinetic drug in critically ill patients? JPEN J Parenter Enteral Nutr. 2015;39(7):763‐766. [DOI] [PubMed] [Google Scholar]
- 6. Lewis K, Alqahtani Z, McIntyre L, et al. The efficacy and safety of prokinetic agents in critically ill patients receiving enteral nutrition: a systematic review and meta‐analysis of randomized trials. Crit Care. 2016;20(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor SJ, Allan K, McWilliam H, et al. A randomised controlled feasibility and proof‐of‐concept trial in delayed gastric emptying when metoclopramide fails: we should revisit nasointestinal feeding versus dual prokinetic treatment: achieving goal nutrition in critical illness and delayed gastric emptying: trial of nasointestinal feeding versus nasogastric feeding plus prokinetics. Clin Nutr ESPEN. 2016;14:1‐8. [DOI] [PubMed] [Google Scholar]
- 8. van Zanten AR. Do we need new prokinetics to reduce enteral feeding intolerance during critical illness? Crit Care. 2016;20(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spapen HD, Duinslaeger L, Diltoer M, Gillet R, Bossuyt A, Huyghens LP. Gastric emptying in critically ill patients is accelerated by adding cisapride to a standard enteral feeding protocol: results of a prospective, randomized, controlled trial. Crit Care Med. 1995;23(3):481‐485. [DOI] [PubMed] [Google Scholar]
- 10. Heyland DK, Tougas G, Cook DJ, Guyatt GH. Cisapride improves gastric emptying in mechanically ventilated, critically ill patients. A randomized, double‐blind trial. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1678‐1683. [DOI] [PubMed] [Google Scholar]
- 11. MacLaren R, Kuhl DA, Gervasio JM, et al. Sequential single doses of cisapride, erythromycin, and metoclopramide in critically ill patients intolerant to enteral nutrition: a randomized, placebo‐controlled, crossover study. Crit Care Med. 2000;28(2):438‐444. [DOI] [PubMed] [Google Scholar]
- 12. MacLaren R, Patrick WD, Hall RI, Rocker GM, Whelan GJ, Lima JJ. Comparison of cisapride and metoclopramide for facilitating gastric emptying and improving tolerance to intragastric enteral nutrition in critically III, mechanically ventilated adults. Clin Ther. 2001;23(11):1855‐1866. [DOI] [PubMed] [Google Scholar]
- 13. Tack J, Camilleri M, Chang L, et al. Systematic review: cardiovascular safety profile of 5‐HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012;35(7):745‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Food and Drug Administation Gastrointestinal Drugs Advisory Committee . Prucalopride for the Treatment of Chronic Idiopathic Constipation—FDA Advisory Committee Meeting Briefing Document 2018. Available from: https://www.fda.gov/media/119034/download Accessed September 2019.
- 15. Reignier J, Boisrame‐Helms J, Brisard L, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomized, controlled, multicentre, open‐label, parallel‐group study (NUTRIREA‐2). Lancet. 2018;391(10116):133‐143. [DOI] [PubMed] [Google Scholar]
- 16. Sim JA, Horowitz M, Summers MJ, et al. Mesenteric blood flow, glucose absorption and blood pressure responses to small intestinal glucose in critically ill patients older than 65 years. Intensive Care Med. 2013;39(2):258‐266. [DOI] [PubMed] [Google Scholar]
- 17. Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;11(2):112‐128. [DOI] [PubMed] [Google Scholar]
- 18. Jones KL, Berry M, Kong MF, Kwiatek MA, Samsom M, Horowitz M. Hyperglycemia attenuates the gastrokinetic effect of erythromycin and affects the perception of postprandial hunger in normal subjects. Diabetes Care. 1999;22(2):339‐344. [DOI] [PubMed] [Google Scholar]
- 19. Czerniak R, Chen Y, Aldairy W, et al. Mo1581—Evaluation of the safety, tolerability, pharmacokinetic and pharmacodynamic profiles of TAK‐954 in a randomized, placebo‐controlled phase 1 study [Abstract]. Gastroenterology. 2019;156(6):S‐789. [Google Scholar]
- 20. Ridley EJ, Peake SL, Jarvis M, et al. Nutrition therapy in Australia and New Zealand intensive care units: an international comparison study. JPEN J Parenter Enteral Nutr. 2018;42(8):1349‐1357. doi: 10.1002/jpen.1163. [DOI] [PubMed] [Google Scholar]
- 21. Deane AM, Chapman MJ, Fraser RJ, et al. Effects of exogenous glucagon‐like peptide‐1 on gastric emptying and glucose absorption in the critically ill: relationship to glycemia. Crit Care Med. 2010;38(5):1261‐1269. [DOI] [PubMed] [Google Scholar]
- 22. Deane AM, Fraser RJ, Chapman MJ. Prokinetic drugs for feed intolerance in critical illness: current and potential therapies. Crit Care Resusc. 2009;11(2):132‐143. [PubMed] [Google Scholar]
- 23. Kar P, Cousins CE, Annink CE, et al. Effects of glucose‐dependent insulinotropic polypeptide on gastric emptying, glycaemia and insulinaemia during critical illness: a prospective, double blind, randomized, crossover study. Crit Care. 2015;19:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kar P, Plummer MP, Chapman MJ, et al. Energy‐dense formulae may slow gastric emptying in the critically ill. JPEN J Parenter Enteral Nutr. 2016;40(7):1050‐1056. [DOI] [PubMed] [Google Scholar]
- 25. Chapman MJ, Besanko LK, Burgstad CM, et al. Gastric emptying of a liquid nutrient meal in the critically ill: relationship between scintigraphic and carbon breath test measurement. Gut. Oct 2011;60(10):1336‐1343. [DOI] [PubMed] [Google Scholar]
- 26. Montori VM, Devereaux PJ, Adhikari NK, et al. Randomized trials stopped early for benefit: a systematic review. JAMA. 2005;294(17):2203‐2209. [DOI] [PubMed] [Google Scholar]
- 27. Wang K, McIlroy K, Plank LD, Petrov MS, Windsor JA. Prevalence, outcomes, and management of enteral tube feeding intolerance: a retrospective cohort study in a tertiary center. JPEN J Parenter Enteral Nutr. 2017;41(6):959‐967. [DOI] [PubMed] [Google Scholar]
- 28. Deane A, Young R, Chapman M, Fraser R. A clinical audit of the efficacy of tegaserod as a prokinetic agent in the intensive care unit. Crit Care Resusc. 2008;10(1):71. [PubMed] [Google Scholar]
- 29. Plummer M, Blaser A, Deane A. Gut dysmotility in the ICU: diagnosis and therapeutic options. Curr Opin Crit care. 2019;25(2):138‐144. [DOI] [PubMed] [Google Scholar]
- 30. Jooste CA, Mustoe J, Collee G. Metoclopramide improves gastric motility in critically ill patients. Intensive Care Med. 1999;25(5):464‐468. [DOI] [PubMed] [Google Scholar]
- 31. Nguyen NQ, Chapman MJ, Fraser RJ, Bryant LK, Holloway RH. Erythromycin is more effective than metoclopramide in the treatment of feed intolerance in critical illness. Crit Care Med. Feb 2007;35(2):483‐489. [DOI] [PubMed] [Google Scholar]
- 32. MacLaren R, Kiser TH, Fish DN, Wischmeyer PE. Erythromycin vs metoclopramide for facilitating gastric emptying and tolerance to intragastric nutrition in critically ill patients. JPEN J Parenter Enteral Nutr. 2008;32(4):412‐419. [DOI] [PubMed] [Google Scholar]
- 33. Hu B, Ye H, Sun C, et al. Metoclopramide or domperidone improves post‐pyloric placement of spiral nasojejunal tubes in critically ill patients: a prospective, multicenter, open‐label, randomized, controlled clinical trial. Crit Care. 2015;19:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen NQ, Chapman M, Fraser RJ, Bryant LK, Burgstad C, Holloway RH. Prokinetic therapy for feed intolerance in critical illness: one drug or two? Crit Care Med. 2007;35(11)2561‐2567. [DOI] [PubMed] [Google Scholar]
- 35. Dickerson RN, Mitchell JN, Morgan LM, et al. Disparate response to metoclopramide therapy for gastric feeding intolerance in trauma patients with and without traumatic brain injury. JPEN J Parenter Enteral Nutr. 2009;33(6):646‐655. [DOI] [PubMed] [Google Scholar]
- 36. Akathisia Anfinson TJ., panic, agoraphobia, and major depression following brief exposure to metoclopramide. Psychopharmacol Bull. Winter 2002;36(1):82‐93. [PubMed] [Google Scholar]
- 37. Parlak I, Atilla R, Cicek M, et al. Rate of metoclopramide infusion affects the severity and incidence of akathisia. Emerg Med J. Sep 2005;22(9):621‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deane AM, Lamontagne F, Dukes GE, et al. Nutrition adequacy therapeutic enhancement in the critically ill: a randomized double‐blind, placebo‐controlled trial of the motilin receptor agonist camicinal (GSK962040): the NUTRIATE study. JPEN J Parenter Enteral Nutr. 2017;42(5):949‐959. [DOI] [PubMed] [Google Scholar]
- 39. van der Meer YG, Venhuizen WA, Heyland DK, van Zanten AR. Should we stop prescribing metoclopramide as a prokinetic drug in critically ill patients? Crit Care. 2014;18(5):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Czerniak R, Chen Y, Aldairy W, et al. Evaluation of the safety, tolerability, pharmacokinetic and pharmacodynamic profiles of Tak–954 in a randomized, placebo‐controlled phase 1 study [poster]. Digestive Disease Week 2019. 2019. Available at: https://ddw.scientificposters.com/epsAbstractDDW.cfm?id=1.
- 41. McHugh S, Lico S, Diamant NE. Cisapride vs metoclopramide. An acute study in diabetic gastroparesis. Dig Dis Sci. 1992;37(7):997‐1001. [DOI] [PubMed] [Google Scholar]
- 42. Rowbotham DJ, Bamber PA, Nimmo WS. Comparison of the effect of cisapride and metoclopramide on morphine‐induced delay in gastric emptying. Br J Clin Pharmacol. 1988;26(6):741‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kar P, Jones KL, Horowitz M, Chapman MJ, Deane AM. Measurement of gastric emptying in the critically ill. Clin Nutr. 2015;34(4):557‐564. [DOI] [PubMed] [Google Scholar]
- 44. Altman DG, Royston P. The cost of dichotomizing continuous variables. BMJ. 2006;332(7549):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arabi YM, Preiser JC. A critical view on primary and secondary outcome measures in nutrition trials. Intensive Care Med. 2017;43(12):1875‐1877. [DOI] [PubMed] [Google Scholar]
- 46. Summers MJ, Chapple LA, McClave SA, Deane AM. Event‐rate and delta inflation when evaluating mortality as a primary outcome from randomized controlled trials of nutritional interventions during critical illness: a systematic review. Am J Clin Nutr. 2016;103(4):1083‐1090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


