Abstract
Objectives
To evaluate recurrence and progression risk after simultaneous endoscopic surgery of bladder cancer and benign prostatic hyperplasia (BPH), as simultaneous surgery is not an unusual scenario and theoretically simultaneous transurethral resection of bladder tumour (TURBT) and transurethral resection of the prostate (TURP) can lead to an increased risk of recurrence in the bladder neck and prostatic urethra (BN/PU).
Methods
We conducted a systematic review and meta‐analysis to assess the risk of recurrence (i.e. whole bladder and/or BN/PU) and tumour progression as outcomes after a simultaneous endoscopic surgery of bladder tumour and BPH, as compared to TURBT alone. We queried PubMed and Web of Science database on 1 January 2020. We used random‐ and/or fixed‐effects meta‐analytic models in the presence or absence of heterogeneity according to the I 2 statistic, respectively.
Results
Nine retrospective and three clinical trial studies were selected after considering inclusion and exclusion criteria. We conducted the meta‐analysis on retrospective and randomised controlled trials (RCTs) separately. Eight retrospective and three RCT studies were included to assess the BN/PU recurrence risk and the summarised risk ratio (RR) was 1.02 (95% confidence interval [CI] 0.74–1.41) and 0.93 (95% CI 0.47–1.84), respectively. Five retrospective and two RCT studies were included to assess the progression risk and the summarised RR was 0.91 (95% CI 0.56–1.48) and 1.16 (95% CI 0.30–4.51), respectively. Eight retrospective and three RCT studies were included to assess the whole bladder recurrence risk and the summarised RR was 0.87 (95% CI 0.78–0.97) and 0.89 (95% CI 0.65–1.21), respectively.
Conclusion
We did not observe any increased risk of total bladder recurrence, BN/PU recurrence, or progression after a simultaneous endoscopic surgery of bladder tumour and BPH, as compared to TURBT alone.
Keywords: bladder cancer, benign prostatic hyperplasia, endoscopic surgery, simultaneous surgery, TURBT, TURP, #BladderCancer, #blcsm
Introduction
Bladder cancer is a multifocal disease. It is potentially associated with synchronous tumours and metachronous recurrence. Two major theories have been proposed regarding its natural history. The older theory is known as the ‘field cancerisation’. According to this theory, the whole bladder urothelium is exposed to carcinogens and consequently this leads to the development of multiple cancers from different cells of origin (multiclonal tumours). The newer theory is known as the ‘clonality’. According to this theory, multiple tumours could arise from the seeding of cells liberated during surgery: intra‐epithelial expansion or spread from a single tumour clone (monoclonal tumours) [1, 2].
Occasionally, we detects a bladder tumour while doing a TURP or find an incidental bladder tumour in patients who have a synchronous symptomatic BPH. Endoscopic interventions could treat these two conditions simultaneously. Simultaneous incidence of bladder tumour and BPH endoscopic surgery was reportedly between 3.9% and 6.4% [3, 4, 5]. However, theoretically, a simultaneous surgery for bladder cancer and BPH could lead to tumour cell seeding and increased risk of recurrence in the bladder neck/prostatic urethra (BN/PU). To test this theory, we performed a systematic review evaluating the recurrence (i.e. whole bladder and/or BN/PU) and progression risk after simultaneous endoscopic surgery for bladder cancer and BPH.
Methods
Eligibility criteria
We only retrieved original articles, and excluded all other types of reports (e.g. case report, letter and editorial report). The search was limited to studies published in English. Our main objective was to test the hypothesis stating that there might exist an increase in the risk of BN/PU recurrence after simultaneous endoscopic surgery of bladder tumour and BPH. The PICO framework items that used to form the question of the study were included: P (population of study) patients with a synchronous bladder tumour and BPH diagnosis; I (intervention group) simultaneous endoscopic surgery for bladder tumour and BPH; C (control group) endoscopic surgery for bladder tumour alone; O, (outcomes) whole bladder recurrence, BN/PU recurrence and progression rates. All current articles that assessed the risk of recurrence after a simultaneous endoscopic treatment of bladder cancer and BPH were eligible for this systematic review. We defined endoscopic treatment as traditional electrocautery resectoscope and/or laser instruments. Inclusion criteria for the quantitative meta‐analysis involved all original research articles that assessed overall and/or BN/PU recurrence and progression rates as treatment outcomes (TURBT + TURP) with a control group that consisted of TURBT alone. Exclusion criteria involved studies without a control group (TURBT alone).
Information Source
PubMed and Web of Science were used to search for specific queries on 1 January 2020. The search query lines and strategies were “(((turp) OR (“transurethral resection of prostate”[All Fields])) AND (“turbt”[All Fields])) and (((turp) OR (“transurethral resection of prostate”[All Fields]))) AND (“transurethral resection of bladder tumor”[All Fields])” in PubMed database and “ALL=((“transurethral resection of bladder tumor” OR “turbt”) AND (“transurethral resection of prostate” OR “turp”))” in Web of Science database for English language.
We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines. Article title and abstract screening were done by two independent reviewers and any disagreements about eligible and ineligible articles were resolved according to Delphi consensus criteria between co‐authors. We used a data extraction sheet developed on the basis of the Cochrane Consumers and the Communication Review Group’s data extraction template (http://cccrg.cochrane.org/author‐resources). We extracted the following data: first author, type of article, year of publication, dates of the data collection or enrolment, study design, sample size, number of individuals in each study group, outcomes, how the outcomes were measured, follow‐up duration, type of effect statistic and corresponding P value. We contacted articles’ corresponding author(s) for additional details to overcome data limitations. Modified Newcastle‐Ottawa Scale criteria were used to assess the quality of the included retrospective studies and the RoB 2 tool (the Cochrane Risk of Bias Tool) was used to assess the risk of bias and the quality of randomised controlled trials (RCTs) [6, 7]. Subsequently, the total recurrence, BN/PU recurrence and progression rates were retrieved and all discrepancies regarding data extraction were resolved according to Delphi consensus criteria with co‐authors.
Statistical analysis
Forest plots were used to assess risk ratio (RR) and summarised them to describe RR of total recurrence, BN/PU recurrence and progression rates in the treatment and control groups. Primary and secondary meta‐analysis were conducted among all studies that reported total recurrence and BN/PU recurrence rates as an outcome and the last meta‐analysis was conducted among studies that reported risk of progression rate as an outcome. The heterogeneity across studies was evaluated using P values, and Q and I 2 statistics [8]. Random‐ and fixed‐effect meta‐analyses were used when the heterogeneity was greater and lower than 50%, respectively. P values < 0.05 were considered statistically significant. All analyses were carried out using Stata Statistical Software, release 14 (StataCorp., College Station, TX, USA).
Result
After initial screening, 29 articles were selected for further assessment. The selection process of papers is shown in Fig. 1. After applying the inclusion and exclusion criteria, 12 studies remained for systematic review. Table 1 lists the characteristics of these 12 studies [3, 4, 5, 9, 10, 11, 12, 13, 14, 15, 16, 17]. We excluded the Tsivian et al. [4] study due to the lack of a control group. The tumour characteristics of patients included in this study are shown in Table 2. The treatment and control groups of all 11 studies were adjusted according to the number of tumours (i.e. solitary or multifocal) and the grade and stage of the tumour. Six studies in this review reported a mean time to recurrence (the first recurrence), which is highlighted in Table 3 [9, 10, 11, 12, 16].
Fig. 1.
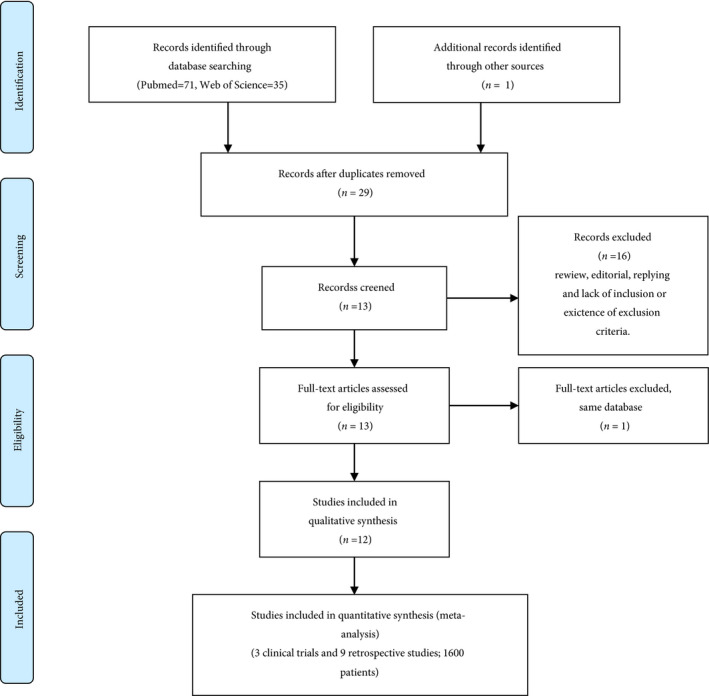
The selection process of the articles to assess the recurrence and progression risk after simultaneous endoscopic surgery of urothelial bladder tumour and BPH.
Table 1.
Characteristics of studies in this systematic review and meta‐analysis.
| Study | Design | N | TG TURBT + TURP, n | CG TURBT, n | TG total recurr., n | CG total recurr., n | TG BN/PU recur., n | CG BN/PU recur., n | TG progress., n | CG progress., n | TG mean FU, months | CG mean FU, months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang et al. (2020) [16] | Retro. | 236 | 118 | 118 | 32 | 38 | 11 | 8 | 9 | 11 | 20.2 | 18.9 |
| Dellabella et al. (2018) [9] | RCT | 85 | 42 | 43 | 22 | 27 | 8 | 9 | NA | NA | 36.91 | 35.16 |
| Li et al. (2014) [10] | RCT | 62 | 25 | 37 | 4 | 7 | 1 | 3 | 1 | 2 | ≥12 | ≥12 |
| Jaidane et al. (2010) [5] | Retro. | 170 | 85 | 85 | 17 | 20 | 1 | 1 | 2 | 2 | 35.2 | 33.1 |
| Singh et al. (2009) [11] | RCT | 48 | 24 | 24 | 12 | 11 | 4 | 3 | 3 | 2 | 35.71 | 37.55 |
| Ham et al. (2009) [3] | Retro. | 203 | 107 | 106 | 31 | 46 | 0 | 0 | 10 | 12 | 48 | 45 |
| Park et al. (2009) [17] | Retro. | 189 | 24 | 165 | 9 | 37 | 1 | 3 | 2 | 10 | 52.2 | 43.8 |
| Ugurlu et al. (2007) [12] | Retro. | 65 | 31 | 34 | 11 | 14 | 1 | 1 | 3 | 3 | 30.6 | 27.4 |
| Tsivian et al. (2003)* [4] | Retro. | 51 | 51 | NA | 35 | NA | 11 | NA | 3 | NA | 37.3 | NA |
| Vicente et al. (1988) [13] | Retro. | 200 | 100 | 100 | 55 | 73 | 10 | 10 | NA | NA | 47 | 46 |
| Laor et al. (1981) [14] | Retro. | 287 | 137 | 150 | 77 | 92 | 21 | 27 | NA | NA | 69 | 96 |
| Greene et al. (1972) [15] | Retro. | 200 | 100 | 100 | 54 | 54 | 17 | 16 | NA | NA | 132 | 132 |
This study was designed without a control group. CG, control group; FU, follow‐up; NA, not available; progress., progression; recurr., recurrence; Retro., retrospective; TG, treatment group.
Table 2.
Tumour characteristics of patients in this systematic review.
| Study | TG solitary/ multiple | CG solitary/ multiple | TG Ta/T1/T2 | CG Ta/T1/T2 |
TG LG/HG or G1/G2/G3 |
CG LG/HG or G1/G2/G3 |
TG/CG CIS existence | TG tumour size, cm | CG tumour size, cm | TG adjuvant therapy or SIIC | CG adjuvant therapy or SIIC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang et al. (2020) [16] | 75/43 | 82/36 | 14/114 | 21/97 | 71/47 | 79/39 | None | 2.4 ± 1.3 | 2.2 ± 0.9 | 118 SIIC and 93 adjuvant (Chemo. or BCG) | 118 SIIC and 89 adjuvant (Chemo. or BCG) |
| Dellabella et al. (2018) [9] | 21/21 | 23/19 | 27/15 | 26/19 | 28/14 | 31/14 | 4/4 | <4 | <4 | 27 adjuvant Chemo. | 26 adjuvant Chemo. |
| Li et al. (2014) [10] | 19/6 | 25/12 | 3/17/5 | 6/22/9 | 20/5 | 29/8 | None | 2.2 ± 0.8 | 2.5 ± 0.7 | 25 SIIC | 37 SIIC |
| Jaidane et al. (2010) [5] | 70/15 | 65/20 | 9/76 | 11/74 | 32/45/8 | 33/44/8 | None | 2 ± 0.92 | 2.2 ± 1.13 | 69; BCG | 70; BCG |
| Singh et al. (2009) [11] | 24/0 | 24/0 | 17/7 | 18/6 | 10/11/3 | 9/11/4 | None | <3 | <3 | None | None |
| Ham et al. (2009) [3] | 58/48 | 56/51 | 21/85 | 19/88 | 60/46 | 59/48 | None | <3 and ≥3 | <3 and ≥3 | 53; BCG | 49; BCG |
| Park et al. (2009) [17] | 12/12 | 98/67 | 8/16 | 43/109 | 13/11 | 81/84 | 5/22 | <3 and ≥3 | <3 and ≥3 | NA | NA |
| Ugurlu et al. (2007) [12] | 31/0 | 34/0 | 25/6 | 25/9 | 26/3/2 | 31/3/0 | None | <3 | <3 | None | None |
| Tsivian et al. (2003)* [4] | 28/23 | NA | 42/7/2 | NA | 8/42/1 | NA | NA | NA | NA | NA | NA |
| Vicente et al. (1988) [13] | 58/42 | 52/48 | 21/79 | 24/76 | 4/78/18 | 18/73/9 | NA | NA | NA | NA | NA |
| Laor et al. (1981) [14] | 112/25 | 124/26 | NA | NA | 34/35/51 | 35/7/57 | NA | NA | NA | NA | NA |
| Greene et al. (1972) [15] | 81/19 | 77/23 | NA | NA | 57/29/14 | 59/23/18 | NA | NA | NA | NA | NA |
| Overall number | 589/254 | 660/302 | 187/422/7 | 193/500/9 | 606 LG or G1,G2/ 220 HG or G3 | 625 LG or G1,G2/ 289 HG or G3 |
This study was designed without a control group. BCG, bacillus Calmette‐Guérin; CG, control group; , preoperative single instillation chemotherapy; Chemo., chemotherapy; G, grade; HG, high grade; LG, low grade; NA, not available; SIIC, single immediate intravesical chemotherapy; TG, treatment group.
Table 3.
Mean time to recurrence among studies in this review.
| Study | Mean time to recurrence in TURBT + TURP group, months | Mean time to recurrence in TURBT group, months | P |
|---|---|---|---|
| Wang et al. (2020) [16] | 20.2 ± 10.4 | 18.9 ± 9.9 | 0.685 |
| Dellabella et al. (2018) [9] | 17.7 (6–48) | 16.64 (5–48) | 0.29 |
| Li et al. (2014) [10] | 13.5 ± 3.6 | 11.6 ± 3.2 | Not available |
| Singh et al. (2009) [11] | 7.33 ± 1.58 | 7 ± 1.54 | 0.54 |
| Ugurlu et al. (2007) [12] | 20.2 (3–59) | 13.7 (4–27) | 0.78 |
| Tsivian et al. (2003) [4] | 14.9 (13.5–18) | No control group | Not available |
We performed meta‐analyses, among studies that assessed the whole bladder recurrence rate, BN/PU recurrence and progression rates between TURBT/TURP and TURBT only. We conducted meta‐analyses using retrospective studies and RCTs, separately. The risk of bias and quality assessment of all studies included in the meta‐analysis are summarised in Tables 4 and 5. The summarised RR of eight retrospective and three RCT studies that assessed whole bladder recurrence risk (Primary outcome) was 0.87 (95% CI 0.78–0.97) and 0.89 (95% CI 0.65–1.21), respectively.
Table 4.
The Newcastle‐Ottawa Scale for all studies that included in the quantitative synthesis.
| Study | Sample size, n | Selection | Comparability | Outcome | Total |
|---|---|---|---|---|---|
| Wang et al. (2020) [16] | 236 | **** | ** | ** | 8 |
| Jaidane et al. (2010) [5] | 170 | **** | ** | ** | 8 |
| Ham et al. (2009) [3] | 203 | *** | ** | ** | 7 |
| Park et al. (2009) [17] | 189 | **** | ** | ** | 8 |
| Ugurlu et al. (2007) [12] | 65 | **** | * | ** | 7 |
| Tsivian et al. (2003)* [4] | 51 | *** | * | ** | 6 |
| Vicente et al. (1988) [13] | 200 | *** | * | ** | 6 |
| Laor et al. (1981) [14] | 287 | ** | ** | ** | 6 |
| Greene et al. (1972) [15] | 200 | *** | * | ** | 6 |
Each asterisk (*) represents an individual criterion within the subsection that was fulfilled.
[Correction added on 14 August 2020, after first online publication: A reference has been amended in this version.]
Table 5.
The risk of bias and quality of evidence for all RCTs included in the systematic review and meta‐analysis.
| Study | Risk of the bias domains | |||||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | Overall | |
| Dellabella et al. (2018) [9] |

|

|

|

|

|

|
| Li et al. (2014) [10] |

|

|

|

|

|

|
| Singh et al. (2009) [11] |

|

|

|

|

|

|
Domains:
D1: Bias arising from the randomization process.
D2: Bias due to deviations from independent intervention.
D3: Bias due to missing outcome data.
D4: Bias in measurement of the outcome.
D5: Bias in selection of the reported results.
Judgement:
 , Some concerns.
, Some concerns.
 , Low.
, Low.
The summarised RR of eight retrospective and three RCT studies that assessed BN/PU recurrence risk (Secondary outcome) was 1.02 (95% CI 0.74–1.41) and 0.93 (95% CI 0.47–1.84), respectively. The summarised RR of five retrospective and two RCT studies that assessed progression risk was 0.91 (95% CI 0.56–1.48) and 1.16 (95% CI 0.30–4.51), respectively. The Forest plots of the meta‐analyses are shown in Figs 2, 3, 4.
Fig. 2.
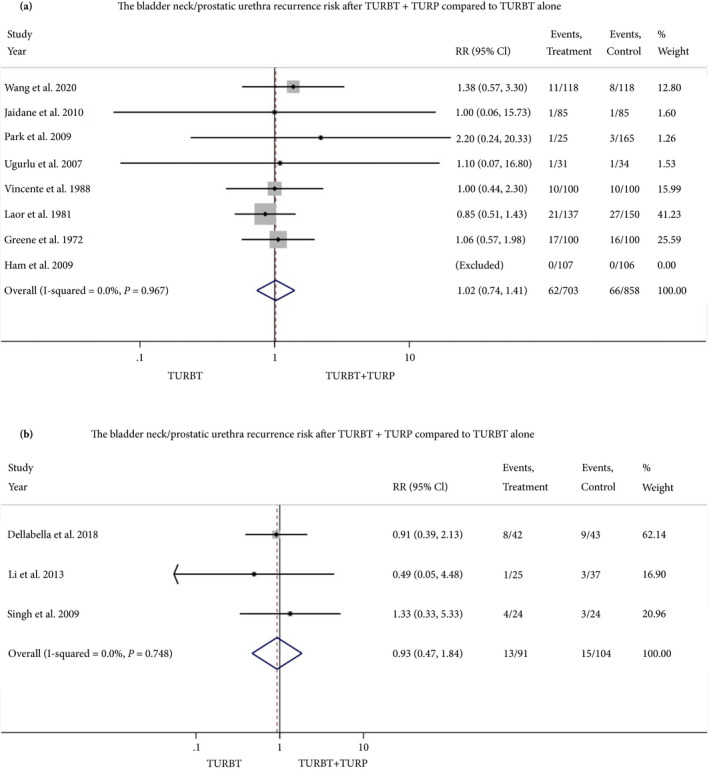
Forest plot, relative risk of BN/PU recurrence. a, retrospective studies, b, RCTs. [Correction added on 14 August 2020, after first online publication: A reference has been amended in this version.]
Fig. 3.
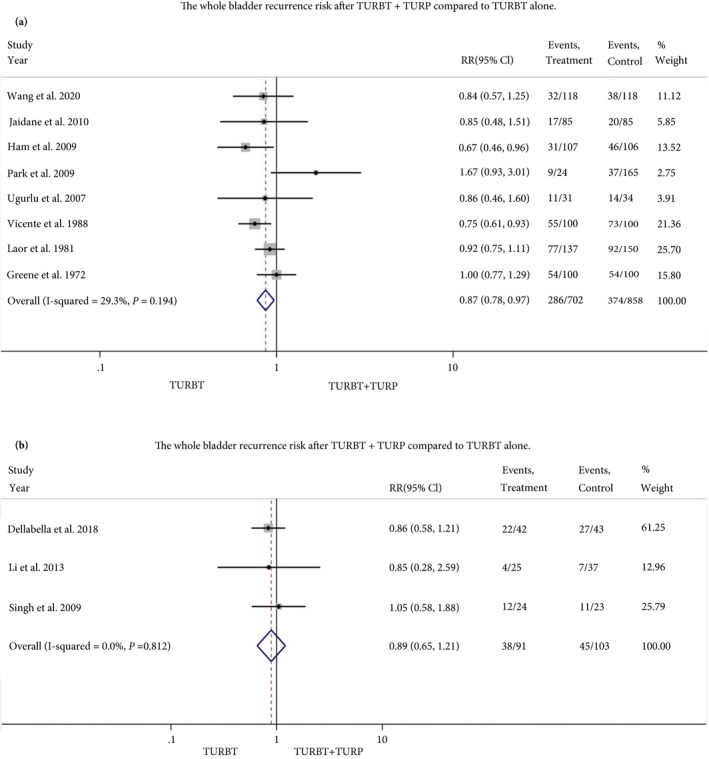
Forest plot, relative risk of total bladder recurrence. a, retrospective studies, B, RCTs. [Correction added on 14 August 2020, after first online publication: A reference has been amended in this version.]
Fig. 4.
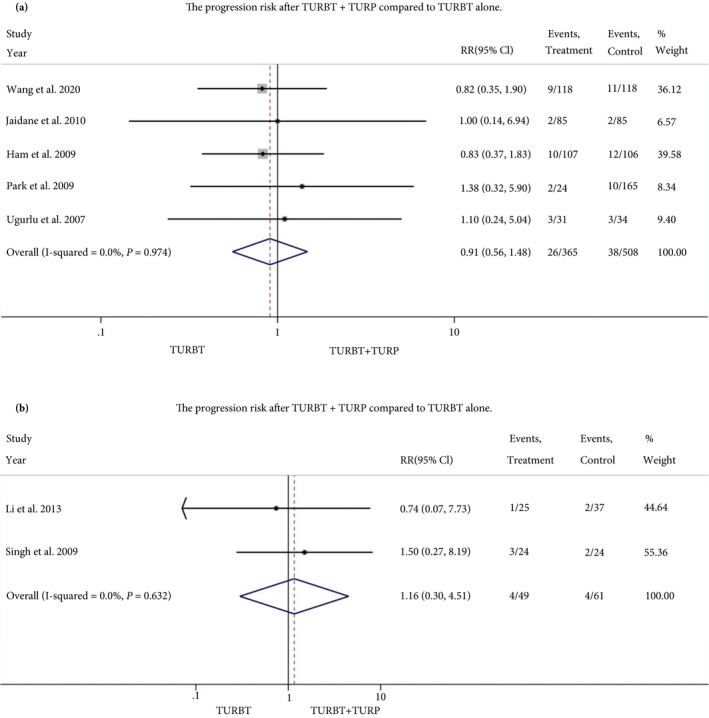
Forest plot, relative risk of tumour progression. a, retrospective studies, b, RCTs. [Correction added on 14 August 2020, after first online publication: A reference has been amended in this version.]
Discussion
There is a dilemma as to whether a simultaneous endoscopic surgery of the bladder tumour and BPH can lead to an increased risk of tumour cell re‐implantation in BN/PU and, consequently, an increased risk of BN/PU recurrence. Currently, the European Association of Urology (EAU) and others do not provide clear recommendations on this issue due to lack of evidence [18]. Their recommendation was mainly based on the systematic review and meta‐analysis by Picozzi et al. [19]. However, their meta‐analysis had some limitations: only one RCT was included in their analysis and the meta‐analysis was conducted among retrospective cohort studies and the RCT together. The present study addressed this void and resulted in several noteworthy findings.
According to the results of the present meta‐analysis, we did not find an increased risk of whole bladder recurrence, BN/PU recurrence, or progression after a simultaneous endoscopic surgery of bladder tumour and BPH compared to TURBT alone. The risk difference of BN/PU recurrence and progression was neither clinically meaningful nor statistically significant between treatment (TURBT + TURP) and control groups (TURBT alone). Our present findings are consistent with those of the meta‐analysis performed by Zhou et al. [20] in terms of tumour recurrence and progression; however, our present meta‐analysis included recently published studies [16].
Contrary to the often propagated opinion, we also found that simultaneous endoscopic surgery of the bladder tumour and BPH significantly reduced the overall bladder cancer recurrence risk compared to TURBT alone. This finding was similar to that of the meta‐analysis performed by Picozzi et al. [19], who reported a lower rate of overall recurrence in patients undergoing simultaneous procedures (odds ratio 0.72, 95% CI 0.57–0.92). This risk reduction could possibly be related to resection of the concurrent carcinoma in situ (CIS) in the BN/PU, as the incidence of concurrent CIS in the BN/PU is estimated at 12% in the presence of T1 high‐grade disease [18, 21]. However, the meta‐analysis result of retrospective studies with 1325 patients did not correlate with the meta‐analysis result of RCT studies with 194 patients, in terms of the overall bladder recurrence risk reduction. Ham et al. [3], found a lower total bladder recurrence rate when simultaneous TURBT and TURP was performed compared to TURBT alone; however, the other studies included in the present review did not report such a risk reduction. It is also possible that a selection bias applies to all available studies on the topic, whereby small single tumours without CIS were predominantly included in the published data.
The overall risk factors of urothelial bladder cancer recurrence and progression [18], as well as the risk factors of PU recurrence [3, 9, 22] were considered in almost all studies included in the present review, except concurrent CIS and proximity to BN/PU. Indeed, 30%, 70% and 29% of all tumours were multifocal, T1 and high grade, respectively (Table 2). These variables (i.e. solitary and/or multifocal tumour, tumour grade and T stage of tumour) were used to perform adjusted analysis in all the 11 studies included in our present meta‐analysis.
Both bladder tumour size ˃3 cm and concurrent CIS are important risk factors for bladder tumour recurrence[18]. Although, among studies that reported patients’ tumour size, almost all of them assessed tumours of ≤3 cm (Table 2), and both Ham et al. [3] and Park et al. [3, 17] included tumours of < and > 3 cm. Additionally, Dellabella et al. [9] included tumours of ≤4 cm. The results of these three studies affirm our present meta‐analysis results. Moreover, they confirmed that recurrence risk (i.e. whole and BN/PU) and progression risk are not influenced by tumour size after a simultaneous bladder tumour and BPH surgery [3, 9, 17]. Bladder tumours with concurrent CIS were excluded in almost all studies except those conducted by Park et al. [3, 17] and Dellabella et al. [9] (Table 2). However, both studies reported that there was not any increased risk of whole bladder and BN/PU recurrence after a simultaneous surgical intervention for bladder tumour and BPH. Although a few studies included bladder tumours of >3 cm and/or bladder tumour with concurrent CIS, their results support our present findings.
Currently, the EAU recommends simultaneous TURBT and TURP only in patients with papillary, small and not extensively multifocal bladder tumours [18]. However, according to the present systematic review (Table 2) and meta‐analysis results, it appears that simultaneous TURBT and TURP for BPH could be considered in patients with concurrent bladder tumour and BPH without exceptions based on bladder tumour characteristics. However, it is unlikely that a TURP will be considered in patients with extensive/multifocal bladder tumours, in whom an eventual cystectomy may be required.
Although most urological surgeons may avoid concomitant single immediate intravesical chemotherapy (SIIC) at TURBT and TURP to avoid extravasation‐related side‐effects of intravesical chemotherapy, two studies (one retrospective and one RCT) reported the use of SIIC after a simultaneous endoscopic surgery for bladder tumour and BPH [10, 16]. Both Li et al. [10, 16] and Wang et al. [10, 16] used laser technology to resect the bladder tumour and enucleation of the prostate adenoma, while SIIC was administered for all patients after surgery. They did not report any adverse events related to extravasation of the chemotherapeutic agents. Probably a good coagulation state and a lower risk of haematuria after simultaneous surgery by the laser technology enabled the use of SIIC in those studies.
The main limitation of the present review was the low number of RCTs. However, the findings of these RCTs correlated with those of the cohort studies. Another limitation was the lack of the standard use of SIIC; it is not clear whether it would lead to more adverse events in cases of TURBT and TURP. Consequently, there is not enough evidence to comment on the usage of SIIC at concurrent TURBT and TURP. Finally, there were few studies with different study design that used SIIC and/or included bladder tumours of ˃3 cm and those that occur along with CIS. Therefore, performing a subgroup analysis was not feasible.
Conclusions
The findings of the present study suggest that there is no increased risk of overall bladder recurrence, BN/PU recurrence and/or tumour progression after concurrent TURBT/TURP vs TURBT alone. Future studies are required to assess potential risk of concurrent TURBT/TURP in more extensive/multifocal bladder tumours, as well as on side‐effects of SIIC.
Conflict of Interest
None declared.
Abbreviations
- BN
bladder neck
- CIS
carcinoma in situ
- EAU
European Association of Urology
- PU
prostatic urethra
- RCT
randomised controlled trial
- RR
risk ratio
- SIIC
single immediate intravesical chemotherapy
- TURBT
transurethral resection of bladder tumour
Supporting information
Supplementary Material
Acknowledgements
None.
References
- 1. Bryan RT, Collins SI, Daykin MC et al. Mechanisms of recurrence of Ta/T1 bladder cancer. Ann R Coll Surg Engl 2010; 92: 519–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanli O, Dobruch J, Knowles MA et al. Bladder cancer. Nat Rev Dis Primer 2017; 3: 17022 [DOI] [PubMed] [Google Scholar]
- 3. Ham WS, Kim WT, Jeon HJ, Lee DH, Choi YD. Long‐term outcome of simultaneous transurethral resection of bladder tumor and prostate in patients with nonmuscle invasive bladder tumor and bladder outlet obstruction. J Urol 2009; 181: 1594–8 [DOI] [PubMed] [Google Scholar]
- 4. Tsivian A, Shtricker A, Sidi AA. Simultaneous transurethral resection of bladder tumor and benign prostatic hyperplasia: hazardous or a safe timesaver? J Urol 2003; 170: 2241–3 [DOI] [PubMed] [Google Scholar]
- 5. Jaidane M, Bouicha T, Slama A et al. Tumor recurrence in prostatic urethra following simultaneous resection of bladder tumor and prostate: a comparative retrospective study. Urology 2010; 75: 1392–5 [DOI] [PubMed] [Google Scholar]
- 6. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010; 25: 603–5 [DOI] [PubMed] [Google Scholar]
- 7. Higgins JP, Altman DG, Gøtzsche PC et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dellabella M, Branchi A, Gasparri L, Claudini R, Castellani D. Oncological safety and quality of life in men undergoing simultaneous transurethral resection of bladder tumor and prostate: results from a randomized controlled trial. World J Urol 2018; 36: 1629–34 [DOI] [PubMed] [Google Scholar]
- 10. Li Z, Hou R, Li J. The efficacy and safety of simultaneous transurethral GreenLight photoselective vaporization of bladder tumor and prostate in patients with bladder tumor and lower urinary tract symptoms. Int Urol Nephrol 2014; 46: 691–4 [DOI] [PubMed] [Google Scholar]
- 11. Singh V, Sinha RJ, Sankhwar SN. Outcome of simultaneous transurethral resection of bladder tumor and transurethral resection of the prostate in comparison with the procedures in two separate sittings in patients with bladder tumor and urodynamically proven bladder outflow obstruction. J Endourol 2009; 23: 2007–11 [DOI] [PubMed] [Google Scholar]
- 12. Ugurlu O, Gonulalan U, Adsan O, Kosan M, Oztekin V, Cetinkaya M. Effects of simultaneous transurethral resection of prostate and solitary bladder tumors smaller than 3 cm on oncologic results. Urology 2007; 70: 55–9 [DOI] [PubMed] [Google Scholar]
- 13. Vicente J, Chéchile G, Pons R, Méndez G. Tumor recurrence in prostatic urethra following simultaneous resection of bladder tumor and prostate. Eur Urol 1988; 15: 40–2 [DOI] [PubMed] [Google Scholar]
- 14. Laor E, Grabstald H, Whitmore WF. The influence of simultaneous resection of bladder tumors and prostate on the occurrence of prostatic urethral tumors. J Urol 1981; 126: 171–5 [DOI] [PubMed] [Google Scholar]
- 15. Greene LF, Yalowitz PA. The advisability of concomitant transurethral excision of vesical neoplasm and prostatic hyperplasia. J Urol 1972; 107: 445–7 [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Zhang Z, Shao J, Lü Y, Li X, Li R. Outcome of simultaneous thulium laser enucleation of bladder tumor and prostate in patients with non‐muscle invasive bladder tumor and benign prostatic hyperplasia: a matched‐pair comparison with a long‐term follow‐up. World J Urol 2020. [Online ahead of print]. DOI: 10.1007/s00345‐020‐03158‐3 [DOI] [PubMed] [Google Scholar]
- 17. Park SH, Kim SI, Kim SJ. Effects of simultaneous transurethral resection of non‐muscle‐invasive bladder cancer and benign prostatic hyperplasia. Korean J Urol 2009; 50: 534–9 [Google Scholar]
- 18. Professionals S‐O . EAU Guidelines: Non‐muscle‐invasive Bladder Cancer [Internet]. Uroweb. Available at: https://uroweb.org/guideline/non‐muscle‐invasive‐bladder‐cancer/#note_130. Accessed March 2020
- 19. Picozzi SC, Ricci C, Gaeta M et al. Is it oncologically safe performing simultaneous transurethral resection of the bladder and prostate? A meta‐analysis on 1,234 patients. Int Urol Nephrol 2012; 44: 1325–33 [DOI] [PubMed] [Google Scholar]
- 20. Zhou L, Liang X, Zhang K. Assessment of the clinical efficacy of simultaneous transurethral resection of both bladder cancer and the prostate: a systematic review and meta‐analysis. Aging Male 2020; 1–12 [DOI] [PubMed] [Google Scholar]
- 21. Palou J, Sylvester RJ, Faba OR et al. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease‐specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette‐Guérin. Eur Urol 2012; 62: 118–25 [DOI] [PubMed] [Google Scholar]
- 22. Mungan MU, Canda AE, Tuzel E, Yorukoglu K, Kirkali Z. Risk factors for mucosal prostatic urethral involvement in superficial transitional cell carcinoma of the bladder. Eur Urol 2005; 48: 760–3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


