ABSTRACT
Background
It is unknown whether individuals with tic disorders are at increased risk for serious transport accidents.
Objectives
The aim of this study was to investigate the risk for injuries or death caused by transport and motor vehicle accidents in individuals with Tourette syndrome or chronic tic disorder.
Methods
This population‐based, sibling‐controlled cohort study included all individuals aged ≥18 years living in Sweden between 1997 and 2013 (N = 6,127,290). A total of 3449 individuals had a registered diagnosis of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. We also identified 2191 families with full siblings discordant for tic disorders. Cox proportional hazards regression modeling was used to estimate the risk for injuries or deaths as a result of transport accidents in individuals with a lifetime diagnosis of Tourette syndrome or chronic tic disorder compared with unexposed individuals and siblings.
Results
Individuals with tic disorders had a higher risk for transport injuries or death compared with the general population (adjusted hazard ratio, 1.50 [95% confidence interval: 1.33–1.69]) and their unaffected siblings (adjusted hazard ratio, 1.41 [95% confidence interval: 1.18–1.68]). The risks were similar across sexes. The exclusion of most psychiatric comorbidities did not alter the magnitude of the estimates. However, the risks were no longer significant after exclusion of individuals with comorbid attention deficit hyperactivity disorder.
Conclusions
The marginally increased risk for serious transport accidents in tic disorders is mainly driven by attention deficit hyperactivity disorder comorbidity. Improved detection and management of attention deficit hyperactivity disorder symptoms in this patient group are warranted. © 2020 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: tic disorders, motor vehicle accidents, mortality, injuries
Road traffic injuries are the leading cause of death for children and young adults aged 5–29 years, and the eighth leading cause of death for all age groups. 1 Although some progress has been made in reducing traffic‐related deaths in middle‐ and high‐income countries, globally, the number of traffic‐related deaths is still increasing steadily, reaching 1.35 million deaths in 2016. 1
Tourette syndrome (TS) and chronic tic disorder (CTD) are childhood‐onset neurodevelopmental movement disorders characterized by multiple motor and/or vocal tics persisting for more than 1 year. 2 It is currently unknown whether persons with TS/CTD are at increased risk for traffic accidents. Anecdotal evidence suggests that tics may interfere with driving in a number of ways; for example, patients with complex tics and/or compulsions may have unpleasant urges to look away from the road, close their eyes, drive toward incoming vehicles, touch items on the dashboard, or take their hands off the steering wheel. 3 At the same time, it is well known that some individuals can suppress their symptoms for long periods, particularly when engaged in activities that demand concentration, such as playing the piano. 4 In this light, many individuals with chronic tics may be able to drive normally with little or no interference from their tics. However, in the absence of relevant research literature, these alternative possibilities remain speculative. In some countries, such as Sweden or the United Kingdom, licensing authorities encourage persons with TS to report their diagnosis if they feel that the condition may affect their ability to drive safely.
Another important consideration is that 70% to 90% of individuals with TS/CTD present with psychiatric comorbidities, such as attention deficit hyperactivity disorder (ADHD), obsessive‐compulsive disorder, or autism spectrum disorders, among others, 5 , 6 which may further increase the risk for traffic accidents. In particular, a substantial body of literature indicates that individuals with ADHD are at increased risk for serious transport accidents. 7 , 8 Some clinical features, such as impulsivity, excessive risk taking, poor emotional control, and increased risk for substance use disorders, may be common to both ADHD and TS/CTD. 9 , 10 Thus, it is possible that individuals with TS/CTD might have an elevated risk for transport accidents, at least partially because of psychiatric comorbidities such as ADHD. However, at this point, it would be premature to rule out the possibility that tic disorders per se are associated with increased risk for serious transport accidents.
In this study, we leveraged the unique Swedish population‐based registers, which contain one of the world's largest cohorts of prospectively followed individuals with TS/CTD, and allow the construction of family pedigrees to adjust for important genetic and environmental confounders with the following goals: (1) to estimate the risk for injuries or deaths attributed to transport accidents and, specifically, motor vehicle accidents in individuals with TS/CTD, compared with the general population and with their unaffected full siblings; and (2) to establish the extent to which the association between TS/CTD and injuries or deaths caused by transport accidents is explained by psychiatric comorbidity in general and ADHD in particular.
Subjects and Methods
The Stockholm Regional Ethical Review Board approved the study. The requirement for informed consent was waived because all individuals included in this register‐based study were deidentified.
The Swedish National Registers
We conducted a population‐based cohort study by linking data across the nationwide Swedish health and administrative registers via the unique personal identification number assigned to all Swedish residents. 11 The following registers were linked: (1) the Total Population Register, which contains demographic and migration data for all inhabitants in Sweden since 1968; 12 (2) the National Patient Register, which captures diagnostic information from all visits to inpatient care (1964 onward and 1973 onward for somatic and psychiatric data, respectively, with nationwide coverage from 1987) and specialist outpatient services (2001 onward); 13 in this register, clinical diagnoses are coded using the Swedish version of the International Classification of Diseases (ICD); (3) the Cause of Death Register, which covers all dates, and the international version of the ICD codes for causes of death of Swedish residents since 1952; 14 (4) the Prescribed Drug Register, which records all prescribed medications dispensed across pharmacies in Sweden since July 2005, using Anatomical Therapeutic Chemical Classification System codes; 15 and (5) the Multi‐Generation Register, which contains information on biological and adopted parents of all individuals who were born in Sweden from 1932 or have ever been registered in the country since 1961. 16
Study Population
The study population comprised all individuals who were born before 1996, living in Sweden anytime between 1997 (when the tenth revision of the ICD manual [ICD‐10] was introduced) and 2013, and had information on biological mothers available from the Multi‐Generation Register (N = 6,854,309). We excluded individuals who had died or emigrated from Sweden before age 18 or prior to 1997 (n = 547,489); had immigrated to Sweden after the start of follow‐up or had a missing date of immigration (n = 21,467); or had a record of a lifetime diagnosis of epilepsy (ICD‐10: G40, G41; n = 82,901), organic brain disorder (ICD‐10: F00‐F09; n = 54,938), or mental retardation (ICD‐10: F70‐F79; n = 20,224). These conditions were excluded because they are known to interfere with driving. The final study cohort included 6,127,290 individuals (Fig. 1) who were followed up from January 1, 1997, or their 18th birthday, whichever occurred last, until the date of the outcome (ie, the registered record of injury or death as a result of a transport accident), emigration, death, or the end of the follow‐up (December 31, 2013), whichever occurred first. In Sweden, a driver's license can be obtained only from age 18 years.
FIG. 1.
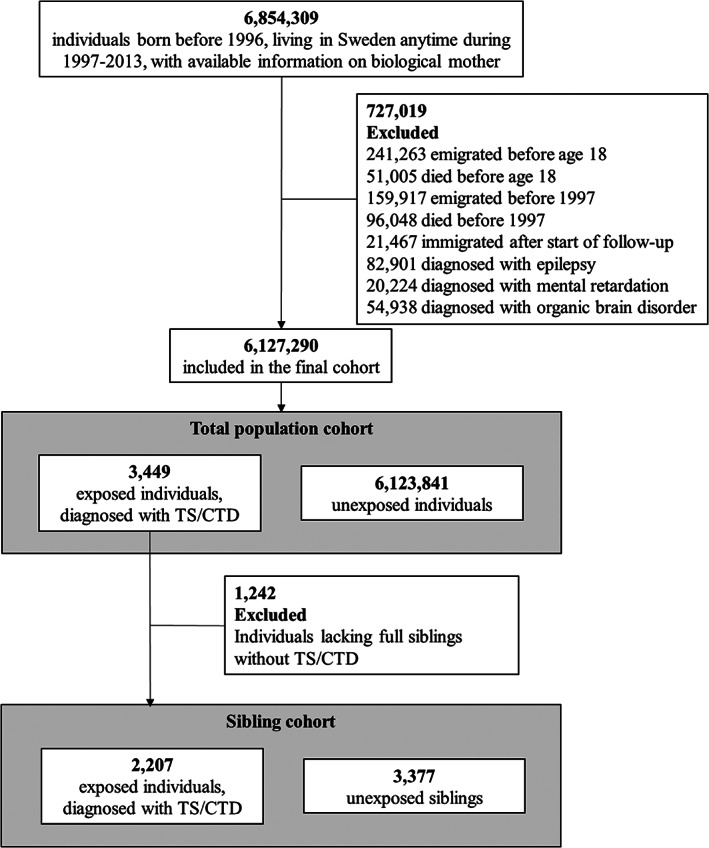
Study cohorts. Among 727,019 excluded persons, individuals may have more than one reason for exclusion. CTD, chronic tic disorder; TS, Tourette syndrome.
Measures
Information on the individuals’ sex and birth year (categorized by 10‐year increments) was retrieved from the Total Population Register. From the National Patient Register, we identified all individuals with an ICD‐10 lifetime record of tic disorders (F95.0 [transient tic disorder], F95.1 [chronic motor or vocal tic disorder], F95.2 [combined vocal and multiple motor tic disorder, de la Tourette], F95.8 [other tic disorders], or F95.9 [tic disorder, unspecified]), if diagnosed at the age of 3 years or older. In line with prior research, 17 , 18 we selected those with a diagnosis of TS or CTD by using an algorithm that minimizes the inclusion of individuals with only transient tics and applied the minimal age limit to avoid diagnostic misclassification. 19 The Swedish codes for TS/CTD diagnoses have been previously validated, with a reported positive predictive value of 97% for ICD‐10. 20
For the main analysis, the records of injuries or deaths as a result of transport accidents were collected from the National Patient Register and the Cause of Death Register, respectively, by the ICD‐10 codes corresponding to transport accidents (V01–V99), if recorded at the age of 18 years or older. This broad category includes transport accidents in general, regardless of whether they are related to motor vehicles (eg, pedestrians or cyclists injured in collision with vehicles). In additional analyses, we specifically focused on the ICD‐10 codes corresponding to a subgroup of motor vehicle accidents (including V20–V29 [motorcycle rider injured in transport accident], V30–V39 [occupant of three‐wheeled motor vehicle injured in transport accident], V40–V49 [car occupant injured in transport accident], V50–V59 [occupant of pickup truck or van injured in transport accident], and V60–V69 [occupant of heavy transport vehicle injured in transport accident]).
Furthermore, from the National Patient Register, we collected data on lifetime psychiatric comorbidities to explore their potential impact on the association between TS/CTD and serious transport accidents. We retrieved ICD‐10 codes for autism spectrum disorders, ADHD (complemented with data from the Prescribed Drug Register on medications used in Sweden for ADHD treatment, as suggested by prior Swedish register studies on ADHD), conduct disorder, anxiety disorders, obsessive‐compulsive disorder, posttraumatic and other stress‐related disorders, eating disorders, depression and other mood disorders, bipolar disorders, schizophrenia and other psychotic disorders, substance use disorders, and dissocial personality disorder (see Supporting Information Table S1 for the ICD‐10 codes and corresponding minimal age limits).
Statistical Analysis
We applied Cox proportional hazards regression modeling to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for the risk for injuries or deaths caused by transport accidents in individuals with a lifetime diagnosis of TS/CTD, compared with unexposed individuals, with age in years as the underlying timescale. The analysis was first conducted for a broad definition of the outcome (ie, all transport accidents; ICD‐10 codes: V01–V99) and then repeated for the subgroup corresponding to motor vehicle accidents (ICD‐10 codes: V20–V69). All models were adjusted for the individuals' sex and birth year. We also conducted the analyses stratified by sex, adjusting for birth year.
For the sibling analyses, we identified all individuals with TS/CTD for whom information on both mother and father was available from the Multi‐Generation Register and then linked to their full siblings (ie, those sharing both parents). Family identification numbers were created for the linkage. By design, the sibling comparison controls for potential familial confounders, which are shared by full siblings, including unmeasured shared environmental factors, such as parental socioeconomic status, and about 50% of the genetic factors. We used stratified Cox proportional hazards regression models where each family was considered a stratum, and all full siblings within a family were compared with each other in a way that unexposed siblings served as controls to the exposed ones. The models controlled for sex and birth year.
To address the second aim, we repeated the main analyses after excluding individuals with different groups of comorbid psychiatric disorders from the whole study cohort (ie, from both exposed and unexposed individuals), one disorder group at a time. Given our particular interest in comorbidity with ADHD, in a first post hoc analysis, we further explored whether individuals with TS/CTD and comorbid ADHD differ in risk for serious transport accidents from individuals with TS/CTD and no comorbid ADHD, compared with individuals unaffected by TS/CTD. To provide a comparative benchmark, we also examined the risk for serious traffic accidents in individuals who had ADHD but no comorbid TS/CTD, compared with individuals without ADHD.
Because no information on TS/CTD severity was available in the National Patient Register, we conducted an additional post hoc analysis limited to a subcohort of individuals who had at least two diagnoses of TS/CTD after the age of 18 years (as a proxy for the severity and long‐term persistence of TS/CTD into adulthood) and compared them with individuals unaffected by TS/CTD. Thus, this analysis excluded individuals diagnosed with TS/CTD but who were no longer seen by a specialist after age 18 years and those who were seen only once after age 18 years. These models also controlled for sex and birth year.
All earlier‐mentioned models were clustered by the identification number of the individual's mother (for the analyses of the whole study population) or stratified by the family identification number (for the sibling comparison), and used a robust sandwich estimator of standard errors to account for nonindependence of observations within families. 22 All tests used two‐tailed significance set at P < 0.05. Data management and analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC) and STATA version 15.1 (StataCorp LLC, College Station, TX), respectively.
Results
Descriptive Statistics
The final population cohort consisted of 6,127,290 individuals (51.12% male) aged 18–65 years at the start of follow‐up (see Table 1 for descriptive data). The mean length of the follow‐up was 13.46 years (SD, 5.35). Within this cohort, 3449 individuals had a diagnosis of TS/CTD in the National Patient Register (75.94% male, n = 2619).
TABLE 1.
Distribution of study characteristics in individuals with TS or CTDs and in unaffected individuals from the general population
| Individuals With TS/CTD (n = 3449) | Unaffected Individuals (n = 6,123,841) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Men | 2619 | 75.94 | 3,129,702 | 51.11 |
| Women | 830 | 24.06 | 2,994,139 | 48.89 |
| Age at start of follow‐up, years a | ||||
| 18–19 | 2684 | 77.82 | 1,969,426 | 32.16 |
| 20–29 | 354 | 10.26 | 1,036,797 | 16.93 |
| 30–39 | 196 | 5.68 | 1,014,092 | 16.56 |
| 40–49 | 133 | 3.86 | 996,119 | 16.27 |
| 50–65 | 82 | 2.38 | 1,107,407 | 18.08 |
| Psychiatric comorbidities b | ||||
| Autism spectrum disorders | 925 | 26.82 | 23,748 | 0.39 |
| Attention deficit hyperactivity disorder | 1738 | 50.39 | 73,820 | 1.21 |
| Conduct disorder | 177 | 5.13 | 4698 | 0.08 |
| Anxiety disorders | 1163 | 33.72 | 345,088 | 5.64 |
| Obsessive‐compulsive disorder | 757 | 21.95 | 24,008 | 0.39 |
| Posttraumatic and other stress‐related disorders | 407 | 11.80 | 148,408 | 2.42 |
| Eating disorders | 81 | 2.35 | 26,593 | 0.43 |
| Depression and other mood disorders | 1001 | 29.02 | 292,868 | 4.78 |
| Bipolar disorders | 220 | 6.38 | 49,644 | 0.81 |
| Schizophrenia and other psychotic disorders | 235 | 6.81 | 50,710 | 0.83 |
| Substance use disorders | 585 | 16.96 | 236,462 | 3.86 |
| Dissocial personality disorder | 24 | 0.70 | 2426 | 0.04 |
Abbreviations: TS, Tourette syndrome; CTD, chronic tic disorder.
Individuals are followed from their 18th birthday or January 1, 1997, whichever occurred last.
The study participants, both exposed and unexposed, may have more than one psychiatric comorbid disorder.
A total of 340,940 individuals in the whole cohort had a record of at least one injury or death caused by a transport accident (271 in the TS/CTD cohort and 340,669 in the unaffected population cohort). Of these, 190,565 (189 in the TS/CTD cohort and 190,376 in the unaffected population cohort) had at least one record of injury or death as a result of motor vehicle accidents.
Risk for Transport Accident Injuries or Deaths
Individuals with TS/CTD had a 50% increased risk for injuries or death as a result of transport accidents compared with the individuals from the general population without a diagnosis of TS/CTD (HR adjusted for sex and birth year [aHR], 1.50 [95% CI: 1.33–1.69]; Table 2). When the outcome was narrowed down to injuries or death caused by motor vehicle accidents only, the results were similar (aHR, 1.58 [95% CI: 1.37–1.82]; Table 2). The risk was comparable for men and women, with no significant sex differences (overlapping CIs) in both outcomes (Table 2).
TABLE 2.
HRs and corresponding 95% CIs for the risk for injury or death as a result of transport accidents and motor vehicle accidents among individuals with TS or CTDs, compared with unaffected individuals from the general population (population cohort) and with their unaffected full siblings (sibling cohort)
| Individuals with TS/CTD (n = 3449) | Unaffected Individuals (n = 6,123,841) | HR (95% CI) Adjusted for Sex and Birth Year | |||
|---|---|---|---|---|---|
| Population Cohort | n | % | n | % | |
| All serious transport accidents | 271 | 7.86 | 340,669 | 5.56 | 1.50 (1.33–1.69) |
| Men | 208 | 7.94 | 190,544 | 6.09 | 1.48 (1.29–1.70) |
| Women | 63 | 7.59 | 150,125 | 5.01 | 1.57 (1.23–2.01) |
| Motor vehicle accidents | 189 | 5.48 | 190,376 | 3.11 | 1.58 (1.37–1.82) |
| Men | 151 | 5.77 | 119,382 | 3.81 | 1.50 (1.28–1.77) |
| Women | 38 | 4.58 | 70,994 | 2.37 | 1.86 (1.35–2.55) |
| Individuals With TS/CTD With Unaffected Full Siblings (n = 2207) | Unaffected Full Siblings of Individuals With TS/CTD (n = 3377) | HR (95% CI) Adjusted for Sex and Birth Year | |||
|---|---|---|---|---|---|
| Sibling Cohort | n | % | n | % | |
| All serious transport accidents | 194 | 8.79 | 238 | 7.05 | 1.41 (1.18–1.68) |
| Motor vehicle accidents | 132 | 5.98 | 161 | 4.77 | 1.27 (1.03–1.57) |
Statistically significant HRs are highlighted in bold. P values for all reported HRs (95% CIs) are P < 0.001, apart from the sibling analysis for motor vehicle accidents (P = 0.026).
Abbreviations: HR, hazard ratio; CI, confidence interval; TS, Tourette syndrome; CTD, chronic tic disorder.
Sibling Comparison
Of the 1,871,590 families who had at least two singleton children, 2191 families included clusters of full siblings who were discordant for TS/CTD, totalling 2207 individuals with TS/CTD and 3377 unaffected siblings. Individuals with a diagnosis of TS/CTD had a higher risk for serious transport accidents and motor vehicle accidents, compared with their unaffected full siblings (aHR, 1.41 [95% CI: 1.18–1.68] and 1.27 [95% CI: 1.03–1.57], respectively; Table 2).
Contribution of Psychiatric Comorbidities
When different groups of psychiatric comorbidities were excluded from the analyses, the risk estimates for serious transport accidents and motor vehicle accidents remained largely similar (Table 3), except when ADHD was excluded, which substantially reduced the risk to nonsignificant for both transport accident injuries or deaths (aHR, 1.20 [95% CI: 0.99–1.44]) and motor vehicle accident injuries or deaths (aHR, 1.18 [95% CI: 0.94–1.49]).
TABLE 3.
Risk for injuries and death as a result of transport accidents and motor vehicle accidents in individuals with TS or CTD, compared with unaffected individuals from the general population, when excluding common comorbid disorders from the whole study cohort (one disorder group at the time)
| HR (95% CI) Adjusted for Sex and Birth Year | ||
|---|---|---|
| All Serious Transport Accidents | Motor Vehicle Accidents | |
| Autism spectrum disorders | 1.67 (1.46–1.91) | 1.82 (1.55–2.13) |
| Attention deficit hyperactivity disorder | 1.20 (0.99–1.44) | 1.18 (0.94–1.49) |
| Conduct disorder | 1.53 (1.35–1.72) | 1.59 (1.37–1.84) |
| Anxiety disorders | 1.40 (1.20–1.65) | 1.42 (1.17–1.72) |
| Obsessive‐compulsive disorder | 1.63 (1.43–1.86) | 1.68 (1.43–1.97) |
| Posttraumatic and other stress‐related disorders | 1.35 (1.18–1.54) | 1.39 (1.18–1.64) |
| Eating disorders | 1.51 (1.34–1.70) | 1.58 (1.37–1.83) |
| Depression and other mood disorders | 1.39 (1.19–1.61) | 1.44 (1.20–1.73) |
| Bipolar disorders | 1.47 (1.30–1.67) | 1.52 (1.30–1.76) |
| Schizophrenia and other psychotic disorders | 1.57 (1.39–1.77) | 1.64 (1.41–1.89) |
| Substance use disorders | 1.35 (1.17–1.56) | 1.43 (1.20–1.69) |
| Dissocial personality disorder | 1.47 (1.31–1.66) | 1.54 (1.33–1.78) |
Statistically significant HRs are highlighted in bold. P values for HRs (95% CIs) in the analyses where attention deficit hyperactivity disorder is excluded are for all serious transport accidents, P = 0.057, and for motor vehicle accidents, P = 0.160. P values for the rest of the reported HRs (95% CIs) are P < 0.001.
Abbreviations: CI, confidence interval; HR, hazard ratio.
In the first post hoc analysis, where we split individuals with TS/CTD by their ADHD comorbidity status and compared them with individuals unaffected by TS/CTD, the increased risks for injury or death caused by transport accidents (aHR, 1.86 [95% CI: 1.59–2.17]; Table 4) and motor vehicle accidents (aHR, 2.02 [95% CI: 1.69–2.42]; Table 4) were notable only for individuals with TS/CTD and comorbid ADHD. By contrast, no significant associations were observed for individuals with TS/CTD who were free of ADHD comorbidity (aHR, 1.18 [95% CI: 0.98–1.42] and 1.16 [95% CI: 0.92–1.46], respectively; Table 4).
TABLE 4.
HRs and corresponding 95% CIs for the risk for injury or death as a result of transport accidents and motor vehicle accidents among individuals with TS or CTD with and without comorbid ADHD, compared with individuals unaffected by TS or CTD from the general population
| Total, n | All Serious Transport Accidents | Motor Vehicle Accidents | |||||
|---|---|---|---|---|---|---|---|
| n | % | HR (95% CI) Adjusted for Sex and Birth Year | n | % | HR (95% CI) Adjusted for Sex and Birth Year | ||
| Unaffected by TS/CTD | 6,123,841 | 340,669 | 5.56 | 1.00 | 190,376 | 3.11 | 1.00 |
| TS/CTD with comorbid ADHD | 1738 | 160 | 9.21 | 1.86 (1.59–2.17) | 118 | 6.79 | 2.02 (1.69–2.42) |
| TS/CTD without comorbid ADHD | 1711 | 111 | 6.49 | 1.18 (0.98–1.42) | 71 | 4.15 | 1.16 (0.92–1.46) |
Statistically significant HRs are highlighted in bold. P values for HR (95% CI) reported for analyses of TS/CTD with comorbid ADHD are P < 0.001. P values for analysis of TS/CTD without comorbid ADHD are P = 0.083 for all serious transport accidents and P = 0.215 for motor vehicle accidents.
Abbreviations: HR, hazard ratio; CI, confidence interval; TS, Tourette syndrome; CTD, chronic tic disorder; ADHD, attention deficit hyperactivity disorder.
To provide a useful benchmark for comparison, we also examined the risk of the outcomes in individuals with ADHD without comorbid TS/CTD (n = 73,820; among which 10.6% had any transport accident and 7.12% had a motor vehicle accident) with the corresponding risks in individuals unaffected by ADHD (n = 6,051,732; among which 5.50% had any transport accident and 3.06% had a motor vehicle accident). The results showed significant associations between exposure to ADHD without comorbid TS/CTD and all transport accidents (HR, 2.01 [95% CI: 1.96–2.05]) and motor vehicle accidents (HR, 2.14 [95% CI: 2.08–2.20]).
In the second post hoc analysis, 1570 individuals with at least two diagnoses of TS/CTD after the age of 18 years (45.52% of the initial TS/CTD cohort) were compared with individuals unaffected by TS/CTD. Among individuals with persistent TS/CTD diagnoses, 10.57% (n = 166) had at least one serious transport accident and 7.45% (n = 117) had a motor vehicle accident under the study period, compared with 5.56% and 3.11%, respectively, of individuals unaffected by TS/CTD. This corresponded to an increased risk for injury or death as a result of all transport accidents (HR, 1.68 [95% CI: 1.45–1.96]) and motor vehicle accidents (HR, 1.82 [95% CI: 1.52–2.19]). Thus, the associations were slightly stronger than, but not significantly different from, those seen for the whole cohort of individuals with TS/CTD.
Discussion
To our knowledge, this is the first study to formally investigate whether TS/CTD are associated with a subsequent risk for severe transport accidents. The main finding was that individuals with TS/CTD had a modestly increased risk (approximately 50%) for injuries or death as a result of accidents,compared with the general population and with their unaffected full siblings. The observed risks were similar among men and women.
In general, the exclusion of most psychiatric disorders did not alter the magnitude of the estimates. However, after exclusion of individuals with ADHD from the whole study cohort, the magnitude of the risk decreased from 50% to 20% for injuries or deaths caused by all transport accidents, and from 58% to 18% for motor vehicle accidents, with the estimates for both outcomes being no longer statistically significant. These results suggest that comorbid ADHD was the single largest contributor to the observed risks. Additional post hoc analyses confirmed this observation: individuals with TS/CTD and comorbid ADHD had an 86% higher risk for transport accidents (and a 102% higher risk for motor vehicle accidents), compared with the general population, whereas individuals with TS/CTD and no comorbid ADHD did not have a significantly elevated risk for either outcome.
Notably, the proportion of persons with TS/CTD involved in transport accidents resulting in injury or death was small, even in ADHD comorbid cases. More than 92% of all individuals with TS/CTD in the cohort (91% of those with comorbid ADHD and 93% of those without comorbid ADHD) did not have a single serious transport accident during the follow‐up period. The corresponding proportion from the general population without TS/CTD was 94%.
Implications for Clinical Practice and Vehicle Licensing Agencies
From a clinical perspective, increased detection and adequate management of comorbid ADHD in driving‐age patients with TS/CTD should be prioritized, because there is mounting evidence that adequate pharmacological treatment of ADHD symptoms is associated with a significantly reduced risk for severe road traffic injuries and deaths. 8 , 23 From a licensing authority perspective, our data suggest that the majority of individuals with TS/CTD will be able to drive safely, and therefore licensing agencies should not necessarily equate TS/CTD with an increased risk for serious transport accidents. Instead, any licensing decisions should be made on an individual basis.
Strengths and Limitations
The main strengths of this study are the large population‐based sample of individuals with TS/CTD; the long follow‐up of up to 17 years; the high validity of the ICD diagnostic codes 20 ; the use of nationwide registers with prospective data collection, which minimizes the risk for selection, recall, surveillance, and report biases; and the use of a sibling design that controls for a number of potentially important confounders. Reverse causation is also unlikely because, by definition, the exposures always preceded the outcomes; that is, the diagnosis of TS/CTD requires an onset of tics before 18 years (even if sometimes the actual diagnosis is recorded after age 18), and in Sweden a driver's license can be obtained only from age 18.
The results should be interpreted in light of some limitations. First, because the registers do not contain information about tic severity at the time of the follow‐up, we could not examine the impact of this potentially important variable on the risks. In a post hoc analysis, we limited the cohort of individuals with TS/CTD to those receiving two or more diagnoses from age 18 years (as a proxy of greater tic severity or persistence), which resulted in similar estimates. It is possible that the results may not generalize to milder or transient forms of tic disorders that do not require or seek contact with the health services. Second, our study focused on outcomes requiring hospital/specialized outpatient clinic visits or deaths attributable to transport accidents. Thus, we had no access to information on milder accidents not requiring such care (eg, individuals treated at primary care). Third, based on the available ICD codes for accidents, we cannot know whether the person involved in the accident was the driver or a passenger. However, we made the assumption that the probability of being involved in a transport accident as a passenger should not be higher for individuals with TS/CTD than for individuals from the general population. Fourth, to our knowledge, there are no published data regarding the probability that individuals with TS/CTD will obtain a drivers' license; if individuals with TS/CTD were less likely to obtain a license or to actually drive, compared with individuals form the general population, that would potentially result in an underestimation of the true risk of serious transport accidents in this patient group. Finally, the results of the sibling analyses need to be interpreted in light of the known limitations of such designs. 24
Conclusions
Individuals with TS/CTD and comorbid ADHD are at increased risk for transport accident injuries or death, compared with the general population, although the overall proportion of individuals with such outcomes is small. Individuals with TS/CTD without comorbid ADHD did not have an elevated risk. The results have important implications both for clinical practice and for vehicle licensing agencies. Increased detection and long‐term management of ADHD symptoms in this patient group is warranted. Licensing agencies should not automatically assume that TS/CTD are associated with increased risks for accidents.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution.
2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique.
3. Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
David Mataix‐Cols: 1A, 1B, 1C, 2A, 2C, 3A.
Gustaf Brander: 1A, 1B, 1C, 2A, 2B, 2C, 3A.
Zheng Chang: 2C, 3B.
Henrik Larsson: 2C, 3B.
Brian M. D'Onofrio: 2C, 3B.
Paul Lichtenstein: 2C, 3B.
Anna Sidorchuk: 1A, 1B, 1C, 2A, 2B, 2C, 3A.
Lorena Fernández de la Cruz: 1A, 1B, 1C, 2A, 2C, 3A.
Financial Disclosures
David Mataix‐Cols receives royalties for contributing articles to UpToDate, Wolters Kluwer Health and for editorial work from Elsevier, all outside the submitted work. Henrik Larsson has served as a speaker for Evolan Pharma, Eli‐Lilly, and Shire, and has received research grants from Shire, all outside the submitted work. Lorena Fernández de la Cruz receives royalties for contributing articles to UpToDate, Wolters Kluwer Health, outside the submitted work. All other authors report no potential conflicts of interest.
Supporting information
Appendix S1. Supporting information.
Relevant conflicts of interest/financial disclosures: Nothing to report.
Full financial disclosures and author roles may be found in the online version of this article.
References
- 1. World Health Organization . Global Status Report on Road Safety. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 2. American Psychiatric Association . The Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 3. BBC Two . Recent horizons: Mad but glad. bbc.co.uk. http://www.bbc.co.uk/sn/tvradio/programmes/horizon/broadband/tx/madbutglad/. 2007. Accessed February 28, 2020.
- 4. Sacks O. Musicophilia: Tales of Music and the Brain. Toronto, Canada: Vintage Canada; 2010. [Google Scholar]
- 5. Hirschtritt ME, Lee PC, Pauls DL, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry 2015;72(4):325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pinto R, Monzani B, Leckman JF, et al. Understanding the covariation of tics, attention‐deficit/hyperactivity, and obsessive‐compulsive symptoms: a population‐based adult twin study. Am J Med Genet B Neuropsychiatr Genet 2016;171(7):938–947. [DOI] [PubMed] [Google Scholar]
- 7. Barkley RA, Cox D. A review of driving risks and impairments associated with attention‐deficit/hyperactivity disorder and the effects of stimulant medication on driving performance. J Safety Res 2007;38(1):113–128. [DOI] [PubMed] [Google Scholar]
- 8. Chang Z, Lichtenstein P, D'Onofrio BM, Sjolander A, Larsson H. Serious transport accidents in adults with attention‐deficit/hyperactivity disorder and the effect of medication: a population‐based study. JAMA Psychiatry 2014;71(3):319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen K, Budman CL, Diego Herrera L, et al. Prevalence and clinical correlates of explosive outbursts in Tourette syndrome. Psychiatry Re 2013;205(3):269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hovik KT, Egeland J, Isquith PK, et al. Distinct patterns of everyday executive function problems distinguish children with Tourette syndrome from children with ADHD or autism spectrum disorders. J Atten Disord 2017;21(10):811–823. [DOI] [PubMed] [Google Scholar]
- 11. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24(11):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31(2):125–136. [DOI] [PubMed] [Google Scholar]
- 13. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brooke HL, Talback M, Hornblad J, et al. The Swedish cause of death register. Eur J Epidemiol 2017;32(9):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wettermark B, Hammar N, Fored CM, et al. The new Swedish prescribed drug register: opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16(7):726–735. [DOI] [PubMed] [Google Scholar]
- 16. Ekbom A. The Swedish multi‐generation register. Methods Mol Biol 2011;675:215–220. [DOI] [PubMed] [Google Scholar]
- 17. Brander G, Isomura K, Chang Z, et al. Association of Tourette syndrome and chronic tic disorder with metabolic and cardiovascular disorders. JAMA Neurol 2019;76(4):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perez‐Vigil A, Fernández de la Cruz L, Brander G, et al. Association of Tourette syndrome and chronic tic disorders with objective indicators of educational attainment: a population‐based sibling comparison study. JAMA Neurol 2018;75(9):1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cesta CE, Oberg AS, Ibrahimson A, et al. Maternal polycystic ovary syndrome and risk of neuropsychiatric disorders in offspring: prenatal androgen exposure or genetic confounding? Psychol Med 2020;50(4):616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rück C, Larsson KJ, Lind K, et al. Validity and reliability of chronic tic disorder and obsessive‐compulsive disorder diagnoses in the Swedish National Patient Register. BMJ Open 2015;5(6):e007520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsson H, Ryden E, Boman M, Langstrom N, Lichtenstein P, Landen M. Risk of bipolar disorder and schizophrenia in relatives of people with attention‐deficit hyperactivity disorder. Br J Psychiatry 2013;203(2):103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams RL. A note on robust variance estimation for cluster‐correlated data. Biometrics 2000;56(2):645–646. [DOI] [PubMed] [Google Scholar]
- 23. Chang Z, Quinn PD, Hur K, et al. Association between medication use for attention‐deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry 2017;74(6):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D'Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. Critical need for family‐based, quasi‐experimental designs in integrating genetic and social science research. Am J Public Health 2013;103:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


