Abstract
Purpose
The effectiveness and safety of surgery for spheno‐orbital meningiomas remains subject of debate, as studies often describe different surgical approaches and reconstruction techniques with very heterogeneous outcomes. We aimed to systematically summarize and analyse the literature on spheno‐orbital meningiomas regarding presenting symptoms, surgical techniques, outcomes and complications.
Methods
Studies were retrieved from eight databases. Original articles were included if in ≥5 patients presenting symptoms, surgical treatment and outcomes were described. Fixed‐ and random‐effects meta‐analysis was performed to estimate weighted percentages with 95%CIs of presenting symptoms, outcomes and complications.
Results
Thirty‐eight articles were included describing 1486 patients. Proptosis was the most common presenting symptom (84%; 95%CI 76–91%), followed by unilateral visual acuity deficits (46%; 95%CI 40–51%) and visual field deficits (31%; 95%CI 20–43%). In 35/38 studies (92%), a pterional craniotomy was used. Decompression of the optic canal (82%) and the superior orbital fissure (66%) was most often performed, and usually dural (47%) and bony defects (76%) were reconstructed. In almost all patients, visual acuity (91%; 95%CI 86–96%), visual fields (87%; 95%CI 70–99%) and proptosis (96%; 95%CI 90–100%) improved. Furthermore, surgery showed improvement in 96% (95%CI 78–100%) for both diplopia and ophthalmoplegia. The most common surgical complications were hypesthesia (19%; 95%CI 10–30%), ptosis and diplopia (both 17%; 95%CI, respectively, 10–26% and 5–33%) and ophthalmoplegia (16%; 95%CI 10–24).
Conclusion
Patients with spheno‐orbital meningioma usually present with proptosis or unilateral decreased visual acuity. Surgery shows to be effective in improving visual acuity and visual field deficits with mostly minor and well‐tolerated complications.
Keywords: cranial nerves, meningioma, neurosurgery, orbit, spheno‐orbital, vision
Introduction
Meningiomas are central nervous system tumours, arising from the meninges (Whittle et al. 2004). Spheno‐orbital meningiomas (SOM) represent 9–18% of all meningiomas (Cushing & Eisenhardt 1939; Maroon et al. 1994; Mirone et al. 2009; Leroy et al. 2016) and are characterized by substantial hyperostosis of the sphenoid wing with an en‐plaque carpet‐like soft‐tissue component (Li et al. 2009). Due to the complex anatomical location in the anterior skull base and the tendency for invasion of the periorbit, intra‐orbital growth, and sometimes the extensive hyperostosis of the optic canal, superior orbital fissure, and other cranial nerve foramina, resection of these tumours is challenging and is associated with possible neurological and visual deficits (Ringel et al. 2007). Due to this growth pattern, the most common presenting symptoms are progressive symptoms of proptosis, unilateral decrease of visual acuity and visual field deficits (Jiranukool et al. 2016). Aim of surgery is to improve or prevent further deterioration of these visual, neurological and cosmetic symptoms.
Studies published on spheno‐orbital meningiomas are mostly small series, describing different surgical approaches and reconstruction techniques with very heterogeneous neurological and visual outcomes. As a result, the effectiveness and safety of spheno‐orbital surgery, especially regarding visual outcomes, remains subject of debate. Therefore, the aim of this systematic review and meta‐analysis was to systematically summarize the literature on used surgical approaches, extent of decompression/resection of hyperostotic bone, management of the periorbit, reconstruction techniques, visual and neurological outcomes, and complications in patients with spheno‐orbital meningioma.
Methods
This systematic review and meta‐analysis were reported according to the PRISMA criteria (Moher et al. 2009).
Search strategy
PubMed, Embase, Web of Science, COCHRANE Library, Emcare, PsychINFO, Academic Search Premier and Science direct were searched for relevant literature on 8‐2‐2018, and the search strategy was updated on 31‐7‐2019. The search strategy included terms for meningioma, sphenoid wing and spheno‐orbital, and derivatives or synonyms of these words. The complete search strategy can be found in the supplements (Table S1).
Inclusion and selection of articles
All articles were screened on title and abstract, and potentially relevant articles were included based on full‐text screening (Fig. 1). Original articles were included if at least five SOM patients were included in the study, and if information regarding the presenting symptoms, or surgical treatment or outcomes was reported. Only English articles were included. Studies presenting aggregated information on various pathologies were excluded. Literature reviews were also excluded. In case of multiple articles describing overlapping cohorts, the article with the largest study population was included. In case of articles describing the exact same cohort with the same study size, the most recent article was included. The selection of articles and the data extraction was done by two independent reviewers (A.H.Z.N. and F.L.F.).
Fig. 1.
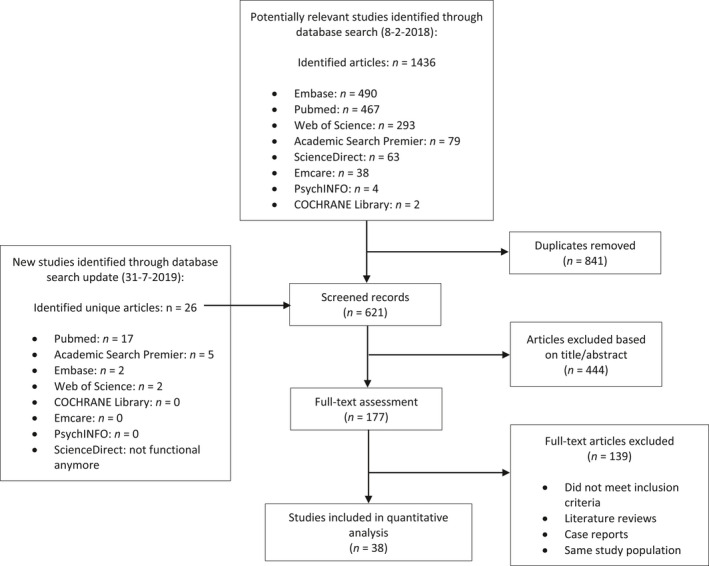
Flow chart of study selection.
Data extraction
The extracted study characteristics consisted of study, patient, tumour and treatment characteristics: the institution, study period, number of patients, age and sex, definition used for hyperostotic SOMs, other tumour location in case of multiple meningioma, WHO grade, SOM tumour diameter, radiological findings of tumour location and invasion, surgical approach, percentage patients who underwent reoperation, extent of decompression and resection of hyperostotic bone, Simpson grade, management of periorbit, reconstruction technique of both the bony and dural defects, previous therapies, and postoperative radiotherapy. The following presenting symptoms and postoperative outcomes were also extracted from the included articles: unspecified visual deficits, visual acuity, visual fields, proptosis, cranial nerve deficits, complications of surgery (specific for SOM surgery and general surgical complications), progression‐free survival, and follow‐up length. As there is no commonly accepted terminology for the anatomy, reconstruction material and visual outcomes, multiple different terms could have been used describing the same concepts. Therefore, we combined some of the terms for surgical approach, visual outcomes and reconstruction materials (Table S2).
Risk of bias
The risk of bias assessment for the individual articles was performed using a modified QUIPS (quality of prognostic factors) scale (Hayden et al. 2013), a standard tool of the Cochrane. Included elements in our assessment were patient population (description of study period, consecutive inclusion of patients and description of baseline demographics), clear description of intervention and outcome measurement (comparison of pre‐ and postoperative outcomes and reporting of reducible percentages), and less than 10% loss to follow‐up (Table S3). An overall low risk of bias was given when seven or more points were scored out of a maximum of nine points. Low risk of bias for the separate components was given if the complete amount of points possible was scored.
Analyses
Estimated weighted percentages of presenting symptoms, clinical outcomes (improved/stable versus worsened) and complications were calculated using random‐effects (n ≥ 5 studies) or fixed‐effects (n < 5 studies) models. Some articles only mentioned improvement of symptoms, and for these studies, only this outcome was pooled. The Freeman–Tukey arcsine transformation was used to prevent exclusion of studies with extreme outcomes (0 or 100%; Nyaga et al. 2014). The I 2 statistic was used for quantification of between‐study heterogeneity for analysis with ≥5 articles. In case of <5 studies, no reliable quantification of between‐study heterogeneity can be estimated (Higgins et al. 2003; Hippel 2015). Pooled results are reported as percentages with 95% confidence intervals (95%CIs). Subgroup analysis was performed on outcomes and complications of studies using the pterional approach in more than 95% of the patients. Analyses were performed with stata version 14.1 (StataCorp LLC, College Station, TX, USA). Reported information on surgical approaches, extent of resection and reconstruction techniques, including the periorbit, were not analysed through meta‐analysis, but systematically summarized and presented. To assess the possible impact in heterogeneity in follow‐up lengths and study period, number of operated cases per year and reporting quality, multiple additional subgroup analyses were performed for the main visual outcomes (proptosis, visual field and visual acuity), only including studies (1) with a minimum mean or median follow‐up length of ≥2 years, (2) published after 2000, and (3) classified as low risk of bias. The cut‐off of ≥2 years follow‐up was based on our own clinical experience, as deterioration in visual outcomes tend to happen after the first two years after surgery.
Results
A total of 621 unique articles were identified on 18‐2‐2018, of which 177 were read full text. Of those, 37 articles were included in the study. With the update of the literature search, 26 new unique articles were identified, of which one was included. Eventually, 38 articles were included in this study (Fig. 1) describing 1486 patients. All studies were retrospective cohort studies. The median sample size was 30 patients (range: 6–130) with a follow‐up range between 3 and 135 months. For a median of 14% of patients (IQR 3–21%), outcomes were described of a reoperation. For all study characteristics, see Table 1.
Table 1.
Study characteristics.
| Name author | Year published | Study period | Study size | Follow‐up period (mean) | Age (mean) | Female | Risk of bias | Re‐resection (%) |
|---|---|---|---|---|---|---|---|---|
| Bonnal | 1980 | – | 21 | 1–8 years* | – | – | Low | NC |
| Maroon | 1994 | 1975–1992 | 15 | 16–95 months* | 46 | 73% | Low | 15 (100) |
| Gaillard | 1996 | 1981–1993 | 20 | 7 years | – | – | High | 5 (25) |
| Honeybul | 2001 | 1991–1998 | 15 | 40 months | 52 | 80% | Low | NC |
| De Jesus | 2001 | 1990–1997 | 6 | 4 years | – | 100% | High | NC |
| Shrivastava | 2005 | 1991–2003 | 25 | 5 years | 51 | 88% | High | 0 (0) |
| Sandalcioglu | 2005 | 1988–2002 | 16 | 68 months | 53 | 94% | Low | 4 (25) |
| Leake | 2005 | 1995–2002 | 22 | 15 months | 53 | 77% | High | 3 (14) |
| Roser | 2005 | – | 82 | 66 months | 52 | 77% | Low | NC |
| Schick | 2006 | 1991–2002 | 67 | 46 months | 58 | 79% | Low | 10 (15) |
| Ringel | 2007 | 1983–2003 | 63 | 54 months | 51 | 79% | Low | 9 (14) |
| Bikmaz | 2007 | 1994–2004 | 17 | 36 months | 72 | 88% | High | 2 (13) |
| Mariniello | 2008 | 1983–2003 | 60 | 116 months | 47 | 85% | High | NC |
| Cannon | 2009 | 2000–2007 | 12 | 31 months | 51 | 92% | Low | NR |
| Heufelder | 2009 | 1997–2006 | 21 | 67 months | 61 | – | High | NC |
| Mirone | 2009 | 1986–2006 | 71 | 77 months | 53 | 87% | Low | NC |
| Scarone | 2009 | 1994–2005 | 30 | 61 months | 51 | 100% | Low | 0 (0) |
| Li | 2009 | 1998–2009 | 37 | 36 months | 46 | 60% | Low | 2 (5) |
| Saeed | 2011 | – | 66 | 102 months | 46 | 92% | High | NC |
| Oya | 2011 | 1994–2009 | 39 | 41 months | 48 | 87% | High | 6 (15) |
| Nochez | 2012 | 1986–2006 | 40 | 7 months | 50 | 93% | High | NR |
| Marcus | 2013 | 2004–2012 | 19 | 5 years | 44 | 90% | Low | 3 (16) |
| Simas | 2013 | 1998–2008 | 18 | 55 months | 52 | 83% | Low | NC |
| Mariniello | 2013 | 1986–2006 | 60 | 5 years | ‐ | ‐ | High | NC |
| Boari | 2013 | 2000–2010 | 40 | 73 months | 53 | 88% | Low | NC |
| Talacchi | 2014 | 1992–2012 | 47 | 52 months | 57 | 55% | Low | NC |
| Forster | 2014 | 2003–2013 | 18 | 40–44 months* | 50 | 100% | Low | 2 (11) |
| Solmaz | 2014 | 2006–2013 | 13 | 26 months | 34 | 23% | High | 0 (0) |
| Amirjamshidi | 2015 | 1979–2013 | 88 | 135 months | 46 | 65% | High | NC |
| Leroy | 2016 | 1995–2012 | 70 | 57 months | 52 | 92% | Low | 0 (0) |
| Jiranukool | 2016 | 2008–2012 | 26 | 52 months | 44 | 96% | Low | 0 (0) |
| Honig | 2017 | 2001–2006 | 30 | 18 months | 54 | 73% | High | 4 (13) |
| Freeman | 2017 | 2000–2016 | 25 | 45 months | 51 | 92% | Low | 7 (28) |
| Belinsky | 2017 | 2000–2016 | 38 | 63 months | 56 | 58% | Low | NR |
| Peron | 2017 | 2013–2014 | 30 | 2 years | 46 | 73% | Low | 0 (0) |
| Gonen | 2018 | 2005–2014 | 27 | 41 months | 53 | 89% | Low | 2 (7) |
| Terrier | 2018 | 1996–2016 | 130 | 6.4 years | 51 | 92% | Low | 19 (14) |
| Nagahama | 2019 | 1996–2017 | 12 | 74.4 months | 49 | 58% | Low | 3 (25) |
NC = not clear; NR = not reported.
No mean follow‐up period could be calculated.
Risk of bias individual studies
The scoring of the risk of bias assessment is shown in Table S4. For description of the patient population, 20/38 (53%) of the articles were classified as low risk of bias, for intervention 28/38 (74%), for outcome measurement 35/38 (92%) and for follow‐up only 8/38 (21%). A total of 24 studies had an overall low risk of bias (24/38; 63%). For the complete scoring of risk of bias, see Table S4.
Presenting symptoms
Presenting symptoms regarding visual symptoms, cranial nerve palsies and other neurological deficits are depicted in Fig. 2.
Fig. 2.
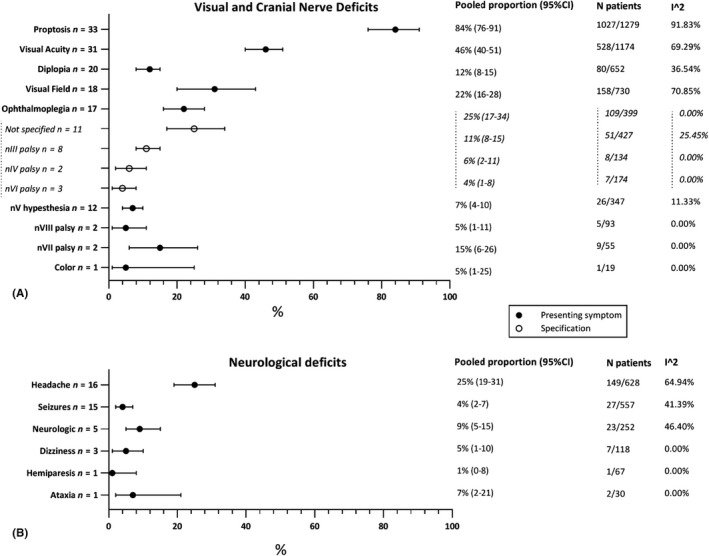
Presenting symptoms: (A) visual and cranial nerve deficits; (B) other neurological deficits.
The most common presenting symptoms were proptosis (84%; 95%CI 76–91%), unilateral visual acuity (VA) (46%; 95%CI 40–51%) and visual field (VF) (31%; 95%CI 20–43%) deficits. Patients suffered from ophthalmoplegia in 22% (95%CI 16–28%) of cases. Patients presented in 9% (95%CI 5–15%) of the cases with cognitive/neurological complaints, like mental change, concentration problems and memory problems. Seizures were reported as the first symptom in 4% (95%CI 2–7%) of the patients.
Surgical approach
Different surgical approaches were used to resect spheno‐orbital meningiomas. The (extended) pterional approach was the most used approach as used in 37 of 38 studies (97%). For a schematic representation of the (extended) pterional approach, see Fig. 3. The surgeon's view after a pterional approach on the sphenoid bone is shown in Fig. 4. One article did not mention their surgical approach (2%). In 2008, the first approach other than pterional was described, the lateral orbitotomy. Since a few years, endoscopic resection in selected SOM patients is also performed and is described in three articles (3/38; 8%). For the complete table of surgical approaches, see Table S5.
Fig. 3.
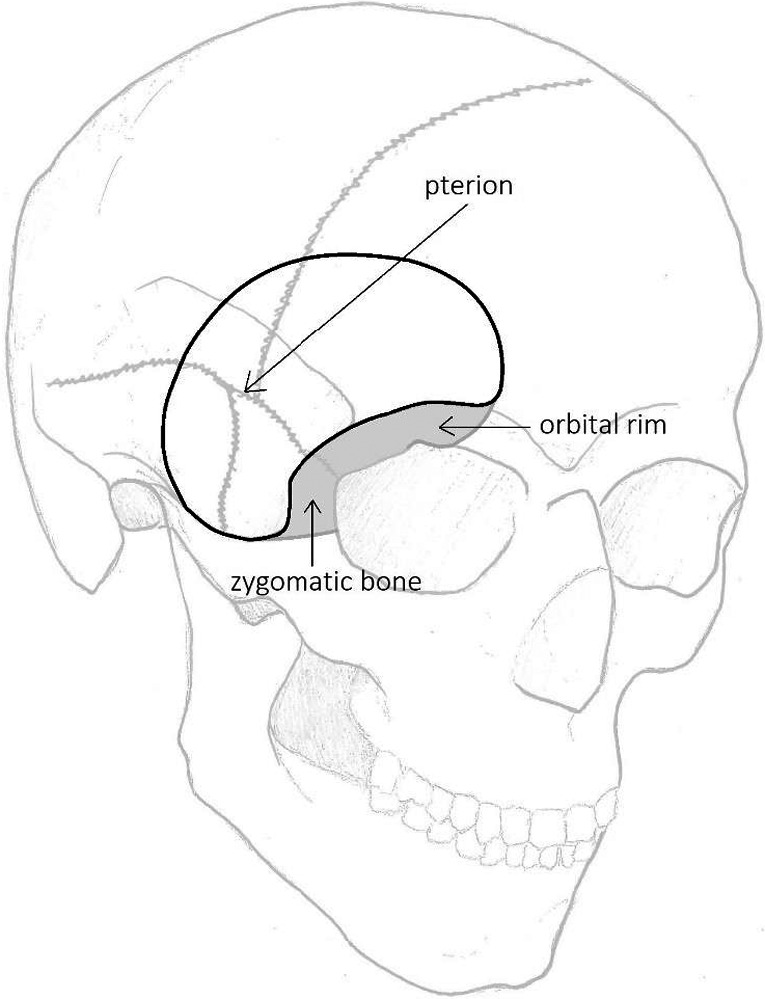
Schematic presentation of the (extended) pterional approach [Inline Image Removed1] Pterional approach; [Inline Image Removed2] Extension of the pterional approach (orbitozygomatic).
Fig. 4.
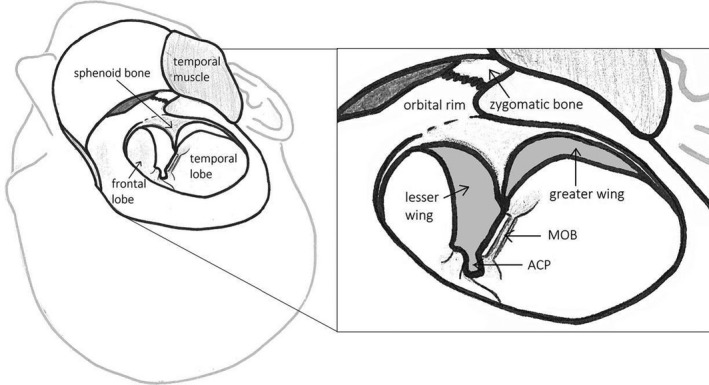
Surgeon’s view of the pterional approach after development of skin‐muscle flap, detaching of part of the temporal muscle and craniotomy. ACP = anterior clinoid process; MOB = meningo‐orbital band.
Decompression/resection of hyperostotic bone
The extent of decompression or resection of hyperostotic bone per article is shown in Fig. 5. The most frequently decompressed structure was the optic canal (31/38; 82%), followed by decompression of the superior orbital fissure (SOF, 25/38; 66%) and resection of the anterior clinoid process (ACP, 22/28; 58%) and the lateral orbital wall (21/38; 55%). There was no trend over the years in the extent of decompression or resection of hyperostotic bone (see Table S6).
Fig. 5.
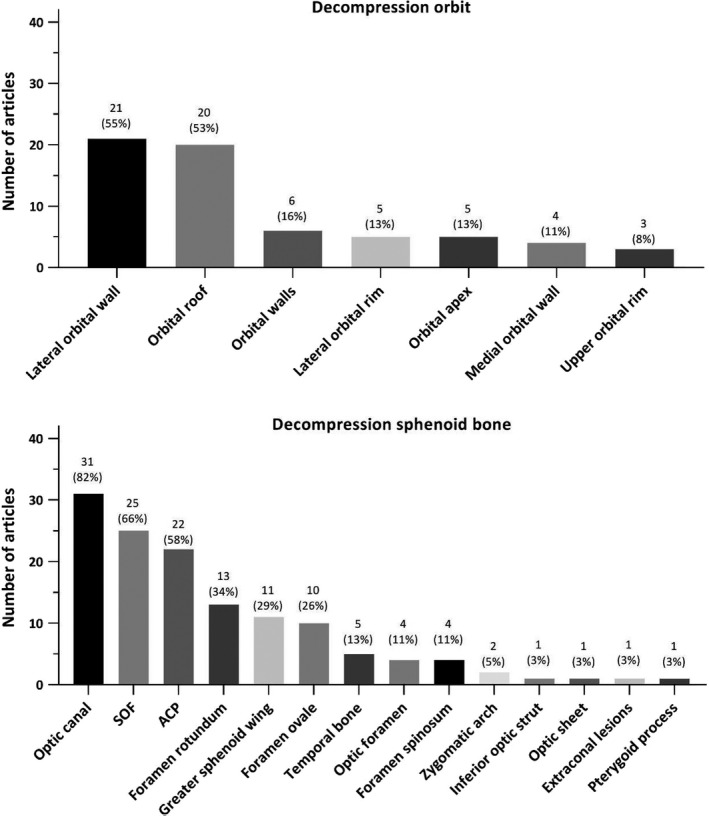
(A) Decompression/resection of the orbit; (B) decompression/resection of the sphenoid bone. In these graphs, no distinction was made in whether decompression of the different structures was always performed or only in selected patients. The percentages represent the fraction of total articles, which performed decompression/resection of the different structures.
Reconstruction
The most used reconstruction materials for dural defects were fascial grafts (7/38; 18%) and pericranium (6/38; 16%). The most used reconstruction materials for bony defects were titanium mesh (14/38; 37%), inner calvarial table grafts (11/38; 29%) and polymethylmethacrylate (10/38; 26%) (Fig. 6). Abdominal fat was used to fill up the remaining cavity in eight articles (9/38; 24%). In Table S7, the usage of reconstruction materials over time is presented. Regarding dural defect reconstruction, in recent years more artificial dural reconstruction materials were used.
Fig. 6.
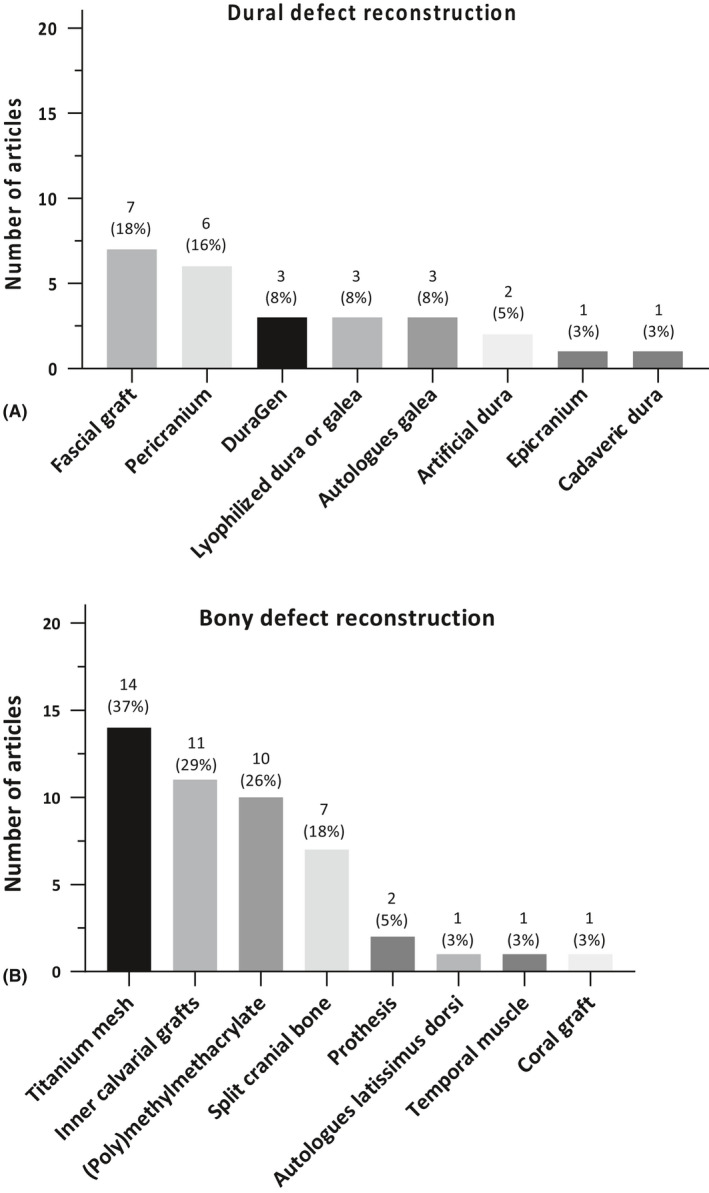
(A) Reconstruction materials for dural defects; (B) reconstruction materials for bony defects. The percentages represent the part of the total articles, which performed this decompression/resection. The numbers given next to the bullets, represent the number of articles using this reconstruction material in this year.
Periorbit
In 22/38 articles (58%), infiltrated periorbit was resected in some cases. Eleven articles (11/38; 29%) reported they (sometimes) opened the periorbit (Fig. 7). It was maintained in only two of 38 articles (5%). Nine articles did not mention their management of the periorbit (24%). In Table S8, it is shown that resection of the periorbit has been performed from the early days on. Only opening the periorbit was first reported in 2005 by Schick et al. (2006).
Fig. 7.
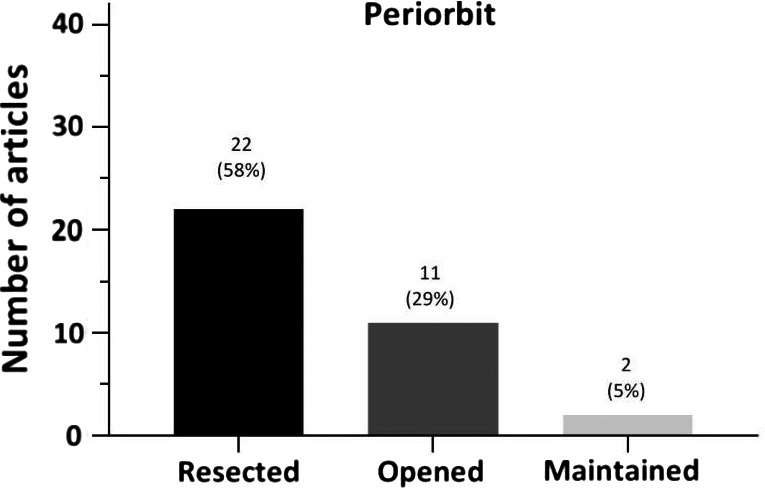
Management of the periorbit.
Clinical outcomes
For clinical outcomes, see Fig. 8. Surgery improved diplopia in 96% of patients (95%CI 78–100%). Ophthalmoplegia was improved in 96% (95%CI 78–100%). Visual acuity deficits improved in 91% of the cases (95%CI 86–96%) and visual field deficits in 87% (95%CI 70–99%). In 96% (95%CI 90–100%), proptosis improved.
Fig. 8.
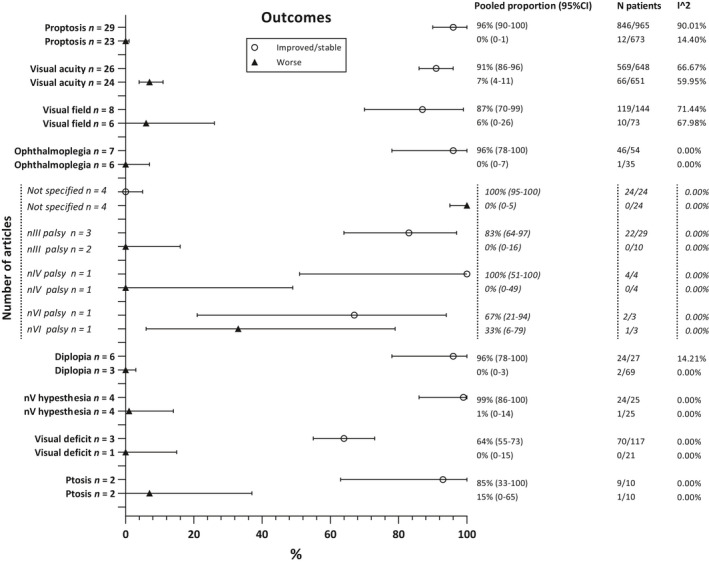
Overall clinical outcomes.
In subgroup analysis only including studies using the (extended) pterional approach (Fig. S1), diplopia improved in 94% of the cases (95%CI 73–100%). In 93% (95%CI 69–100%), ophthalmoplegia improved. Existing visual acuity deficits improved in 91% (95%CI 86–95%) and visual field deficits in 84% (95%CI 60–99%) of patients. Proptosis improved in 96% of the patients (95%CI 89–100%).
Outcomes of sensitivity analysis only including articles published after 2000, with a follow‐up ≥2 years, and scored with a low risk of bias were overall similar to the main analysis (Figs S2–S4).
Complications
Hypesthesia of CN V is the most common complication with an occurrence of 19% (95%CI 10–30%), followed by ptosis (17%; 95%CI 10–26%), unspecified CN deficit (17%; 95%CI 11–25%), diplopia (17%; 95%CI 5–33%) and ophthalmoplegia (16%; 95%CI 10–24%). Complications regarding visual acuity and visual field occurred in respectively 9% (95%CI 2–18%) and 4% (95%CI 1–8%) of the patients (see Fig. 9).
Fig. 9.
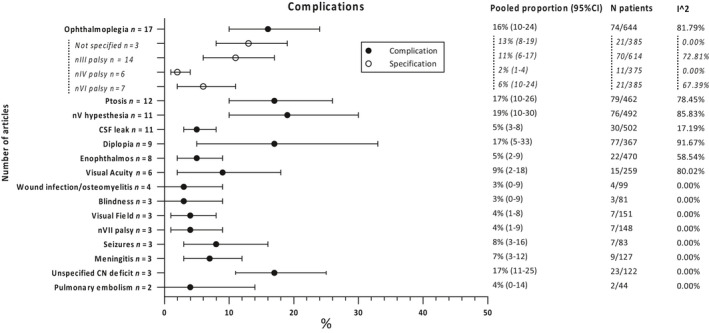
Overall complications.
In subgroup analysis only including studies using the (extended) pterional approach, unspecified CN deficits occur most as a complication in 23% of patients (95%CI 14–32%), followed by ophthalmoplegia (20%; 95%CI 13–29%) and diplopia (17%; 95%CI 5–33%), ptosis (17%; 95%CI 8–28) and CN V hypesthesia (15%; 95%CI 8–24%). Complications regarding visual acuity and visual field in this group occurred in respectively 5% (95%CI 1–10%) and 4% (95%CI 1–8%) of the cases (Fig. S5).
For a table of comparison of the complete analysis and subgroup analysis, regarding clinical outcomes and complications, see Table S9.
Discussion
Resection of spheno‐orbital meningioma is a safe and effective therapeutic option, as it results in excellent improvement of visual and neurological symptoms with low complication rates. Therefore, it is stimulated and advised to refer these patients for surgery to prevent further progression of their presenting visual and neurological symptoms. Over the years, there is no clear trend in change of surgical approach and reconstruction technique, except for opening of the periorbit instead of periorbit resection and the use of endoscopic and multiportal approaches in recent years for selected cases.
Surgical decompression and resection
While different surgical approaches are possible for SOMs, the aim of surgery should always be a maximum safe resection with improvement or retainment of patient’s HRQoL (Zamanipoor Najafabadi et al. 2017). Maximum safe decompression and resection of hyperostotic bone should be performed to improve presenting visual deficits or to prevent further deterioration, while minimizing the risk for complications such as development of new cranial nerve deficits, possibly leading to an impaired HRQoL (Mirone et al. 2009; Gonen et al. 2018). Nevertheless, subtotal resection of affected bone increases the risk for recurrence and the possible need for second operation or radiotherapy with its potential risks. In addition, recent PET‐CT studies reported presence of pathological hyperostotic cells in areas not identified by surgeons, supporting the need for a maximum resection (Kunz et al. 2017).
There is especially strong evidence for the safety and effectiveness of the pterional approach. The pterional approach is specifically a useful approach, as it enables access to the middle cranial fossa, anterior cranial fossa and the orbit through one approach. Recently, transorbital approaches have been reported, primarily for decompression of hyperostotic bone for optimal cosmetic results and in combination with other approaches (i.e. multiportal approaches including transnasal approach) for optimal multi‐angle surgical resection and decompression of hyperostotic bone (Zimmer & Theodosopoulos 2009; Dallan et al. 2015).
Reconstruction techniques
The primary goal of reconstruction of the orbital walls is to prevent enophthalmos, especially pulsatile enophthalmos, which after reconstruction occurs between 2,3% and 30,0% of patients (Gaillard et al. 1997; Honeybul et al. 2001; Bikmaz et al. 2007; Ringel et al. 2007; Mirone et al. 2009; Saeed et al. 2011; Amirjamshidi et al. 2015; Terrier et al. 2018). Reconstruction of dural defects is done to prevent CSF leaks, and reduce the risk of wound infection and meningitis (Talacchi et al. 2014; Leroy et al. 2016), which after reconstruction occurs in respectively 5%, 3% and 6% of patients. The periorbit can be opened or removed to gain access to intra‐orbital tumour extension. However, possible complications for intra‐orbital tumour resection are ophthalmoplegia (Maroon et al. 1994). Therefore, in the majority of cases the periorbit is only resected/opened when invaded. In these cases, resection of periorbit may be necessary to minimize the risk of recurrence and get significant proptosis reduction. Terrier et al also suggested that there is a correlation between opening the periorbita and the reduction of proptosis after surgery (Terrier et al. 2018).
Clinical outcomes, complications and predictors of outcomes
Results of this meta‐analysis show strong evidence for improvement of most symptoms, especially proptosis and cranial nerve deficits which improved in almost all patients, but also visual acuity and visual field deficits which improved respectively in 91% and 87% of patients. While ophthalmoplegia is one of the most occurring complications in approximately 16%, almost all patients with ophthalmoplegia as a presenting symptom showed improvement (96%). Accordingly, the presence of these symptoms and the aim to improve these symptoms seem a proper indication for surgery. There is no clear evidence in literature on the effect of timing of surgery on visual and neurological outcomes. However, Bikmaz et al. reports that surgery in an early stage will stabilize the condition of the patient, with a small risk for permanent visual complications (Bikmaz et al. 2007).
There are several predictors for postoperative visual outcomes, which should be taken into account during surgical decision‐making. Invasion of the optic canal is a negative predictor for both postoperative visual acuity and visual field deficits. Extension into the periorbit is a negative predictor for postoperative visual acuity, and intracranial soft‐tissue component for postoperative visual field (Yannick et al. 2012; Forster et al. 2014). Excision of the periorbit seems to have a positive effect on the reduction of proptosis, while radiological involvement of the optic canal is a predictor for residual postoperative proptosis (Yannick et al. 2012; Terrier et al. 2018).
Strengths and limitations of this study
This is the first systematic review and meta‐analysis systematically evaluating surgical aspects and clinical outcomes of SOM patients. While we performed an extensive and systematic literature search, there were no studies reporting results separately for other approaches than the pterional approach, and therefore, we could not compare outcomes of different surgical approaches. However, our systematic review showed that the pterional approach is the most used approach and sound analysis could be conducted to present outcomes for this approach. In addition, the articles described heterogenous case series regarding extent of resection and decompression, reconstruction techniques, and management of the periorbit, which therefore could not be analysed separately. Also, it was not possible to distinguish between transient and permanent complications and between transient and permanent improvements of signs and symptoms, because not all articles specified this in their results. Similarly, it was not possible to estimate outcomes separately for patients who underwent a first operation or reoperation. Similarly, most articles did not report separate percentages for patients with improved and normalized symptoms. Nevertheless, we could assess whether over the years there was a trend of change in these surgical aspects. Although multiple subgroup analyses were performed to assess the impact of between‐study heterogeneity, which added to the robustness of the reported results, we were not able to perform additional analyses to assess the impact of important prognostic variables, such as tumour size, degree of hyperostosis and comorbidities, as these variables were scarcely reported in the included studies. International collaboration is needed to harmonize data collection and increase patient numbers of clinical studies to assess the impact of important prognostic variables on surgical outcomes.
Implications and future perspective
This systematic review and meta‐analysis showed that there is firm evidence that surgery, especially the pterional approach, is a rather safe and effective treatment option for spheno‐orbital meningioma patients presenting with visual or neurological deficits. While complications occur frequently, up to one in five patients, they are minor and well tolerated. We therefore encourage ophthalmologist to refer these patients for surgery. Over the last 40 years, no consensus emerged in the details of surgery; still a large variety is reported in the extent of resection or decompression of hyperostotic bone, management of the periorbit, and dural and bone reconstruction techniques. Also, the role of radiotherapy has not been clarified yet. The preferred surgical techniques and treatment strategies can therefore not yet be determined. Future studies are needed to assess how spheno‐orbital meningioma surgery can be optimized regarding these topics. As there is paucity in HRQoL data, these studies should not only focus on conventional outcomes, but also patient‐reported outcomes (Zamanipoor Najafabadi et al. 2017).
Supporting information
Figure S1. Outcomes subgroup (pterional + extended approach).
Figure S2. Sensitivity analysis only including articles published after 2000.
Figure S3. Sensitivity analysis only including articles with a follow‐up ≥2 years.
Figure S4. Sensitivity analysis only including articled scores with a low risk of bias.
Figure S5. Complications subgroup (pterional + extended approach).
Table S1. Search strategies used for the systematic review.
Table S2. Combined terms (surgical approaches, presenting symptoms and reconstruction materials).
Table S3. Description of risk of bias assessment.
Table S4. Risk of bias assessment.
Table S5. Surgical approach of included studies, presented per year of publication.
Table S6. Decompression/resection over time.
Table S7. Reconstruction materials over time.
Table S8. Management of the periorbit over time.
Table S9. Improved outcomes and complications of the whole patient population and of the subgroup (pterional + extended approach).
We would like to acknowledge Jesse Weeda for his help in the screening of articles for inclusion in this systematic review and meta‐analysis.
References
- Amirjamshidi A, Abbasioun K, Amiri RS, Ardalan A & Hashemi SM (2015): Lateral orbitotomy approach for removing hyperostosing en plaque sphenoid wing meningiomas. Description of surgical strategy and analysis of findings in a series of 88 patients with long‐term follow up. Surg Neurol Int 6: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky I, Murchison AP, Evans JJ et al. (2018): Spheno‐orbital meningiomas: an analysis based on World Health Organization classification and Ki‐67 proliferative index. Ophthalmic Plast Reconstr Surg 34: 143–150. [DOI] [PubMed] [Google Scholar]
- Bikmaz K, Mrak R & Al‐Mefty O (2007): Management of bone‐invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg 107: 905–912. [DOI] [PubMed] [Google Scholar]
- Boari N, Gagliardi F, Spina A, Bailo M, Franzin A & Mortini P (2013): Management of spheno‐orbital en plaque meningiomas: clinical outcome in a consecutive series of 40 patients. Br J Neurosurg 27: 84–90. [DOI] [PubMed] [Google Scholar]
- Bonnal J, Thibaut A, Brotchi J & Born J (1980): Invading meningiomas of the sphenoid ridge. J Neurosurg 53: 587–599. [DOI] [PubMed] [Google Scholar]
- Cannon PS, Rutherford SA, Richardson PL, King A & Leatherbarrow B (2009): The surgical management and outcomes for spheno‐orbital meningiomas: a 7‐year review of multi‐disciplinary practice. Orbit 28: 371–376. [DOI] [PubMed] [Google Scholar]
- Cushing H & Eisenhardt L (1939): Meningiomas: Their classification, regional behaviour, life history, and surgical end results. JAMA 112: 175. [Google Scholar]
- Dallan I, Castelnuovo P, Locatelli D, Turri‐Zanoni M, AlQahtani A, Battaglia P, Hirt B & Sellari‐Franceschini S (2015): Multiportal combined transorbital transnasal endoscopic approach for the management of selected skull base lesions: preliminary experience. World Neurosurg 84: 97–107. [DOI] [PubMed] [Google Scholar]
- De Jesús O & Toledo MM (2001): Surgical management of meningioma en plaque of the sphenoid ridge. Surg Neurol 55: 265–269. [DOI] [PubMed] [Google Scholar]
- Forster MT, Daneshvar K, Senft C, Seifert V & Marquardt G (2014): Sphenoorbital meningiomas: surgical management and outcome. Neurol Res 36: 695–700. [DOI] [PubMed] [Google Scholar]
- Freeman JL, Davern MS, Oushy S, Sillau S, Ormond DR, Youssef AS & Lillehei KO (2017): Spheno‐orbital meningiomas: a 16‐year surgical experience. World Neurosurg 99: 369–380. [DOI] [PubMed] [Google Scholar]
- Gaillard S, Pellerin P, Dhellemmes P, Pertuzon B, Lejeune JP & Christiaens JL (1997): Strategy of craniofacial reconstruction after resection of spheno‐orbital “en plaque” meningiomas. Plast Reconstr Surg 100: 1113–1120. [DOI] [PubMed] [Google Scholar]
- Gonen L, Nov E, Shimony N, Shofty B & Margalit N (2018): Sphenoorbital meningioma: surgical series and design of an intraoperative management algorithm. Neurosurg Rev 41: 291–301. [DOI] [PubMed] [Google Scholar]
- Hayden JA, van der Windt DA, Cartwright JL, Côté P & Bombardier C (2013): Assessing bias in studies of prognostic factors. Ann Intern Med 158: 280–286. [DOI] [PubMed] [Google Scholar]
- Heufelder MJ, Sterker I, Trantakis C, Schneider JP, Meixensberger J, Hemprich A & Frerich B (2009): Reconstructive and ophthalmologic outcomes following resection of spheno‐orbital meningiomas. Ophthalmic Plast Reconstr Surg 25: 223–226. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ & Altman DG (2003): Measuring inconsistency in meta‐analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel PT (2015): The heterogeneity statistic I(2) can be biased in small meta‐analyses. BMC Med Res Methodol 15: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeybul S, Neil‐Dwyer G, Lang DA, Evans BT & Ellison DW (2001): Sphenoid wing meningioma en plaque: a clinical review. Acta Neurochir (Wien) 143: 749–757; discussion 758. [DOI] [PubMed] [Google Scholar]
- Honig S, Trantakis C, Frerich B, Sterker I, Schober R & Meixensberger J (2010): Spheno‐orbital meningiomas: outcome after microsurgical treatment: a clinical review of 30 cases. Neurol Res 32: 314–325. [DOI] [PubMed] [Google Scholar]
- Jiranukool J, Iampreechakul P, Dhanachai M & Tirakotai W (2016): Outcomes of surgical treatment and radiation therapy in en plaque sphenoid wing meningioma. J Med Assoc Thai 99(Suppl 3): S54–S61. [PubMed] [Google Scholar]
- Kunz WG, Jungblut LM, Kazmierczak PM et al. (2017): Improved detection of transosseous meningiomas using 68Ga‐DOTATATE PET/CT compared with contrast‐enhanced MRI. J Nucl Med 58: 1580–1587. [DOI] [PubMed] [Google Scholar]
- Leake D, Gunnlaugsson C, Urban J & Marentette L (2005): Reconstruction after resection of sphenoid wing meningiomas. Arch Facial Plast Surg 7: 99–103. [DOI] [PubMed] [Google Scholar]
- Leroy HA, Leroy‐Ciocanea CI, Baroncini M, Bourgeois P, Labreuche J, Duhamel A & Lejeune JP (2016): Internal and external spheno‐orbital meningioma varieties: different outcomes and prognoses. Acta Neurochir (Wien). 158: 1587–1596. [DOI] [PubMed] [Google Scholar]
- Li Y, Shi JT, An YZ, Zhang TM, Fu JD, Zhang JL & Zhao JZ (2009): Sphenoid wing meningioma en plaque: report of 37 cases. Chin Med J (Engl). 122(20): 2423–2427. [PubMed] [Google Scholar]
- Marcus H, Schwindack C, Santarius T, Mannion R & Kirollos R (2013): Image‐guided resection of spheno‐orbital skull‐base meningiomas with predominant intraosseous component. Acta Neurochir (Wien) 155: 981–988. [DOI] [PubMed] [Google Scholar]
- Mariniello G, Maiuri F, Strianese D, Donzelli R, Juliano A, Tranfa F, de Divitiis E & Bonavolantà G (2008): Spheno‐orbital meningiomas: surgical approaches and outcome according to the intraorbital tumor extent. Zentralbl Neurochir 69: 175–181. [DOI] [PubMed] [Google Scholar]
- Mariniello G, Bonavolontà G, Tranfa F & Maiuri F (2013): Management of the optic canal invasion and visual outcome in spheno‐orbital meningiomas. Clin Neurol Neurosurg 115: 1615–1620. [DOI] [PubMed] [Google Scholar]
- Maroon JC, Kennerdell JS, Vidovich DV, Abla A & Sternau L (1994): Recurrent spheno‐orbital meningioma. J Neurosurg 80: 202–208. [DOI] [PubMed] [Google Scholar]
- Mirone G, Chibbaro S, Schiabello L, Tola S & George B. (2009): En plaque sphenoid wing meningiomas: recurrence factors and surgical strategy in a series of 71 patients. Neurosurgery 65(6 Suppl): 100–108; discussion 108–109. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J & Altman DG (2009): Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol 62(10): 1006–1012. [DOI] [PubMed] [Google Scholar]
- Nagahama A, Goto T, Nagm A et al. (2019): Spheno‐orbital meningioma: surgical outcomes and management of recurrence. World Neurosurg 126: e679–e687. [DOI] [PubMed] [Google Scholar]
- Nyaga VN, Arbyn M & Aerts M (2014): Metaprop: a Stata command to perform meta‐analysis of binomial data. Arch Public Health 72: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya S, Sade B & Lee JH (2011): Sphenoorbital meningioma: surgical technique and outcome. J Neurosurg 114: 1241–1249. [DOI] [PubMed] [Google Scholar]
- Peron S, Cividini A, Santi L, Galante N, Castelnuovo P & Locatelli D (2017): Spheno‐orbital meningiomas: when the endoscopic approach is better. Acta Neurochir Suppl 124: 123–128. [DOI] [PubMed] [Google Scholar]
- Ringel F, Cedzich C & Schramm J. (2007): Microsurgical technique and results of a series of 63 spheno‐orbital meningiomas. Neurosurgery 60(4 Suppl 2): 214–221; discussion 221–222. [DOI] [PubMed] [Google Scholar]
- Roser F, Nakamura M, Jacobs C, Vorkapic P & Samii M (2005): Sphenoid wing meningiomas with osseous involvement. Surg Neurol 64: 37–43; discussion 43. [DOI] [PubMed] [Google Scholar]
- Saeed P, van Furth WR, Tanck M, Freling N, van der Sprenkel JW, Stalpers LJ, van Overbeeke JJ & Mourits MP (2011): Surgical treatment of sphenoorbital meningiomas. Br J Ophthalmol 95: 996–1000. [DOI] [PubMed] [Google Scholar]
- Sandalcioglu IE, Gasser T, Mohr C, Stolke D & Wiedemayer H (2005): Spheno‐orbital meningiomas: interdisciplinary surgical approach, resectability and long‐term results. J Craniomaxillofac Surg 33: 260–266. [DOI] [PubMed] [Google Scholar]
- Scarone P, Leclerq D, Héran F & Robert G (2009): Long‐term results with exophthalmos in a surgical series of 30 sphenoorbital meningiomas. Clinical article. J Neurosurg 111: 1069–1077. [DOI] [PubMed] [Google Scholar]
- Schick U, Bleyen J, Bani A & Hassler W (2006): Management of meningiomas en plaque of the sphenoid wing. J Neurosurg 104: 208–214. [DOI] [PubMed] [Google Scholar]
- Shapey J, Jung J, Barkas K, Gullan R, Barazi S, Bentley R, Huppa C & Thomas NW (2019): A single centre's experience of managing spheno‐orbital meningiomas: lessons for recurrent tumour surgery. Acta Neurochir (Wien) 161: 1657–1667. [DOI] [PubMed] [Google Scholar]
- Shrivastava RK, Sen C, Costantino PD & Della Rocca R (2005): Sphenoorbital meningiomas: surgical limitations and lessons learned in their long‐term management. J Neurosurg 103: 491–497. [DOI] [PubMed] [Google Scholar]
- Simas NM & Farias JP (2013): Sphenoid Wing en plaque meningiomas: surgical results and recurrence rates. Surg Neurol Int 4: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmaz I, Tehli O, Temiz C, Kural C, Hodaj I, Kutlay M, Gonul E & Daneyemez MK (2014): Surgical strategies for the removal of sphenoorbital meningiomas. Turk Neurosurg 24: 859–866. [DOI] [PubMed] [Google Scholar]
- Talacchi A, De Carlo A, D'Agostino A & Nocini P (2014): Surgical management of ocular symptoms in spheno‐orbital meningiomas. Is orbital reconstruction really necessary? Neurosurg Rev 37: 301–309; discussion 309–310. [DOI] [PubMed] [Google Scholar]
- Terrier LM, Bernard F, Fournier HD, Morandi X, Velut S, Hénaux PL, Amelot A & François P (2018): Spheno‐orbital meningiomas surgery: multicenter management study for complex extensive tumors. World Neurosurg 112: e145–e156. [DOI] [PubMed] [Google Scholar]
- Whittle IR, Smith C, Navoo P & Collie D (2004): Meningiomas. Lancet 363: 1535–1543. [DOI] [PubMed] [Google Scholar]
- Yannick N, Patrick F, Samuel M, Erwan F, Pierre‐Jean P, Michel J & Stéphane V (2012): Predictive factors for visual outcome after resection of spheno‐orbital meningiomas: a long‐term review. Acta Ophthalmol 90: e663–e665. [DOI] [PubMed] [Google Scholar]
- Zamanipoor Najafabadi AH, Peeters MCM, Dirven L et al. (2017): Impaired health‐related quality of life in meningioma patients‐a systematic review. Neuro Oncol 19: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanipoor Najafabadi AH, Peeters MCM, Lobatto DJ et al. (2017): Health‐related quality of life of cranial WHO grade I meningioma patients: are current questionnaires relevant? Acta Neurochir (Wien) 159: 2149–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer LA & Theodosopoulos PV (2009): Anterior skull base surgery: open versus endoscopic. Curr Opin Otolaryngol Head Neck Surg 17: 75–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Outcomes subgroup (pterional + extended approach).
Figure S2. Sensitivity analysis only including articles published after 2000.
Figure S3. Sensitivity analysis only including articles with a follow‐up ≥2 years.
Figure S4. Sensitivity analysis only including articled scores with a low risk of bias.
Figure S5. Complications subgroup (pterional + extended approach).
Table S1. Search strategies used for the systematic review.
Table S2. Combined terms (surgical approaches, presenting symptoms and reconstruction materials).
Table S3. Description of risk of bias assessment.
Table S4. Risk of bias assessment.
Table S5. Surgical approach of included studies, presented per year of publication.
Table S6. Decompression/resection over time.
Table S7. Reconstruction materials over time.
Table S8. Management of the periorbit over time.
Table S9. Improved outcomes and complications of the whole patient population and of the subgroup (pterional + extended approach).


