Abstract
Background
Primary Sjögren's syndrome (pSS) is an autoimmune disease characterized by a lymphocytic infiltrate in salivary glands driving to epithelial damage. The pSS patients present heterogenic clinical and serological characteristics. This heterogenicity could be due to the cytokine microenvironment. Cytokine levels have been analyzed and reported individually, showing controversial results; for that reason, we considered essential to evaluate a cluster of cytokines and relate them with antibody levels and clinical characteristics to find pSS subgroups.
Methods
Ninety‐nine pSS patients, diagnosed by the 2016 ACR/EULAR classification criteria, and 76 control subjects (CS) were included. Cytokine quantification was performed by Multiplex assay. Principal component analysis (PCA) was realized, and the K‐mean test was used to identify clusters/groups. Groups were analyzed by the Kruskal‐Wallis test and the Bonferroni test.
Results
Higher IFN‐γ, IL‐17F, IL‐21, IL‐23, IL‐4, and IL‐31 levels were observed in pSS patients in comparison with control subjects. PCA analysis showed three groups. The severe group was characterized by higher cytokine concentrations as well as an increase in clinical parameters such as antibody levels, damage index score, and others. The moderate group presented intermediate severity; meanwhile, the mild group presented the lowest severity.
Conclusion
Cluster analysis revealed three groups that were different in cytokine levels and clinical parameters in which the mild group was defined by lower severity, the moderate group with intermediate severity, and the severe group with higher severity. This analysis could help subclassify the primary Sjögren syndrome patients for a better understanding of the clinical phenotype that impacts the treatment approach.
Keywords: cytokine levels, PCA analysis, primary Sjögren's syndrome, pSS groups
Principal component analysis of 14 cytokines was realized to determine the cytokine groups in primary Sjögren´s syndrome patients. The mild group was characterized by less severity of the disease with low cytokine levels and fewer clinical parameters; the moderate group included patients with intermediate severity, presented higher cytokine levels than the mild group but less than severe group. Patients of the severe group showed higher severity, higher cytokine levels, and clinical parameters.

1. INTRODUCTION
Primary Sjögren's syndrome (pSS) is an autoimmune disease characterized by a lymphocytic infiltrate in exocrine glands, and circulating levels of anti‐Ro and anti‐La antibodies. T helper (Th) are the predominant T‐cell subsets infiltrated and are active mediators of disease activity promoting proinflammatory cytokines in addition to B‐cell recruitment. 1
Cytokines play an essential role in pSS pathogenesis, mediating cell response and promoting tissue damage. Th1, Th2, and Th17 cells and their cytokine profiles have been implied in the pathogenesis of pSS as well as reported in saliva, serum, and labial salivary gland (LSG), with different results. 2 , 3
Th1 cells produce IL‐1, TNF‐α, and its hallmark, IFN‐γ, considered the main proinflammatory cytokine in the physiopathology of pSS, inducing tissue damage and activation of other immune cells perpetuating salivary inflammation. 2 , 3 , 4 , 5 , 6 Circulating levels of IFN‐γ are controversial. Tripp et al reported high levels in pSS patients associated with lower fatigue. 4 Besides, Szodoray et al showed no differences. 5
Conversely, Th2 cells release mainly IL‐4, IL‐5, and IL‐13, important in humoral immune responses. The Th2 profile role in pSS pathogenesis is unclear. IL‐4 has been described in salivary glands in mild, early, or advanced stages of the disease, associated with the lymphocytic infiltrate degree. 6 , 7 Soluble IL‐4 levels showed no differences with the clinical association, 4 , 5 only correlation of the focus score with TNF‐α/IL‐4 ratio. 8 About inductors and maintainers of Th2 response, IL‐33 was identified at glandular and soluble expression without clinical association in pSS. 9 , 10 Moreover, high IL‐25 mRNA expression from LSG associated with the lymphocytic infiltration was reported. 11
Th17 cells are characterized by secretion of IL‐17, and also IL‐6, IL‐8, IL‐21, IL‐22, and IL‐26. Increased levels of IL‐17 have been reported in tears, saliva, LSG, and serum associated with antibody production, immunoglobulin G levels (IgG), and disease activity in pSS. 7 , 12 , 13 , 14 , 15 , 16 Also, the correlation of IL‐21 and IL‐22 with anti‐Ro titers and IgG levels in pSS was described, 13 suggesting an essential role of Th17 in the pathogenesis of the disease.
Primary Sjögren syndrome patients display clinical and serological heterogeneity, which could be influenced by the cytokine microenvironment. Cytokine levels have been analyzed and reported individually in pSS patients. The study aimed to evaluate cytokine profiles and relate them with the antibody levels and clinical characteristics to determine groups of pSS patients.
2. MATERIALS AND METHODS
2.1. Study group
The study included 99 pSS Mexican patients classified according to the 2016 American College of Rheumatology/European League Against Rheumatism classification criteria 14 recruited from the Hospital General de Occidente, Guadalajara, Jalisco, México. Ninety‐eight percent of them had a positive biopsy of labial minor salivary glands (focus score ≥ 1 foci/4 mm2). Sjögren's Syndrome Disease Activity Index (SSDAI), 15 and Sjögren's Syndrome Disease Damage Index (SSDDI) 15 were evaluated. Seventy‐six healthy Mexican subjects not related were included. All individuals signed confidence consent. The project was performed following the Helsinki Declaration, and it was approved by the ethics committee (CI/037/2016).
2.2. Multiplex analysis
Tubes containing spray‐coated silica and a polymer gel were used for serum separation (BD Vacutainer® SSTTM). Cytokine quantification (IL‐1β, IL‐4, IL‐6, IL‐10, IL‐17A, IL‐17F, IL‐21, IL‐22, IL‐23, IL‐25, IL‐31, IL‐33, IFN‐γ, and TNF‐α) was performed by Bio‐Plex Pro™ Human Th17 Cytokine Panel 15‐Plex #171AA001M kit (Bio‐Rad Laboratories. Inc©) according to the manufacturer instructions and analyzed using a Bio‐Plex® MAGPIXTM Multiplex Reader (Bio‐Rad Laboratories. Inc©).
2.3. Statistical analysis
Parametrical and non‐parametrical tests were performed according to the sample distribution. Spearman test was performed to analyze cytokine correlations. Principal component analysis (PCA) was realized, and the K‐mean test was used to identify clusters (groups). The Kruskal‐Wallis test analyzed patient groups and as a post hoc the Bonferroni test. P < .05 was considered significant. Data were analyzed using IBM SPSS® statistics version 25, GraphPad Prism version 8, and R version 3.6.1 software.
3. RESULTS
3.1. Clinical and demographic characteristics
A total of 175 individuals were included in this study, 99 pSS patients, and 76 CS, all of them female. Patients showed a mean of 55 ± 11 years of age and 2.5 years (1.0p25‐5.0p75 years) of disease duration. The demographic and clinical characteristics are shown in Table 1. None of the patients were under biological treatment. The population showed low positivity to anti‐Ro (13.3%) and anti‐La (11.1%) antibodies.
TABLE 1.
Demographic and clinical characteristics
| Variables | pSS patients (n = 99) | Control subjects (n = 76) |
|---|---|---|
| Demographic characteristics | ||
| Male/Female | 0/99 | 0/76 |
| Age (years) | 55 ± 11 | 46 ± 11 |
| Inflammation markers | ||
| ESR (mm/h) | 23.0 (15.0‐33.0) | 19.5 (12.2‐27.0) |
| CRP (mg/L) | 5.9 (2.5‐9.1) | ‐ |
| Antibodies | ||
| RF (%) | 20.3 | ‐ |
| Anti‐Ro (%) | 13.3 | ‐ |
| Anti‐La (%) | 11.1 | ‐ |
| ANA (%) | 51.7 | ‐ |
| Clinical characteristics | ||
| Disease duration (years) | 2.5 (1.0‐5.0) | ‐ |
| Schirmer test (mm/5 min) | 3.0 (2.0‐5.0) | ‐ |
| Focus number (foci in 4 mm2) | 2.0 (1.0‐3.0) | ‐ |
| SSDAI (score) | 2.0 (1.0‐3.0) | ‐ |
| SSDDI (score) | 1.0 (1.0‐2.0) | ‐ |
| Treatment | ||
| Prednisone (%) | 10.2 | ‐ |
| Chloroquine (%) | 55.5 | ‐ |
| Methotrexate (%) | 22.4 | ‐ |
| Azathioprine (%) | 21.4 | ‐ |
Data distribution was analyzed by the Kolmogorov‐Smirnov test with Lilliefors correction. Data were shown in the median with 25th and 75th percentiles or mean ± SD.
Abbreviations: ANA, Anti‐nuclear antibodies; CRP, C Reactive Protein; ESR, Erythrocyte Sedimentation Rate; RF, Rheumatoid Factor; SSDAI, Sjögren's Syndrome Disease Activity Index; SSDDI, Sjögren's Syndrome Disease Damage Index.
3.2. Cytokine levels
Higher cytokine levels were observed in pSS patients in comparison with control subjects. Patients presented higher levels of IFN‐γ than control subjects (median: 22.7 pg/mL and 11.4 pg/mL, P = .001, respectively), and higher IL‐1β levels were observed in pSS patients respect to CS (median: 0.23 and 0.12 pg/mL, P < .0001, respectively) (Figure 1).
FIGURE 1.
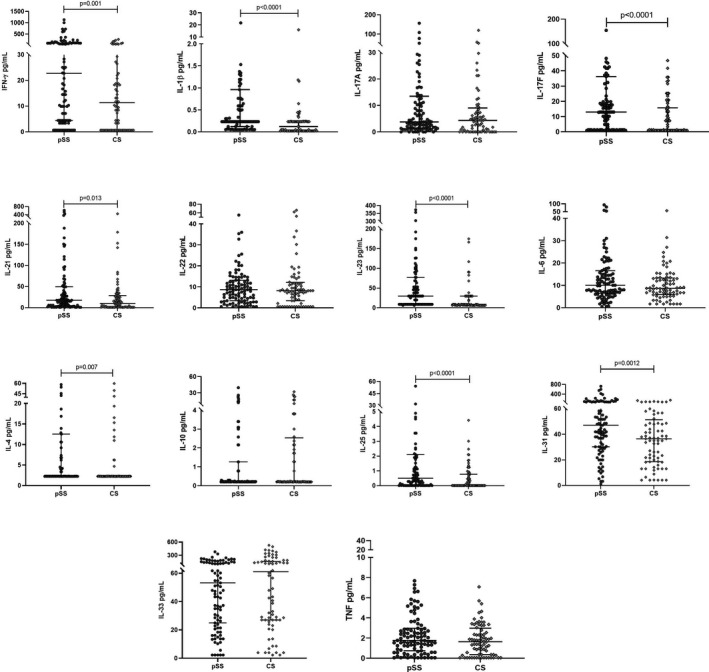
Cytokine levels in pSS and CS. Cytokine levels in pSS patients and CS were quantified. Primary Sjögren syndrome patients presented higher cytokine levels of IFN‐γ, IL‐1β, IL‐17F, IL‐21, IL‐23, IL‐25, and IL‐31, the rest of the cytokines showed similar levels in both groups. Data were analyzed with the Mann‐Whitney U test
The IL‐4, IL‐25, and IL‐31 cytokine levels also were higher in pSS patients than control subjects. Regarding IL‐4, the median was similar in both groups but pSS patients showed higher dispersion levels [pSS: median: 2.3 (2.3p25‐12.5p75) pg/mL vs CS: median: 2.3 (2.3p25‐2.3p75) pg/mL, P = .007]. Despite that IL‐25 levels showed a significant difference (Figure 1), the median does not exceed 1 pg/mL in any group (pSS: 0.5 pg/mL and CS: 0.009 pg/mL, P < .0001). The levels of IL‐31 were as follows: 47.1 pg/mL in pSS patients and 36.5 pg/mL in CS (P = .0012).
Differences in median levels of Th17 cytokines were observed in IL‐17F (pSS: 13.0 pg/mL vs CS: 1.3 pg/mL, P < .0001), IL‐21 (pSS: 17.6 pg/mL vs CS: 10.2 pg/mL, P = .0013), and IL‐23 (pSS: 29.9 pg/mL vs CS: 9.0 pg/mL, P < .0013); the rest of the cytokines showed similar levels in both groups (Figure 1).
3.3. Principal component analysis and clustering
A heatmap that represents the cytokine correlations was performed (Figure 2A). Most of the cytokines correlated strong (Rho (ρ) > 0.400) between them except IL‐22, which did not correlate with almost all of the cytokines. Especially, IFN‐γ, IL‐17A/F, IL‐1β, IL‐21, and IL‐25 had a strong correlation with practically all of the cytokines (ρ > 0.600, P < .0001).
FIGURE 2.
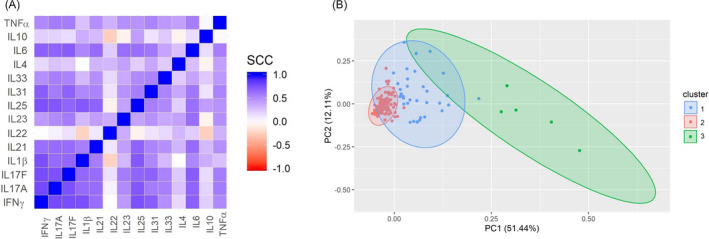
Cytokine correlations and PCA analysis. (A) A heatmap of the cytokine correlations was performed. A strong correlation was observed in almost all of the cytokines between themselves. B, PCA graphic of the two principal components and the clusters observed according to cytokine levels. SCC, Spearman correlation coefficient
Principal component analysis of 14 cytokines was realized to determine the cytokine groups. It was observed that these cytokines were represented in two principal components, which explain 63.55% of all the variance. Three clusters were obtained with the Kaiser criteria: Cluster 1 was composed of 34 individuals (22 pSS patients and 12 CS), cluster 2 by 136 (72 pSS and 64 CS), and cluster 3 by five pSS patients (Figure 2B).
3.4. Cytokine analysis by groups in pSS patients
The cytokines were analyzed in 3 groups represented by the clusters. Even when the cluster analysis involved pSS patients and CS, the cytokine analysis by groups was performed only in the pSS patients. In general, differences were observed in the three groups. Group 3 (severe group) presented higher levels of all the cytokines except for IL‐22 (Figure 3).
FIGURE 3.
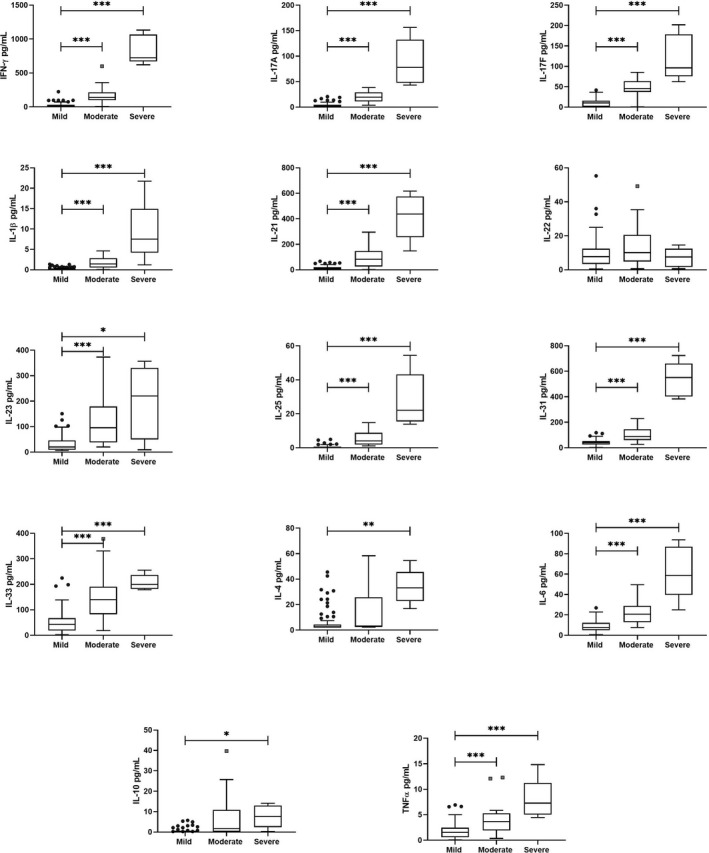
Cytokine levels according pSS groups. Cytokines distribution according to the 3 groups were different. The severe group showed the higher cytokine levels in comparison with the mild and moderate groups; meanwhile, the mild group presented the lower levels. Kruskal‐Wallis test with the Bonferroni correction was performed to analyzed groups. *** P < .0001, ** P ≤ .001, *P < .05
On the other hand, group 2 (mild group) showed lower cytokine levels of the whole cytokines analyzed. In the clustering, cluster 2 included most of the control subjects; in this sense, the patients of this group had similar cytokine levels as control subjects. Group 1 (moderate group) presented higher levels of almost all of the cytokines in comparison with group 2 (mild), but it had lower levels than group 3 (severe). Thus, group 1 showed moderate cytokine levels (Figure 3). Cytokines detailing of serum levels by groups are showed in Table S1.
3.5. Clinical characteristics of pSS patient cluster groups
Primary Sjögren's syndrome patients were stratified in three groups, in concordance with the phenotypic‐driven prognostic classification proposed by Brito‐Zeron et al 16 , and according to cytokine levels showed by PCA. The mild group (the low‐risk group of systemic/severe disease) included patients with elderly‐onset diagnosis, seronegative, and La carriers. The moderate group (moderate risk of systemic/severe disease) included patients young‐onset diagnosis, and Ro‐carriers, while the severe group (high risk of systemic/severe disease) included pSS patients with high focus score or presence of germinal centers in histopathological studies, RF‐carriers, cryoglobulinemic, and hypocomplementemic patients. 16
Our results showed that the mild group was characterized by less severity of the disease with low cytokine levels and fewer clinical parameters; the moderate group included patients with intermediate severity presented higher cytokine levels than the mild group but less than the severe group. Patients of the severe group showed higher severity, higher cytokine levels, and clinical parameters (Figure 4).
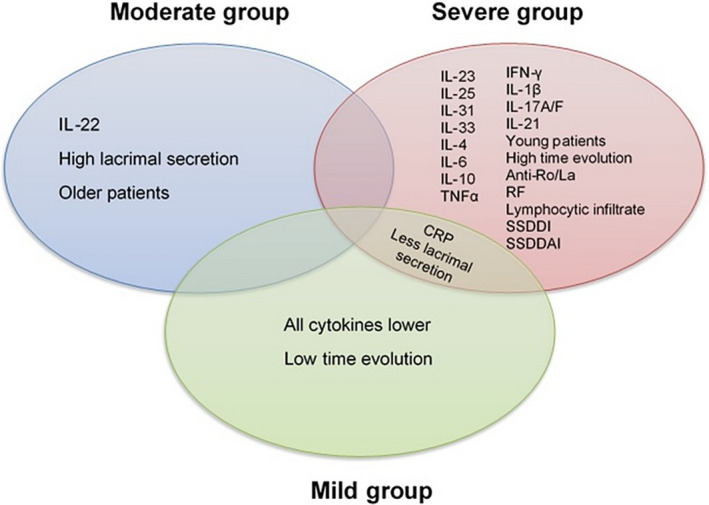
FIGURE 4.
Summary of the different pSS groups. As could be observed, patients belonged to the severe group presented high cytokines and clinic characteristics; meanwhile, the mild group presented these features lower than the severe group except for CRP levels. Although the moderate group only presented the highest IL‐22 levels, this group presents higher cytokine levels than the mild group, but lower than the severe group. Those results could help to subclassify the patients and improve the personal treatment
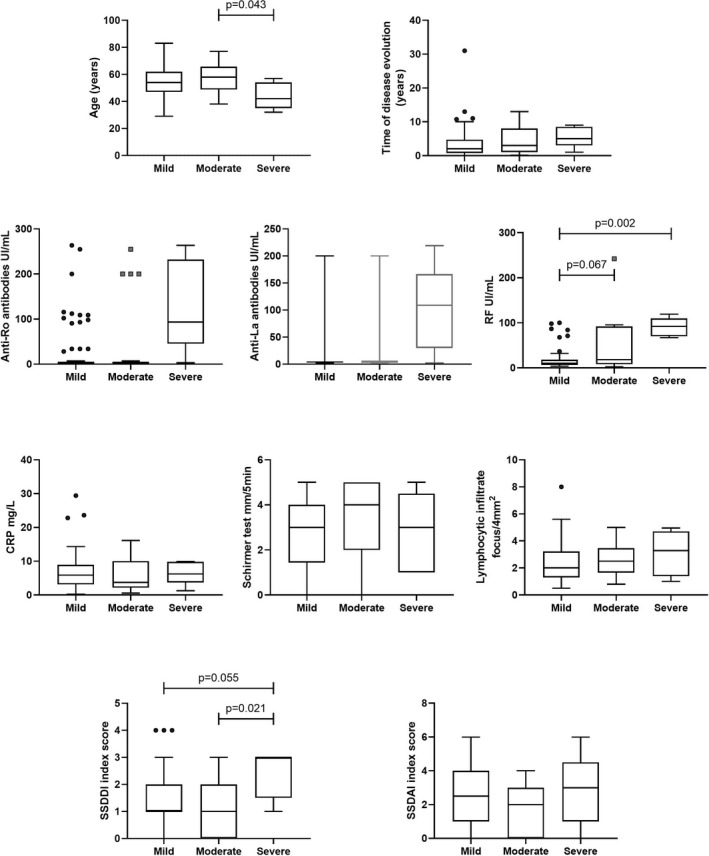
Regarding the clinical characteristics of pSS patients, it was observed that patients in the severe group presented higher RF levels in comparison with the mild group, high damage score evaluated by SSDDI index, and younger age than the moderate group (Figures 4 and 5). Interestingly, a tendency to high time of disease evolution, more elevated anti‐Ro/La antibodies, lymphocytic infiltrate, and disease activity score (SSDDAI index) without statistical significance. Besides, 60% of positivity to ANA, 80% of them presented extra‐glandular manifestations (80% fatigue and 60% arthralgia).
FIGURE 5.
Clinical parameters of pSS groups. The clinical characteristics of the patients were different in the groups. The severe group showed higher RF levels and damage (SSDDI) in comparison with the mild and moderate groups, meanwhile, the mild group presented the lower levels of RF but higher damage than the moderate group. Anti‐Ro/La levels were higher in the severe group but without significant difference (P = .065 and P = .070). Kruskal‐Wallis test with the Bonferroni correction was performed to analyzed groups. RF: Rheumatoid Factor, CRP: C Reactive Protein, SSDAI: Sjögren's Syndrome Disease Activity Index, SSDDI: Sjögren's Syndrome Disease Damage Index
Heterogenic characteristics were observed in moderate and mild groups. Patients of the moderate group presented higher RF levels in comparison with the mild group; meanwhile, these patients showed higher damage and activity scores than the moderate group, anti‐Ro/La levels were similar in both groups, even with more variability in mild group. Both groups showed related lymphocytic infiltration and age, while moderate group presented a tendency to higher lacrimal secretion (evaluated by Schirmer test) and lower time of disease evolution. ANA positivity was similar in both groups (50% and 55%, respectively). Respect to extra‐glandular manifestation, in the moderate group, sixty‐eight percent presented systemic manifestations, and the mild group showed 79% (fatigue and arthralgia were the most frequent in both groups) (Table 2).
TABLE 2.
Clinical parameters of pSS groups
| Parameter | Mild group n = 72 | Moderate group n = 22 | Severe group n = 5 |
|---|---|---|---|
| Age (years) | 58.0 (48.7‐65.7) | 54 (47.0‐62.0) | 42 (35‐54) |
| Time of disease duration (years) | 3.0 (1.0‐8.0) | 2.0 (0.8‐4.7) | 5.0 (3.0‐8.5) |
| Anti‐Ro antibodies (UI/mL) | 3.2 (2.0‐4.6) | 3.4 (2.2‐4.7) | 93.3 (45.3‐231.8) |
| Anti‐La antibodies (UI/mL) | 3.8 (2.2‐6.5) | 3.9 (2.5‐5.0) | 108.9 (29.8‐166.4) |
| ANA (positivity %) | 50 | 55 | 60 |
| RF (UI/mL) | 18.0 (8.2‐91.8) | 10.0 (6.1‐18.1) | 92.3 (70.1‐109.5) |
| CRP (mg/mL) | 3.7 (2.1‐10.0) | 5.9 (3.1‐8.9) | 6.2 (3.7‐9.8) |
| Schirmer test (mm/5 min) | 4.0 (2.0‐5.0) | 3.0 (1.4‐4.0) | 3.0 (1.0‐4.5) |
| Lymphocytic infiltrate (focus/ 4 mm2) | 2.5 (1.6‐3.5) | 2.0 (1.3‐3.2) | 3.3 (1.4‐4.7) |
| SSDDI index (score) | 1.0 (0.0‐2.0) | 1.0 (1.0‐2.0) | 3.0 (1‐3.0) |
| SSDAI index (score) | 1.0 (0.0‐3.0) | 2.0 (1.0‐4.0) | 3.0 (1.0‐4.0) |
| Extra‐glandular manifestations (positivity %) | 68 | 79 | 80 |
| Prednisone (%) | 4.5 | 9.9 | 40 |
| Methotrexate (% | 9.1 | 23.9 | 60 |
| Azathioprine (%) | 18.2 | 22.5 | 20 |
| Chloroquine (%) | 63.6 | 54.2 | 40 |
Data were shown in the median with 25th and 75th percentiles or mean ± SD.
Abbreviations: ANA, Anti‐nuclear antibodies; CRP, C Reactive Protein; RF, Rheumatoid Factor; SSDAI, Sjögren's Syndrome Disease Activity Index; SSDDI, Sjögren's Syndrome Disease Damage Index.
4. DISCUSSION
Cytokines play a crucial role in pSS pathogenesis, modulating the response of different cellular lineages and inducing the secretion of more critical cytokines. The T CD4 cells are the predominant lineage in this pathogenesis due to the secretion of cytokines as well as recruitment and activation of B, NK, macrophages, and other cells. 1
The main T helper cytokine profiles in pSS patients were observed. In the Th1 profile, higher levels of IFN‐γ were found and also IL‐1β, but the levels were too low that it could not have a biological effect. In the Th2 profile, IL‐4 and IL‐31 also showed higher levels in pSS patients than CS. These results reflect the cytokine profile network that involved our patients. The Szodoray study 5 reported similar IFN‐γ and IL‐4 levels, higher IL‐1β, IL‐6, IL‐10, and TNF‐α in comparison with CS, which some of them are in agreement with our study.
Other Th2 cytokines that showed results not previously reported in pSS are the IL‐25 and IL‐31. IL‐25 has been only reported in pSS at the local level: high mRNA expression in LSG biopsy, and it had been related to inducing type 2 innate lymphoid cells. The precise effect at the systemic level is unknown. 11 Nevertheless, IL‐25 levels presented a median value than was not exceed to 1 pg/mL in both groups (pSS: 0.5 pg/mL and CS: 0.009 pg/mL), and these levels should be taken carefully. IL‐31 had been reported in pSS neither locally or systemic. The effect of this cytokine in this pathology is entirely unknown. IL‐31 is a proinflammatory cytokine that regulates cell proliferation, induces other proinflammatory cytokines (IL‐6 and IL‐8) and tissue remodeling, and also participates in the inhibition of proliferation and apoptosis in epithelial cells. 17
Regarding the Th17 profile, a high concentration of IL‐17F was observed; meanwhile, IL‐17A levels were similar to CS; IL‐21 and IL‐23 levels were observed high also in pSS patients. Respect to IL‐17A and IL‐17F, our results are similar to the Gan group that reported high IL‐17F levels in pSS patients associated with increased IgG, ANA, and anti‐Ro antibodies. No difference in IL‐17A levels was observed. 18 On the other hand, IL‐21 and IL‐23 are not widely studied, but our results were according to Tripp, Kang, and Katsifis groups. 4 , 12 , 13
Several studies tried to stratify pSS patients according to clinical parameters, such as antibody positivity, levels of immunoglobulins, or presence of ectopic germinal centers to obtain a better knowledge of the disease that impacts in the treatment approach. 19 , 20 Some reports analyzed cytokines in multiplex assays, but even when they quantified many cytokines, the analysis was performed individually, and most of these studies did not find any correlation with clinical parameters. Szodoray et al stratified the pSS patients regarding extra‐glandular manifestations, positivity to antibodies, high levels of IgG, and according to focus score without differences. 5 Tripp et al analyzed some cytokines and associated them with fatigue; these results were contrary with our results in which the patients positive to fatigue of the mild group (45 pSS patients) presented lower levels of IFN‐γ and TNF‐α; meanwhile, the severe group presented the highest levels of these cytokines (data not shown). 4 Gan et al, analyzed IL‐17A and IL‐17F, they stratified according to IL‐17A/F levels and reported that patients with higher IL‐17F levels have more antibody titers, positivity to ANA, positive correlations with IgG levels, disease activity score, and RF levels. 18 Moutsopoulos et al, on the other hand, analyzed cytokines according to Th profile but without association with clinical parameters. 21
Cytokines are molecules that have several functions in the pSS pathogenesis. Therefore, in pSS patients, the analysis performed by individual cytokines could not explain the appropriate effect; for this reason, it is more viable to analyze cytokines by groups. 22 , 23
Correlation analysis showed that most of the cytokines in pSS correlated strongly with themselves (IL‐22 as an exception). Cluster analysis revealed three groups that presented remarkable results. The mild group that was composed of 76 pSS patients showed the lower levels in almost all cytokines without predominance of some Th profile (IL‐22 showed similar levels with the severe group). The majority of the patients were in this group, most of the CS (64/76) (CS were not analyzed). It suggests that the mild group, in particular, presented cytokine levels similar to control subjects. The moderate group that was formed by 22 pSS patients showed moderated cytokine levels, Th17 inductors as well as Th2 cytokines and Th2 inductors predominance, in comparison with the mild group and the severe group. On the other hand, the severe group was composed of only pSS patients (5 pSS), characterized by higher levels of almost all cytokines, mainly the Th17 profile, in comparison with the moderate and mild groups.
These pSS groups also presented different clinical characteristics that could help to subclassify them. The patients of the severe group showed high RF levels and high damage scores evaluated by the SSDDI index. Interestingly, patients of this group were younger than the moderate and mild groups and also presented the highest time of disease duration, which means that this group has the particularity to develop the disease younger and could have a more severe course of the disease. Even when no differences were observed in other clinical manifestations, this group showed a tendency to higher anti‐Ro/La antibody levels, ANA, lymphocytic infiltrate, and higher disease activity (SSDAI). All these characteristics could be due to the most elevated proinflammatory cytokines that were observed, which could increase the damage and the severity of this disease; therefore, these patients could need a different therapeutic decision. The positivity to ANA and RF is associated with patients that present an increase of systemic features. 24 , 25 The mild and moderate groups showed different characteristics in comparison with the severe group.
There are few studies that try to classify pSS patients according to cluster analysis. Tarn et al made a symptom‐based stratification in which they found 4 clusters groups: low symptom burden group, high symptom burden group, dryness dominant with fatigue group (DDF), and pain dominant with fatigue group in 3 different pSS cohorts. They reported differences in clinic characteristics in the 4 groups, although heterogeneity was observed in the groups and even in the 3 cohorts of patients. The dryness domain with fatigue groups seems to be the group with higher clinic characteristics and showed improvement with biological treatment in comparison with the other groups. 26
Although Tarn et. al. found higher clinical parameters in the DDF group than the high symptom burden group (which are the patients with more symptoms), our results were different: the severe group presented higher clinical parameters (eg, antibody levels, cytokines levels, damage, and activity score) in comparison with the other 2 groups. These differences exhibit the heterogeneity in pSS patients and the relevance of the cluster analysis.
Briton‐Zeron et al 16 made a phenotypic‐driven prognostic classification which is in concordance with our results. The severe group was conformed by patients with high cytokine levels, high antibody titers, and the higher activity of the disease, while the mild group was the older age and seronegative patients.
Although our study found remarkable and well‐characterized groups of pSS, it has some limitations. First, even when cytokines levels showed a difference between groups, the sample is relatively small, and the results could be replicated in further studies; second, the proportions of the groups are quite markedly different (72 in the mild group and 5 in the severe group), it is necessary to replicate these analyses in a homogeneous cohort study; third, the evaluation of other parameters such as IgG, C3, and C4 levels could improve the results and need to be evaluated in future analysis; fourth the low activity and damage score that pSS patients presented may limit the differences that were observed. Finally, all of the patients had a treatment background that could influence cytokine levels.
In conclusion, our results showed the complexity, heterogeneity, and the effects of cytokines in pSS physiopathology. Higher cytokine levels were observed in pSS patients. Cluster analysis revealed three groups that were different in cytokine levels and clinical parameters in which the mild group was defined by lower severity, the moderate group with intermediate severity, and the severe group with higher severity. This analysis showed interesting results because the pSS patients have been characterized by clinical and immunological heterogeneity. Cluster analysis could help to subclassify the primary Sjögren syndrome patients for a better understanding of the clinical phenotype that impacts the treatment approach.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Table S1
ACKNOWLEDGMENT
This work was supported by the CONACYT (Fondo Sectorial SSA/IMSS/ISSSTE‐CONACYT, México‐Universidad de Guadalajara) under grant No. 273324 to EOR.
Fabiola López‐Villalobos E, Muñoz‐Valle JF, Azucena Palafox‐Sánchez C, et al. Cytokine profiles and clinical characteristics in primary Sjögren´s syndrome patient groups. J Clin Lab Anal.2021;35:e23629 10.1002/jcla.23629
REFERENCES
- 1. Verstappen GM, Corneth OBJ, Bootsma H, Kroese FGM. Th17 cells in primary Sjögren's syndrome: Pathogenicity and plasticity. J Autoimmun. 2018;87:16‐25. [DOI] [PubMed] [Google Scholar]
- 2. Retamozo S, Flores‐Chavez A, Consuegra‐Fernández M, Lozano F, Ramos‐Casals M, Brito‐Zerón P. Cytokines as therapeutic targets in primary Sjögren syndrome. Pharmacol Ther. 2018;184:81‐97. [DOI] [PubMed] [Google Scholar]
- 3. Jin J‐O, Yu Q. T cell‐Associated Cytokines In The Pathogenesis of Sjögren's syndrome. J Clin Cell Immunol. 2013;S1(9):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tripp NH, Tarn J, Natasari A, et al. Fatigue in primary Sjögren's syndrome is associated with lower levels of proinflammatory cytokines. RMD Open. 2016;2(2):e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Szodoray P, Alex P, Brun JG, Centola M, Jonsson R. Circulating cytokines in primary Sjogren's syndrome determined by a multiplex cytokine array system. Scand J Immunol. 2004;59(6):592‐599. [DOI] [PubMed] [Google Scholar]
- 6. Moriyama M. Cytokine chemokine profiles contribute to understanding thepathogenesis and diagnosis of primary Sjögren's syndrome. Clin Exp Immunol. 2012;169(1):17‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boumba D, Skopouli FN, Moutsopoulos HM. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjögren's syndrome. Rheumatology. 1995;34(4):326‐333. [DOI] [PubMed] [Google Scholar]
- 8. Kang Kwi, Kim Hyun‐Ok, Kwok Seung‐Ki, et al. Impact of interleukin‐21 in the pathogenesis of primary Sjogren's syndrome: increased serum levels of interleukin‐21 and its expression in the labial salivary glands. Arthritis Research & Therapy. 2011;13 (5):R179 10.1186/ar3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jung SM, Lee J, Baek SY, et al. The Interleukin 33/ST2 axis in patients with primary Sjögren syndrome: expression in serum and salivary glands, and the clinical association. J Rheumatol. 2015;42(2):264‐271. [DOI] [PubMed] [Google Scholar]
- 10. Margiotta DP. The IL33/ST2 axis in Sjogren syndrome in relation to disease activity. Eur Rev Med Pharmacol Sci. 2016;20(7):1295‐1299. [PubMed] [Google Scholar]
- 11. Guggino G, Lin X, Rizzo A, et al. Interleukin‐25 axis is involved in the pathogenesis of human primary and experimental murine Sjögren's syndrome. Arthritis Rheumatol. 2018;70(8):1265‐1275. [DOI] [PubMed] [Google Scholar]
- 12. Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin‐17 and linked cytokines associated with Sjögren's syndrome immunopathogenesis. Am J Pathol. 2009;175(3):1167‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lavoie TN, Carcamo WC, Wanchoo A, et al. IL‐22 regulation of functional gene expression in salivary gland cells. Genomics Data. 2016;7:178‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American college of rheumatology/European league against rheumatism classification criteria for primary Sjögren's syndrome. Arthritis Rheumatol. 2017;69(1):35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vitali C, Palombi G, Baldini C, et al. Sjögren's syndrome disease damage index and disease activity index: Scoring systems for the assessment of disease damage and disease activity in Sjögren's syndrome, derived from an analysis of a cohort of Italian patients. Arthritis Rheum. 2007;56(7):2223‐2231. [DOI] [PubMed] [Google Scholar]
- 16. Brito‐Zerón P, Retamozo S, Ramos‐Casals M. Phenotyping Sjögren's syndrome: towards a personalised management of the disease. Clin Exp Rheumatol. 2018;12:198‐209. [PubMed] [Google Scholar]
- 17. Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL‐1 to IL‐38), interferons, transforming growth factor β, and TNF‐α: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984‐1010. [DOI] [PubMed] [Google Scholar]
- 18. Gan Y, Zhao X, He J, et al. Increased interleukin‐17F is associated with elevated autoantibody levels and more clinically relevant than interleukin‐17A in primary Sjögren's syndrome. J Immunol Res. 2017;2017:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baldini C, Ferro F, Elefante E, Bombardieri S. Biomarkers for Sjögren's syndrome. Biomark Med. 2018;12(3):275‐286. [DOI] [PubMed] [Google Scholar]
- 20. Beckman KA, Luchs J, Milner MS, Ambrus JL. The potential role for early biomarker testing as part of a modern, multidisciplinary approach to Sjögren's syndrome diagnosis. Adv Ther. 2017;34(4):799‐812. [DOI] [PubMed] [Google Scholar]
- 21. Moutsopoulos NM, Katsifis GE, Angelov N, et al. Lack of efficacy of etanercept in Sjogren syndrome correlates with failed suppression of tumour necrosis factor and systemic immune activation. Ann Rheum Dis. 2008;67(10):1437‐1443. [DOI] [PubMed] [Google Scholar]
- 22. Reynolds JA, McCarthy EM, Haque S, et al. Cytokine profiling in active and quiescent SLE reveals distinct patient subpopulations. Arthritis Res Ther. 2018;20(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bournia V‐K, Vlachoyiannopoulos PG. Subgroups of Sjögren syndrome patients according to serological profiles. J Autoimmun. 2012;39(1–2):15‐26. [DOI] [PubMed] [Google Scholar]
- 24. Martel C, Gondran G, Launay D, et al. Active immunological profile is associated with systemic Sjögren's syndrome. J Clin Immunol. 2011;31(5):840‐847. [DOI] [PubMed] [Google Scholar]
- 25. Ramos‐Casals M, Solans R, Rosas J, et al. Primary Sjögren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine (Baltimore). 2008;87(4):210‐219. [DOI] [PubMed] [Google Scholar]
- 26. Tarn JR, Howard‐Tripp N, Lendrem DW, et al. Symptom‐based stratification of patients with primary Sjögren's syndrome: multi‐dimensional characterisation of international observational cohorts and reanalyses of randomised clinical trials. Lancet Rheumatol. 2019;1(2):e85‐e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


