Abstract
Background
To evaluate the role of Tp‐e and (Tp‐e)/QT ratio in differentiating benign ventricular premature complex (VPC) and malignant polymorphic ventricular tachycardia (PVT).
Methods
From January 2017 to December 2017, patients with documented polymorphic ventricular tachycardia (PVT) or ventricular fibrillation (VF) were consecutive included and classified as PVT/VF group. Sixty age‐ and sex‐matched healthy individuals were recruited as comparative control and subdivided into non‐VPC and VPC group. Clinical characteristics and Tp‐e and Tp‐e/QT ratio between the three groups were compared.
Results
Tp‐e and (Tp‐e)/QT ratio were significantly higher in patients of PVT/VF group compared with the other two groups (P < .001). Episodes of syncope were more frequent in patients with PVT/VF (P < .05). The sensitivity and specificity of a Tp‐e interval ≥86 ms for malignant arrhythmias triggered by VPCs were 88% and 66%, respectively, while the sensitivity and specificity of the Tp‐e/QT ratio ≥0.24 were 82% and 70%, respectively. Five patients complained recurrence of syncope in the PVT/VF group and 1 patient died with mean follow‐up of 18 months.
Conclusion
Tp‐e interval and the Tp‐Te/QT ratio is significantly increased in patients with PVT/VF and may be used as a novel non‐invasive marker of differentiating malignant and benign VPC.
Keywords: (Tp‐e)/QT, Tp‐e, ventricular fibrillation, ventricular premature complexes
To evaluate the role of Tp‐e and (Tp‐e)/QT ratio in differentiating benign ventricular premature complex (VPC) and malignant polymorphic ventricular tachycardia (PVT).
![]()
1. INTRODUCTION
Ventricular premature complexes (VPCs), most originating from outflow tract ventricular tachycardia (VT), are frequently encountered in subjects without structural heart disease and usually considered to be a benign arrhythmia in healthy adults. 1 , 2 , 3 Haissaguerre et al 4 reported that the conventional benign outflow tract extrasystoles might develop malignant polymorphic VT without the organic heart disease and inherited ion channelopathies, such as the long‐QT, short‐QT, and Brugada syndromes, as well as catecholaminergic polymorphic ventricular tachycardia (CPVT). Therefore, distinguishing patients with benign idiopathic monomorphic VT from those with these malignant polymorphic forms has been a clinical problem.
Ventricular repolarization abnormality in the form of transmural dispersion of repolarization (TDR) has been reported to play a role in the development of life‐threatening ventricular arrhythmias. 5 , 6 The interval from the peak of the T wave to the end of the T wave (Tpeak to Tend interval [Tp‐e]) on 12‐lead ECG could reflect the transmural dispersion of myocardial repolarization. 7 , 8 A prolonged Tpeak‐Tend interval and (Tp‐e)/QT ratio have been considered as a non‐invasive marker of arrhythmogenesis of malignant polymorphic VT development in different clinical settings. 9 , 10 , 11 However, there are limited data regarding Tp‐e interval and (Tp‐e)/QT ratio as the potential risk marker in differentiating benign VPC and malignant polymorphic VT. Therefore, this study was aimed to evaluate the role of Tp‐e and (Tp‐e)/QT ratio in distinguishing benign VPC and malignant PVT.
2. METHODS
2.1. Study population
From January 2017 to December 2017, patients with documented polymorphic ventricular tachycardia (PVT) or ventricular fibrillation (VF) from the center of NanFang Hospital, Guangzhou, China, were consecutive included and classified as PVT/VF group. Age‐ and sex‐matched healthy individuals without structured heart disease were recruited in the study as controls and subdivided into two subgroups, non‐VPC group and VPC group.
The inclusion criteria were as follows: (a) patients with primary idiopathic PVT or VF with preceding monomorphic ventricular premature complex (VPC); (b) patients experienced at least 1 significant event (syncope, electrical storm, or aborted sudden cardiac death) with documented PVT/VF on a 12‐lead electrocardiogram (ECG). The exclusion criteria were as follows: (a) structure heart diseases diagnosed by echocardiogram, a maximal exercise stress test and cardiac catheterization with coronary angiography and right and left ventriculography; (b) channelopathies defined as normal QRST complexes in V1 to V3 at rest or under intravenous ajmaline (1 mg/kg body weight) drug administration and a corrected QT interval between 340 and 440 ms by use of the Bazett formula. Polymorphic ventricular tachycardia (PVT) was defined as more than five consecutive beats with different QRS morphology and terminating spontaneously. 12 Ventricular fibrillation was defined as a PVT with a hemodynamic decompensation requiring direct cardioversion for termination according to 2017 AHA/ACC/HRS Guideline. 12
2.2. Electrocardiogram and Measurement of Tp‐e, QT, and QTc intervals (Figure 1)
FIGURE 1.
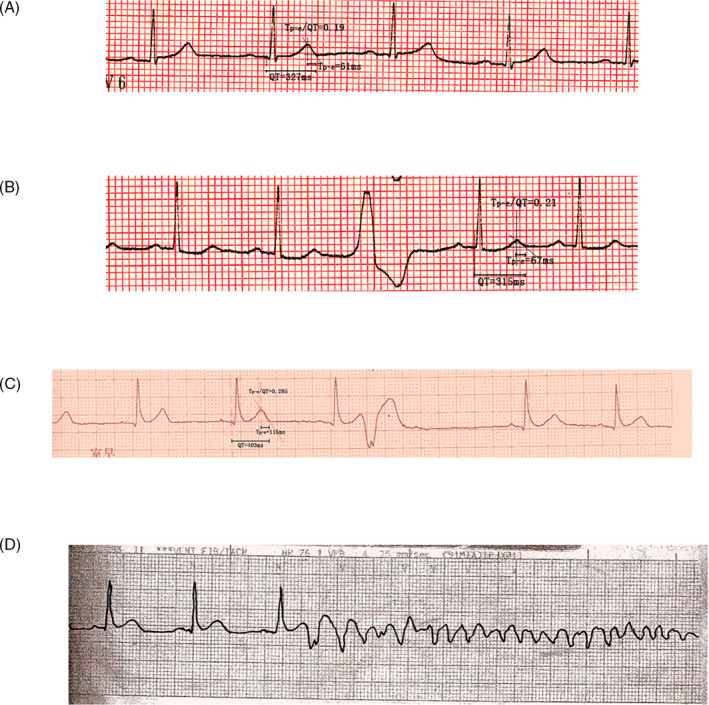
Electrocardiogram. A, ECG representative example from the control group. Note that the Tp‐e was 61 ms; B, A representative example from the benign VPC pattern group. Note that the Tp‐e was 67 ms; C, A representative example from the PVT/VF group. Note that the Tp‐e was 116 ms; D, VF recorded by a monitoring ECG in a patient with the malignant form of idiopathic VT
All the recruited individuals were discontinued with anti‐arrhythmia drugs for at least 2 weeks before analysis. We used a standard 12‐lead ECG tracing at 25‐mm/s article speed and 10‐mm/mV amplitude. Tp‐e was measured from Tpeak to Tend. The QT interval was measured for a single complex from the beginning of the QRS complex to the point at which the tangent of the maximal downslope of the descending limb of the T wave crossed the isoelectric baseline. Tp‐e and QT intervals were measured in lead V5. The QT interval was corrected with the Bazett formula QTc = QT/(RR)1/2. The Tp‐e/QTc ratio was calculated as the ratio of Tp‐e which leaded to the corresponding QTc interval. The Tp‐e and Tp‐e/QTc ratio was compared among the three groups.
2.3. Follow‐up
A mean follow‐up of 18 months was conducted in patients with VF. The end points of the study included syncope, aborted sudden death, and/or documented VT/VF. Patients without complaint were followed up by telephone every 3 months. For patients complained syncope, more closely follow‐up were carried out including implantable cardioverter defibrillator (ICD) interrogation and holter examination.
2.4. Statistical analysis
Data were expressed as mean ± standard deviation. Categorical data were analyzed using chi‐squared test. Multigroup differences were analyzed by one‐way ANOVA followed by Scheffé's multiple comparison test. To examine prognostic value from Tp‐e and Tp‐e dispersion and determine cutoff values, analysis of receiver‐operating characteristic (ROC) curves was made. A P‐value < .05 was considered as significant difference. All data were analyzed using SPSS version 13.0.
3. RESULTS
3.1. Basic characteristics
Seventeen patients were included in the PVT/VF group with an average age of 44.9 ± 14.1 years old. Sixty age‐ and gender‐matched healthy individuals without structured heart disease were recruited in the study as comparative controls and subdivided into two subgroups. The non‐VPC group included 30 healthy subjects (12 males, mean age 39.5 ± 11.5 years). The VPC group included another 30 healthy subjects (11 males, mean age 44.8 ± 13.3 years). There were no significant differences of age and gender among the three groups (Table 1).
Table 1.
Baseline clinical and electrocardiography data of subjects
| Category | Total subjects (n = 77) | P | ||
|---|---|---|---|---|
| Normal (n = 30) | VPC without VF (n = 30) | VPC with VF (n = 17) | ||
| Age, y | 39.5 ± 11.5 | 44.8 ± 13.3 | 44.9 ± 14.1 | .33 |
| Male sex | 12 (60%) | 11 (52.3%) | 10 (58.8%) | .88 |
| Syncope, n (%) | 0 (0%) | 2 (9.5%) | 11 (64.7%) | <.05 |
| Tp‐e, ms | 79.6 ± 4.5 | 78.7 ± 7.2 | 99.1 ± 11.1 | <.001 |
| QT, ms | 351.8 ± 26.8 | 361.4 ± 30.6 | 369.8 ± 32.7 | .20 |
| QTc, ms | 388.2 ± 32.1 | 390.7 ± 30.2 | 401.4 ± 38.9 | .46 |
| Tp‐e/QT | 0.23 ± 0.02 | 0.22 ± 0.02 | 0.27 ± 0.03 | <.001 |
| ICD implanted | 0 (0%) | 0 (0%) | 12 (70.5%) | <.05 |
Categorical data are presented as absolute and relative frequencies, while continuous variables are presented as means + standard deviations.
3.2. Measurement of Tp‐e, QT, and QTc intervals
QT and QTc intervals were similar among the three groups (P > .05). However, Tp‐e and (Tp‐e)/QT ratio was significantly higher in the patients of PVT/VF group compared with the other two groups (P < .05). Episodes of syncope were more frequent in patients with PVT/VF (P < .05). No patients were implanted of ICD in the non‐VPC and VPC groups, while it was 70.5% in the PVT/VF group.
3.3. Patients with PVT/VF and ROC curve
A total of 17 patients met the inclusion criteria of PVT/VF group, while 11 patients experienced syncope as the first symptom at the time of inclusion (Table 2). Cardioverter defibrillator was implanted in 12 patients. We examined the predictive ability of each ECG parameter separately by plotting ROC curves (Figure 2). The sensitivity and specificity of a Tp‐e interval ≥86 ms for malignant arrhythmias triggered by VPCs were 88% and 66%, respectively. The sensitivity and specificity of a Tp‐e/QT ratio ≥ 0.24 were 82% and 70%, respectively.
Table 2.
Clinical Characteristics of 17 Patients with PVT/VF
| Patient No. | Age (y) | Gender | Spontaneous VF | Symptom | Holter ECG findings | |||
|---|---|---|---|---|---|---|---|---|
| Isolated PVC (/day) | QT (ms) | CI (ms) | Tp‐e (ms) | |||||
| 1 | 35 | F | 2 | Syncope | 40 730 | 331 | 320 | 115 |
| 2 | 21 | F | 2 | Syncope | 3425 | 412 | 320 | 113 |
| 3 | 51 | M | 1 | Syncope | 20 013 | 317 | 410 | 92 |
| 4 | 59 | F | 1 | Syncope | 25 130 | 414 | 520 | 115 |
| 5 | 60 | M | 0 | Non | 6789 | 427 | 470 | 108 |
| 6 | 63 | F | 1 | Presyncope | 7355 | 335 | 378 | 104 |
| 7 | 37 | M | 1 | Syncope | 21 674 | 361 | 400 | 94 |
| 8 | 47 | M | 0 | Presyncope | 42 781 | 400 | 390 | 100 |
| 9 | 48 | F | 1 | Syncope | 4576 | 374 | 390 | 101 |
| 10 | 55 | M | 0 | Non | 5079 | 366 | 378 | 89 |
| 11 | 59 | F | 1 | Syncope | 11 793 | 388 | 378 | 105 |
| 12 | 23 | F | 0 | Syncope | 5193 | 363 | 520 | 98 |
| 13 | 36 | M | 0 | Presyncope | 8973 | 379 | 400 | 110 |
| 14 | 42 | F | 0 | Presyncope | 29 634 | 342 | 385 | 85 |
| 15 | 58 | M | 1 | Syncope | 36 771 | 360 | 400 | 90 |
| 16 | 24 | F | 0 | Syncope | 5074 | 390 | 520 | 87 |
| 17 | 48 | F | 1 | Syncope | 8789 | 327 | 395 | 79 |
FIGURE 2.
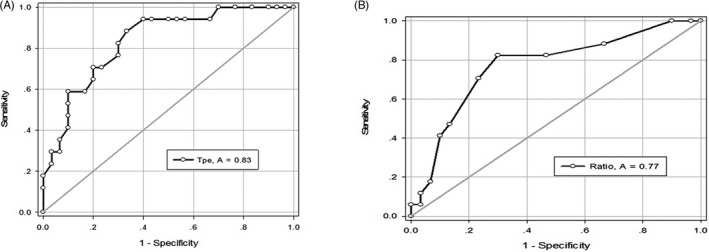
ROC curves. Tp‐e (A) and Tp‐e/QT ratio (B) receiver‐operating characteristic (ROC) curve. The cut point was for Tp‐e values > 86 ms and Tp‐e/QT ratio > 0.24, respectively. The areas under the ROC curves were 0.83 for Tp‐e and 0.77 for Tp‐e/QT ratio
3.4. Follow‐up
Sixteen of 17 patients with PVT successful achieved a mean follow‐up of 18 months. Five patients complained recurrence of syncope. Three of them were implanted of ICD, and the electrogram showed episode of VF with appropriate shock. One patient died of ventricular arrhythmia storms.
4. DISCUSSION
Though idiopathic VT, mostly originating from RVOT, is generally considered as a benign arrhythmia, 13 and sudden death have been reported. 4 , 14 It is important to distinguish potential lethal malignant VPC from structural normal heart. We showed that the Tp‐e interval and Tp‐e/QT ratio was significantly increased in patients with malignant VPC compared with control subjects including VPC patients and non‐VPC subjects. The findings of current study showed that Tp‐e could be potentially used as a non‐invasive index to differentiate malignant arrhythmia and idiopathic VPC. Prolonged Tp‐e interval was associated with increased risk of sudden cardiac death in patients with VPC.
Previous study showed that the Tp‐e interval was proposed to be prolonged in identifying high‐risk populations in congenital LQTS, 15 Brugada syndrome, 16 ERS, 17 and hypertrophic cardiomyopathy. 18 Panikkath et al 19 reported Tp‐e on the resting 12‐lead ECG was prolonged in sudden cardiac death (SCD) cases compared with controls and was associated with SCD independent of age, sex, QTc, QRSD, and LV dysfunction. In this study, we found that ECG markers, Tp‐e and Tp‐e/QT ratio might also be predictors of VT/VF in subjects with VPC. Studies have explored the genesis of Tp‐e as well as the potential mechanisms that resulted in increased risk of malignant ventricular arrhythmia in different clinical condition. From a canine myocardial wedge preparation model, Antzelevitch et al 20 showed that the peak of the T wave coincided with epicardial repolarization and the end of the T wave with repolarization of the M cells, so that the Tp‐e interval provided a measure of the transmural dispersion of repolarization (TDR). Prolonged Tp‐e corresponding with enhance TDR leaded to re‐entry and its perpetuation, resulting in polymorphic ventricular tachycardia or ventricular fibrillation. 21 , 22 , 23 However, Tobias et al 24 argued that Tp‐e was not correlated with TDR, but related to an index of total dispersion of repolarization using transmural mapping of the intact heart. Although controversy remained in whether Tp‐e on the surface ECG might be equivalent to TDR, studies suggested it might provide an index of TDR and thus being used as a prognostic factor of life‐threatening arrhythmia. 22 , 25 , 26
Idiopathic VPC usually showed a monomorphic pattern of tachycardia and has been shown to be caused by triggered activity, mediated by delayed afterdepolarizations. Earlier study reported that such form of VPC might also commonly initiated malignant VF or polymorphic VT by with a very short coupling interval (CI). Haissaguerre et al 4 found that 27 patients with idiopathic VF and VPCs were successfully eliminated by radiofrequency catheter ablation with a relatively short CI of 280 ± 26 ms Viskin et al 27 reported that the CI of VPC in idiopathic VF (300 ± 40 ms) was shorter than that of VPC in benign RVOT VT (427 ± 76 ms) (P < .05). However, Noda et al 28 showed that the CI of initiating VPC in malignant VT (409 ± 62 ms) was not significantly different compared with the CI in benign RVOT (428 ± 65 ms). Thus, CI of initiating VPC seems to be a potential useful index to differentiate malignant VPC and benign ones though the result remains divergence. We suspected that both benign and potential life‐threatening VPC were due to triggered activity arising from a single focus, but different in proarrhythmia matrix.
Transmural dispersion of repolarization was thought to give rise to phase 2 re‐entry, which provided the arrhythmogenetic matrix that precipitated episodes of rapid polymorphic VT and more vulnerable to the closely coupled extrasystole. Compared to CI, Tp‐e and Tp‐e/QT ratio was relatively new index reflexing TDR; hence, they were more sensitive index of arrhythmogenesis. Similar with our results, Karim et al 29 reported Tp‐e/QT ratio in J Wave Syndromes was significant longer than the control group. Panikkath et al 19 observed that prolonged Tp‐e interval and Tp‐e/QT ratio on the resting ECG was associated with increased risk of sudden cardiac death in a community‐based study.
In our study, recurrent events were more frequent in patients with prolonged Tp‐e. Radiofrequency catheter ablation and ICD implanted were performed to protect SCD in patients with malignant VPC. As potential life‐threatening arrythmia were occasionally observed in patients initially diagnosed with benign VPC, the need of careful follow‐up and using non‐invasive tool to identify high‐risk patients was required.
In conclusion, Tp‐e interval and the Tp‐Te/QT ratio might be used as a novel non‐invasive marker of differentiating malignant and benign VPC and predicting the occurrence of life‐threatening arrhythmic events in patients with idiopathic VF.
Zhao D, Liang B, Peng J, et al. Tp‐e and (Tp‐e)/QT ratio as a non‐invasive risk factors for malignant ventricular arrhythmia in patients with idiopathic ventricular premature complexes. J Clin Lab Anal.2021;35:e23636 10.1002/jcla.23636
All authors have participated in the work and have reviewed and agree with the content of the article.
None of the article contents are under consideration for publication in any other journal or have been published in any journal.
No portion of the text has been copied from other material in the literature (unless in quotation marks, with citation).
Funding informationThis work was supported by a grant (201904030730) from Zhaoqing Science Program Project and Southern Medical University Clinical Research Project (LC2016ZD002).
REFERENCES
- 1. Morady F, Kadish AH, DiCarlo L, et al. Long‐term results of catheter ablation of idiopathic right ventricular tachycardia. Circulation. 1990;82:2093‐2099. [DOI] [PubMed] [Google Scholar]
- 2. Kamakura S, Shimizu W, Matsuo K, et al. Localization of optimal ablation site of idiopathic ventricular tachycardia from right and left ventricular outflow tract by body surface ECG. Circulation. 1998;98:1525‐1533. [DOI] [PubMed] [Google Scholar]
- 3. Coggins DL, Lee RJ, Sweeney J, et al. Radiofrequency catheter ablation as a cure for idiopathic tachycardia of both left and right ventricular origin. J Am Coll Cardiol. 1994;23:1333‐1341. [DOI] [PubMed] [Google Scholar]
- 4. Haïssaguerre M, Shoda M, Jaïs P, et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106:962‐967. [DOI] [PubMed] [Google Scholar]
- 5. Yan GX, Lankipalli RS, Burke JF, Musco S, Kowey PR. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol. 2003;42:401‐409. [DOI] [PubMed] [Google Scholar]
- 6. Yan GX, Martin J. Electrocardiographic T wave: a symbol of transmural dispersion of repolarization in the ventricles. J Cardiovasc Electrophysiol. 2003;14:639‐640. [DOI] [PubMed] [Google Scholar]
- 7. Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm. 2007;4:964‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antzelevitch C. T peak‐Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest. 2001;31:555‐557. [DOI] [PubMed] [Google Scholar]
- 9. Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp‐Te interval and its diagnostic value. J Electrocardiol. 2008;41:575‐580. [DOI] [PubMed] [Google Scholar]
- 10. Topilski I, Rogowski O, Rosso R, et al. The morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmias. J Am Coll Cardiol. 2007;49:320‐328. [DOI] [PubMed] [Google Scholar]
- 11. Haarmark C, Hansen PR, Vedel‐Larsen E, et al. The prognostic value of the Tpeak‐Tend interval in patients undergoing primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction. J Electrocardiol. 2009;42:555‐560. [DOI] [PubMed] [Google Scholar]
- 12. Al‐Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91‐e220. [DOI] [PubMed] [Google Scholar]
- 13. Belhassen B, Viskin S. Idiopathic ventricular tachycardia and fibrillation. J Cardiovasc Electrophysiol. 1993;4:356‐368. [DOI] [PubMed] [Google Scholar]
- 14. Haïssaguerre M, Extramiana F, Hocini M, et al. Mapping and ablation of ventricular fibrillation associated with long‐QT and Brugada syndromes. Circulation. 2003;108:925‐928. [DOI] [PubMed] [Google Scholar]
- 15. Kanters JK, Haarmark C, Vedel‐Larsen E, et al. T(peak)T(end) interval in long QT syndrome. J Electrocardiol. 2008;41:603‐608. [DOI] [PubMed] [Google Scholar]
- 16. Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, et al. Tpeak‐Tend and Tpeak‐Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828‐1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Letsas KP, Charalampous C, Korantzopoulos P, et al. Novel indexes of heterogeneity of ventricular repolarization in subjects with early repolarization pattern. Europace. 2012;14:877‐881. [DOI] [PubMed] [Google Scholar]
- 18. Shimizu M, Ino H, Okeie K, et al. T‐peak to T‐end interval may be a better predictor of high‐risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol. 2002;25:335‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panikkath R, Reinier K, Uy‐Evanado A, et al. Prolonged Tpeak‐to‐tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 2011;4:441‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. The M cell. Circ Res. 1991;68:1729‐1741. [DOI] [PubMed] [Google Scholar]
- 21. Antzelevitch C, Shimizu W, Yan GX, et al. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10:1124‐1152. [DOI] [PubMed] [Google Scholar]
- 22. Emori T, Antzelevitch C. Cellular basis for complex T waves and arrhythmic activity following combined I(Kr) and I(Ks) block. J Cardiovasc Electrophysiol. 2001;12:1369‐1378. [DOI] [PubMed] [Google Scholar]
- 23. Watanabe N, Kobayashi Y, Tanno K, et al. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol. 2004;37:191‐200. [DOI] [PubMed] [Google Scholar]
- 24. Opthof T, Coronel R, Janse MJ. Is there a significant transmural gradient in repolarization time in the intact heart?: repolarization gradients in the intact heart. Circ Arrhythm Electrophysiol. 2009;2:89‐96. [DOI] [PubMed] [Google Scholar]
- 25. Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long‐QT syndrome. Circulation. 1998;98:1928‐1936. [DOI] [PubMed] [Google Scholar]
- 26. Barbhaiya C, Po JR, Hanon S, Schweitzer P. Tpeak ‐ Tend and Tpeak ‐ Tend /QT ratio as markers of ventricular arrhythmia risk in cardiac resynchronization therapy patients. Pacing Clin Electrophysiol. 2013;36:103‐108. [DOI] [PubMed] [Google Scholar]
- 27. Viskin S, Rosso R, Rogowski O, Belhassen B. The, "short‐coupled" variant of right ventricular outflow ventricular tachycardia: a not‐so‐benign form of benign ventricular tachycardia? J Cardiovasc Electrophysiol. 2005;16:912‐916. [DOI] [PubMed] [Google Scholar]
- 28. Noda T, Shimizu W, Taguchi A, et al. Malignant entity of idiopathic ventricular fibrillation and polymorphic ventricular tachycardia initiated by premature extrasystoles originating from the right ventricular outflow tract. J Am Coll Cardiol. 2005;46:1288‐1294. [DOI] [PubMed] [Google Scholar]
- 29. Karim Talib A, Sato N, Sakamoto N, et al. Enhanced transmural dispersion of repolarization in patients with J wave syndromes. J Cardiovasc Electrophysiol. 2012;23:1109‐1114. [DOI] [PubMed] [Google Scholar]


