Abstract
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy among other endocrine tumors, and BRAF V600E is a frequent genetic mutation occurring in the disease. Although different molecular techniques, most importantly sequencing has been widely recognized as a gold standard but molecular diagnosis remains an expensive, laborious, and time‐intensive process. Recently, immunohistochemistry (IHC) with anti‐BRAF V600E (VE1) antibody has increased practical utility and implemented clinically for the detection of BRAF V600E mutation. Therefore, the study aimed to evaluate diagnostic accuracy of VE1 IHC for detecting the BRAF V600E mutation frequency and clinical implementation in diagnostic laboratories. In this study, 72 formalin fixed paraffin‐embedded tissues (FFPE) were used to determine the BRAF V600E mutation status using IHC and Sanger sequencing. The mutation was found in 29% and 28% cases using IHC and Sanger sequencing, respectively. Furthermore, the results showed 100% sensitivity, 98.07% specificity, 95.2% positive predictive value, and 100% negative predictive value. Notably, significant associations were found between BRAF V600E status and tumor stage, tumor focality, and extrathyroidal extensions, respectively. VE1 IHC was found to be a highly sensitive, specific, and diagnostically accurate method in this cohort. Therefore, BRAF V600E detection through IHC has been considered as the best tailored technique for routine pathology laboratories.
Keywords: BRAFV600E, diagnostic accuracy, papillary thyroid carcinoma, sanger sequencing, VE1 immunohistochemistry
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy among other endocrine tumors and BRAF V600E (v‐Raf murine sarcoma viral oncogene homolog B) is a frequent genetic mutation occurring in the disease. Although different molecular techniques, most importantly sequencing has been widely recognized as a gold standard but molecular diagnosis remains an expensive, laborious and time intensive process. Recently, immunohistochemistry (IHC) with anti‐BRAF V600E (VE1) antibody has increased practical utility and implemented clinically for the detection of BRAF V600E mutation. Therefore, the study aimed to evaluate diagnostic accuracy of VE1 IHC for detecting the BRAF V600E mutation frequency for clinical implementation in diagnostic laboratories. In this study, 72 formalin fixed paraffin‐embedded tissues (FFPE) were used to determine the BRAF V600E mutation status using IHC and Sanger sequencing. The mutation was identified in 29% and 28% cases by IHC and Sanger sequencing, respectively. Furthermore, results indicated 100% sensitivity, 98.07% specificity, 95.2% positive predictive value, and 100% negative predictive value. Notably, significant associations were found between BRAF V600E status and tumor stage, tumor focality, and extrathyroidal extensions, respectively. VE1 IHC was found to be a highly sensitive, specific, and diagnostically accurate method in this cohort. Therefore, BRAF V600E detection through IHC is considered as the best tailored technique for routine pathology laboratories.

1. INTRODUCTION
Papillary thyroid carcinoma (PTC) is the most frequent type of thyroid cancer (TC) that accounts for greater than 80% of thyroid malignancies. 1 , 2 Recently, molecular target therapies based on specific oncogenic genetic aberrations have yielded promising results for the treatment of PTC. Enormous efforts have uncovered aberrations involved in the development and progression of PTC. 3 BRAF mutations are the most common oncogenic driver mutations correlated with thyroid cancer. 4 Among all BRAF mutations identified, BRAF V600E accounts for more than 90% of those mutations. It encodes a serine threonine protein kinase, belonged to mitogen activated protein kinase signaling (MAPK) pathway. 5 The missense mutation is present in exon 15 of gene and located at chromosome 7q34. BRAF T>A transversion (thymine to adenine) at nucleotide position 1799 (c.T1799>A) results in substitution of valine (V) into glutamic acid (E) at codon 600. 6 , 7
The rate of BRAF V600E mutation in PTC patients mostly depends on the target population and clinicopathological characteristics, including gender, age, tumor stage, tumor focality, lymphovascular invasions, and extrathyroidal extensions. 8 The mutation is found in approximately 50% of PTC cases among western series descended from the USA. 9 , 10 Overall, the prevalence rate varies in different countries, primarily Asian populations have a higher BRAF rate than Western countries. 11 However, the prevalence is more heterogeneous in Asian populations, spanning from 28.2% to 90% mutation. 12 , 13 , 14
In routine molecular pathology laboratories, different molecular methodologies such as Sanger sequencing, allele‐specific PCR (AS‐PCR), droplet digital PCR (ddPCR) and polymerase chain reaction‐restriction fragment length polymorphism (PCR‐RFLP) are employed for the detection of BRAF V600E mutation 7 , 15 , 16 , 17 , 18 . Although different techniques have different performance rates and sensitivities, the technique performs to detect the mutation may have significant correlation with prevalance of BRAF V600E. Sanger sequencing has been widely acknowledged as a gold standard to determine the BRAF V600E mutation, but molecular examination remains laborious, time intensive and an expensive process. 19 , 20 , 21 Additionally, molecular methods require established molecular pathology laboratories, to determine BRAF V600E mutation which however is not always possible in resource constraint settings. 22 , 23
Over the past 5 to 10 years, the clinical utility of IHC with mutation‐specific antibodies has increased considerably and implemented clinically for the detection of mutation. 24 , 25 Several studies have been reported on the performance of VE1 IHC technique to detect the BRAF V600E mutation, and most of these reports showed exceptional concordance between this method and molecular genotyping, thus IHC can be used as an alternative method to Sanger sequencing. 1 , 26 , 27 The IHC technique consumes less time to perform multiple tests as compared to molecular methods, consequently decreasing the turnaround time. 8
The foremost purpose of this study was to evaluate concordance rate between VE1 IHC and Sanger sequencing to determine the BRAF V600E mutation in our cohort, in correlation to various clinicopathological characteristics of PTC patients.
2. MATERIALS AND METHODS
2.1. Study design
This was a hospital‐based retrospective study conducted by the Department of Biological Sciences, International Islamic University, Islamabad and Department of General Surgery, Pakistan Institute of Medical Sciences (PIMS), Islamabad, Pakistan from 2016 to 2019. The present study was approved by Institution Review Board (IRB) of University and Ethical Review Board (ERB) of Pakistan Institute of Medical Sciences (PIMS), Islamabad. Consent form was signed and obtained from each patient.
2.2. Sample size and sample collection
We evaluated the clinicopathological information of one hundred (n = 100) TC patients who underwent total/hemi‐thyroidectomy at PIMS Islamabad, Pakistan. Surgically operated and histologically confirmed classic PTC and follicular variants of PTC patients were involved in the study. A total of 72 consecutive PTC patients who accomplished inclusion criteria were registered for this study, and their formalin fixed tissue blocks were used for DNA extraction and IHC (Figure 1). All hematoxylin and eosin slides (H&E) of enrolled cases were reviewed independently by two experienced histopathologists, and cases were classified according to diagnostic standards and terminologies of World health Organization. 28
Figure 1.
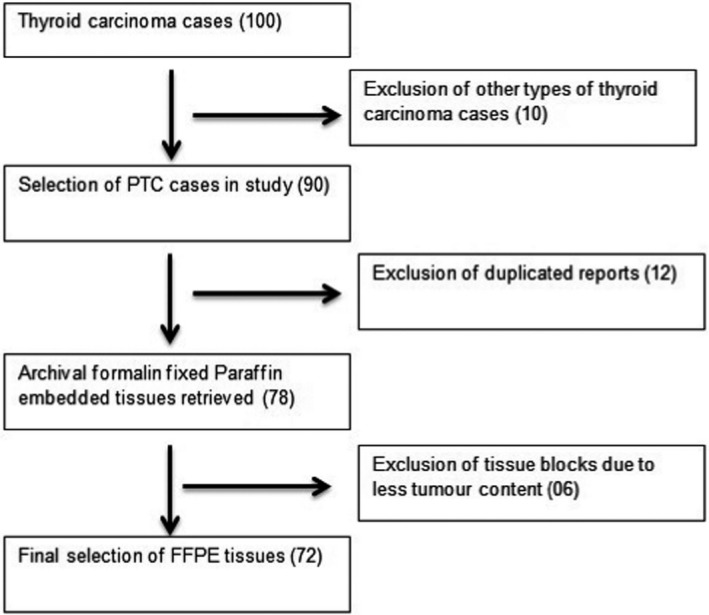
Flowchart showing the inclusions and exclusions of study subjects for final analysis
2.3. Preparation of tissue samples and DNA isolation
Thin‐section slides of FFPE tissue blocks were prepared at the time of diagnosis. The area containing tumor cells on H&E slides was marked by histopathologists, and unstained slides were deparaffinized, depending on the size of selected tumor area. Then, slides were soaked twice in xylene (each time 15 minutes) and subsequently in 96% alcohol for 5 minutes. Slides were once again soaked in distilled water. Tissues were scraped with the disposable needle and transferred to Eppendorf tubes. Total DNA was isolated using FFPE tissue extraction kit ‘’QIAamp DNA’’ (Syngen, Wroclaw, Poland). Quality of isolated DNA was measured using NanoDrop spectrophotometer.
2.4. BRAF V600E mutation analysis
BRAF V600E mutation analysis was done for all 72 cases. After DNA isolation, exon 15 of BRAF gene genomic was amplified using primers; Forward 5′‐GCTTGCGCTGATAGAATAATGAG ‐3′, Reverse 5′‐GATACTCAGCACGATCCTTGG‐3′ (Sigma Aldrich) giving rise to 224bp amplicon. Sanger sequencing of the amplified product was performed using automated DNA sequencer (ABI sequencer, Applied Biosystems).
2.5. Detection of BRAF V600E mutation using IHC
IHC was performed on 5 µm thick sections of FFPE tissue blocks. Automated Ventanna BenchMark immunostainer was used for analysis. Tissue samples were incubated with the clone VE1 (mouse monoclonal antibody) at 38°C for 5 minutes. VE1 immunoreactivity was visualized using optiview IHC DAB kit (Ventanna Medical Systems). The samples were counterstained with bluing reagent and hematoxylin for 4 minutes. Normal thyroid tissue was used as a negative control tissue. The IHC scoring was independently done by two pathologists by considering H‐scoring system, who were unaware to the BRAF sequencing status. H‐score is a semi quantitative scoring system that is obtained by both intensity of positive cells (0, no staining; 1+, weak; 2+, moderate; 3+, strong) and proportion (0%–100%, increase in 5% increments), as previously explicated. 8 , 29
2.6. Statistical analysis
SPSS statistical software package version 23.0 (SPSS, Chicago, IL) was used to perform Descriptive statistics, chi‐square, and Student's t test. P ≤ 0.05 was considered as significant. Formulas used for calculating diagnostic accuracy parameters were adopted, as described previously. 30
3. RESULTS
3.1. Clinicopathological features of PTC patients
The demographic and clinicopathological characteristics of PTC cases are described in Table 1. In the present study, 72 PTC tissues were analyzed and their clinicopathological characteristics were noted. Average age of patients at the time of diagnosis was 46 ± 14 years (range, 32 to 60 years). Most of the PTC patients were females (75%) as compared to males. Moreover, younger age patients were more predisposed to PTC than elders in this cohort. Cancer staging was in accordance to the guidelines of American Joint Committee on Cancer (AJCC), 8th edition. 31 Histopathological investigation of PTC tissues showed capsular, lymphovascular invasions, and extrathyroidal extensions in 57%, 58%, and 54% of cases, respectively (Table 1).
Table 1.
Demographic and Clinicopathological features of PTC patients
| Characteristics | Patients, N = 72 (%) |
|---|---|
| Age at diagnosis (years), mean ± SD | 46 ± 14 |
| Age in years | |
| Age < 55 years | 54 (75) |
| Age ≥ 55 years | 18 (25) |
| Gender | |
| Male, n (%) | 22 (30.6) |
| Female, n (%) | 50 (69.4) |
| Staging, Age < 55 years | |
| Stage I | 21 (29.2) |
| Stage II | 33 (45.8) |
| Staging, Age ≥ 55 years | |
| Stage I + II | 7 (9.7) |
| Stage III & above | 11 (15.3) |
| Tumor focality | |
| Multifocal | 32 (44.4) |
| Unifocal | 40 (55.6) |
| Capsular invasion | |
| Absent | 41 (56.9) |
| Present | 31 (43.1) |
| Lymphovascular invasion | |
| Absent | 42 (58.3) |
| Present | 30 (41.7) |
| Extrathyroidal extension | |
| Absent | 39 (54.2) |
| Present | 43 (45.8) |
Data are represented in percentages (%), except age in mean + SD.
3.2. BRAF V600E mutational analysis using IHC and correlation with clinicopathological features PTC patients
The representative pictures showing diffuse and focal staining on performing VE1 IHC are depicted in Figure 2. In terms of IHC, BRAF V600E mutation was present in 29.0% (21 of 72) of enrolled PTC patients and significantly associated with tumor stage, tumor focality and extrathyroidal extension (P ≤ 0.05). However, BRAF V600E mutation was not significantly associated with patient age and gender (P > 0.05). Capsular and lymphovascular invasions were also not significantly correlated to BRAF V600E positive cases (P > 0.05) (Table 2).
Figure 2.
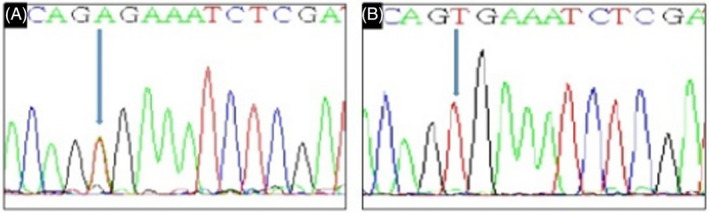
Mutation Sequencing: A, Partial electropherogram (forward) of mutant in exon 15 of the BRAF gene codon 600 (T→A; transversion); B, Partial electropherogram (forward) of an adjacent normal
Table 2.
Correlation of BRAF V600E mutation status with clinicopathological features of PTC patients
| Characteristics | VE1 IHC, (n = 72) | P value | |
|---|---|---|---|
| Positive 21 (29.0%) | Negative 51 (71.0%) | ||
| Age | |||
| <55 years | 15 (27.8) | 39 (72.2) | 0.653 |
| ≥55 years | 06 (33.3) | 12 (66.7) | |
| Gender | |||
| Male | 09 (41) | 13 (59) | 0.1 |
| Female | 12 (24) | 38 (76) | |
| Tumor staging, <55 years | |||
| Stage I | 09 (5.8) | 12 (15.2) | 0.048 |
| Stage II | 06 (9.2) | 27 (23.8) | |
| Tumor staging, ≥55 years | |||
| Stage I + II | 02 (2.3) | 05 (4.7) | 0.05 |
| Stage III + above | 04 (3) | 07 (7.3) | |
| Tumor focality | |||
| Unifocal | 08 (20) | 32 (80) | 0.05 |
| Multifocal | 13 (41) | 19 (59) | |
| Lymphovascular invasion | |||
| Present | 15 (35.7) | 27 (64.3) | 0.19 |
| Absent | 06 (20) | 24 (80) | |
| Capsular Invasion | |||
| Present | 15 (37) | 26 (63) | 0.1 |
| Absent | 06 (19) | 25 (81) | |
| Extrathyroidal extension | |||
| Present | 16 (41) | 23 (59) | 0.01 |
| Absent | 05 (15.2) | 28 (84.8) | |
3.3. Evaluation of concordance between VE1 IHC and sequencing
The partial electropherograms showing T to A transversion in case of BRAF V600E mutation, as depicted in Figure 3. In this study, the BRAF V600E mutation was positive in 21/72 cases (29%) using IHC while BRAF V600E mutation was positive in 20/72 cases (28%) using Sanger sequencing. When the results of both methods were compared, 20 cases found true positive, while 51 cases were true negative. There were no false negative results. However, one case with positive BRAF V600E mutation using VE1 IHC was negative using sequencing (false positive). Overall, the rate of concordance was 98.6% between IHC and sequencing (Table 3). We found sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of VE1 IHC as 100%, 98.07%, 95.2%, and 100%, respectively.
Figure 3.
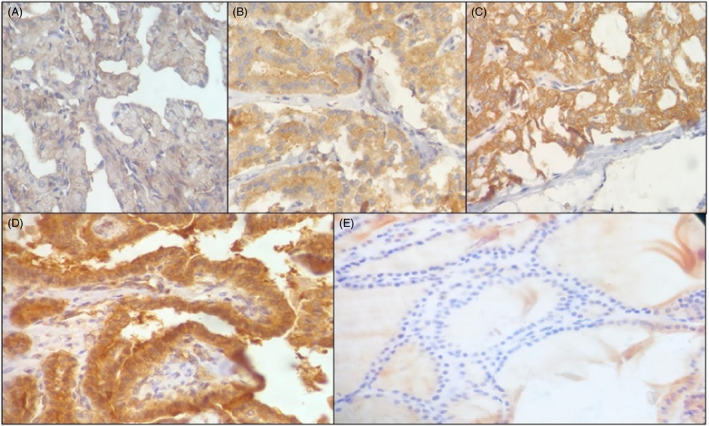
Representative images of BRAFV600E mutant VE1 immunostaining. PTC is shown with diffuse cytoplasmic staining A, 0, negative; B, 1+, weak; C, 2+, moderate; D, 3+, strong; E, normal thyroid tissue
Table 3.
Comparison of IHC and sequencing for determining BRAF V600E mutation status
| IHC | Sequencing | Total | Concordance rate | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | ||||
| 1+ | 2 | 1 | 3 | 2/3 (67%) |
| 2+ | 8 | 0 | 8 | 8/8 (100%) |
| 3+ | 10 | 0 | 10 | 10/10 (100%) |
| Negative | ||||
| 0 | 0 | 51 | 51 | 51/51 (100%) |
| Total | 20 | 52 | 72 | 71/72 (98.6%) |
4. DISCUSSION
The BRAF V600E mutation is a well‐known diagnostic and prognostic marker for PTC among other subtypes of thyroid carcinoma, and an important target for the BRAF V600E specific inhibitors. At present time, usefulness of IHC for the BRAF V600E mutation analysis is under immense discussion among scientists. In this study, we confirmed practical utility of IHC method for the BRAF V600E mutation detection and analyzed concordance with Sanger sequencing, a gold standard in this study. Moreover, the BRAF V600E mutation was associated with indicators of poor prognosis factors in PTC. 22 , 32 In this study, the BRAF V600E mutation rate was found to be 29.0% which is comparable, if not exact, to prior studies. 8 , 13 , 21 The possible explanations of difference in the BRAF V600E mutation rate relate to the selection bias in samples and most importantly due to heterogeneity in ethnic characteristics. 7 , 17 , 33 PTC patients demonstrated significant correlations of the BRAF V600E mutation determined by IHC with adverse prognostic factors such as tumor stage, tumor focality, and extrathyroidal extension. Extrathyroidal extension has been known as an important prognostic factor related to recurrence and disease persistence. 22 In contrast to this study, some previous studies reported lack of association of BRAFV600E mutation determined by IHC and adverse clinical characteristics such as extrathyroidal extensions. 33 , 34 The bias in clinical outcome may be due to heterogeneity in patients demographic data, the size of study samples, and histological subtypes of PTC tissues obtained for analysis. 35 Thyroid cancer is the only cancer found in young patients particularly in females due to hormonal effects. But in the current study, significant difference (P > 0.05) in the BRAF V600E rate was not detected in terms of age and gender, which was inconsistent with previous study. 36
VE1 IHC indicated excellent analytic performance and the high concordance with Sanger sequencing for the detection of mutation. The high sensitivity and specificity of results were determined, with no false negative and only one false positive case. The reason of false positive result may be due to sample contamination or antigen cross reactivity. 35 , 36 In this study, the VE1 IHC method was able to detect low tumor cellularity, high tumor heterogeneity, and low mutant allele frequency. Additionally, to the best of our knowledge, decalcification does not obstruct with the results of IHC test. However, prior decalcification of samples is not appropriate for Sanger sequencing. Several reports suggested that VE1 immunostaining successfully detected BRAF V600E mutation when applied to small sized tissue samples such as fine needle aspirates and core biopsy samples before surgery. 37 , 38 , 39
In former studies, different molecular methods such as real‐time PCR, sequencing and SNaPshot PCR have been employed as gold standards to compare with the results of VE1 immunostaining. However, some of these methods reported more discordant cases when compared to VE1 IHC which could either be due to difference in IHC protocol used or sensitivity of techniques. 22 , 35 Interestingly, most of the studies addressed discordant cases either by re‐performing IHC and genotyping or by employing of more sensitive molecular methods. 19 , 35 , 40
There are various limitations in the current study. Firstly, different histological types of thyroid carcinoma, including tall cell variant PTC, anaplastic TC and microcarcinomas, were not included in the study which could be a reason for bias in clinical correlation analysis. Secondly, high quality FFPE tissue samples were acquired for our study, which however cannot always be possible in clinical study. Most of the PTC samples for diagnostic testing were obtained from core needle biopsy (CNB) and fine‐needle aspiration (FNA) with low tumor content. These types of samples may not be suitable for Sanger sequencing, and hence, diagnostic validity parameters including sensitivity and specificity may bias the results. However, several studies have highlighted the superior performance of highly sensitive ddPCR, to detect mutation from FNA and low‐abundance DNA mutation samples. 41 Thirdly, mutations with less fractional abundance (from 5% to 10%) were reported as negative in our clinical settings because it could not be detected by Sanger sequencing, while only ≥10% fractional abundance would have been reported as positive. Fourthly, due to limited resource settings, single type of molecular test may increase the risk of false positive and false negative results. Therefore, more sensitive and combination of molecular techniques are required to validate discordant cases. Lastly, this is a single‐center‐based study with a small series of patients; hence, large sample size is warranted to confirm the clinical utility of IHC in BRAF V600E testing.
5. CONCLUSIONS
In our cohort, IHC using VE1 antibody was found to be strongly concordant with the Sanger sequencing. Taking everything into consideration, BRAF IHC can be consider as an initial or alternative tool for BRAF V600E mutation analysis. Thus, due to high diagnostic accuracy, this technique probably is used instead of Sequencing for clinical implementation in routine diagnostic laboratories.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS’ CONTRIBUTIONS
Conceptualization, FR; Methodology, FR, MK, MA, and HA; Validation, ST; Formal Analysis, FR and MK; Investigation, FR; Resources, SSA and TK; Writing—Original Draft Preparation, FR and ST; Writing—Review and Editing, MK, SSA and HA; Supervision, ST and SSA; Project Administration, ST.
ACKNOWLEDGEMENTS
We would like to thanks technical staff of Histopathology department (PIMS hospital) for providing access to pathology slides.
Rashid FA, Tabassum S, Khan MS, et al. VE1 immunohistochemistry is an adjunct tool for detection of BRAF V600E mutation: Validation in thyroid cancer patients. J Clin Lab Anal.2021;35:e23628 10.1002/jcla.23628
[Correction added on 18 December 2020, after first online publication: Affiliation number 6 and figures legends for Figure 2 and 3 has been corrected.]
Contributor Information
Sobia Tabassum, Email: sobia.tabasum@iiu.edu.pk.
Syed Sameer Aga, Email: agasy@ngha.med.sa.
REFERENCES
- 1. Qiu T, Lu H, Guo L, et al. Detection of BRAF mutation in Chinese tumor patients using a highly sensitive antibody immunohistochemistry assay. Sci Rep. 2015;5:9211 10.1038/srep09211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bychkov A, Jung CK, Liu Z, Kakudo K. Noninvasive Follicular Thyroid Neoplasm with Papillary‐Like Nuclear Features in Asian Practice: Perspectives for Surgical Pathology and Cytopathology. Endocr Pathol. 2018;29:276‐288. 10.1007/s12022-018-9519-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Research Network . Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949‐954. [DOI] [PubMed] [Google Scholar]
- 5. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245‐262. [DOI] [PubMed] [Google Scholar]
- 6. Cantwell‐Dorris ER, O'Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10:385‐394. [DOI] [PubMed] [Google Scholar]
- 7. Dvorak K, Aggeler B, Palting J, McKelvie P, Ruszkiewicz A, Waring P. Immunohistochemistry with the anti‐BRAF V600E (VE1) antibody: impact of pre‐analytical conditions and concordance with DNA sequencing in colorectal and papillary thyroid carcinoma. Pathology. 2014;46:509‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choden S, Keelawat S, Jung CK, Bychkov A. VE1 Immunohistochemistry improves the limit of genotyping for detecting BRAF(V600E) mutation in papillary thyroid cancer. Cancers (Basel). 2020;12:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375:1054‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bychkov A. Prevalence of BRAF(V600E) mutation in Asian patients with thyroid cancer. Malays J Pathol. 2017;39:95‐96. [PubMed] [Google Scholar]
- 12. Sedliarou I, Saenko V, Lantsov D, et al. The BRAFT1796A transversion is a prevalent mutational event in human thyroid microcarcinoma. Int J Oncol. 2004;25:1729‐1735. [PubMed] [Google Scholar]
- 13. Kim YH, Yim H, Lee YH, et al. Evaluation of the VE1 antibody in thyroid cytology using ex vivo papillary thyroid carcinoma specimens. J Pathol Transl Med. 2016;50:58‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oh HS, Kwon H, Park S, et al. Comparison of immunohistochemistry and direct sanger sequencing for detection of the BRAF(V600E) mutation in thyroid neoplasm. Endocrinol Metab. 2018;33:62‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33:42‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu X, Ma X, Zhang X, et al. Detection of BRAF V600E mutation in fine‐needle aspiration fluid of papillary thyroid carcinoma by droplet digital PCR. Clin Chim Acta. 2019;491:91‐96. [DOI] [PubMed] [Google Scholar]
- 17. Hong AR, Lim JA, Kim TH, et al. The frequency and clinical implications of the BRAF(V600E) mutation in papillary thyroid cancer patients in korea over the past two decades. Endocrinol Metab. 2014;29:505‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Z, Sun K, Jing C, Cao H, Ma R, Wu J. Comparison of droplet digital PCR and direct Sanger sequencing for the detection of the BRAFV600E mutation in papillary thyroid carcinoma. J Clin Lab Anal. 2019;33(6):e22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Na JI, Kim JH, Kim HJ, et al. VE1 immunohistochemical detection of the BRAF V600E mutation in thyroid carcinoma: a review of its usefulness and limitations. Virchows Arch. 2015;467:155‐168. [DOI] [PubMed] [Google Scholar]
- 20. Zhu X, Luo Y, Bai Q, et al. Specific immunohistochemical detection of the BRAF V600E mutation in primary and metastatic papillary thyroid carcinoma. Exp Mol Pathol. 2016;100:236‐241. [DOI] [PubMed] [Google Scholar]
- 21. Kim JK, Seong CY, Bae IE, et al. Comparison of Immunohistochemistry and direct sequencing methods for identification of the BRAF(V600E) mutation in papillary thyroid carcinoma. Ann Surg Oncol. 2018;25:1775‐1781. [DOI] [PubMed] [Google Scholar]
- 22. Zagzag J, Pollack A, Dultz L, et al. Clinical utility of immunohistochemistry for the detection of the BRAF v600e mutation in papillary thyroid carcinoma. Surgery. 2013;154:1199‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Routhier CA, Mochel MC, Lynch K, Dias‐Santagata D, Louis DN, Hoang MP. Comparison of 2 monoclonal antibodies for immunohistochemical detection of BRAF V600E mutation in malignant melanoma, pulmonary carcinoma, gastrointestinal carcinoma, thyroid carcinoma, and gliomas. Hum Pathol. 2013;44:2563‐2570. [DOI] [PubMed] [Google Scholar]
- 24. Gow CH, Hsieh MS, Lin YT, Liu YN, Shih JY. Validation of immunohistochemistry for the detection of BRAF V600E‐mutated lung adenocarcinomas. Cancers (Basel). 2019;11:866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ritterhouse LL, Barletta JA. BRAF V600E mutation‐specific antibody: a review. Semin Diagn Pathol. 2015;32:400‐408. [DOI] [PubMed] [Google Scholar]
- 26. Fisher KE, Neill SG, Ehsani L, Caltharp SA, Siddiqui MT, Cohen C. Immunohistochemical Investigation of BRAF p. V600E mutations in thyroid carcinoma using 2 separate BRAF antibodies. Appl Immunohistochem Mol Morphol. 2014;22:562‐567. [DOI] [PubMed] [Google Scholar]
- 27. Ilie MI, Lassalle S, Long‐Mira E, et al. Diagnostic value of immunohistochemistry for the detection of the BRAF(V600E) mutation in papillary thyroid carcinoma: comparative analysis with three DNA‐based assays. Thyroid. 2014;24:858‐866. 10.1089/thy.2013.0302 [DOI] [PubMed] [Google Scholar]
- 28. Jung CK, Kim Y, Jeon S, Jo K, Lee S, Bae JS. Clinical utility of EZH1 mutations in the diagnosis of follicular‐patterned thyroid tumors. Hum Pathol. 2018;81:9‐17. [DOI] [PubMed] [Google Scholar]
- 29. Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625‐627. [DOI] [PubMed] [Google Scholar]
- 30. Simundic AM. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19:203‐211. [PMC free article] [PubMed] [Google Scholar]
- 31. Lamartina L, Grani G, Arvat E, et al. 8th edition of the AJCC/TNM staging system of thyroid cancer: what to expect (ITCO#2). Endocr Relat Cancer. 2018;25:L7‐L11. [DOI] [PubMed] [Google Scholar]
- 32. Boursault L, Haddad V, Vergier B, et al. Tumor homogeneity between primary and metastatic sites for BRAF status in metastatic melanoma determined by immunohistochemical and molecular testing. PLoS One. 2013;8:e70826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koperek O, Kornauth C, Capper D, et al. Immunohistochemical detection of the BRAF V600E‐mutated protein in papillary thyroid carcinoma. Am J Surg Pathol. 2012;36:844‐850. [DOI] [PubMed] [Google Scholar]
- 34. Abd Elmageed ZY, Sholl AB, Tsumagari K, et al. Immunohistochemistry as an accurate tool for evaluating BRAF‐V600E mutation in 130 samples of papillary thyroid cancer. Surgery. 2017;161:1122‐1128. [DOI] [PubMed] [Google Scholar]
- 35. Szymonek M, Kowalik A, Kopczynski J, et al. Immunohistochemistry cannot replace DNA analysis for evaluation of BRAF V600E mutations in papillary thyroid carcinoma. Oncotarget. 2017;8:74897‐74909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gown AM. Diagnostic immunohistochemistry: what can go wrong and how to prevent it. Arch Pathol Lab Med. 2016;140:893‐898. [DOI] [PubMed] [Google Scholar]
- 37. Zimmermann AK, Camenisch U, Rechsteiner MP, Bode‐Lesniewska B, Rossle M. Value of immunohistochemistry in the detection of BRAF(V600E) mutations in fine‐needle aspiration biopsies of papillary thyroid carcinoma. Cancer Cytopathol. 2014;122:48‐58. [DOI] [PubMed] [Google Scholar]
- 38. Crescenzi A, Guidobaldi L, Nasrollah N, et al. Immunohistochemistry for BRAF(V600E) antibody VE1 performed in core needle biopsy samples identifies mutated papillary thyroid cancers. Horm Metab Res. 2014;46:370‐374. [DOI] [PubMed] [Google Scholar]
- 39. Jung CK, Baek JH, Na DG, Oh YL, Yi KH, Kang HC. 2019 practice guidelines for thyroid core needle biopsy: a report of the clinical practice guidelines development committee of the korean thyroid association. J Pathol Transl Med. 2020;54:64‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang X, Wang L, Wang J, et al. Immunohistochemistry is a feasible method to screen BRAF V600E mutation in colorectal and papillary thyroid carcinoma. Exp Mol Pathol. 2018;105:153‐159. [DOI] [PubMed] [Google Scholar]
- 41. Li X, Du H, Luo J, et al. Comparison of the clinical validity of droplet digital PCR to ARMS‐PCR for BRAF V600E mutation detection in thyroid nodules. J Clin Lab Anal. 2020; 10.1002/jcla.23458 [DOI] [PMC free article] [PubMed] [Google Scholar]


