Abstract
Epithelial cells are typically connected through different types of cell junctions that are localized from the apical membrane to the basal surface. In this way, epithelium cells form the first barrier against pathogenic microorganisms and prevent their entry into internal organs and the circulatory system. Recent studies demonstrate that bacterial pathogens disrupt epithelial cell junctions through targeting junctional proteins by secreted virulence factors. In this review, we discuss the diverse strategies used by common bacterial pathogens, including Pseudomonas aeruginosa, Helicobacter pylori, and enteropathogenic Escherichia coli, to disrupt epithelial cell junctions during infection. We also discuss the potential of targeting the pathogenic mechanisms in the treatment of pathogen‐associated diseases.
Keywords: adherens junction, bacterial pathogen, cell junction, epithelial cell, epithelium, infection, tight junction, virulence factor
Epithelial cells are typically connected through different types of cell junctions that are localized from the apical membrane to the basal surface. Bacterial pathogens disrupt epithelial cell junctions through targeting junctional proteins by secreted virulence factors.
1. INTRODUCTION
The epithelium is a continuous sheet of interconnected cells that forms a barrier between the internal and external environments. The two main epithelial types are simple squamous (eg, pulmonary) epithelium and simple columnar (eg, gastric mucosa and intestinal villous) epithelium. The protective function of this cell monolayer depends on a highly organized structure that is maintained by cell junctions connecting adjacent cells and various cytoskeletal components. There are five types of cell junctions, namely tight junctions, adherens junctions, gap junctions, desmosomes, and hemidesmosomes. 1 , 2
The mucosal epithelium consists of one or more cell layers with specialized apical and basolateral surfaces separated by tight junctions that regulate the exchange of substances between the extracellular environment and lumen and between adjacent cells, respectively. 3 Claudins and occludin are the two main transmembrane proteins of tight junctions. 4 These transmembrane proteins directly bind to cytoplasmic adaptor proteins, such as the zonula occludens (ZO) proteins (ZO‐1, ZO‐2, and ZO‐3) that interact with the actin cytoskeleton. 5 ZO‐1 links tight and adherens junctions by binding to α‐catenin and afadin. 6 Classical cadherins, including E‐cadherin, are the main type of adherens junction protein. 7 , 8 Desmosomes are composed of desmoglein and desmocollin, which interact with the armadillo family proteins, plakoglobin and plakophilin. 9 , 10
The epithelium forms the first barrier against the microbiota and pathogenic microbes. 11 , 12 Under normal conditions, mucosal epithelial cells, mucus‐secreting cells, and immune cells form a protective barrier against invading pathogens. 13 Epithelial cell junctions maintain cell polarity and control diffusion through the epithelium by allowing the selective permeation of substances and excluding bacterial and other types of toxin. Their disruption (eg, under conditions of disease) increases intercellular permeability, resulting in the entry of bacteria, viruses, endotoxins, and macromolecules into the systemic circulation. 8 , 14 , 15 , 16 This review focuses on the different mechanisms of how exposure of epithelial cells to bacterial pathogens perturb cell junctions, which will be facilitated on the selection of therapeutic target to prevent the invasion of pathogens.
2. PSEUDOMONAS AERUGINOSA, HELICOBACTER PYLORI, AND ENTEROPATHOGENIC ESCHERICHIA COLI
The world's annual death toll from infectious diseases accounts for about a quarter of deaths. Some of these diseases are caused by pathogenic bacterial infections. 17 For example, acute nosocomial infections like pneumonia are mainly caused by Pseudomonas aeruginosa infections. 18 In general, Helicobacter pylori and its secretory factors will destroy the gastric barrier and cause the inflammation and proliferation of gastric cell. The excessive cell proliferation increases the risk of gastric cancer. 19 The mortality rate of infantile diarrhea is mainly caused by pathogenic Escherichia coli. 20 These bacterial pathogens interact with epithelial cells to cause pathology.
3. PSEUDOMONAS AERUGINOSA SECRETES VIRULENCE EFFECTORS TO DISRUPT CELL JUNCTIONS
During infection, P. aeruginosa enters epithelial cells of the mucosal barrier and endothelial cells of the vascular lumen by modulating their cytoskeleton, 21 , 22 , 23 thereby evading immune surveillance and creating a microenvironment that promotes its proliferation and invasion into deeper tissues. The destruction of the epithelial barrier integrity by changing cell junction components facilitates the intercellular transport of P. aeruginosa. 23 Meanwhile, P. aeruginosa virulence factors destroy tight and adherens junctions between epithelial cells, which alter cytoskeletal structure and epithelial cell polarity and increase mucosal permeability, potentially leading to disease. 22 , 24
Effectors of the type II secretion system (T2SS), including proteases along with quorum sensing system signals in bacteria, contribute to the destruction of cell junctions (Figure 1). The extracellular protease LasB, released through the T2SS, degrades junctional proteins between endothelial cells, such as vascular endothelial cadherin and occludin. 25 P. aeruginosa elastase (PE) increases paracellular permeability and transiently blocks the association between the tight junction proteins claudin‐1 and claudin‐4, occludin, and tricellulin. 26 However, ZO‐1, ZO‐2, and the adherens junction proteins E‐cadherin and β‐catenin are unaltered in human nasal epithelial cells treated with PE. On the other hand, exposure to the quorum sensing system signal 3‐oxo‐C12‐HSL decreases total intracellular ZO‐1, ZO‐3, and junctional adhesion molecule levels and induces occludin dephosphorylation, resulting in tight junction disruption. 27 , 28 In addition, 3‐oxo‐C12‐HSL reduces the level of E‐cadherin/β‐catenin in the cell through phosphorylation‐dependent mechanism, thereby destroying the adherens junction. 29
Figure 1.
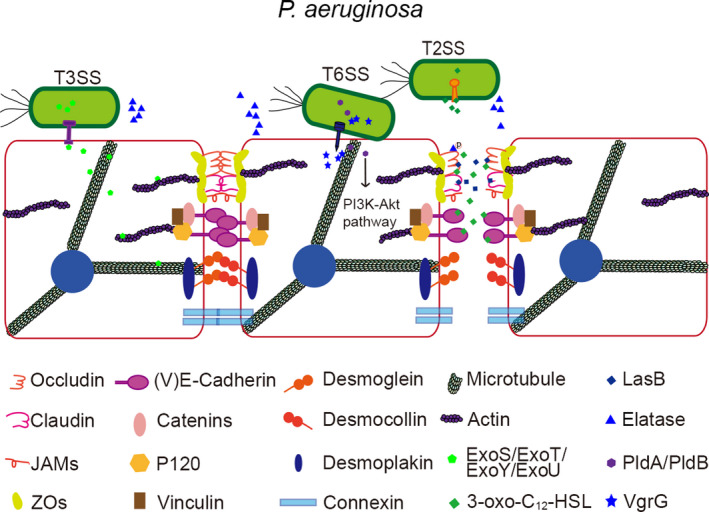
Virulence factors of P. aeruginosa target epithelial cell junctions during infection. The extracellular protease LasB released through T2SS temporarily blocks the binding of tight junction proteins. The quorum sensing system signal 3‐oxo‐C12‐HSL induces the removal of occludin phosphorylation. The secreted T3SS effectors ExoS, ExoT, ExoY, and ExoU act on the cytoskeleton. The secreted T6SS effector VgrG2b interacts with α‐tubulin, β‐tubulin, and the γ‐tubulin ring complex. PldA and PldB activate PI3K‐Akt signaling to infiltrate epithelial cells
The T3SS undermines the epithelial cell barrier by various means. Pathogenic bacteria inject T3SS toxins through the apical surface of host epithelial cells. 30 The secreted T3SS effectors ExoS, ExoT, ExoY, and ExoU destroy the cytoskeleton and cause cell retraction or rounding, eventually leading to cell shedding. 31 , 32 , 33 Additionally, ExoU induces cell death. 34 , 35 The T6SS also plays an important role in the pathogenic mechanism of P. aeruginosa. 36 Phospholipase D type A (PldA) and PldB activate phosphatidylinositol 3‐kinase (PI3K)‐Akt signaling to enable epithelial cell invasion. Recent studies have shown that the effector, VgrG2b, simultaneously interacts with α‐tubulin, β‐tubulin, and the microtubule‐organizing center γ‐tubulin ring complex to alter the microtubule structure. 21 , 37 However, the mechanisms by which P. aeruginosa T6SS compromises the epithelial barrier are not fully understood. The secretory systems of P. aeruginosa especially T2SS and T3SS toxins play a vital role in disturbing the cytoskeletons and cell junctions. Both the secretory systems are positively regulated by quorum sensing systems, so the quorum sensing systems and its signal molecules can be selected as targets to downregulate the level of secretory toxins generally.
4. HELICOBACTER PYLORI SYSTEMATICALLY DESTROYS CELL JUNCTIONS
Helicobacter pylori colonizes gastric mucosa in about 50% of the human population. Its discovery more than 30 years ago altered the diagnosis and treatment of gastroduodenal disease. 38 The perturbation of adherens junctions by H pylori infection is a risk factor for gastric cancer, 39 which is characterized by the downregulation of E‐cadherin. H pylori has three major virulence factors that weaken the gastric epithelial barrier, including the cytotoxin VacA, the secreted T4SS system, and effector CagA (Figure 2). 40
Figure 2.
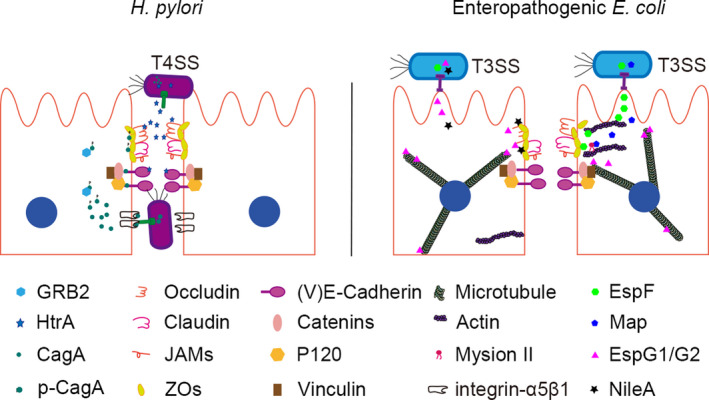
Disruption of cell junctions by H pylori and enteropathogenic E coli. A, The T4SS effector serine protease HtrA cleaves E‐cadherin and acts on the tight junction proteins occludin and claudin‐8. The effector CagA destroys polarized gastric epithelial cells by interacting with E‐cadherin. Phosphorylated CagA (p‐CagA) interacts with GRB2 and ZO‐1. B, EspF and Map are secreted through the T3SS effector system. EspF binds to the ZO‐1 and ZO‐2 scaffold proteins. The interaction between Map and myosin II regulates the tight junction. In addition, the T3SS effector EspG1 and its homolog EspG2 destroy tight junctions by targeting microtubules, and NleA may destroy occludin and ZO‐1
Genes that encoding CagA and T4SS components are both located in the Cag pathogenicity island of pathogenic H pylori strains. 41 T4SS facilitates contact‐dependent cytoplasmic translocation of CagA and membrane‐associated transporter complexes into gastric epithelial cells via interaction with integrin α5β1, which is expressed at the basolateral surface and is protected by tight and adherens junctions. 42 The serine protease HtrA mediates this interaction while displacing E‐cadherin and the interaction between the tight junction proteins occludin and claudin‐8, as demonstrated by in vitro experiments using recombinant proteins. 43 The HtrA inhibitor, P1 peptide, present at E‐cadherin cleavage sites, blocks CagA translocation and phosphorylation and consequently, H pylori transmigration. 44
CagA participates in multiple intracellular processes including inflammation and prevents actin‐mediated intracellular trafficking as well as intercellular tight junction maintenance. 45 CagA activity is regulated by phosphorylation. Non‐phosphorylated CagA disrupts adherens junctions in polarized gastric epithelial cells by interacting with E‐cadherin, which prevents formation of the E‐cadherin/β‐catenin complex. This leads to cytoplasmic and nuclear accumulation and activation of β‐catenin, followed by malignant transformation of the cells. 46 , 47 Phosphorylated CagA interacts with host proteins such as growth factor receptor‐bound protein 2 (GRB2) and ZO‐1, resulting in the over‐proliferation and migration of gastric epithelial cells. 48 Most of the effective virulence factors of H pylori are protease. Screening the specific inhibitor of these proteases is a potential strategy to inhibit the intruding of H pylori.
5. ENTEROPATHOGENIC E COLI DISRUPTS CELL JUNCTIONS THROUGH TTSS EFFECTORS
As a leading cause of infant diarrhea in developing countries, enteropathogenic E coli colonizes intestinal epithelial cells and uses T3SS to inject virulence‐associated effector proteins into host cells (Figure 2). 20 Genes encoding T3SS components are present in the locus for enterocyte effacement (LEE) pathogenicity island. 49 One T3SS‐dependent virulence mechanism of enteropathogenic E coli involves the perturbation of intestinal tight junctions, which is associated with multiple events in host cells, including decreased transepithelial resistance of polarized cell monolayers, phosphorylation of myosin light chain and ezrin, dephosphorylation of occludin, dissociation of occludin and ZO‐1 from tight junctions, and migration of basolateral proteins such as β1 integrin to the apical membrane. 50 , 51
The LEE‐encoded T3SS effectors EspF and Map are required for enteropathogenic E coli‐mediated tight junction destruction. 52 EspF associates with ZO‐1 and ZO‐2 scaffold proteins to sequester profilin and prevent actin polymerization, with subsequent rearrangement of occludin and claudin. 50 , 53 The interaction between microtubule‐associated protein and myosin II regulates the assembly and disassembly of tight junctions, ultimately increasing membrane permeability. 54 In contrast, the T3SS effectors EspG1 and its homolog EspG2 disrupt tight junctions by targeting microtubules. 55 , 56 Like Map and EspF, EspG1/G2 also induces the translocation of occludin from the membrane to the cytosol, which alters tight junction structure. 50 During enteropathogenic E coli infection, EspG1/G2 can prevent the restoration of tight junctions and the epithelial barrier through targeting calcium switch of calmodulins. 55 NleA may play a role in this process, which is along with anti‐inflammatory factors that disrupt the tight junction proteins occludin and ZO‐1. 57 , 58 T3SS effectors are the mainly factors that involved in the destruction of tight junctions. Inhibition the releases of these effectors through damaging the normal structure of T3SS is a strategy to reduce toxic reaction on epithelial cells.
6. CONCLUDING REMARKS
It is essential that in the process of preventing microbial infections, epithelial cells can separate the inside of the host from the external environment. 59 Mucosal epithelia are targeted by pathogenic microorganisms as a means of adhesion, internalization, and exploitation of host cell properties. 60 During infection, pathogens release virulence factors that destroy tight and adherens junctions, which increases paracellular permeability and compromises epithelial barrier functions through modulation of microtubule and actin filaments. Additionally, interaction with cell junctions allows pathogens to persist at the epithelial cell surface and activate receptors that induce inflammation and promote host cell invasion (Table 1). 61 Tight junctions are also involved in immunity; activation of mucosal immune cells and inflammation have been implicated in the development of pathogen‐mediated diseases. 60
Table 1.
The role of common bacterial pathogens in disrupting epithelial cell junctions during infection
| Bacterial Pathogens | The role of bacterial pathogens during infection |
|---|---|
| Pseudomonas aeruginosa |
Alter cytoskeletal structure and epithelial cell polarity; changing cell junction components to destruct the epithelial barrier integrity and increase mucosal permeability |
| Helicobacter pylori |
Inhibition of actin‐mediated intracellular trafficking; interact with E‐cadherin to prevent formation of junctional complex; weaken the gastric epithelial barrier |
|
Enteropathogenic Escherichia coli |
Decreases transepithelial resistance of polarized cell monolayers; disrupt the formation of junctional complex; interrupt the migration of basolateral proteins to the apical membrane |
Clarifying the mechanisms by which bacteria disrupt cell junctions can provide insight into the etiology of many diseases and a basis for the development of more effective treatments. For the pathogens that possessing various virulence factors, the upstream regulation system of these factors is a potential therapeutic target, such as the quorum sensing (QS) system in P. aeruginosa. Overexpression of the QS quenching system and degradation of the signaling molecules of QS are feasible to reduce the levels of virulence factors which will inhibit the hijack of tight junctions. Some pathogens destroy cell junctions through proteases which are interacted with tight junctions related proteins directly. For example, HtrA and CagA interact with E‐cadherin directly during the invasion of H pylori. Small molecules or proteins that block this interaction can be selected as a potential target in the treatment of H pylori associated diseases. Secretory systems are involved in many virulence factors that participated in the destroy of tight junction. Obstruction of the normal secretory systems may interfere this process during the pathogens invasion. Changes in cell junctions may be an important step in the pathogenesis of infectious diseases. Future research needs to target the virulence factors that related to the junctions disruption rather than the microbes, which may be more sensitive and effective. 59
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
MZ wrote the manuscript and drew the figures. SS and ML conceived the study and revised the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the Natural Science Foundation of Shandong Province (ZR2018BC002) and the China Postdoctoral Science Foundation (2017M622257).
Zheng M, Sun S, Zhou J, Liu M. Virulence factors impair epithelial junctions during bacterial infection. J Clin Lab Anal.2021;35:e23627 10.1002/jcla.23627
Contributor Information
Shuang Sun, Email: sunshuang0916@163.com.
Min Liu, Email: minliu@sdnu.edu.cn.
REFERENCES
- 1. Garcia MA, Nelson WJ, Chavez N. Cell–cell junctions organize structural and signaling networks. Cold Spring Harb Perspect Biol. 2018;10(4):a029181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang Y, Xin G, Zhao LM, et al. Novel insights into host‐pathogen interactions of large yellow croakers ( Larimichthys crocea) and pathogenic bacterium Pseudomonas plecoglossicida using time‐resolved dual RNA‐seq of infected spleens. Zool Res. 2020;41:314‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun S, Zhou J. Phase separation as a therapeutic target in tight junction‐associated human diseases. Acta Pharmacol Sin. 2020;41(10):1310‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahn C, Shin D‐H, Lee D, et al. Expression of claudins, occludin, junction adhesion molecule A and zona occludens 1 in canine organs. Mol Med Rep. 2016;14:3697‐3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Itallie CM, Tietgens AJ, Anderson JM. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO‐1. Mol Biol Cell. 2017;28:524‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol. 2014;36:157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braga V. Spatial integration of E‐cadherin adhesion, signalling and the epithelial cytoskeleton. Curr Opin Cell Biol. 2016;42:138‐145. [DOI] [PubMed] [Google Scholar]
- 8. Xie W, Yang Y, Gao S, et al. The tumor suppressor CYLD controls epithelial morphogenesis and homeostasis by regulating mitotic spindle behavior and adherens junction assembly. J Genet Genomics. 2017;44:343‐353. [DOI] [PubMed] [Google Scholar]
- 9. Broussard JA, Getsios S, Green KJ. Desmosome regulation and signaling in disease. Cell Tissue Res. 2015;360:501‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kowalczyk AP, Green KJ. Structure, function and regulation of desmosomes. Prog Mol Biol Transl Sci. 2013;116:95‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang MX, Song TZ, Zheng HY, et al. Superior intestinal integrity and limited microbial translocation are associated with lower immune activation in SIVmac239‐infected northern pig‐tailed macaques (Macaca leonina). Zool Res. 2019;40:522‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang DC, Chen R, Cai YH, Wang JJ, Yin C, Zou K. Hyperactive reactive oxygen species impair function of porcine Sertoli cells via suppression of surface protein ITGB1 and connexin‐43. Zool Res. 2020;41:203‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruch TR, Engel JN. Targeting the mucosal barrier: How pathogens modulate the cellular polarity network. Cold Spring Harb Perspect Biol. 2017;9(6):a027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong D, Xie W, Liu M. Alteration of cell junctions during viral infection. Thorac Cancer. 2020;11:519‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie W, Li D, Dong D, et al. HIV‐1 exposure triggers autophagic degradation of stathmin and hyperstabilization of microtubules to disrupt epithelial cell junctions. Signal Transduct Target Ther. 2020;5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song T, Zhou J. The primary cilium in corneal development and disease. Zool Res. 2020;41(5):495‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Welch MD. Why should cell biologists study microbial pathogens? Mol Biol Cell. 2015;26:4295‐4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Curran CS, Bolig T, Torabi‐Parizi P. Mechanisms and targeted therapies for Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med. 2018;197:708‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chmiela M, Kupcinskas J. Review: pathogenesis of Helicobacter pylori infection. Helicobacter. 2019;24(Suppl 1):e12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh AP, Aijaz S, Enteropathogenic E coli: breaking the intestinal tight junction barrier. F1000Res. 2015;4:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sana TG, Baumann C, Merdes A, et al. Internalization of Pseudomonas aeruginosa strain PAO1 into epithelial cells is promoted by interaction of a t6ss effector with the microtubule network. MBio. 2015;6:e00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Che P, Wagener BM, Zhao X, et al. Neuronal Wiskott‐Aldrich syndrome protein regulates Pseudomonas aeruginosa‐induced lung vascular permeability through the modulation of actin cytoskeletal dynamics. FASEB J. 2020;34:3305‐3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Engel J, Eran Y. Subversion of mucosal barrier polarity by Pseudomonas aeruginosa . Front Microbiol. 2011;2:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berube BJ, Rangel SM, Hauser AR Pseudomonas aeruginosa: breaking down barriers. Curr Genet. 2016;62:109‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Golovkine G, Faudry E, Bouillot S, Voulhoux R, Attree I, Huber P. VE‐cadherin cleavage by LasB protease from Pseudomonas aeruginosa facilitates type III secretion system toxicity in endothelial cells. PLoS Pathog. 2014;10:e1003939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nomura K, Obata K, Keira T, et al. Pseudomonas aeruginosa elastase causes transient disruption of tight junctions and downregulation of PAR‐2 in human nasal epithelial cells. Respir Res. 2014;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vikström E, Tafazoli F, Magnusson KE Pseudomonas aeruginosa quorum sensing molecule N‐(3 oxododecanoyl)‐l‐homoserine lactone disrupts epithelial barrier integrity of Caco‐2 cells. FEBS Lett. 2006;580:6921‐6928. [DOI] [PubMed] [Google Scholar]
- 28. Vikström E, Bui L, Konradsson P, Magnusson KE. Role of calcium signalling and phosphorylations in disruption of the epithelial junctions by Pseudomonas aeruginosa quorum sensing molecule. Eur J Cell Biol. 2010;89:584‐597. [DOI] [PubMed] [Google Scholar]
- 29. Vikström E, Bui L, Konradsson P, Magnusson KE. The junctional integrity of epithelial cells is modulated by Pseudomonas aeruginosa quorum sensing molecule through phosphorylation‐dependent mechanisms. Exp Cell Res. 2009;315:313‐326. [DOI] [PubMed] [Google Scholar]
- 30. Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 2009;7:654‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geiser TK, Kazmierczak BI, Garrity‐Ryan LK, Matthay MA, Engel JN Pseudomonas aeruginosa ExoT inhibits in vitro lung epithelial wound repair. Cell Microbiol. 2001;3:223‐236. [DOI] [PubMed] [Google Scholar]
- 32. Huber P, Bouillot S, Elsen S, Attrée I. Sequential inactivation of Rho GTPases and lim kinase by Pseudomonas aeruginosa toxins ExoS and ExoT leads to endothelial monolayer breakdown. Cell Mol Life Sci. 2014;71:1927‐1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Engel J, Balachandran P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol. 2009;12:61‐66. [DOI] [PubMed] [Google Scholar]
- 34. Foulkes DM, McLean K, Haneef AS, et al. Pseudomonas aeruginosa toxin ExoU as a therapeutic target in the treatment of bacterial infections. Microorganisms. 2019;7(12):707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Golovkine G, Reboud E, Huber P Pseudomonas aeruginosa takes a multi‐target approach to achieve junction breach. Front Cell Infect Microbiol. 2017;7:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen L, Zou Y, She P, Wu Y. Composition, function, and regulation of T6SS in Pseudomonas aeruginosa . Microbiol Res. 2015;172:19‐25. [DOI] [PubMed] [Google Scholar]
- 37. Wettstadt S, Wood TE, Fecht S, Filloux A. Delivery of the Pseudomonas aeruginosa phospholipase effectors PldA and PldB in a VgrG‐ and H2–T6SS‐dependent manner. Front Microbiol. 2019;10:1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Costa AM, Leite M, Seruca R, Figueiredo C. Adherens junctions as targets of microorganisms: a focus on Helicobacter pylori . FEBS Lett. 2013;587:259‐265. [DOI] [PubMed] [Google Scholar]
- 39. Wroblewski LE, Peek RM Jr. Targeted disruption of the epithelial‐barrier by Helicobacter pylori . Cell Commun Signal. 2011;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sgouras D, Tegtmeyer N, Wessler S. Activity and functional importance of Helicobacter pylori virulence factors. Adv Exp Med Biol. 2019;1149:35‐56. [DOI] [PubMed] [Google Scholar]
- 41. Backert S, Tegtmeyer N, Fischer W. Composition, structure and function of the Helicobacter pylori cag pathogenicity island encoded type IV secretion system. Future Microbiol. 2015;10:955‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Backert S, Blaser MJ. The role of CagA in the gastric biology of Helicobacter pylori . Cancer Res. 2016;76:4028‐4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Backert S, Bernegger S, Skórko‐Glonek J, Wessler S. Extracellular HtrA serine proteases: an emerging new strategy in bacterial pathogenesis. Cell Microbiol. 2018;20:e12845. [DOI] [PubMed] [Google Scholar]
- 44. Harrer A, Boehm M, Backert S, Tegtmeyer N. Overexpression of serine protease HtrA enhances disruption of adherens junctions, paracellular transmigration and type IV secretion of CagA by Helicobacter pylori . Gut Pathog. 2017;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Naumann M, Sokolova O, Tegtmeyer N, Backert S Helicobacter pylori: A paradigm pathogen for subverting host cell signal transmission. Trends Microbiol. 2017;25:316‐328. [DOI] [PubMed] [Google Scholar]
- 46. Backert S, Tegtmeyer N. Type IV secretion and signal transduction of Helicobacter pylori CagA through Interactions with host cell receptors. Toxins (Basel). 2017;9:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Murata‐Kamiya N, Kurashima Y, Teishikata Y, et al. Helicobacter pylori CagA interacts with E‐cadherin and deregulates the beta‐catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617‐4626. [DOI] [PubMed] [Google Scholar]
- 48. Alzahrani S, Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol. 2014;20:12767‐12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Slater SL, Sågfors AM, Pollard DJ, Ruano‐Gallego D, Frankel G. The Type III secretion system of pathogenic Escherichia coli . Curr Top Microbiol Immunol. 2018;416:51‐72. [DOI] [PubMed] [Google Scholar]
- 50. Ugalde‐Silva P, Gonzalez‐Lugo O, Navarro‐Garcia F. Tight junction disruption induced by Type 3 secretion system effectors injected by enteropathogenic and enterohemorrhagic Escherichia coli . Front Cell Infect Microbiol. 2016;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li HZ, Li N, Wang JJ, et al. Dysbiosis of gut microbiome affecting small intestine morphology and immune balance: a rhesus macaque model. Zool Res. 2020;41:20‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singh AP, Sharma S, Pagarware K, et al. Enteropathogenic E coli effectors EspF and Map independently disrupt tight junctions through distinct mechanisms involving transcriptional and post‐transcriptional regulation. Sci Rep. 2018;8:3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peralta‐Ramírez J, Hernandez JM, Manning‐Cela R, et al. EspF interacts with nucleation‐promoting factors to recruit junctional proteins into pedestals for pedestal maturation and disruption of paracellular permeability. Infect Immun. 2008;76:3854‐3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One. 2007;2:e658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Glotfelty LG, Zahs A, Hodges K, et al. coli effectors EspG1/G2 disrupt microtubules, contribute to tight junction perturbation and inhibit restoration. Cell Microbiol. 2014;16:1767‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Glotfelty LG, Hecht GA, Enteropathogenic E coli effectors EspG1/G2 disrupt tight junctions: new roles and mechanisms. Ann N Y Acad Sci. 2012;1258:149‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thanabalasuriar A, Koutsouris A, Weflen A, Mimee M, Hecht G, Gruenheid S. The bacterial virulence factor NleA is required for the disruption of intestinal tight junctions by enteropathogenic Escherichia coli . Cell Microbiol. 2010;12:31‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alvarez C‐S, Giménez R, Cañas M‐A, et al. Extracellular vesicles and soluble factors secreted by Escherichia coli Nissle 1917 and ECOR63 protect against enteropathogenic E coli‐induced intestinal epithelial barrier dysfunction. BMC Microbiol. 2019;19:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tapia R, Kralicek SE, Hecht GA. Modulation of epithelial cell polarity by bacterial pathogens. Ann N Y Acad Sci. 2017;1405:16‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wittekindt OH. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch. 2017;469:135‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]


