Abstract
Aims
To assess the effectiveness of brief interventions in primary care aimed at reducing or discontinuing long‐term benzodiazepine/Z‐drug (BZRA) use.
Method
Systematic review of randomized controlled trials of brief interventions in primary care settings aimed at reducing or discontinuing long‐term BZRA use in adults taking BZRAs for ≥ 3 months. Four electronic databases were searched: PubMed, EMBASE, PsycINFO and CENTRAL. The primary outcome was BZRA use, classified as discontinuation or reduction by ≥ 25%. The Theoretical Domains Framework (TDF) was used to retrospectively code behavioural determinants targeted by the interventions. The Behaviour Change Technique (BCT) Taxonomy was used to identify the interventions’ active components. Study‐specific estimates were pooled, where appropriate, to yield summary risk ratios (RRs) and 95% confidence intervals (CIs). Pearson's correlations were used to determine the relationship between intervention effect size and the results of both the TDF and BCT coding.
Results
Eight studies were included (n = 2071 patients). Compared with usual care, intervention patients were more likely to have discontinued BZRA use at 6 months (eight studies, RR = 2.73, 95% CI = 1.84–4.06) and 12 months post‐intervention (two studies, RR = 3.41, 95% CI = 2.22–5.25). TDF domains ‘knowledge’, ‘memory, attention and decision processes’, ‘environmental context and resources’ and ‘social influences’ were identified as having been included in every intervention. Commonly identified BCTs included ‘information about health consequences’, ‘credible source’ and ‘adding objects to the environment’. There was no detectable relationship between effect size and the results of either the TDF or BCT coding.
Conclusion
Brief interventions delivered in primary care are more effective than usual care in reducing and discontinuing long‐term benzodiazepine/Z‐drug use.
Keywords: Benzodiazepines, Z‐drugs, brief interventions, systematic review, Theoretical Domains Framework, primary care, behaviour change techniques, meta‐analysis
Introduction
Benzodiazepines have multiple clinical indications, including anxiety and insomnia. Guidelines recommend restricting benzodiazepine prescriptions to short‐term use (≤ 4 weeks) to minimize adverse outcomes, such as dependence and withdrawal symptoms [1, 2]. Similar recommendations apply to Z‐drugs (e.g. zopiclone, zolpidem), a group of non‐benzodiazepine hypnotics which are also available to treat insomnia [3]. However, guidelines are often not adhered to, as long‐term use of these medications, which are collectively referred to as benzodiazepine receptor agonists (BZRAs), persists world‐wide [4, 5]. Numerous countries have reported no notable changes, or only modest decreases, in benzodiazepine use in recent years [6, 7, 8, 9]. In some instances, changes in benzodiazepine prescribing have been offset by increased Z‐drug prescribing [5, 9, 10, 11] for which evidence of a more favourable risk profile is lacking [3].
The estimated prevalence of long‐term BZRA use (> 6 months) among the general population varies throughout countries (range = 6–15%), with the highest prevalence reported among older people (range = 22–55%), typically defined as ≥ 65 years [12, 13, 14]. Long‐term benzodiazepine use is one of the most commonly identified indicators of potentially inappropriate prescribing in older people [15, 16, 17, 18, 19, 20]. This is concerning, as age‐related physiological changes impact on the drugs’ pharmacokinetics and pharmacodynamics, thereby making older adults most susceptible to BZRAs’ adverse effects such as falls, fractures and cognitive impairment [21, 22]. Only in recent years have prescribing tools to assess quality use of medicines incorporated Z‐drugs (e.g. Beers criteria [23]). Hence, comparatively fewer studies have examined potentially inappropriate Z‐drug prescribing and associated risks [24, 25]. The ongoing issue of long‐term BZRA use has called existing guidelines into question [26]. As long‐term BZRA prescribing also occurs in middle‐aged populations (typically defined as 45–64 years) [7, 27], the problem is likely to persist for generations to come without targeted interventions.
Various interventions aimed at discontinuing long‐term benzodiazepine use have been evaluated and, in recent years, efforts have been made to systematically review and pool existing evidence [28, 29, 30, 31, 32]. However, previous research has largely overlooked Z‐drugs. Previously evaluated interventions range from brief interventions (i.e. written letters, self‐help information or short consultations with health‐care professionals directed towards the specific goal of reducing or discontinuing patients’ long‐term use of the medication) to more complex interventions involving cognitive–behavioural therapy (CBT) and pharmacological treatment (e.g. anticonvulsants) [28, 29, 30, 31].
An existing Cochrane review on interventions targeting benzodiazepine dependence and abuse specifically highlighted brief interventions as an area warranting further research [33]. Brief interventions have been described as a family of interventions which can be directed at different target groups and vary in length, structure, media of communication and personnel responsible for their delivery [34]. Brief interventions have been extensively reviewed in the context of alcohol use [35] and defined as ‘in‐person, time‐limited efforts to provide information or advice, increase motivation to avoid substance use, or to teach behaviour change skills with the aim of reducing substance use and the likelihood of experiencing negative consequences’ [36]. Brief interventions are applicable in targeting long‐term BZRA use. For example, the stepped‐care approach (Supporting information, S1), which was developed for treating benzodiazepine dependence, advocates for brief intervention‐based approaches before progressing to more intensive interventions (e.g. CBT) if required [37]. However, a number of previous related reviews have not explicitly defined the concept of a brief intervention as it applies to long‐term BZRA use or elaborated on the intervention's core components beyond the information that was outlined to patients about BZRAs and whether the interventions were provided in person (e.g. short consultations) or through written communication (e.g. letters advising patients to reconsider their long‐term use of the medication) [29, 30, 31].
Despite evidence of effectiveness for some existing intervention approaches, a lack of theoretical underpinning has been identified in this field of research [38]. This limits our understanding of the causal mechanisms underlying the interventions’ effects. To address this, determinants of the target behaviour (i.e. long‐term BZRA use) need to be examined, together with the interventions’ active components.
The Theoretical Domains Framework (TDF) can be used to identify the determinants of the target behaviour [39]. The TDF distils psychological theory relevant to behaviour change into theoretical domains which are considered to be mediators (i.e. barriers and facilitators) of behaviour change [40, 41]. Identifying key mediators involved in changing target behaviours provides a theoretical basis for informing a rigorous and systematic intervention development process [42]. In cases where intervention development is not based on explicit theories, it is likely that intervention developers nevertheless had an implicit idea of how change was to occur that informed the implicit mediators they targeted. It is therefore possible to retrospectively identify and code factors (or in this case, domains) that interventions may have targeted using a sufficiently broad framework such as the TDF [43]. This can help understanding of the behavioural determinants targeted in order for an intervention to elicit behaviour change and can contribute to a cumulative evidence base for designing future interventions [43].
It is also possible to identify and describe interventions’ active components using standardized terminology. For example, the Behaviour Change Technique Taxonomy (version 1, BCTTv1) [44] consists of 93 BCTs, and has been applied in a number of systematic reviews to help identify component BCTs of interventions and to explore their impact on effectiveness [45, 46, 47]. Applying the taxonomy in this way can facilitate intervention replication and evidence synthesis [47].
Aim and objectives
A systematic review was undertaken which aimed to evaluate the evidence‐base for brief interventions targeting long‐term BZRA use in primary care settings.
The objectives were to:
examine the effectiveness of brief interventions targeting long‐term BZRA use in primary care in terms of the pre‐determined primary and secondary outcomes,
assess risk of bias (including publication bias),
assess reporting of intervention development and evaluation in published manuscripts,
explore behavioural determinants targeted by the interventions using the TDF [41] and
identify BCTs present in the interventions using the BCTTv1 [44].
Methods
A systematic review was conducted of randomized controlled trials (RCTs), including cluster RCTs (cRCTs), evaluating brief interventions targeting long‐term BZRA use in primary care settings (e.g. general practice, community pharmacy) against usual care. The primary research question and analysis plan were not pre‐registered on a publicly available platform. For the purpose of this review, a brief intervention was defined as an intervention comprising oral or written communication that involved discussion, negotiation or encouragement for reduction or discontinuation of long‐term BZRA use, with or without additional support or follow‐up. This definition was adapted from the National Institute of Health and Care Excellence (NICE) [48]. As brief interventions are considered time‐limited efforts to deliver information and promote behaviour change [36], eligible interventions had to be delivered in the context of routine clinical consultations (i.e. for interventions involving face‐to‐face consultations) and/or with minimal impact on routine clinical practice (i.e. for interventions involving written information sent directly to patients). It was anticipated that some interventions would include advice on gradual dosage reduction strategies, and therefore no limit was set on the number of additional follow‐up consultations.
Interventions were eligible for inclusion if they were directed at changing patients’ long‐term BZRA use behaviour, delivered within or through primary care settings, and specifically targeted adult patients (≥ 18 years) prescribed BZRAs on a long‐term basis. There is no consensus as to the duration that constitutes long‐term BZRA use [13]. Therefore, study definitions of long‐term use were accepted provided that the mean (or median) duration was at least 3 months, which is consistent with previous related reviews [29, 30, 31]. Interventions focusing on CBT were excluded, as were interventions targeting long‐term BZRA use as part of a wider initiative to address potentially inappropriate prescribing. This review was conducted and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (Supporting information, S2) [49].
The primary outcome was BZRA use, and was classified as either complete discontinuation or reduction by ≥ 25%. There is no established threshold for a clinically significant BZRA dosage reduction. Previous studies have arbitrarily defined reduction using thresholds ranging between 25 and 50% [50, 51]. It has been suggested that a trial dosage reduction, without an obligation to completely stop the medication, may enhance motivation among patients who are reluctant to consider a change to their current BZRA use [52]. For the purpose of this review, dosage reduction was defined as ≥ 25% to provide a threshold that may have described a meaningful reduction in patients’ BZRA use as opposed to a random fluctuation. Secondary outcomes comprised: health‐related quality of life; withdrawal symptoms; anxiety; sleep quality; depression; and health‐care utilization (i.e. GP visits, hospital admissions, use of other medications).
Search strategy and data extraction
The following electronic databases were searched from inception to February 2019: PubMed, EMBASE, PsycINFO and CENTRAL. The search strategy (Supporting information, S3) was developed with assistance from a research librarian using relevant keywords and medical subject heading (MeSH) terms. These included: benzodiazepines, Z‐drugs, brief intervention, withdrawal, reduction and discontinuation. Validated sensitivity‐maximizing filters were applied [53]. Abstracts were screened for inclusion by two authors (T.L., C.A.C.) working independently. If studies appeared to meet inclusion criteria, full‐text articles were retrieved and assessed for inclusion. Searches were restricted to English language publications. Conference abstracts were not included. Any disagreements were resolved by discussion between authors and consultation with a third author (C.R.) if necessary.
Data extraction was conducted independently by two authors (T.L., C.A.C.) using a purposefully designed data extraction form which was piloted on one of the included studies. Data were extracted relating to study design, participants, interventions, control groups and outcomes. Information was also extracted in relation to included studies’ risk of bias using the Cochrane Collaboration's risk of bias tool [54]. The reporting of intervention development and evaluation was assessed using the Workgroup for Intervention Development and Evaluation Research (WIDER) checklist [55, 56]. Inconsistencies were resolved by discussion between authors.
Analysis
Primary outcome data from individual studies were combined through meta‐analysis where possible (comparable interventions and outcomes) by calculating the risk ratio (RR) and 95% confidence interval (CI) using a random‐effects model. For studies that assessed more than one brief intervention, intervention group outcome data were pooled where the intervention groups involved modest variations of the core brief intervention (as evidenced by the findings of the BCT coding exercise outlined below) and no detectable differences were observed between the different forms/variations of the brief interventions for outcome assessments relating to BZRA use. This allowed one single comparison against the control arm, as recommended in the Cochrane Handbook [57]. For each of the cRCTs, results were adjusted for clustering using the study's reported intra‐class correlation coefficient to calculate the effective sample size based on the recommended methods outlined in the Cochrane Handbook [58]. The Cochrane Handbook's definitions of heterogeneity were used to assist interpretation: potentially not important (I 2 = 0–40%), moderate (I 2 = 30–60%), substantial (I 2 = 50–90%) and considerable heterogeneity (I 2 = 75–100%) [59]. If outcome data could not be pooled, a narrative summary was provided. Funnel plots were to be used to assess publication bias if comparable data were available from at least 10 studies [60].
Intervention coding
The behavioural determinants that interventions may have targeted were explored using the TDF following established methods [43], as summarized below. Verbatim descriptions of intervention and control groups were extracted. Study authors were e‐mailed to verify the reported descriptions and asked for any additional supporting information. Intervention and control groups were coded using the 14 domains from TDF version 2 [41] as the coding framework. A coding manual was developed by the research team, which included established definitions of each domain [41] together with examples of items to code under each domain (Supporting information, S4). The coding manual was developed using a sample of brief intervention studies included in previous related reviews [29, 30, 31]. All TDF coding was conducted independently by two members of the research team (T.L., C.A.C.). Any inconsistencies were resolved by discussion and consultation with a third reviewer (C.R.).
Pearson's correlations (two‐tailed) were used to determine the relationship between the intervention effect size for the primary review outcomes and the number of different domains coded (maximum score of 14). For three‐armed studies involving more than one brief intervention, the average number of domains coded across intervention groups was used. Coding of domains in the control groups was subtracted from coding of domains in the intervention groups. A sensitivity analysis was subsequently performed in which the coding in the control groups was not subtracted.
BCTs included in the interventions were explored using the BCTTv1 [44] based on the method previously described by Presseau et al. [47] and using the same extracted information for the TDF analysis. Preliminary coding was conducted by two members of the research team (C.A.C., Z.M.v.A.) using the entire taxonomy. Based on the results of this analysis, a coding manual was developed which included established definitions for the subset of identified BCTs and examples of items to code under each BCT (Supporting information, S5). BCT coding was then conducted independently by two members of the research team (T.L., C.A.C) using the coding manual. Any inconsistencies were resolved by discussion and consultation with a third reviewer (Z.M.v.A.). Pearson's correlations were used to determine the relationship between the number of different BCTs coded and intervention effect size for the primary review outcomes.
Results
Results of the search
Figure 1 summarizes the search results. Six RCTs [50, 61, 62, 63, 64, 65] and two cRCTs [66, 67] were included, four of which were three‐armed trials that compared different types of brief interventions against usual care [50, 62, 64, 67]. In total, 2071 patients were involved, most of whom were female (71.2%). Participants’ mean age ranged from 59 to 75 years. Only two studies reported that patients were prescribed Z‐drugs [64, 67]. The studies were conducted in general practice [50, 61, 62, 63, 67], integrated health‐care delivery systems [64, 65] and community pharmacies [66] throughout four countries: Canada [66], Spain [63, 67], United Kingdom [50, 61, 62] and United States [64, 65].
Figure 1.
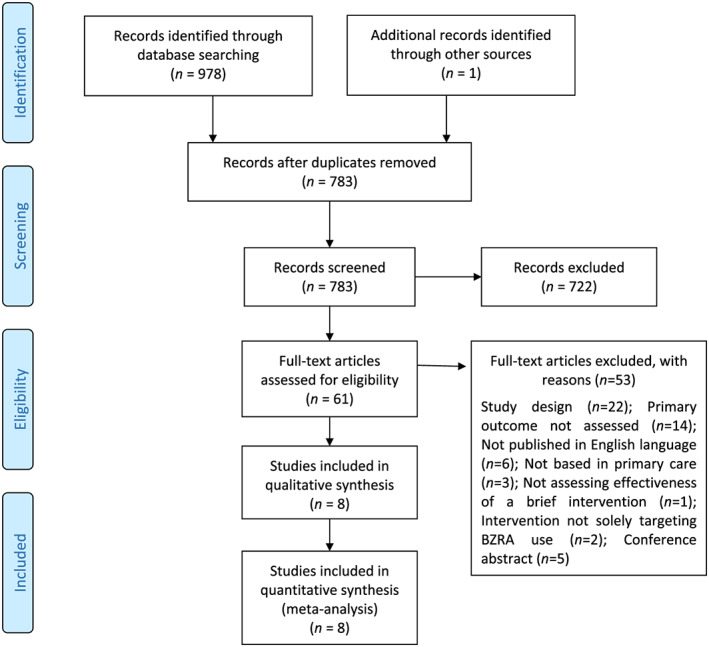
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram. [Colour figure can be viewed at wileyonlinelibrary.com]
Description of the interventions
Table 1 provides an overview of each intervention which consisted primarily of written letters signed by patients’ prescribers or a clinical pharmacist and short consultations provided by health‐care professionals [general practitioners (GPs), practice pharmacists, practice nurses] recommending reduction/discontinuation of the medications. One study used a patient empowerment‐based educational resource consisting of a personalized booklet that was posted to patients [66]. The booklet contained a self‐assessment detailing risks of benzodiazepine use and knowledge statements designed to create cognitive dissonance about the perceived safety of benzodiazepine use. A peer champion story was also included that was designed to encourage participants to attempt discontinuation using the included gradual dose reduction (GDR) protocol.
Table 1.
Overview of included studies.
| Study ID and location | Design | Participants and setting | Follow‐up | Intervention and control group descriptions | Primary review outcomes |
|---|---|---|---|---|---|
|
Bashir et al [61]
United Kingdom |
Two‐armed RCT |
109 patients from 11 general practices 61.5% female Age (mean, range) = 62 (32–86) years |
6 months post‐intervention |
Intervention (n = 51): during a consultation, GPs highlighted the risks associated with benzodiazepine use and advised patients to reduce and then stop the medication. Patients were provided with a self‐help booklet and encouraged to follow the advice within it. The booklet included basic information about benzodiazepines and practical advice on stopping, including techniques on coping with fears and anxieties
Control (n = 58): no intervention received |
Reduction in benzodiazepine use (defined as stopping or reducing benzodiazepine use at 6‐months post‐intervention) Discontinuation of benzodiazepine use (defined as discontinuation of benzodiazepine use at 6 months post‐intervention) |
|
Cormack et al [62]
United Kingdom |
Three‐armed RCT |
209 patients from three general practices 79.4% female Age (mean, range) = 69 (34–102) years |
6 months post‐intervention |
Intervention group 1 [letter group] (n = 65): patients received a letter from their GP asking them to reduce or stop their benzodiazepine use and advising that this should be conducted gradually Intervention group 2 [letter plus information sheets] (n = 75): patients received the same letter as intervention group 1, followed at monthly intervals by four information sheets giving advice about reducing benzodiazepines, including practical suggestions for coping without benzodiazepines Control (n = 69): no intervention received |
Reduction in benzodiazepine use (defined as reduction to half or less of original benzodiazepine use)
Discontinuation of benzodiazepine use (defined as no benzodiazepine prescriptions after the intervention) |
|
Heather et al [50]
United Kingdom |
Three‐armed RCT |
284 patients from 7 general practices 74% female Age (mean, standard deviation) = 69 (11.5) years |
6 months post‐intervention |
Intervention group 1 [consultation group] (n = 98): patients were sent a letter inviting them to see their GP for a medication review. Guidelines were produced on information that should be provided to patients which included information about benzodiazepines, reasons why it might be beneficial to reduce the medication and a timetable that could be used to plan dosage reduction. Patients also received a self‐help booklet on benzodiazepine discontinuation and a leaflet on sleeping problems Intervention group 2 [letter group] (n = 93): patients were sent a letter by their GP which advised them to consider reducing or stopping benzodiazepines and that this should be conducted gradually. Patients in this group were not sent the self‐help booklet or leaflet Control (n = 93): patients received usual care |
Reduction in benzodiazepine use (defined as reduction in benzodiazepine use by ≥ 25%) Discontinuation of benzodiazepine use (defined as stopping benzodiazepine use) |
|
Kuntz et al [64]
United States |
Three‐armed RCT |
149 patients who were members of an integrated health‐care delivery system
66.4% female Age (mean) = 70 years |
6 months post‐intervention |
Intervention group 1 [educational intervention] (n = 50): patients received a letter from their prescriber encouraging them to reconsider their Z‐drug use. This was supplemented by an educational brochure which presented evidence on the risks of Z‐drug use together with suggestions for other treatment options (pharmacological and non‐pharmacological) and a tapering schedule. A self‐assessment quiz on the risks of Z‐drug use was also included which reinforced information in the brochure Intervention group 2 [educational intervention and follow‐up telephone call] (n = 49): patients received the same intervention as group 1, which was supplemented by a telephone call from a clinical pharmacist 2–4 weeks later. During the call, the pharmacist discussed and reinforced information from the educational mailing, assessed barriers to Z‐drug discontinuation and provided advice on tapering, alternatives to Z‐drugs and recommendations for care co‐ordination through other specialties (e.g. sleep medicine). The pharmacist had prescriber approval to implement a protocol to switch patients to alternative sleep medications Control (n = 50): patients received usual care |
Discontinuation of Z‐drug use (defined as no Z‐drug dispensing during the 6‐month follow‐up period) |
|
Navy et al [65]
United States |
Two‐armed RCT |
346 patients who were members of an integrated health‐care delivery system 64% female Age (mean) = 73 years |
6 months post intervention |
Intervention (173): patients received a letter from a clinical pharmacist highlighting the risks of long‐term alprazolam use. Patients were advised to call the clinical pharmacist to discuss reducing alprazolam and other potential treatment options. Patients were advised not to stop alprazolam prior to consulting the clinical pharmacist. The pharmacist collaborated with patients’ primary care physician in developing individualized gradual dosage reduction plans for patients. The pharmacist monitored patients’ progress through follow‐up telephone calls Control (173): patients received usual care |
Discontinuation of alprazolam use (defined as no alprazolam dispensing at any time during the 6‐month follow‐up) Reduction of alprazolam use (defined as ≥ 50% dose reduction during the 6‐month follow‐up) |
|
Tannenbaum et al [66]
Canada |
Two‐armed cRCT |
303 patients from 30 community pharmacies that were part of a chain 69% female Age (mean, standard deviation) = 75 (6.3) years |
6 months post‐intervention |
Intervention (n = 148): a personalized educational booklet was mailed to patients. The booklet contained a self‐assessment detailing risks of benzodiazepine use and knowledge statements designed to create cognitive dissonance about the perceived safety of benzodiazepine use. A peer champion story was also included that was designed to encourage participants to attempt discontinuation using the included gradual dosage reduction protocol Control (n = 155): received usual care and then received educational intervention 6 months after the intervention group |
Reduction in benzodiazepine use (defined as ≥ 25% reduction in benzodiazepine dose compared with baseline and sustained for ≥ 3 consecutive months) Discontinuation of benzodiazepine use (defined as an absence of any benzodiazepine prescription renewal at the time of the 6‐month follow‐up that was sustained for ≥ 3 consecutive months) |
|
Vicens et al [63]
Spain |
Two‐armed RCT |
139 patients recruited from 3 public primary care centres 82% female Age (mean, standard deviation) = 59 (11.4) years |
6 months post intervention |
Intervention (n = 73): patients received a consultation from their GP with a standardized message on benzodiazepines, covering risks of long‐term use and information on discontinuing benzodiazepines. Patients underwent a 10–25% dosage reduction every 2 weeks at follow‐up visits Control (n = 66): patients did not receive the structured intervention. Patients were managed according to usual practice and informed of the convenience of reducing benzodiazepine use |
Reduction in benzodiazepine use (defined as ≥ 50% reduction in initial benzodiazepine dose)
Discontinuation of benzodiazepine use (defined as no benzodiazepine use or using benzodiazepines no more than once every 15 days) |
|
Vicens et al [67]
Spain |
Three‐armed cRCT |
532 patients across 21 primary care centres 72% female Age (median, interquartile range) = 64 (55–72) years |
6, 12 and 36 months post‐intervention |
Intervention group 1 [structured educational intervention with follow‐up consultation] (n = 191). During a consultation, GPs provided information on the risks of long‐term benzodiazepine/Z‐drug use and reassurance about reducing the medication. A self‐help leaflet for improving sleep quality was provided to patients with insomnia. Patients underwent gradual dosage reduction at follow‐up consultations (10–25% reduction every 2–3 weeks). GPs could switch patients suffering from withdrawal symptoms to longer‐acting benzodiazepines to aid dosage reduction process Intervention group 2 [structured education interventions with written follow‐up] (n = 168): Patients received the same initial GP consultation as intervention group 1. They were then provided with written instructions reinforcing educational information they received together with a tailored gradual dosage reduction plan. No follow‐up visits were scheduled. However, patients could request an appointment with their GP when needed Control (n = 173): patients received routine care |
Discontinuation of benzodiazepine use (defined as no benzodiazepine use or using fewer than four doses of benzodiazepines in the previous month) |
RCT = randomized controlled trial; GP = general practitioner.
All interventions advocated GDR to patients. However, the guidance provided on GDR varied. For example, in some studies patients were given general advice on GDR (e.g. by taking the medication only when needed [62] or by taking half a tablet as opposed to a full one [50]). In other studies, more detailed tapering schedules were provided. In a number of studies, interventions were supplemented with additional consultations, telephone calls and written educational resources (e.g. information sheets, self‐help booklets).
Primary outcomes
Pooled data from all eight studies (1168 intervention participants, 796 control participants) showed that intervention participants were more likely to discontinue BZRA use 6 months post‐intervention compared to control group participants (RR = 2.73, 95% CI = 1.84–4.06) (Fig. 2). Moderate heterogeneity was observed among the studies (I 2 = 55%, P = 0.03).
Figure 2.
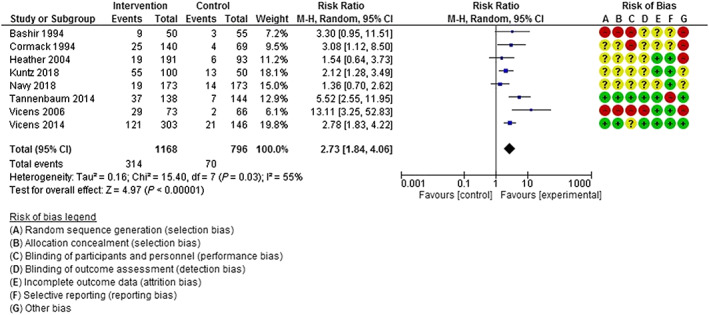
Discontinuation of benzodiazepine/Z‐drug use 6 months post‐intervention. [Colour figure can be viewed at wileyonlinelibrary.com]
Pooled data from two studies [63, 67] (376 intervention participants, 212 control participants) showed that intervention participants were more likely to discontinue BZRA use 12 months post‐intervention compared to control group participants (RR = 3.41, 95% CI = 2.22–5.25) (Fig. 3). Potentially unimportant heterogeneity was observed across studies (I 2 = 18%, P = 0.27).
Figure 3.
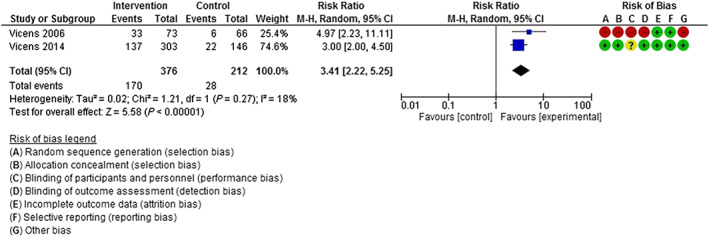
Discontinuation of benzodiazepine/Z‐drug use at 12 months post‐intervention. [Colour figure can be viewed at wileyonlinelibrary.com]
Reduction in BZRA use
Pooled outcome data from five studies [50, 61, 62, 65, 66] (692 intervention participants, 534 control participants) showed that intervention participants were more likely to reduce benzodiazepine use 6 months post‐intervention compared to control group participants (RR = 1.68, 95% CI = 1.03, 2.75) (Fig. 4). Considerable heterogeneity was observed across studies (I 2 = 63%, P = 0.03). No study included in this meta‐analysis involved Z‐drug patients.
Figure 4.
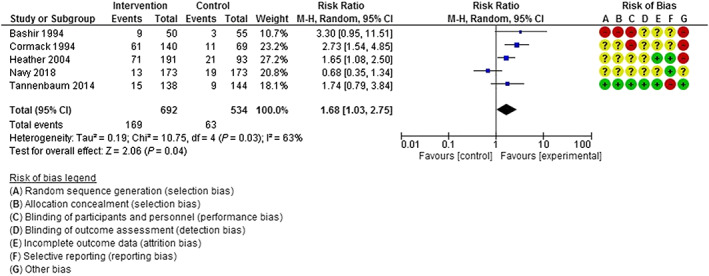
Reduction in benzodiazepine use at 6 months post‐intervention. [Colour figure can be viewed at wileyonlinelibrary.com]
Only one study [63] reported reduction of BZRA use at 12 months post‐intervention, therefore meta‐analysis was not possible. The study showed that 16 (21.9%) intervention participants reduced BZRA use compared with 11 (16.7%) control group participants.
Secondary outcomes
An outline of secondary outcomes reported among included studies together with an overview of the assessment tools that were used is provided in Supporting information, S6–S7. Due to differences in the assessment and reporting of secondary outcomes, meta‐analysis was not possible. Limited reported data for secondary outcome assessments among studies also made it difficult to provide a narrative summary of results. For example, both studies that reported assessments of anxiety used different assessment scales: the Hospital Anxiety and Depression Scale [63] and Part B of the General Health Questionnaire [50]. Outcome data were not clearly reported for one of the studies [50] which precluded any meaningful synthesis.
Risk of bias
The risk‐of‐bias assessments are shown in Fig. 5. Only two studies showed a low risk of bias among most domains. Four studies were at high risk of contamination bias (‘other bias’) whereby those delivering the intervention also cared for control group participants.
Figure 5.
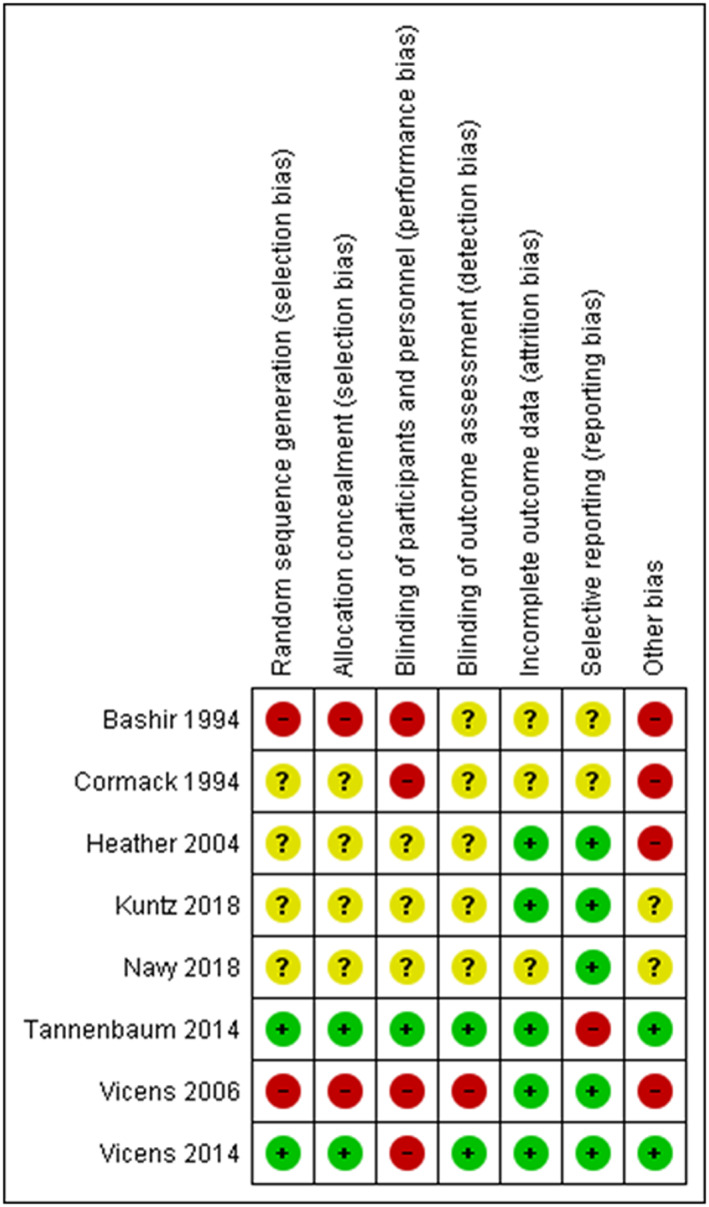
Risk of bias assessment of included studies. [Colour figure can be viewed at wileyonlinelibrary.com]
Publication bias
Given that fewer than 10 studies were included in each meta‐analysis, assessments of funnel plot asymmetry were not conducted because the power of the statistical tests was too low to distinguish real asymmetry from chance [60].
Intervention development and evaluation in published manuscripts
Table 2 shows the results of the WIDER checklist assessment. Most studies outlined the mode of delivery (n = 6) and characteristics of intervention recipients (n = 7). Four studies provided detailed descriptions of the intervention and content delivered. Only one study [66] described intervention development and referred to the use of formal theory, which included social constructivist learning theory and self‐efficacy theory.
Table 2.
Workgroup for Intervention Development and Evaluation Research (WIDER) checklist assessments.
| Cormack et al. 1994 [62] | Bashir et al. 1994 [61] | Heather et al. 2004 [50] | Vicens et al. 2006 [63] | Vicens et al. 2014 [67] | Tannenbaum et al. 2014 [66] | Kuntz et al. 2018 [64] | Navy et al. 2018 [65] | |
|---|---|---|---|---|---|---|---|---|
| 1. Detailed description of interventions in published papers | ||||||||
| Detailed description provided? | ||||||||
| Characteristics of those delivering the intervention | No | Yes | No | Unclear | Yes | Unclear | No | Unclear |
| Characteristics of the recipients | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| The setting (e.g. work‐site, time and place of intervention) | Unclear | Unclear | Yes | Unclear | Unclear | Unclear | Yes | Unclear |
| The mode of delivery (e.g. face‐to‐face) | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear | Yes |
| The intensity (e.g. contact time) | No | Unclear | Unclear | Yes | Yes | Unclear | Unclear | No |
| The duration (e.g. number of sessions and their spacing over a given period) | No | Unclear | Unclear | Yes | Yes | Unclear | No | No |
| Adherence/fidelity to delivery protocols | No | No | Unclear | Unclear | No | Unclear | No | No |
| Detailed description of the intervention content provided for each study group | Unclear | Unclear | Yes | Yes | Yes | Yes | No | Unclear |
| 2. Clarification of assumed change process and design principles | ||||||||
| Cormack et al. 1994 | Bashir et al.1994 | Heather et al. 2004 | Vicens et al. 2006 | Vicens et al. 2014 | Tannenbaum et al. 2014 | Kuntz et al. 2018 | Navy et al. 2018 | |
| Detailed description provided? | ||||||||
| Description of intervention development | No | Unclear | No | Unclear | No | Yes | Unclear | No |
| Description of the change techniques used in the intervention | No | No | No | No | Unclear | Yes | No | No |
| Description of the causal processes targeted by these change techniques | No | No | No | No | No | Unclear | No | No |
| 3. Access to intervention manuals/protocols | ||||||||
| Cormack et al. 1994 | Bashir et al.1994 | Heather et al. 2004 | Vicens et al. 2006 | Vicens et al. 2014 | Tannenbaum et al. 2014 | Kuntz et al. 2018 | Navy et al. 2018 | |
| Detailed description provided? | ||||||||
| Has the intervention protocol been published? | No | No | No | No | Yes | Yes | No | No |
| Is a manual describing the intervention available? | Unclear | No | Unclear | No | No | Yes | No | Unclear |
| 4. Detailed description of active control conditions | ||||||||
| Cormack et al. 1994 | Bashir et al.1994 | Heather et al. 2004 | Vicens et al. 2006 | Vicens et al. 2014 | Tannenbaum et al. 2014 | Kuntz et al. 2018 | Navy et al. 2018 | |
| Detailed description provided? | ||||||||
| Details provided of the content of active control group? (i.e. what did usual care involve?) | No | No | Unclear | Unclear | Unclear | No | No | No |
| Is a similar level of description of the content active control group provided to that of the intervention itself? | No | No | No | No | No | No | No | No |
Two studies published study protocols with one also including an intervention manual. None of the studies provided detailed descriptions of control conditions. One study identified an unexpected deviation from the protocol whereby a practice pharmacist and a nurse delivered interventions instead of the GP [50].
Barriers and enablers targeted and behaviour change techniques identified
Twelve theoretical domains were identified as being targeted across the interventions (Table 3). The total number of domains identified per intervention varied (range = 6–11). ‘Knowledge’, ‘memory, attention and decision processes’, ‘environmental context and resources’ and ‘social influences’ were identified in every intervention. The only domains that were not evident in interventions were ‘emotion’ and ‘social/professional role and identity’.
Table 3.
Results of Theoretical Domains Framework (TDF) coding.
| TDF domains | Identified domains | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Cormack et al. 1994 [62]
Three‐armed trial |
Bashir et al. 1994 [61]
Two‐armed trial |
Heather et al. 2004 [50]
Three‐armed trial |
Vicens et al. 2006 [63]
Two‐armed trial |
Vicens et al. 2014 [67]
Three‐armed trial |
Tannenbaum et al. 2014 [66]
Two‐armed trial |
Kuntz et al. 2018 [64]
Three‐armed trial |
Navy et al. 2018 [65]
Two‐armed trial |
||
| Knowledge | CG | Unclear | Unclear | Unclear | Yes | Yes | Unclear | Unclear | Unclear |
| IG1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| IG2 | Yes | NA | Yes | NA | Yes | NA | Yes | NA | |
| Skills | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | |
| IG2 | Yes | NA | Yes | NA | Yes | NA | Yes | NA | |
| Social/professional role and identity | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | No | No | No | No | No | No | No | No | |
| IG2 | No | NA | No | NA | No | NA | No | NA | |
| Beliefs about capabilities | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | No | No | Yes | No | No | Yes | No | No | |
| IG2 | No | NA | No | NA | No | NA | No | NA | |
| Optimism | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | No | No | Yes | No | No | Yes | No | No | |
| IG2 | No | NA | Yes | NA | No | NA | No | NA | |
| Beliefs about consequences | CG | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Unclear | Unclear |
| IG1 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | |
| IG2 |
Yes |
NA | Yes | NA | Yes | NA | No | NA | |
| Reinforcement | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | Yes | No | Yes | Yes | Yes | No | Yes | Yes | |
| IG2 | Yes | NA | Yes | NA | Yes | NA | Yes | NA | |
| Intentions | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | Yes | No | No | No | No | No | No | No | |
| IG2 | Yes | NA | Yes | NA | No | NA | No | NA | |
| Goals | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | Yes | No | No | Yes | Yes | No | No | No | |
| IG2 | Yes | NA | Yes | NA | Yes | NA | No | NA | |
| Memory, attention and decision process | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| IG2 | Yes | NA | Yes | NA | Yes | NA | Yes | NA | |
| Environmental context and resources | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| IG2 | Yes | NA | Yes | NA | Yes | NA | Yes | NA | |
| Social influences | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| IG2 | Yes | NA | Yes | NA | Yes | NA | Yes | NA | |
| Emotion | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | No | No | No | No | No | No | No | No | |
| IG2 | No | NA | No | NA | No | NA | No | NA | |
| Behavioural regulation | CG | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| IG1 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | |
| IG2 | Yes | NA | Yes | NA | Yes | NA | Yes | NA | |
| TDF coding | |||||||||
| Total number of different domains identified | CG | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 |
| IG1 | 10 | 7 | 10 | 9 | 9 | 9 | 6 | 7 | |
| IG2 | 10 | NA | 11 | NA | 9 | NA | 7 | NA | |
CG = control group; IG1 = intervention group 1; IG2 = intervention group 2;
NA = not applicable if the study only had one intervention group;
Cormack 1994, IG1 = letter group, IG2 = letter plus information sheet;
Heather 2004, IG1 = consultation group, IG2 = letter group;
Vicens 2014, IG1 = structured educational intervention with follow‐up consultations; IG2 = structured educational intervention with written follow‐up;
Kuntz 2018, IG1 = educational intervention; IG2 = educational intervention plus pharmacist telephone call.
There was no detectable relationship between intervention effect size for BZRA discontinuation or reduction at 6 months and the number of different domains coded (Table 4). A sensitivity analysis (whereby control group domains were not subtracted) showed similar results.
Table 4.
Relationship between intervention effect size and number of different domains identified from the Theoretical Domains Framework.
| Outcome | Number of studies included in meta‐analysis | Pearson correlation value | P‐value |
|---|---|---|---|
| Discontinuation at 6 months follow‐up | 8 | −0.219 | 0.603 |
| Reduction at 6 months follow‐up | 5 | 0.102 | 0.870 |
Seventeen BCTs were identified across the studies (Table 5). The number of BCTs per intervention varied (range = 4–8). The most commonly identified BCTs were ‘information about health consequences’, ‘credible source’ and ‘adding objects to the environment’. No detectable relationship was found between intervention effect size for BZRA discontinuation or reduction at 6 months post‐intervention and the number of identified BCTs (Table 6).
Table 5.
Behaviour change techniques (BCTs) identified in intervention groups.
| BCTS identified | Included studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Cormack et al. 1994 [62]
Three‐armed trial |
Bashir et al.1994 [61]
Two‐armed trial |
Heather et al. 2004 [50]
Three‐armed trial |
Vicens et al. 2006 [63]
Two‐armed trial |
Vicens et al. 2014 [67]
Three‐armed trial |
Tannenbaum et al. 2014 [66]
Two‐armed trial |
Kuntz et al. 2018 [64]
Three‐armed trial |
Navy et al. 2018 [55]
Two‐armed trial |
||
| 1.2 Problem‐solving | IG1 | No | No | Yes | No | Yes | Yes | No | No |
| IG2 | Yes | NA | No | NA | Yes | NA | No | NA | |
| 1.3 Goal setting (outcome) | IG1 | Yes | Yes | No | No | No | No | No | No |
| IG2 | Yes | NA | Yes | NA | No | NA | No | NA | |
| 1.4 Action planning | IG1 | Yes | No | No | No | No | No | No | No |
| IG2 | Yes | NA | No | NA | No | NA | No | NA | |
| 3.1 Social support (unspecified) | IG1 | Yes | No | No | No | No | Yes | No | No |
| IG2 | Yes | NA | Yes | NA | Yes | NA | No | NA | |
| 3.2 Social support (practical) | IG1 | No | No | No | No | Yes | No | No | Yes |
| IG2 | No | NA | No | NA | No | NA | Yes | NA | |
| 4.1 Instruction on how to perform the behaviour | IG1 | Yes | Yes | No | No | No | No | Yes | Yes |
| IG2 | Yes | NA | No | NA | No | NA | Yes | NA | |
| 5.1 Information about health consequences | IG1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| IG2 | Yes | NA | Yes | NA | Yes | NA | Yes | NA | |
| 5.6 Information about emotional consequences | IG1 | No | No | Yes | No | No | No | No | No |
| IG2 | No | NA | No | NA | No | NA | No | NA | |
| 6.2 Social comparison | IG1 |
No |
No | No | No |
No |
Yes | No | No |
| IG2 | No | NA | No | NA | No | NA | No | NA | |
| 8.7 Graded tasks | IG1 | No | No | Yes | Yes | Yes | Yes | No | No |
| IG2 | No | NA | Yes | NA | Yes | NA | No | NA | |
| 9.1 Credible source | IG1 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| IG2 | Yes | NA | Yes | NA | Yes | NA | Yes | NA | |
| 10.4 Social reward | IG1 | No | No | No | Yes | No | No | No | No |
| IG2 | No | NA | No | NA | No | NA | No | NA | |
| 11.1 Pharmacological support | IG1 | No | No | No | No | Yes | No | No | Yes |
| IG2 | No | NA | No | NA | Yes | NA | Yes | NA | |
| 11.2 Reduce negative emotions | IG1 | No | Yes | No | No |
No |
No | No | No |
| IG2 | No | NA | No | NA | No | NA | No | NA | |
| 12.5 Adding objects to the environment | IG1 | Yes | No | No | No | No | Yes | Yes | Yes |
| IG2 | Yes | NA | Yes | NA | No | NA | Yes | NA | |
| 13.3 Incompatible beliefs | IG1 | No | No | No | Yes | No | No | No | No |
| IG2 | No | NA | No | NA | No | NA | No | NA | |
| 15.3 Focus on past success | IG1 | No | No | No | No | Yes | No | No | No |
| IG2 | No | NA | No | NA | No | NA | No | NA | |
| BCT identification | |||||||||
| Number of different BCTs per intervention | IG1 | 7 | 5 | 5 | 5 | 7 | 6 | 4 | 6 |
| IG2 | 8 | NA | 6 | NA | 6 | NA | 6 | NA | |
Table 6.
Relationship between intervention effect size and number of different behaviour change techniques identified.
| Outcome | Number of studies included in meta‐analysis | Pearson correlation value | P‐value |
|---|---|---|---|
| Discontinuation at 6 months follow‐up | 8 | −0.551 | 0.157 |
| Reduction at 6 months follow‐up | 5 | −0.007 | 0.991 |
Insufficient numbers of studies reported on discontinuation and reduction at 12 months post‐intervention to allow correlation between effect size and the results of the TDF and BCT coding to be examined.
Discussion
This review identified eight studies that evaluated brief interventions targeting long‐term BZRA use in primary care [50, 61, 62, 63, 64, 65, 66, 67]. The pooled effect estimates showed that, compared to usual care, patients who received a brief intervention were more likely to reduce or discontinue long‐term BZRA use at 6 and 12 months post‐intervention. This is consistent with previous related reviews [29, 30, 31]. The review offers support for the first stage of the aforementioned ‘stepped‐care’ approach [37]. The additional assessments undertaken through application of the WIDER checklist [55] and intervention coding involving the TDF [41] and BCTTv1 [44] have provided detailed insight into intervention content, development and reporting that has, to date, been lacking. For example, a scoping review of interventions targeting discontinuation of long‐term BZRA use among community‐dwelling adults by Pollman et al. [38] applied the Behaviour Change Wheel to identify broad categorizations of intervention functions (e.g. enablement, training) [68]. Given its scoping nature, the review included a broader range of interventions and study designs than the current review and did not look to pool outcome data among included studies. The identified intervention functions did not provide insights into the behavioural determinants that were targeted by the interventions or the specific BCTs that were used to elicit behaviour change. The TDF and BCT coding exercises in the current review have helped to address this. Pollman et al. [38] also reported that intervention reporting was poor across included studies. However, as no specific reporting tool was applied to the study reports, it was not possible to identify which specific aspects of intervention reporting needed to be improved. The application of the WIDER checklist in the current review has helped to address this. The current review advances this previous work and will help to build a more cumulative and replicable evidence base.
A variety of brief interventions were evaluated, comprising discontinuation letters, short consultations with health‐care professionals and written educational information, delivered alone or in combination. The WIDER assessments [55] showed that intervention development was often poorly described. In recent years, the UK Medical Research Council's complex intervention framework [69] has drawn considerable attention to intervention development and evaluation processes. The framework advocates using evidence and theory during intervention development to inform selection of relevant components, prior to feasibility testing. This precedes definitive evaluations of intervention effectiveness. Although a number of studies had considered existing evidence by adapting interventions from previous research the role of theory was largely overlooked, whereby only one study reported incorporating theory into the intervention development process [66]. A realist evaluation that was conducted alongside the main trial of this intervention enabled the researchers to investigate the mechanisms and contexts underlying its effects from patients’ perspectives [70]. This evaluation found that targeting patients’ motivation and capacity to discontinue BZRAs yielded successful outcomes where health‐care providers were supportive, and patients did not have internal competing desires to continue BZRA use. These findings helped to refine the initial theoretical understanding of how the intervention worked and provided important insights that could help to inform implementation of such interventions on a wider scale. The lack of underpinning theory among the other included studies limits our understanding of why the interventions were successful and how they exerted their effects [71].
In addition to considering intervention development processes, it is also important to examine their content and delivery. All included studies recommended GDR; however, the specific instructions provided to patients varied. For example, patients were advised to reduce their benzodiazepine use by taking the medication only when needed [62] or by taking half a tablet as opposed to a full one [50]. These suggestions could prompt overly rapid dosage reductions (e.g. reduction by 50% or even abrupt discontinuation) which could precipitate withdrawal symptoms and hinder patients’ success in discontinuing long‐term use. GDR regimens vary in the literature and an optimal GDR schedule has yet to be identified [22, 29]. Therefore, a personalized and flexible approach is preferable which allows patients to balance dosage reduction against the emergence of any withdrawal symptoms [22]. The extent to which benzodiazepine‐related GDR processes are transferrable to Z‐drugs has previously been questioned [38]. However, the findings of the single study that specifically targeted Z‐drug patients indicate that personalized GDR regimens are also helpful to this patient cohort [64].
The included studies highlighted the potential to include health‐care professionals other than prescribers in intervention delivery (e.g. pharmacists [50, 64, 65]). A follow‐up evaluation of the study by Heather et al. that identified a deviation from the trial protocol, whereby a practice pharmacist had delivered interventions instead of the GP, found the pharmacist's involvement to be a potentially cost‐effective means of reducing long‐term benzodiazepine use [72]. This warrants further investigation and could help in maximizing efficiency of intervention delivery.
Outcome evaluations were generally limited to 6 and 12 months post‐intervention. One study reported that approximately 70% of patients that discontinued BZRAs at 12 months post‐intervention maintained this at 36 months follow‐up [73]. This is a positive finding in relation to the interventions’ long‐term effects. Furthermore, a follow‐up study of a non‐RCT evaluation of a brief intervention involving a discontinuation letter found that maintaining benzodiazepine discontinuation for the first 2 years post‐intervention was a significant predictor of sustaining that change in behaviour 10 years later [74].
The risk of bias assessments highlighted a number of issues with the design of included studies which affected their internal validity. Therefore, the pooled effect estimates should be interpreted cautiously. These assessments are not directly comparable to previous reviews [29, 30, 31] because different methods were used to assess study quality (e.g. quality scales). One key limitation with the older studies which was not raised in previous reviews was the potential for contamination, whereby patients randomized to intervention and control arms were attending the same practice or health‐care professional. To overcome this, future studies would benefit from appropriate cRCT designs.
Given the identified lack of theory underpinning the interventions, application of the TDF helped to provide insight into mediators of long‐term BZRA use that may have been targeted. For example, ‘knowledge’, ‘skills’ and ‘beliefs about consequences’ were frequently coded, suggesting that study authors believed that educating patients about the risks associated with long‐term BZRA use and providing them with appropriate skills to undertake GDR were important in changing their existing behaviour. These are potentially important domains to target, as a lack of knowledge and concern about the risks associated with these medications have previously been identified as reasons for long‐term BZRA use [75]. The TDF coding also highlighted domains such as ‘optimism’, ‘emotion’ and ‘beliefs about capabilities’ which were infrequently coded or not coded at all. Previous qualitative research has reported negative patient emotions and perceptions towards discontinuation of long‐term BZRA use [75]. Appropriate targeting of these domains may help to address important patient‐level barriers, thereby facilitating optimization of interventions aimed at discontinuation of long‐term BZRA use.
Application of the BCTTv1 [44] helped in identifying the interventions’ potential active components. ‘Information about health consequences’ was the only BCT that was common among all interventions. This is consistent with the ‘beliefs about consequences’ domain being commonly identified across the interventions as per the results of a previous mapping exercise linking BCTs to TDF domains [76]. Only three interventions included ‘problem solving’, which may be important to consider for inclusion in future interventions, as patients have reported barriers to discontinuing long‐term BZRA use (e.g. experience of withdrawal symptoms) for which solutions could be generated [77]. However, further prospective studies of interventions involving clearly specified BCTs are needed to identify which BCTs are most effective in discontinuing long‐term BZRA use. For example, it remains to be seen whether providing patients with detailed GDR plans with specified increments of when and how to reduce their current dose (coded as ‘graded tasks’) is more effective than simply providing general information on GDR (coded as ‘instruction on how to perform the behaviour’).
Given the differences in the results of the TDF and BCT coding exercises across the interventions, it is difficult to speculate as to which domains and BCTs were critical to the interventions’ effects. The additional exploratory analyses demonstrated a lack of detectable relationship between intervention effect size and the results of both of these coding exercises. A more detailed exploratory analysis involving meta‐regression was not possible due to the relatively small number of included studies [66]. However, another systematic review that conducted a similar TDF coding exercise identified an inverse relationship [40] which suggests that targeting multiple domains may not necessarily result in more effective interventions. Instead, specific domains should be targeted that are relevant to both the target population and behaviour [39, 40]. Further research into the most effective BCTs to use in brief interventions targeting long‐term BZRA use will help to optimize already effective interventions. Describing intervention components using the BCTTv1 [44] could enhance transparency of reporting, thereby ensuring that interventions can be replicated and applied in other settings.
This review provides a comprehensive overview of existing evidence for brief interventions targeting long‐term BZRA use in primary care and includes more studies than any previous review. The application of the WIDER checklist [55], together with the intervention coding involving the TDF [41] and BCTTv1 [44] represent novel aspects of the review that have provided additional insights into intervention content, development and reporting that have, to date, been lacking. The limitations of this review were that it focused on studies published in the English language and did not include grey literature.
Conclusion
This review shows that brief interventions delivered in primary care were more effective than usual care in reducing and discontinuing long‐term BZRA use. By retrospectively coding the interventions using the TDF and BCTTv1, it has been possible to identify key domains targeted by the interventions and the component BCTs. The review findings may help to optimize the development and evaluation of future brief interventions targeting long‐term BZRA use in primary care.
Declaration of interests
None.
Supporting information
Data S1. Supporting information.
Acknowledgements
T.L. was supported by a Clement Archer Scholarship from the School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland. The authors are grateful to Grainne McCabe, Assistant Librarian, RCSI Library for her assistance in reviewing the search strategy.
Lynch, T. , Ryan, C. , Hughes, C. M. , Presseau, J. , van Allen, Z. M. , Bradley, C. P. , and Cadogan, C. A. (2020) Brief interventions targeting long‐term benzodiazepine and Z‐drug use in primary care: a systematic review and meta‐analysis. Addiction, 115: 1618–1639. 10.1111/add.14981.
References
- 1. Benzodiazepine Committee . Benzodiazepines: Good Practice Guidelines for Clinicians. Dublin: Department of Heath and Children; 2002. [Google Scholar]
- 2. Royal College of General Practitioners . Prescribing Drugs of Dependence in General Practice, Part B—Benzodiazepines. Victoria: Royal College of General Practitioners; 2015. [Google Scholar]
- 3. National Institue for Health and Clinical Excellence . Guidance on the Use of Zaleplon, Zolpidem and Zopiclone for Short‐Term Management of Insomnia. London: National Institute for Health and Care Excellence; 2004. [Google Scholar]
- 4. Donoghue J., Lader M. Usage of benzodiazepines: a review. Int J Psychiatry Clin Pract 2010; 14: 78–87. [DOI] [PubMed] [Google Scholar]
- 5. Weymann D., Gladstone E. J., Smolina K., Morgan S. G. Long‐term sedative use among community‐dwelling adults: a population‐based analysis. Can Med Assoc J Open 2017; 5: E52–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marra E. M., Mazer‐Amirshahi M., Brooks G., van den Anker J., May L., Pines J. M. Benzodiazepine prescribing in older adults in U.S. ambulatory clinics and emergency departments (2001–10). J Am Geriatr Soc 2015; 63: 2074–2081. [DOI] [PubMed] [Google Scholar]
- 7. Bénard‐Laribière A., Noize P., Pambrun E., Bazin F., Verdoux H., Tournier M. et al Trends in incident use of benzodiazepines and Z‐drugs in France from 2006 to 2012: a population‐based study. Pharmacoepidemiol Drug Saf 2017; 26: 162–169. [DOI] [PubMed] [Google Scholar]
- 8. Moriarty F., Hardy C., Bennett K., Smith S. M., Fahey T. Trends and interaction of polypharmacy and potentially inappropriate prescribing in primary care over 15 years in Ireland: a repeated cross‐sectional study. BMJ Open 2015; 5: e008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alessi‐Severini S., Bolton J. M., Enns M. W., Dahl M., Collins D. M., Chateau D. et al Use of benzodiazepines and related drugs in Manitoba: a population‐based study. Can Med Assoc J Open 2014; 2: E208–E216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cadogan C. A., Ryan C., Cahir C., Bradley C. P., Bennett K. Benzodiazepine and Z‐drug prescribing in Ireland: analysis of national prescribing trends from 2005–2015. Br J Clin Pharmacol 2018; 84: 1354–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaufmann C. N., Spira A. P., Alexander G. C., Rutkow L., Mojtabai R. Trends in prescribing of sedative‐hypnotic medications in the USA: 1993–2010. Pharmacoepidemiol Drug Saf 2016; 25: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng J. S., Huang W. F., Lin K. M., Shih Y. T. Characteristics associated with benzodiazepine usage in elderly outpatients in Taiwan. Int J Geriatr Psychiatry 2008; 23: 618–624. [DOI] [PubMed] [Google Scholar]
- 13. Kurko T. A., Saastamoinen L. K. Tahkapaa S., Tuulio‐Henriksson A., Taiminen T., Tiihonen J. et al Long‐term use of benzodiazepines: definitions, prevalence and usage patterns—a systematic review of register‐based studies. Eur Psychiatry 2015; 30: 1037–1047. [DOI] [PubMed] [Google Scholar]
- 14. Preville M., Bosse C., Vasiliadis H. M., Voyer P., Laurier C., Berbiche D. et al Correlates of potentially inappropriate prescriptions of benzodiazepines among older adults: results from the ESA study. Can J Aging 2012; 31: 313–322. [DOI] [PubMed] [Google Scholar]
- 15. Ryan C., O'Mahony D., Kennedy J., Weedle P., Byrne S. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol 2009; 68: 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryan C., O'Mahony D., Kennedy J., Weedle P., Cottrell E., Heffernan M. et al Potentially inappropriate prescribing in older residents in Irish nursing homes. Age Ageing 2013; 42: 116–120. [DOI] [PubMed] [Google Scholar]
- 17. De Wilde S., Carey I. M., Harris T., Richards N., Victor C., Hilton S. R. et al Trends in potentially inappropriate prescribing amongst older UK primary care patients. Pharmacoepidemiol Drug Saf 2007; 16: 658–667. [DOI] [PubMed] [Google Scholar]
- 18. Ubeda A., Ferrandiz L., Maicas N., Gomez C., Bonet M., Peris J. E. Potentially inappropriate prescribing in institutionalised older patients in Spain: the STOPP‐START criteria compared with the beers criteria. Pharm Pract 2012; 10: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Popovic B., Quadranti N. R., Matanovic S. M., Lisica I. D., Ljubotina A., Duliba D. P. et al Potentially inappropriate prescribing in elderly outpatients in Croatia. Eur J Clin Pharmacol 2014; 70: 737–744. [DOI] [PubMed] [Google Scholar]
- 20. Nishtala P. S., Bagge M. L., Campbell A. J., Tordoff J. M. Potentially inappropriate medicines in a cohort of community‐dwelling older people in New Zealand. Geriatr Gerontol Int 2014; 14: 89–93. [DOI] [PubMed] [Google Scholar]
- 21. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol 2014; 77: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ng B. J., Le Couteur D. G., Hilmer S. N. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging 2018; 35: 493–521. [DOI] [PubMed] [Google Scholar]
- 23. American Geriatrics Society Beers Criteria Update Expert Panel American Geriatrics Society updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60: 616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Narayan S. W., Nishtala P. S. Prevalence of potentially inappropriate medicine use in older new Zealanders: a population‐level study using the updated 2012 beers criteria. J Eval Clin Pract 2015; 21: 633–641. [DOI] [PubMed] [Google Scholar]
- 25. Hwang H.‐J., Kim S.‐H., Lee K. S. Potentially inappropriate medications in the elderly in Korean long‐term care facilities. Drugs 2015; 2: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neutel C. I., Skurtveit S., Berg C. What is the point of guidelines? Benzodiazepine and z‐hypnotic use by an elderly population. Sleep Med 2012; 13: 893–897. [DOI] [PubMed] [Google Scholar]
- 27. Cooper J. A., Moriarty F., Ryan C., Smith S. M., Bennett K., Fahey T. et al Potentially inappropriate prescribing in two populations with differing socio‐economic profiles: a cross‐sectional database study using the PROMPT criteria. Eur J Clin Pharmacol 2016; 72: 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Denis C., Fatseas M., Lavie E., Auriacombe M. Pharmacological interventions for benzodiazepine mono‐dependence management in outpatient settings. Cochrane Database Syst Rev 2006; 3: CD005194. [DOI] [PubMed]
- 29. Voshaar R. C., Couvee J. E., van Balkom A. J., Mulder P. G., Zitman F. G. Strategies for discontinuing long‐term benzodiazepine use: meta‐analysis. Br J Psychiatry 2006; 189: 213–220. [DOI] [PubMed] [Google Scholar]
- 30. Parr J. M., Kavanagh D. J., Cahill L., Mitchell G., Mc D. Y. R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta‐analysis. Addiction 2009; 104: 13–24. [DOI] [PubMed] [Google Scholar]
- 31. Mugunthan K., McGuire T., Glasziou P. Minimal interventions to decrease long‐term use of benzodiazepines in primary care: a systematic review and meta‐analysis. Br J Gen Pract 2011; 61: e573–e578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gould R. L., Coulson M. C., Patel N., Highton‐Williamson E., Howard R. J. Interventions for reducing benzodiazepine use in older people: meta‐analysis of randomised controlled trials. Br J Psychiatry 2014; 204: 98–107. [DOI] [PubMed] [Google Scholar]
- 33. Darker C. D., Sweeney B. P., Barry J. M., Farrell M. F., Donnelly‐Swift E. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev 2015; 5: CD009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCambridge J., Cunningham J. A. The early history of ideas on brief interventions for alcohol. Addiction 2014; 109: 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Donnell A., Anderson P., Newbury‐Birch D., Schulte B., Schmidt C., Reimer J. et al The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol 2013; 49: 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Young M. M., Stevens A., Galipeau J., Pirie T., Garritty C., Singh K. et al Effectiveness of brief interventions as part of the screening, brief intervention and referral to treatment (SBIRT) model for reducing the nonmedical use of psychoactive substances: a systematic review. Syst Rev 2014; 3: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lader M., Russell J. Guidelines for the prevention and treatment of benzodiazepine dependence: summary of a report from the Mental Health Foundation. Addiction 1993; 88: 1707–1708. [DOI] [PubMed] [Google Scholar]
- 38. Pollmann A. S., Murphy A. L., Bergman J. C., Gardner D. M. Deprescribing benzodiazepines and Z‐drugs in community‐dwelling adults: a scoping review. BMC Pharmacol Toxicol 2015; 16: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Francis J. J., O'Connor D., Curran J. Theories of behaviour change synthesised into a set of theoretical groupings: introducing a thematic series on the theoretical domains framework. Implement Sci 2012; 7: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michie S., Johnston M., Abraham C., Lawton R., Parker D., Walker A. et al GroupMaking psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005; 14: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cane J., O'Connor D., Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012; 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. French S. D., Green S. E., O'Connor D. A., McKenzie J. E., Francis J. J., Michie S. et al Developing theory‐informed behaviour change interventions to implement evidence into practice: a systematic approach using the theoretical domains framework. Implement Sci 2012; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Little E. A., Presseau J., Eccles M. P. Understanding effects in reviews of implementation interventions using the theoretical domains framework. Implement Sci 2015; 10: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Michie S., Richardson M., Johnston M., Abraham C., Francis J., Hardeman W. et al The Behavior Change Technique Taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013; 46: 81–95. [DOI] [PubMed] [Google Scholar]
- 45. Cradock K. A., Ol G., Finucane F. M., Gainforth H. L., Quinlan L. R., Ginis K. A. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: a systematic review and meta‐analysis. The International Journal of Behav Nutr Phys Activity 2017; 14: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lawrenson J. G., Graham‐Rowe E., Lorencatto F., Burr J., Bunce C., Francis J. J. et al Interventions to increase attendance for diabetic retinopathy screening. Cochrane Database Syst Rev 2018; 1: CD012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Presseau J., Ivers N. M., Newham J. J., Knittle K., Danko K. J., Grimshaw J. M. Using a Behaviour Change Techniques Taxonomy to identify active ingredients within trials of implementation interventions for diabetes care. Implement Sci 2015; 10: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. National Institute for Clincal Health and Care Excellence (NICE) NICE Guidance, Lifestyle and Wellbeing, Behaviour Change. London: NICE; 2014. [Google Scholar]
- 49. Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heather N., Bowie A., Ashton H., McAvoy B., Spencer I., Brodie J. et al Randomised controlled trial of two brief interventions against long‐term benzodiazepine use: outcome of intervention. Addict Res Theory 2004; 12: 141–154. [Google Scholar]
- 51. Heather N., Paton J., Ashton H. Predictors of response to brief intervention in general practice against long‐term benzodiazepine use. Addict Res Theory 2011; 19: 519–527. [Google Scholar]
- 52. Ashton H. The treatment of benzodiazepine dependence. Addiction 1994; 89: 1535–1541. [DOI] [PubMed] [Google Scholar]
- 53. Manheimer E.G.J. Chapter 6: Searching for studies . In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at: www.cochrane‐handbook.org (accessed 20 April 2017).
- 54. Higgins J. A. D., Sterne J.A.C. Chapter 8: Assessing risk of bias in included studies . In: J.P.T. Higgins, Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at www.handbook.cochrane.org (accessed 15 May 2017).
- 55. Albrecht L., Archibald M., Arseneau D., Scott S. D. Development of a checklist to assess the quality of reporting of knowledge translation interventions using the workgroup for intervention development and evaluation research (WIDER) recommendations. Implement Sc 2013; 8: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Workgroup for Intervention Development and Evaluation Research (WIDER) . Improving reporting of behavioural interventions: WIDER consensus statement. 2009.
- 57. Higgins J.P.T., Eldridge S., Li T Chapter 23: Including variants on randomized trials. In: J.P.T. Higgins, Thomas J., Chandler J. et al, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available at: www.training.cochrane.org/handbook (accessed 12 November 2019).
- 58. Julian P.T. Higgins S.E., Li Tianjing. Chapter 23: Including variants on randomized trials. In J.P.T. Higgins, Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at: www.handbook.cochrane.org (accessed 12 November 2019).
- 59. Deeks J.J., Higgins J.P.T. and Altman D.G. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins J.P.T., Deeks, J.J., Altman D.G., editors. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at: www.handbook.cochrane.org (accessed 4 April 2019).
- 60. Sterne JAC EM, Moher D. Chapter 10: Addressing reporting biases. In: J.P.T. Higgins, Green S., editors. Cochrane Handbook for Systematic Reviews of Intervention, version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at www.handbook.cochrane.org (accessed 12 November 2019).
- 61. Bashir K., King M., Ashworth M. Controlled evaluation of brief intervention by general practitioners to reduce chronic use of benzodiazepines. Br J Gen Pract 1994; 44: 408–412. [PMC free article] [PubMed] [Google Scholar]
- 62. Cormack M. A., Sweeney K. G., Hughes‐Jones H., Foot G. A. Evaluation of an easy, cost‐effective strategy for cutting benzodiazepine use in general practice. Br J Gen Pract 1994; 44: 5–8. [PMC free article] [PubMed] [Google Scholar]
- 63. Vicens C., Fiol F., Llobera J., Campoamor F., Mateu C., Alegret S. et al Withdrawal from long‐term benzodiazepine use: randomised trial in family practice. Br J Gen Pract 2006; 56: 958–963. [PMC free article] [PubMed] [Google Scholar]
- 64. Kuntz J. L., Kouch L., Christian D., Hu W., Peterson P. L. Patient education and pharmacist consultation influence on nonbenzodiazepine sedative medication deprescribing success for older adults. Perm J 2019; 23: 18–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Navy H. J., Weffald L., Delate T., Patel R. J., Dugan J. P. Clinical pharmacist intervention to engage older adults in reducing use of alprazolam. Consult Pharm 2018; 33: 711–722. [DOI] [PubMed] [Google Scholar]
- 66. Tannenbaum C., Martin P., Tamblyn R., Benedetti A., Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174: 890–898. [DOI] [PubMed] [Google Scholar]
- 67. Vicens C., Bejarano F., Sempere E., Mateu C., Fiol F., Socias I. et al Comparative efficacy of two interventions to discontinue long‐term benzodiazepine use: cluster randomised controlled trial in primary care. Br J Psychiatry 2014; 204: 471–479. [DOI] [PubMed] [Google Scholar]
- 68. Michie S., van Stralen M. M., West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011; 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Craig P., Dieppe P., Macintyre S., Michie S., Nazareth I., Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud 2013; 50: 587–592. [DOI] [PubMed] [Google Scholar]
- 70. Martin P., Tannenbaum C. A realist evaluation of patients’ decisions to deprescribe in the EMPOWER trial. BMJ Open 2017; 7: e015959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stewart D., Klein S. The use of theory in research. Int J Clin Pharm 2016; 38: 615–619. [DOI] [PubMed] [Google Scholar]
- 72. Godfrey C., Heather N., Bowie A., Brodie J., Parrott S., Ashton H. et al Randomised controlled trial of two brief interventions against long‐term benzodiazepine use: cost‐effectiveness. Addict Res Theory 2008; 16: 309–317. [Google Scholar]
- 73. Vicens C., Sempere E., Bejarano F., Socias I., Mateu C., Fiol F. et al Efficacy of two interventions on the discontinuation of benzodiazepines in long‐term users: 36‐month follow‐up of a cluster randomised trial in primary care. Br J Gen Pract 2016; 66: e85–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. de Gier N. A., Gorgels W. J., Lucassen P. L., Oude V. R., Mulder J., Zitman F. Discontinuation of long‐term benzodiazepine use: 10‐year follow‐up. Fam Pract 2011; 28: 253–259. [DOI] [PubMed] [Google Scholar]
- 75. Sirdifield C., Chipchase S. Y., Owen S., Siriwardena A. N. A systematic review and meta‐synthesis of patients’ experiences and perceptions of seeking and using benzodiazepines and Z‐drugs: towards safer prescribing. Patient 2017; 10: 1–15. [DOI] [PubMed] [Google Scholar]
- 76. Cane J., Richardson M., Johnston M., Ladha R., Michie S. From lists of behaviour change techniques (BCTs) to structured hierarchies: comparison of two methods of developing a hierarchy of BCTs. Br J Health Psychol 2015; 20: 130–150. [DOI] [PubMed] [Google Scholar]
- 77. Parr J. M., Kavanagh D. J., Young R. M., McCafferty K. Views of general practitioners and benzodiazepine users on benzodiazepines: a qualitative analysis. Soc Sci Med 2006; 62: 1237–1249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.


