Abstract
Background
As the face is known for its extreme variation in vascular anatomy and the number of filler‐associated complications due to intra‐arterial injection is increasing, we are in need of a method to visualize anyone’s individual arterial anatomy of the face in a completely harmless way.
Aims
The different medical imaging methods and a recently developed MRA protocol are reviewed.
Methods
The literature of the last twenty years—with special attention for the last five years—concerning the different medical imaging modalities of the facial arteries was reviewed.
Results
A harmless visualisation of the facial arteries is currently only possible with US or MRA. US may identify single vessels but never the complete arterial network. A combination of IR “heat enhancement” and a MRA 3D‐TOF sequence might make it feasible to visualize a large number of facial arteries in a risk‐free, radiation‐free, contrast‐free and non‐invasive way.
Conclusion
Currently, a new combination of IR “heat enhancement” and a MRA 3D‐TOF sequence might be the only method to visualize a large number of facial arteries.
Keywords: cosmetic procedure, filler, MRI, ultrasound
1. INTRODUCTION
Due to the incremental use of soft tissue fillers (STF) for facial rejuvenation, mostly hyaluronic acid (HA) 1 —an increasing number of adverse reactions related to vascular compression or intravascular injection is observed. 2 In spite of the potential reversibility of the inadvertent HA injection with hyaluronidase (which has the ability to dissolve HA), 3 vascular occlusion may persist. Even in the hands of experienced practitioners respecting the safety procedures, 4 , 5 intra‐arterial injection of STF may cause embolization and may lead to localized skin necrosis, 6 blindness, 7 and even cerebral artery embolism. 8 It is clear that a thorough knowledge of the complex anatomy of the facial arteries is essential when planning any facial procedure, whether it be for aesthetic or reconstructive purposes. 9 , 10 However, the vascular anatomy of the face is extremely variable. Not only is the arterial course very tortuous, the localization of the facial arteries may vary from person to person. 11 So performing any surgical procedure of the face with standard text book anatomy is—although essential—often not enough. This is especially true for STF injections, where the risk of injection‐related complications is mostly related to the variability in vascular facial anatomy and the inability to see the individual arterial anatomy when performing percutaneous injections. Therefore, there is a high need for a (preferably harmless) imaging technique to visualize these individual anatomical variations in facial rejuvenation procedures with STF due to the increasing number of complications. 12
2. TECHNIQUES
In order to procure a reliable image of the important facial arteries during the planning of facial surgery or STF injections, several techniques have been proposed.
High‐resolution ultrasonography (US) is a non‐invasive imaging technique. Color Doppler and spectral analysis may be added in order to facilitate the visualization of the small facial arteries, and depth measurements may allow a detailed local analysis of (a small part of) the vessel (Figure 1). However, US cannot visualize a wide area, nor the complex 3D structure of the vasculature, it is operator‐dependent and also very time‐consuming. 13 The branches of the facial arteries show tortuous courses along different planes and in all directions, and they have slow flow rates. Hence, obtaining a complete 3D overview of the facial vascular anatomy using US is currently not feasible. Until now, no 3D US probes have been developed that are able to visualize the tortuous facial artery network, which makes is currently impossible to see more than one vessel at the time. Also, performing an US to look for blood vessels and doing an STF injection simultaneously is tedious. Only in experienced hands, it is sometimes feasible to visualize both the tip of the injection needle or cannula and the tortuous vessel in one image and even then the 2D imaging is often insufficient. Moreover, injecting STF yields an immediate result, which is hard to appreciate with the US probe held onto the skin. Additionally, the sterility of an STF injection might be compromised as the needle or cannula often touches the non‐sterile US probe. US guidance might however be a very useful tool for the treatment of intravascular STF injections, as the obstructed vessel might be retrieved and injected with hyaluronidase in case that HA is used as an STF, 14 although it requires a certain degree of experience.
FIGURE 1.
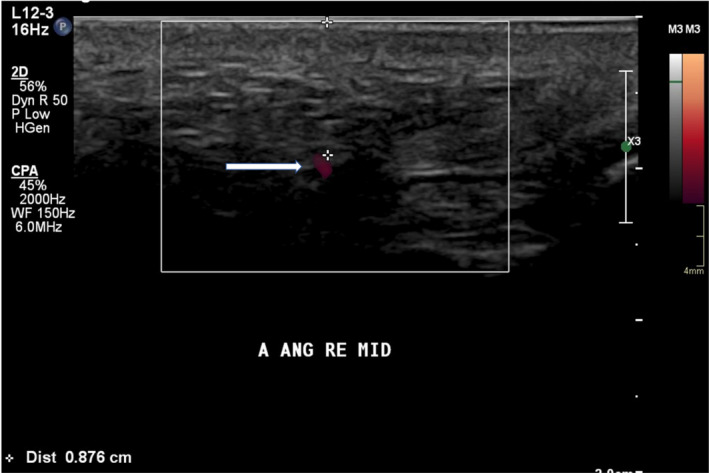
US with Doppler analysis of a part of the right angular artery. The exact depth of the artery may be measured on the cross‐sectional image
The most precise evaluation technique for the facial arteries remains conventional angiography (CA) using contrast medium. During this procedure, a small catheter is inserted in an artery (mostly) of the groin and threaded through the vascular network to reach the facial artery. However, this is not a riskless procedure, as bleeding at the puncture site, damage to the vessel wall and thrombus formation may occur. Moreover, the risk of a focal neurological deficit caused by CA ranges between 0.14% and 0.5%. 15 This means that up to 1 in 200 patients may suffer from a cerebral attack during a CA.
CT angiography (CTA) is also a very useful tool to illustrate the 3D structure of the vessels. Although vessel catheterization is avoided, there is still a risk of complications due to the use of iodine contrast medium and the radiation exposure (overall patient risk per CTA procedure ranges between 15 and 36 cancer risks per 1 million procedures). 16
Magnetic resonance angiography (MRA) may use IV contrast to enhance the vessels, but the need for an IV gadolinium injection entails potential risk for the patient.
However, in bright‐blood methods the signal from the moving protons is accentuated relative to the stationary protons of the surrounding tissue. 17 , 18 These flow‐based techniques include mainly Time of Flight (TOF), Phase Contrast (PC), and Fast Spin Echo Imaging (FSE). 19 TOF is the most time‐efficient method for obtaining MRA images. 20 , 21 A single measurement is performed, with the stationary tissue signal suppressed relative to the flowing tissue signal. 22 Maximum intensity projections (MIP) are used to visualize the MRA data. TOF MRA has until recently never been successfully used for the visualization of all the facial blood vessels. 23 This is mainly due to the tortuous arterial courses along different planes and directions, which challenges the use of TOF to a greater extent.
However, a harmless MRA 24 would ideally be the method of choice for the visualization of the facial arterial network. Recently, a combined technique of infrared (IR) facial heating and MRA (3D‐TOF MOTSA) 25 was proposed. Images may be acquired on a 1.5 or 3 Tesla (T) full‐body MR system, using a dedicated head coil. Additionally, a flexible wrap‐around surface coil may be mounted on top of the head coil (Figure 2) in order to increase the signal reception from the facial vessels. Before the 3D TOF MRA examination, which is known to be flow‐dependent, the patient is positioned with closed eyes in front of an IR light source (300 W with an UV filter) during 10 minutes. This should induce vasodilatation and enhance vascular flow. At the same time, the patient is asked to stimulate the facial muscles by slowly moving the lips and forehead and switching between several facial expressions during the exposure time in order to further enhance the arteries (by vascular dilatation and increased flow speed due to muscle activation). A 3D‐TOF MOTSA MRA sequence is acquired in an oblique coronal plane (Figure 3). The MRA protocol is summarized in Table 1. During the acquisition, the patient is asked to remain completely still. A multislab technique is used to reduce the saturation effect of the inflowing blood signal. MIP images are made in a sagittal plane. The MRA allows visualization of the important facial arteries (facial (F); angular (A); superior (SL) and inferior labial (IL); lateral nasal (LN); dorsal nasal (DN); supratrochlear (STr); supraorbital (SO); and superficial temporal (ST) artery) (Figure 4).
FIGURE 2.
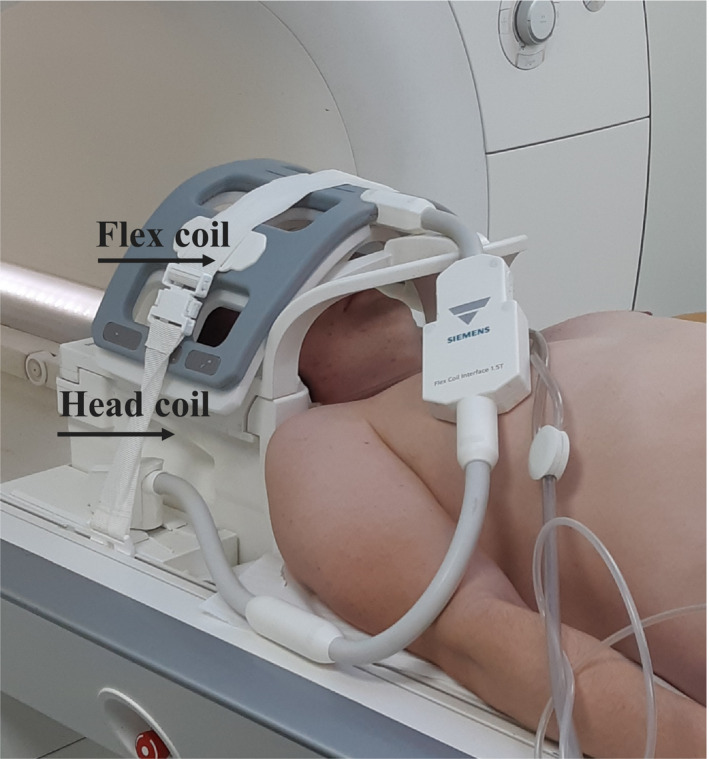
Position of the head and flex coil for the MRA
FIGURE 3.
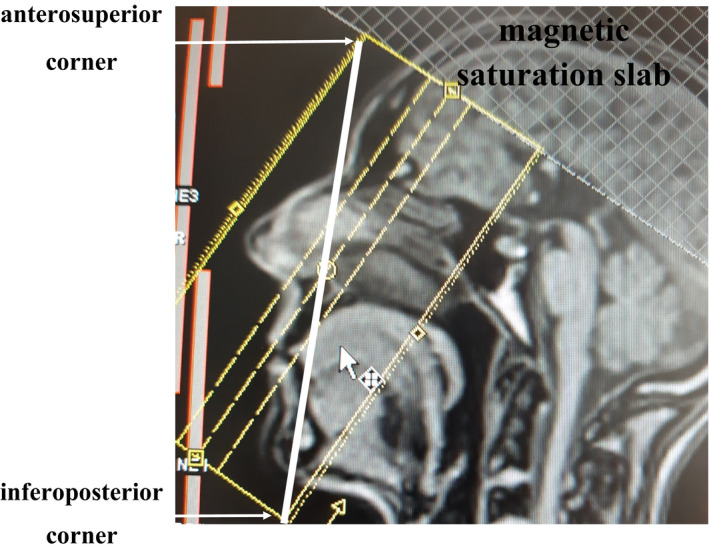
Positioning of the 3D‐TOF MOTSA MRA slabs block on the localizer. The line drawn from the glabella to the chin transects the slabs position block from the anterosuperior corner to the inferoposterior corner. A magnetic saturation slab is positioned above the slabs block
TABLE 1.
3D TOF MOTSA 1.5 T sequence
| TR | 30 ms |
| TE | 6.8 ms |
| Acquisitions | 1 |
| FOV | 180 mm |
| Flip angle | 30° |
| Matrix | 180 × 180 pixels |
| Slice thickness | 0.5 mm |
| Averages | 2 |
| SNR | 1.0 |
| Voxel size | 0.4 × 0.4 × 0.5 mm |
| Time of acquisition | 16 min 14 s |
Gradient echo sequence with 5 overlapping (17.5%) slabs.
Abbreviations: TE, time of echo; TR, time of repetition.
FIGURE 4.
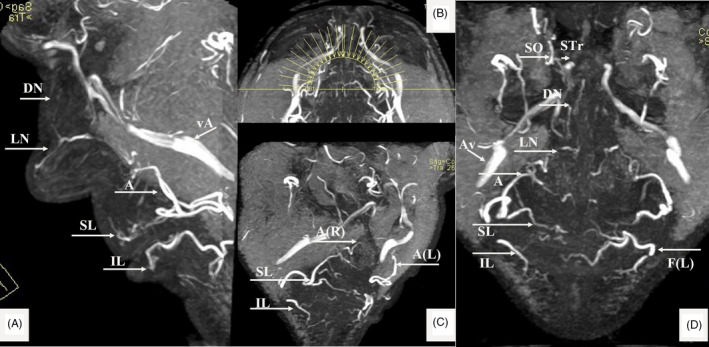
MRA findings (MIP of 3D‐TOF) in a 58‐y‐old male. Superior (SL) and Inferior Labial (IL), Angular (A); Lateral Nasal (LN); Dorsal Nasal (DN); Supratrochlear (STr); Supraorbital (SO) and Facial artery (F); Angular vein (Av); (R) Right and (L) Left. (A) Lateral view. (B) The MIP—reconstruction levels shown on the axial view. (C) Right oblique view with details of the labial arteries. (D) AP view of the (annotated) arteries
Peri‐orbital artifacts may hinder the visualization of the peri‐orbital vessels, and in patients with dental wires (after having braces), the perioral vessels may be hard to see. There is some evidence in the literature that also calcium hydroxylapatite‐based dermal fillers (CaHA) are visible on MRI immediately after injection as a heterogeneous intermediate signal intensity on both T1‐ and T2‐weighted sequences. 26 However, in one case report, there was no signal on MRI anymore after 2.5 years 27 due to the complete biodegradability of CaHA. 1.5 T MRA also are more susceptible to motion artifacts due to the longer examination time. Although the reaction of the skin (red color) to the IR exposure will be visible, no adverse reactions due to the “IR enhancement” are noted, nor mentioned. The only (sometimes confusing) venous structure—running more posteriorly and laterally outside of the field of interest—is the large angular vein (Figure 4).
3D PC MRA (Figure 5) may be an alternative, but the mixture of venous and arterial structures makes PC MRA less suitable for this specific purpose. Moreover, the high variability in blood flow in the different large and small arterial vessels of the face speed may hinder the choice of an optimal velocity encoding (Venc) factor.
FIGURE 5.
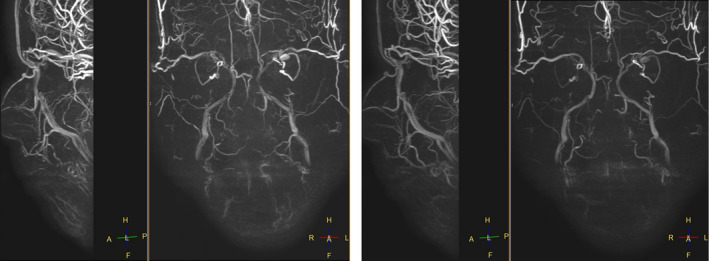
3D PC MRA: a lot of venous interference with low speed factor selection (Right Venc 5) and less obvious visualization of low flow speed arteries at higher speed factor selection (Left Venc 10)
MRA images may be repeatedly projected on the face of the patient in preparation of or during the STF injections. At the moment, there are no prospective cohort studies looking at the arterial anatomy of the face. There is some evidence that small vessels might become more tortuous with age, but their location and anatomical variants do not change. It is certainly advisable to repeat the MRI after extensive facial modifications such as extensive facial trauma, tumor surgery, or deep plane face lifts. We intend to perform a new MRI on our test cohort of patients in 7‐10 years in order to verify the location and possible changes in anatomy.
Currently, as the MRI is projected on the patients' face, unfortunately the 3D depth aspect of the information is lost. We are currently working on a better visualization system to keep this additional information during the injection (work in progress).
Reimbursement of these MRI examinations depends on the country and the local insurance system. In some countries, preventive radiologic exams may be reimbursed by the health system as a safety procedure. This may increase as the awareness of the number of complications is growing. 28 However, if privately funded, an MRI will cost on average about 300 to 600 USD, which represents approximately the mean price of one filler.
3. CONCLUSION
A harmless visualization of the facial arteries is currently only possible with US or MRA. High‐resolution US may identify single vessels but never the complete 3D arterial network of the face. Moreover, it is quite operator‐dependent and simultaneous use of US and STF injections remains cumbersome. In order to fill in the high need for information about the individual patient's anatomy prior to facial rejuvenation and therefore better plan and execute these procedures, a combination of IR “heat enhancement” and a MRA 3D‐TOF sequence might make it feasible to visualize a large number of facial arteries in a risk‐free, radiation‐free, contrast‐free, and non‐invasive way.
CONFLICT OF INTEREST
None.
Mespreuve M, Hendrickx B, Waked K. Visualization techniques of the facial arteries. J Cosmet Dermatol.2021;20:386–390. 10.1111/jocd.13477
REFERENCES
- 1. La Gatta A, De Rosa M, Frezza MA, Catalano C, Meloni M, Schiraldi C. Biophysical and biological characterization of a new line of hyaluronan‐based dermal fillers: a scientific rationale to specific clinical indications. Mater Sci Eng C. 2016;68:565‐572. [DOI] [PubMed] [Google Scholar]
- 2. Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447‐458. [DOI] [PubMed] [Google Scholar]
- 3. Abduljabbar MH, Basendwh MA. Complications of hyaluronic acid fillers and their managements. J Dermatology Dermatologic Surg. 2016;20:100‐106. [Google Scholar]
- 4. Scheuer JF, Sieber DA, Pezeshk RA, Gassman AA, Campbell CF, Rohrich RJ. Facial danger zones: techniques to maximize safety during soft‐tissue filler injections. Plast Reconstr Surg. 2017;139:1103‐1108. [DOI] [PubMed] [Google Scholar]
- 5. Van Loghem JAJ, Fouché JJ, Thuis J. Sensitivity of aspiration as a safety test before injection of soft tissue fillers. J Cosmet Dermatol. 2018;17:39‐46. [DOI] [PubMed] [Google Scholar]
- 6. Inoue K, Sato K, Matsumoto D, Gonda KD, Yoshimura K. Arterial embolization and skin necrosis of the nasal ALA following injection of dermal fillers. Plast Reconstr Surg. 2008;121:128. [DOI] [PubMed] [Google Scholar]
- 7. Thanasarnaksorn W, Cotofana S, Rudolph C, Kraisak P, Chanasumon N, Suwanchinda A. Severe vision loss caused by cosmetic filler augmentation: case series with review of cause and therapy. J Cosmet Dermatol. 2018;17:712‐718. [DOI] [PubMed] [Google Scholar]
- 8. Hong JH, Ahn SJ, Woo SJ, et al. Central retinal artery occlusion with concomitant ipsilateral cerebral infarction after cosmetic facial injections. J Neurol Sci. 2014;346:310‐314. [DOI] [PubMed] [Google Scholar]
- 9. von Arx T, Tamura K, Yukiya O, Lozanoff S. The face – a vascular perspective. A literature review. Swiss Dent J. 2018;128:382‐392. [DOI] [PubMed] [Google Scholar]
- 10. Cotofana S, Lachman N. Arteries of the face and their relevance for minimally invasive facial procedures: an anatomical review. Plast Reconstr Surg. 2019;143:416‐426. [DOI] [PubMed] [Google Scholar]
- 11. Pilsl U, Anderhuber F, Neugebauer S. The facial artery‐the main blood vessel for the anterior face? Dermatol Surg. 2016;42(2):203‐208. [DOI] [PubMed] [Google Scholar]
- 12. DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthetic Surg J. 2014;34:584‐600. [DOI] [PubMed] [Google Scholar]
- 13. Safran T, Gorsky K, Viezel‐Mathieu A, Kanevsky J, Gilardino MS. The role of ultrasound technology in plastic surgery. J Plast Reconstr Aesthetic Surg. 2018;71:416‐424. [DOI] [PubMed] [Google Scholar]
- 14. Schelke LW, Decates TS, Velthuis PJ. Ultrasound to improve the safety of hyaluronic acid filler treatments. J Cosmet Dermatol. 2018;17(6):1019‐1024. [DOI] [PubMed] [Google Scholar]
- 15. Heiserman JA, Dean BL. Interventional neurovascular disease: avoidance and management of complications and review of the current literature. J Neurosurg Sci. 2011;55:233‐242. [PubMed] [Google Scholar]
- 16. Alkhorayef M, Babikir E, Alrushoud A, Al‐Mohammed H, Sulieman A. Patient radiation biological risk in computed tomography angiography procedure. Saudi J Biol Sci. 2017;24:235‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartung MP, Grist TM, François CJ. Magnetic resonance angiography: current status and future directions. J Cardiovasc Magn Reson. 2011;13:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanaka T, Oda M, Kito S, et al. Noninvasive identification of peripheral vessels of oral and maxillofacial regions by using electrocardiography‐triggered three‐dimensional fast asymmetric spin‐echo sequences. Oral Surgery Oral Med Oral Pathol Oral Radiol Endodontology. 2011;112:493‐501. [DOI] [PubMed] [Google Scholar]
- 19. Sumi T, Sumi M, Van Cauteren M, Kimura Y, Nakamura T. Parallel imaging technique for the external carotid artery and its branches: comparison of balanced turbo field echo, phase contrast, and time‐of‐flight sequences. J Magn Reson Imaging. 2007;25:1028‐1034. [DOI] [PubMed] [Google Scholar]
- 20. Oda M, Tanaka T, Kito S, et al. Magnetic resonance angiography using fresh blood imaging in oral and maxillofacial regions. Int J Dent. 2012;2012:865369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jia JM, Guo H, Huo WJ, et al. Preoperative evaluation of patients with hemifacial spasm by three‐dimensional time‐of‐flight (3D‐TOF) and three‐dimensional constructive interference in steady state (3D‐CISS) sequence. Clin Neuroradiol. 2016;26:431‐438. [DOI] [PubMed] [Google Scholar]
- 22. Brown MA, Semelka RC. MRI: Basic Principles and Applications (2nd ed). New York, NY: Wiley‐Liss; 1999. [Google Scholar]
- 23. van den Berg R, Wasser MN, Van Gils AP, van der Mey AG, Hermans J, van Buchem MA. Vascularization of head and neck paragangliomas: comparison of three MR angiographic techniques with digital subtraction angiography. Am J Neuroradiol. 2000;21:162‐170. [PMC free article] [PubMed] [Google Scholar]
- 24. Koban KC, Cotofana S, Frank K, et al. Precision in 3‐dimensional surface imaging of the face: a handheld scanner comparison performed in a cadaveric model. Aesthetic Surg J. 2019;39:NP36‐NP44. [DOI] [PubMed] [Google Scholar]
- 25. Hendrickx B, Waked K, Mespreuve M. Infrared thermally enhanced 3D‐TOF MRA imaging for the visualization of the arteries of the face. Aesthet Surg J Open Forum. 2020:ojaa020 10.1093/asjof/ojaa020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feeney JN, Fox JJ, Akhurst T. Radiological impact of the use of calciumhydroxylapatite dermal fillers. Clin Radiol. 2009;64:897‐902. [DOI] [PubMed] [Google Scholar]
- 27. Pavicic T. Complete biodegradable nature of calcium hydroxylapatite after injection for malar enhancement: an MRI study. Clin Cosmet Investig Dermatol. 2015;8:19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Girolamo M, Mattei M, Signore A, Grippaudo RF. MRI in the evaluation of facial dermal fillers in normal and complicated cases. Eur Radiol. 2015;25:1431‐1442. [DOI] [PubMed] [Google Scholar]


