Abstract
Background
Whether decreased physical functioning of patients with mitochondrial disease (MD) is related to altered body composition or low protein intake needs clarification at the background of the nutrition state.
Methods
In this 2‐site cross‐sectional study, MD patients were age‐, body mass index (BMI)–, and gender‐matched to controls. Body composition was assessed by dual‐energy x‐ray absorptiometry. Physical functioning was measured by handgrip strength, 6‐minute walking test, 30‐second sit‐to‐stand test (30SCT), and 6‐minute mastication test. Total daily protein intake was calculated by 3‐day food records. Malnutrition was assessed by Patient‐Generated Subjective Global Assessment and the Global Leadership Initiative on Malnutrition (GLIM) criteria and sarcopenia by the 2018 consensus. Data were analyzed using independent samples t‐tests, Fisher exact test, and Spearman and Pearson correlation coefficients.
Results
Thirty‐seven MD patients (42 ± 12 years, BMI: 23 ± 4 kg/m2, 59% females) and 37 matched controls were included. Handgrip strength was moderate, inversely related to fat mass index in both MD patients and controls, whereas it correlated with fat‐free mass index in controls solely. Protein intake was associated with muscle strength (handgrip strength and 30SCT) in MD patients but not in controls. Twenty‐seven MD patients (73%) were malnourished, and 5 (14%) were classified as sarcopenic.
Conclusions
Muscle strength is related to body composition and protein intake in MD patients. This, in combination with the high incidence of both malnutrition and sarcopenia, warrants individual nutrition assessment in MD patients.
Keywords: BIA, body composition, DXA, handgrip strength, malnutrition, mitochondrial disease, nutrition assessment, physical functioning, sarcopenia
Clinical Relevancy Statement
Physical functioning is chronically impaired in adult patients with mitochondrial disease, implicating decreased quality of life. Insights in the relation between protein intake, body composition, and physical functioning in these patients and the prevalence of malnutrition and sarcopenia could help to improve patient care.
Introduction
Approximately 1 out of 5000 persons worldwide is confronted with mitochondrial disease (MD), 1 a genetic neuromuscular chronic disorder causing intracellular energy (adenosine triphosphate) shortage. 1 As MD patients experience a wide range of complaints, such as fatigue, muscular weakness, gastrointestinal complaints, dysphagia, and exercise intolerance, 2 , 3 treatment focuses on symptom relief, securing physical functioning, and quality of life. 2
Poor physical functioning is predicted by a body weight that is either too low or too high, 4 , 5 , 6 , 7 conditions that are frequently observed in MD patients. 8 , 9 , 10 , 11 , 12 Also, body composition is known to be an important factor in physical functioning in the general population as well as in neuromuscular disorders in particular. 6 , 7 , 13 , 14 Decreased physical functioning was related to an increased fat mass in patients with myotonic dystrophy 13 or with decreased muscle mass in patients with muscular dystrophy. 6 , 7 , 15 In patients with chronic symptoms similar to MD, like kidney disease, 16 lower fat‐free mass and decreased muscle strength were associated. An increased muscle mass and strength may also be related to lower diabetes risk. 17 Altered body composition in MD patients has been reported, 9 , 12 and higher skeletal muscle mass index in these patients was correlated with higher muscle strength. 12 However, whether physical functioning is related to fat mass has not been established yet.
It is known that physical activity improves physical function and body composition. 18 The decreased physical activity in MD patients 10 is likely to play a role in body composition and physical functioning in these patients. However, it is not known whether MD patients’ physical functioning is decreased because of altered body composition and/or if this possibly is influenced by nutrition intake. It is know that physical function in MD patients can be improved by physical exercise 19 ; therefore, exercise is part of standard care in MD patients. 20
The combination of low muscle mass and low muscle strength seen in MD patients may be defined as sarcopenia. 21 , 22 Sarcopenia significantly reduces functioning, as well as survival. 21 , 23 Therefore, it is relevant to know whether sarcopenia is also present in MD patients in order to adjust treatment accordingly. 24
The low physical functioning in MD patients may also be related to alterations in nutrition intake and nutrition status. Recent observational studies suggested that MD patients have inadequate protein intake and are at risk of malnutrition. 9 , 24 , 25 , 26 This could affect both body composition and physical functioning. Diagnosing malnutrition in MD patients is challenging. 9 , 27 Standard screening tools aimed at screening acute malnutrition are not applicable in MD patients, as these patients suffer from chronic malnutrition. 24 Recent literature advises to perform nutrition assessment to determine nutrition state in MD patients, 9 , 24 , 26 but it is not known which measurements are valid or which cutoff values should be applied.
The primary goal of this study is to explore the association between physical functioning, protein intake, and body composition in adult MD patients. Additionally, the prevalence of malnutrition and sarcopenia in this MD population is assessed using various nutrition assessment tools. Finally, the diagnostic accuracy of bioelectrical impedance analysis (BIA) to determine body composition in MD patients was tested.
Methods
In this 2‐site, cross‐sectional study, the associations between physical functioning, protein intake, and body composition were examined in MD patients compared with age‐, body mass index (BMI)–, and gender‐matched controls. The study protocol was approved by the ethics committee of the Nijmegen‐Arnhem region (NL58262.091.16/2016‐2667).
Primary and Secondary End Points
The primary end point was the association between physical functioning, protein intake and body composition in MD patients. The secondary end point is the prevalence of malnutrition and sarcopenia in MD patients.
Study Population
Genetically confirmed MD patients and healthy controls were included if they (1) were ≥18 years of age and (2) had signed informed consent. Controls were matched with included MD patients for age (±5 years), BMI (±2 kg/m2), and gender. Exclusion criteria were (1) a pacemaker or implant, (2) pregnancy or lactation, (3) disordered hydration status (edema, dehydration), (4) diagnosis of a (chronic) disease interfering with the nutrition assessment, or (5) acute illness and fever. Additionally, MD patients were excluded in case of (1) non–genetically confirmed MD diagnosis, (2) diagnosis of none myopathic MD phenotype, or (3) unavailability of dual‐energy x‐ray absorptiometry (DXA) and handgrip‐strength data.
A sample size of 37 MD patients and 37 controls was calculated using data of MD patients (n = 24) and compared with the reference values of Dodds 28 to show a difference of physical functioning between MD patients and controls with a power of 0.80 and an α of 0.05 based on an estimated dropout rate of 30%, using the handgrip strength as primary outcome variable for physical functioning.
Data Collection
MD patients’ data were prospectively obtained from the Radboudumc's MD expertise center (Radboud Centre for Mitochondrial Medicine [RCMM]) from March 2015 until January 2017. MD patients were measured during a 4‐day multidisciplinary evaluation program (as usual care) in the internal medicine ward. Controls were measured at the Nutritional Assessment Lab of the HAN University of Applied Sciences between August 2017 and July 2018.
Demographic and Disease Characteristics
Age (years) and gender were collected from electronic records in MD patients or obtained at day of measurement in controls. In addition, MD patients’ genotype and phenotype as well as the presence of dysphagia or gastrointestinal problems were registered.
Physical Functioning
Physical functioning was assessed according to the applicable standard operating procedures using 4 tools relevant for MD patients. Muscle strength was measured by handgrip strength (kg) 21 , 29 and 30‐second sit‐to‐stand test (30SCT; number of sit‐to‐stands). 30 Endurance was measured by the 6‐minute walk test (6MWT; meters) 21 , 31 and the 6‐minute mastication test (6MMT; number of chew cycles). 32
Anthropometry and Body Composition
Height (cm), weight (kg), and waist circumference (cm) were measured to the nearest 1 decimal point. Total fat mass (kg), total appendicular skeletal muscle mass (kg), and regional lean tissue mass (kg) were determined by whole‐body DXA (Radboudumc: Hologic, model Discovery A S/N 85606; HAN: Hologic, model Horizon W S/N 200103). 33 Fat mass, appendicular skeletal muscle mass, and regional lean tissue mass were normalized by dividing total mass (kg) by height (m) squared into fat mass index (kg/m2), skeletal muscle index (kg/m2), and regional lean tissue mass index (kg/m2), respectively.
Additionally, BIA measurements were performed according to clinical practice (Bodystat MDD 1500, 50 Hz). Fat‐free mass (kg) was calculated twice using (1) Kyle formula, 34 having the smallest standard error and highest R 2, 34 and (2) Dey, being validated in the elderly. 35
The diagnostic accuracy of the BIA was tested using the DXA as the golden standard. The sensitivity and specificity were determined using the cutoff point of <15 kg/m2 and <17 kg/m2 for fat‐free mass index in women and men, respectively, 36 and obesity as fat percentage >30% for women and >25% for men. 37
Nutrition Intake
Nutrition intake was assessed by either a 3‐day food record or, in case missing in clinic, a dietary history using the nutrition calculation program Madows. Mean energy (kcal/d) and protein (g/kg body weight/d) intake were calculated. Number of persons on a specific diet were registered.
Malnutrition and Sarcopenia
Malnutrition was assessed by Patient‐Generated Subjective Global Assessment (PG‐SGA) (score of ≥4 was interpreted as risk for malnutrition and ≥9 as malnourished) 38 and the global leadership initiative on malnutrition (GLIM) criteria. 29 Sarcopenia was classified using handgrip strength. The cutoff point for 70‐year‐olds 21 as well as the actual age cutoff point for low handgrip strength 28 was applied, as the majority of MD patients are younger than 70 years. Sarcopenic obesity was diagnosed according to Baumgartner. 39
Data Management
Data were entered encoded in an online case report form (Castor, CIWIT B.V., Amsterdam, the Netherlands) and double‐checked visually by 2 researchers.
Statistical Analysis
Data were reported as means ± SD, median and interquartile range, or frequencies and percentage of the group or total population, if applicable. Normal distribution of the variables was assessed by Shapiro‐Wilk tests. Differences between MD patients and controls were tested using the independent t‐test or Mann‐Whitney U tests for continuous variables and Pearson χ2 test or Fisher exact test for categorical variables.
Pearson or Spearman correlation coefficients were determined to assess the relations between physical functioning, (regional) body composition, and protein intake. Because fat‐free mass index and skeletal muscle mass index were associated, only fat‐free mass index was used in association analysis. Statistical analyses were performed using SPSS statistics (IBM Statistics 23). To correct for multiple testing, P‐values ≤ .02 were considered statistically significant for all analyses.
Results
Thirty‐seven MD patients and 37 matched controls (all white) were included in this study (Figure 1). Mean age was 42 ± 12 years, and 59% were female (Table 1). Despite having similar BMI, MD patients had a shorter stature compared with controls. Compared with controls, MD patients were on a (diabetes) diet and experienced gastrointestinal problems or dysphagia more often. The majority of the MD patients were diagnosed with the m.3243A>G mutation (78%) (Table 2).
Figure 1.
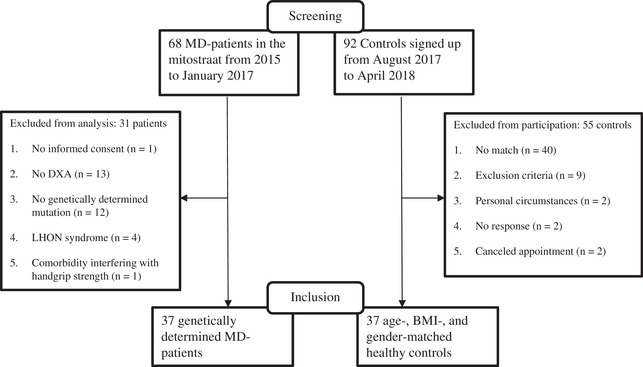
Screening flowchart of MD patients and controls in the DYNAMO study. BMI, body mass index; DXA, dual‐energy x‐ray assessment; LHON, Leber's hereditary optic neuropathy; MD, mitochondrial disease
Table 1.
Demographics of MD Patients and Controls
| Demographics | MD patients (n = 37) | Controls (n = 37) | P‐value |
|---|---|---|---|
| Age, y (mean ± SD) | 42 ± 13 | 42 ± 12 | .84 |
| Female (n/%) | 22 (59%) | 22 (59%) | |
| BMI, kg/m b , (median: IQR) | 21.9: 19.9–24.5 | 23.0: 21.3–25.0 | .54 |
| BMI categories (n/%) | |||
| <18.5, kg/m b | 2 (5%) | 0 (0%) | |
| <20, kg/m 2 | 9 (24%) | 4 (11%) | |
| 20–25, kg/m 2 | 20 (54%) | 23 (62%) | |
| 25–30, kg/m 2 | 6 (16%) | 10 (27%) | |
| >30, kg/m 2 | 2 (5%) | 0 (0%) | |
| Weight, kg (mean ± SD) | 66.6 ± 12.3 | 72.2 ± 8.9 | .04 |
| Weight loss a (n/%) | 4 (11%) | 2 (5%) | .39 |
| Height, cm (mean ± SD) | 170.8 ± 1.0 | 176.4 ± 0.9 | .01 |
| Waist circumference, cm, (mean ± SD) | 84.3 ± 11.5 | 81.8 ± 8.0 | .30 |
| High waist circumference b (n/%) | 14 (38%) | 12 (32%) | .46 |
| Diet (n/%) | 25 (68%) | 2 (5%) | <.001 |
| Gastrointestinal problems (n/%) | 28 (76%) | 5 (13.5%) | <.001 |
| Dysphagia (n/%) | 18 (49%) | 0 (0%) | <.001 |
P‐values ≤.02 (in bold) were considered significant.
BMI, body mass index; IQR, interquartile range; MD, mitochondrial disease.
Weight loss according to Global Leadership Initiative on Malnutrition (GLIM) criteria: >5% within past 6 months or >10% beyond 6 months. 29
High waist circumference >94 cm (women) and >80 cm (men) according to World Health Organization (WHO) 2008. 43
Table 2.
Disease Characteristics of Mitochondrial Disease Patients (n = 37)
| Disease Characteristics | Frequency (n) | Proportion (%) |
|---|---|---|
| Genotype | ||
| m.3243A>G | 29 | 78 |
| Point mutations (mtDNA) | 5 | 14 |
| nDNA mutation | 2 | 5 |
| Deletion mtDNA | 1 | 3 |
| Phenotype | ||
| Mitochondrial myopathy | 14 | 38 |
| MIDD | 13 | 35 |
| MELAS | 4 | 11 |
| CPEO | 3 | 8 |
| Leigh syndrome | 2 | 5 |
| MERRF | 1 | 3 |
CPEO, chronic progressive external ophthalmoplegia; MELAS, mitochondrial encephalomyopathy, lactic acidosis, and stroke‐like episodes; MERRF, myoclonus epilepsy with ragged‐red fibers; MIDD, maternally inherited diabetes and deafness; mtDNA, mitochondrial DNA; nDNA, nuclear DNA.
Physical Functioning
Physical functioning in MD patients was lower compared with that of controls, based on all physical functioning tests (Table 3).
Table 3.
Physical Functioning, Body Composition, Nutrition Intake, Malnutrition, and Sarcopenia in MD Patients and Controls
| Physical functioning, body composition, nutritional intake, malnutrition and sarcopenia | MD patients (n = 37) | Controls (n = 37) | P‐value* |
|---|---|---|---|
| Physical functioning test | |||
| Handgrip strength, kg, (mean ± SD) | 28 ± 10 | 43 ± 9 | <.001 |
| Too low handgrip strength a (n/%) | 6 (16) | 0 (0) | |
| Too low handgrip strength b (n/%) | 15 (41) | 0 (0) | |
| 6MMT (n chewing cycles) (mean ± SD) | 396 ± 130 (n = 29) | 577 ± 141 (n = 36) | <001 |
| 30SCT (n sit‐to‐stands) (mean ± SD) | 12 ± 4 (n = 22) | 17 ± 4 (n = 36) | <.001 |
| 6MWT (distance in m) (median; IQR) | 441: 426–427 (n = 20) | 681: 635–639 | <.001 |
| 6MWT < 400 m (n/%) | 3 (15) | 0 (0) | |
| Total body composition | |||
| FMI, kg/m2, (median; IQR) | 7.7: 6.7–8.7 | 7.0: 6.3–7.7 | .25 |
| Fat percentage (%) (mean ± SD) | 22 ± 7 | 16 ± 7 | .21 |
| High fat percentage c (n/%) | 9 (24) | 7 (19) | .21 |
| ASM, kg, (mean ± SD) | 17.6 ± 4.0 | 19.8 ± 3.8 | .02 |
| FFMI, kg/m2, (mean ± SD) | 15.2 ± 1.9 | 15.5 ± 1.6 | .38 |
| SMI, kg/m2, (mean ± SD) | 6.0 ± 1.0 | 6.3 ± 0.9 | .11 |
| Too low SMI d (n/%) | 25 (68) | 21 (57) | .34 |
| Bone density, g/cm2, (mean ± SD) | 0.31 ± 0.83 | 0.27 ± 0.80 | .99 |
| Osteopenia e (n/%) | 3 (8) | 0 (0) | .08 |
| Regional LTMI, kg/m2, (mean ± SD) | |||
| Average arm | 1.3 ± 0.30 | 1.3 ± 0.3 | .42 |
| Trunk | 12.7 ± 2.0 | 13.1 ± 1.5 | .34 |
| Average leg | 3.8 ± 0.7 | 4.3 ± 0.6 | .004 |
| Nutrition intake | |||
| Protein intake, g/kg/d, (median; IQR) | 1.1: 0.9–1.4 | 1.2: 1.1–1.7 | .07 |
| Too low protein intake f (n/%) | 25 (68%) | 4 (12%) | <.01 |
| Energy, kcal/d | 1663 ± 500 | 2322 ± 644 | <.001 |
| Energy intake (% of calculated needs, mean ± SD) | 81% ± 23.8% | 98% ± 25.1% | .03 |
| Too low energy intake g (n/%) | 25 (68%) | 17 (46%) | |
| PG‐SGA h | <.001 | ||
| PG‐SGA h 0–4 (n/%) | 5 (14%) | 35 (95%) | |
| PG‐SGA h 4–9 (n/%) | 16 (43%) | 1 (3%) | |
| PG‐SGA h ≥9 (n/%) | 16 (43%) | 1 (3%) | |
| Malnutrition i (n/%) | 17 (46%) | 10 (27%) | .09 |
| Severe malnutrition i (n/%) | 1 (3%) | 0 (0%) | |
| Sarcopenia j (n/%) | 5 (14%) | 0 (0%) | .02 |
| Sarcopenic obesity k (n/%) | 4 (11%) | 3 (8%) | .7 |
30SCT, 30‐second sit‐to‐stand test; 6MMT, 6‐minute mastication test; 6MWT, 6‐minute walk test; ASM, appendicular muscle mass; FFMI, fat‐free mass index; FMI, fat mass index; GLIM, Global Leadership Initiative on Malnutrition; IQR, interquartile range; LTMI, lean tissue mass index; PG‐SGA, Patient‐Generated Subjective Global Assessment; SMI, skeletal muscle index.
P‐values ≤. 02 (in bold) were considered significant.
Too low handgrip strength = <16 kg for women and <27 kg for men based on Dodds reference at age 70 28 according to the sarcopenia consensus 2018. 22
High fat percentage according to the sarcopenic obesity criteria of Baumgartner = >28% for men and >40% for women. 40
Too low SMI <7 kg/m2 for men and <6 kg/m2 for women according to the recommendations from European Working Group on Sarcopenia in Older People 2 (EWGSOP2). 22 , 30
Ostopenia = t‐score between −1 and −2.5.
Too low protein intake = <1.2 g/kg/d for MD patients at risk for malnutrition = PG‐SGA ≥4 and/or malnutrition according to GLIM criteria (n = 34; 92% off MD patients) and 0.8 g/kg/d for controls and MD patients not at risk for malnutrition.
Too low energy intake <90% of calculated energy needs = resting energy expenditure according to the Harris and Benedict formula (1984) and an activity factor of 1.4 for mobile MD patients, 1.2 for immobile MD patients, and 1.5 for controls. 10
PG‐SGA: 0–1 does not require nutrition input, 2–3 requires nutrition education, 4–8 requires specialized nutrition intervention, ≥9 indicates in critical need of symptom management together with specialized nutrition intervention/malnutrition. 39
Malnutrition and severe malnutrition according to GLIM criteria. 30
Sarcopenia according to 2018 consensus. 22
Sarcopenic obesity according to Baumgartner 40 low SMI and high fat percentage.
Body Composition
Total appendicular skeletal muscle mass was lower in MD patients compared with controls (P < .02) (Table 3), whereas no differences in total body fat or muscle mass were observed. Also, leg lean tissue mass index was lower in MD patients compared with controls (Table 3).
Association Between Physical Functioning and Body Composition
Handgrip strength was increased with higher fat‐free mass index in controls (Pearson r = 0.67, P < .001) but not in MD patients (Pearson r = 0.30, P = .08) (Figure 2A). In all MD patients together, no significant association between handgrip strength and skeletal muscle mass index was observed; however, a significant association for the m.3243A>G mutation genotype subgroup (Spearman r = 0.31, P = .06, and r = 0.44, P < .02, respectively) was observed (Table S1). Handgrip strength declined in both MD patients and controls with higher fat mass index (Spearman r = −0.61 and r = −0.49, respectively; P < .01) (Figure 2B).
Figure 2.
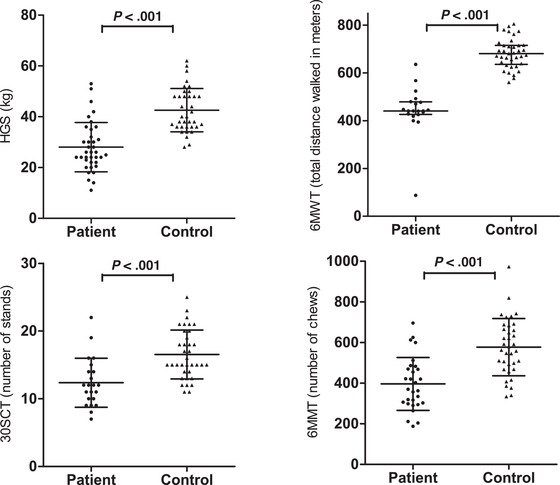
Association between HGS, 30SCT, fat mass index, fat‐free mass index, and protein intake. P‐values < .02 (in bold) were considered significant. 6MMT, 6‐minute mastication test; 6MWT, 6‐minute walking test; 30SCT, 30‐second sit‐to‐stand test; HGS, handgrip strength.
Nutrition Intake
Energy intake was significantly lower in MD patients compared with controls (Table 3). Moreover, significantly more patients had an energy and protein intake below the recommended intake (Table 3).
Association Between Physical Functioning and Protein Intake
Protein intake was inversely correlated with fat mass index in MD patients (Spearman r = −0.55, P < .01) and controls (Spearman r = −0.39, P = .02) (Figure 2C). Physical function was increased with higher protein intake in MD patients, as measured by the 30SCT (r = 0.57, P = .006) (Figure 2D) and handgrip strength (Spearman r = 0.39, P = .02) (Table S2), but not in controls.
Other Associations
Protein intake and handgrip strength correlated with arm lean tissue mass index in MD patients and controls, respectively (MD patients: Pearson r = 0.53, P < .01 and controls: Pearson r = 0.81, P < .001). No correlations were found between either the 6MWT or the 6MMT and body composition in MD patients and controls. The 30SCT correlated with fat mass index (r = 0.41, P = .01) but not with fat‐free mass index (r = 0.01, P = .96) in controls solely. No correlations were found between the 30SCT and 6MWT with leg lean tissue mass index. The 6MMT correlated moderately with protein intake (Spearman r = 0.45, P = .02) in MD patients (Table S2).
Malnutrition and Sarcopenia
According to the PG‐SGA, 32 MD patients (86%) were in need of a nutrition intervention, whereas this was only the case in 2 controls (6%) (Table 3). According to the GLIM criteria, 46% of the MD patients were malnourished, whereas 43% were malnourished according to the PG‐SGA (Table 3). If the PG‐SGA data were combined with the GLIM data, 27 MD patients (73%) were classified as malnourished. Sarcopenia was observed in 14% or 27% of the MD patients using either the consensus criterion or the actual age cutoff point for low handgrip strength, respectively.
Diagnostic Accuracy of BIA vs the DXA
The BIA‐derived fat‐free mass formulas of both Kyle and Dey show good correlation with DXA fat‐free mass in MD patients (Kyle r 2 = 0.9, Dey r 2 = 0.8) as well as in controls (Kyle r 2 = 0.94). BIA tends to overestimate fat‐free mass compared with DXA (mean difference = 1.8 kg, P = .01; 95% CI, −3.8 to 7.4 kg). BIA sensitivity (66%) and specificity (57%) to diagnose obesity are lower than to diagnose malnutrition (sensitivity 77%, specificity 93%) (Table S3).
Discussion
The main finding of this study is that muscle strength is related with body composition and protein intake in MD patients.
Hou et al (2019) 12 found a positive correlation between skeletal muscle mass index and muscle strength (r = 0.4), which is consistent with our hypothesis but was not significant in our cohort; this may be due to smaller numbers. However, a positive association between skeletal mass index and handgrip strength was found in the m.3243A>G genotype subgroup (Table S1), and a moderate association between handgrip strength and arm lean tissue mass index was found.
Surprisingly, sarcopenia existed in MD patients only, whereas no difference in muscle mass between MD patients and controls was observed. This might imply that handgrip strength and body composition are differently associated in MD patients compared with controls. Our results confirm a different association in fat‐free mass index with handgrip strength between controls (Pearson r = 0.67, P < .001) and MD patients (Pearson r = 0.30, P = .08, Figure 2A).
A high prevalence of malnutrition in MD patients (73%) was confirmed. 9 According to the GLIM criteria, 46% of the MD patients were malnourished, whereas 43% were malnourished according to the PG‐SGA (Table 3). This seems to be a consistent result; however, Figure 3 shows low comparability in malnutrition between the 2 methods. A better comparability was seen between sarcopenia and malnutrition because all patients diagnosed with sarcopenia were also classified as malnourished (Figure 3). This low comparability underlines the challenges of diagnosing malnutrition and the conclusions of Aubry et al (2017) 9 that nutrition assessment should be part of patient care in all adult MD patients. For measuring body composition, DXA should be preferably used instead of BIA because of the higher accuracy. For physical functioning, it is advisable to measure handgrip strength and use the actual age cutoff point of the Dodds reference. 28 The incidence of sarcopenia with this actual age cutoff point (27%) is very similar to the results of Hou et al (2019), 12 who observed sarcopenia in 24.7% of the MD patients. The risk for malnutrition according to the PG‐SGA is higher in the current study compared with the slightly overlapping cohort from the DINAMITE study 26 —89% vs 55%. The DINAMITE study cohort consisted of outpatients, whereas the DYNAMO study cohort was more severely affected, which justified a 4‐day hospital admission. According to Hou et al, 12 body composition is a sensitive biomarker of disease severity in MD patients. As low muscle mass is an indicator for malnutrition, the difference in malnutrition risk observed in the 2 studies is probably due to differences in disease severity.
Figure 3.
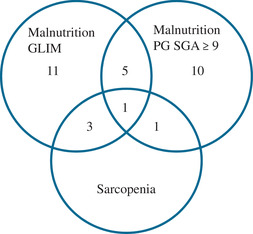
Venn diagram malnutrition according to GLIM criteria and PG‐SGA score ≥ 9 and Sarcopenia consensus 2018. GLIM, Global Leadership Initiative on Malnutrition; PG‐SGA, Patient‐Generated Subjective Global Assessment.
The observed decreased physical functioning in MD patients confirms previous studies. 12
Except for lower stature, lower appendicular skeletal muscle mass, and lower leg lean tissue mass index, body composition in MD patients was not different compared with that of controls. Although not significant and less profound, our data showed a trend toward higher fat mass and lower lean mass in MD patients, which is similar to other studies. 9 , 12 In the study by Aubry et al (2017), 9 MD patients had higher BMI with comparable weight than MD patients had in the current study. Moreover, their MD‐patient group consisted of more males and had a higher mean age compared with that of the current study. 9 As height and body composition are known to be gender‐ and age‐related, 7 , 13 this may have contributed to the (larger) difference in body composition observed in the study of Aubry et al (2017). 9 When comparing the individual MD patients’ and controls’ body composition in the current study with reference values, 40 a low skeletal muscle index in both MD patients and controls (68% vs 57%) was observed. This result was anticipated for the MD patients, but not for the controls, and might indicate that either the controls do not represent a healthy status of body composition or the reference data are not representative for the healthy Dutch population.
A lower protein intake (g/kg/d) could not be confirmed in MD patients compared with controls (P = .07). However, relative to their protein needs, more MD patients have a protein intake that is too low (68%) compared with that of controls (12%).
The matched‐control design, together with the accurate measurement of whole and regional body composition using DXA, is a strength of this study. The small study sample size is a shortcoming. However, taking into account the low prevalence of MD, a total of 37 MD patients is considerable.
Data collection bias may have occurred because multiple professionals performed the measurements, although standard operating procedures have been applied. Validity of the DXA measurements may be influenced by the use of 2 scanners. However, that the body composition was not different in MD patients compared with controls could not be explained by this, because the body composition measured by BIA confirms that there was no difference between body composition in MD patients compared with controls (Table S3). Furthermore, underreporting or overreporting of nutrition intake might have occurred.
Causal relationships could not be established in this study. The associations observed were moderate at best, indicating confounding or influencing factors. Handgrip strength is known to be dependent by physical strength exercise, and also, gender and age play a big role. 41 Gender and age cannot be changed, but exercise and nutrition intake is something that can be influenced.
Although fat mass did not differ between MD patients and controls, MD patients frequently have a high fat percentage (24%, Table 3) and high waist circumference (38%, Table 1). As patients with neuromuscular disease are at risk for developing metabolic syndrome, 42 monitoring body composition is recommended. If DXA is not available, and because BIA has low sensitivity and specificity of diagnosing high fat percentage (Table S3), waist circumference in MD patients is the preferred method. Nutrition strategies aiming at improving physical functionality and preventing metabolic syndrome are recommended. Improving protein intake seems a good start because a positive moderate association between protein and muscle strength was found as well as a moderate negative association between fat mass and protein intake. Prospective intervention studies are recommended to investigate whether adjusting protein intake may improve functioning and muscle mass.
Conclusion
In conclusion, MD patients have decreased physical functioning. There is a moderate inverse association between handgrip strength and fat mass index in MD patients and controls, between handgrip strength and fat‐free mass index in controls only, and between protein intake and muscle strengths in MD patients only. This, in combination with the high prevalence of both malnutrition and sarcopenia, warrants nutrition assessment in MD patients. Future intervention studies on improving protein intake are recommended.
Statement of Authorship
H. E. E. Zweers drafted the manuscript. H. E. E. Zweers, S. Leij‐Halfwerk, V. Bordier, and J. in ‘t Hulst contributed to the acquisition, analysis and the interpretation of the data. H. E. E. Zweers and S. Leij‐Halfwerk equally contributed to the conception and design of the research. M. C. H. Janssen and G. J. A. Wanten contributed to the design of the research and the interpretation of the data. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Acknowledgments
We thank the patients and healthy volunteers for their time and effort. We thank R. Akkermans PhD, for his statistical assistance. We thank our students L. Boegborn BSc, S. Martens MSc, J. Moens BSc, I. Evers MSc, L. Martens MSc, and M. de Jong MSc, for their help in data collection and analyses. Finally, we thank Mr. G. Markwalder for designing the DYNAMO logo.
Financial disclosure: None declared.
Conflicts of interest: None declared.
References
- 1. Schaefer AM, Taylor RW, Turnbull DM, Chinnery PF. The epidemiology of mitochondrial disorders–past, present and future. Biochim Biophys Acta. 2004;1659(2‐3):115‐120. [DOI] [PubMed] [Google Scholar]
- 2. de Laat P, Koene S, van den Heuvel LPWJ, Rodenburg RJ, Janssen MCH, Smeitink JAM. Clinical features and heteroplasmy in blood, urine and saliva in 34 Dutch families carrying the m.3243A>G mutation. J Inherit Metab Dis. 2012;35(6):1059‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mancuso M, Angelini C, Bertini E, et al. Fatigue and exercise intolerance in mitochondrial diseases. Literature revision and experience of the Italian network of mitochondrial diseases. Neuromuscular Disorders: NMD. 2012;22 (suppl 3):S226‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferreira RS, da Silva Coqueiro R, Barbosa AR, Pinheiro PA, Fernandes MH. Relationship between BMI and physical performance among older adults. Geriatr Nurs. 2013;34(6):465‐468. [DOI] [PubMed] [Google Scholar]
- 5. Visser M, Harris T, Langlois J, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53(3):M214‐M221. [DOI] [PubMed] [Google Scholar]
- 6. Skalsky AJ, Abresch RT, Han JJ, Shin CS, McDonald CM. The relationship between regional body composition and quantitative strength in facioscapulohumeral muscular dystrophy (FSHD). Neuromuscul Disord. 2008;18(11):873‐880. [DOI] [PubMed] [Google Scholar]
- 7. Palmieri GM, Bertorini TE, Griffin JW, Igarashi M, Karas JG. Assessment of whole body composition with dual energy x‐ray absorptiometry in Duchenne muscular dystrophy: correlation of lean body mass with muscle function. Muscle Nerve. 1996;19(6):777‐779. [DOI] [PubMed] [Google Scholar]
- 8. de Laat P, Zweers H, Knuijt S, Smeitink JAM, Wanten GJA, Janssen MCH. Dysphagia, malnutrition and gastrointestinal problems in patients with mitochondrial disease caused by the m3243A>G mutation. Neth J Med. 2015;73(1):30‐36. [PubMed] [Google Scholar]
- 9. Aubry E, Aeberhard C, Bally L, et al. Are patients affected by mitochondrial disorders at nutritional risk? Nutrition. 2018;47:56‐62. [DOI] [PubMed] [Google Scholar]
- 10. Apabhai S, Gorman GS, Sutton L, et al. Habitual physical activity in mitochondrial disease. PLoS One. 2011;6(7):e22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boal RL, Ng YS, Pickett SJ, et al. Height as a clinical biomarker of disease burden in adult mitochondrial disease. J Clin Endocrinol Metab. 2019;104(6):2057‐2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou Y, Xie Z, Zhao X, Yuan Y, Dou P, Wang Z. Appendicular skeletal muscle mass: a more sensitive biomarker of disease severity than BMI in adults with mitochondrial diseases. PLoS One. 2019;14(7):e0219628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pruna L, Chatelin J, Pascal‐Vigneron V, Kaminsky P. Regional body composition and functional impairment in patients with myotonic dystrophy. Muscle Nerve. 2011;44(4):503. [DOI] [PubMed] [Google Scholar]
- 14. Nau KL, Dick AR, Peters K, Schloerb PR. Relative validity of clinical techniques for measuring the body composition of persons with amyotrophic lateral sclerosis. J Neurol Sci. 1997;152(suppl 1):S36‐42.[CrossRef] [DOI] [PubMed] [Google Scholar]
- 15. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well‐functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324‐333. [DOI] [PubMed] [Google Scholar]
- 16. Aguilera AI, Hsiao S‐M, Tsai Y‐C, et al. Association of fluid status and body composition with physical function in patients with chronic kidney disease. PLoS One. 2016;11(10):e0165400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeung CHC, Au Yeung SL, Fong SSM, Schooling CM. Lean mass, grip strength and risk of type 2 diabetes: a bi‐directional Mendelian randomisation study. Diabetologia. 2019;62(5):789‐799. [DOI] [PubMed] [Google Scholar]
- 18. Ten Haaf DSM, Eijsvogels TMH, Bongers C, et al. Protein supplementation improves lean body mass in physically active older adults: a randomized placebo‐controlled trial. J Cachexia Sarcopenia Muscle. 2019;10(2):298‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jeppesen TD, Schwartz M, Olsen DB, et al. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain. 2006;129(12):3402‐3412. [DOI] [PubMed] [Google Scholar]
- 20. Bates MG, Newman JH, Jakovljevic DG, et al. Defining cardiac adaptations and safety of endurance training in patients with m.3243A>G‐related mitochondrial disease. Int J Cardiol. 2013;168(4):3599‐3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cruz‐Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008;43(1):24‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang SF, Lin PL. Systematic literature review and meta‐analysis of the association of sarcopenia with mortality. Worldviews Evid Based Nurs. 2016;13(2):153‐162. [DOI] [PubMed] [Google Scholar]
- 24. Rinninella E, Pizzoferrato M, Cintoni M, Servidei S, Mele MC. Nutritional support in mitochondrial diseases: the state of the art. Eur Rev Med Pharmacol Sci. 2018;22(13):4288‐4298. [DOI] [PubMed] [Google Scholar]
- 25. Zweers H, Janssen MC, Leij S, Wanten G. Patients with mitochondrial disease have an inadequate nutritional intake. JPEN J Parenter Enteral Nutr. 2018;42(3):581‐586. [DOI] [PubMed] [Google Scholar]
- 26. Zweers H, Smit D, Leij S, Wanten G, Janssen MCH. Individual dietary intervention in adult patients with mitochondrial disease due to the m.3243A>G mutation: the DINAMITE study, a randomized controlled trial. Nutrition. 2019;69:110544. [DOI] [PubMed] [Google Scholar]
- 27. de Laat P, Zweers HEE, Knuijt S, Smeitink JAM, Wanten GJA, Janssen MCH. Dysphagia, malnutrition and gastrointestinal problems in patients with mitochondrial disease caused by the m3243A>G mutation. Neth J Med. 2015;73(1):30‐36. [PubMed] [Google Scholar]
- 28. Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9(12):e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jensen GL, Cederholm T, Correia M, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. JPEN J Parenter Enteral Nutr. 2019;43(1):32‐40. [DOI] [PubMed] [Google Scholar]
- 30. Jones CJ, Rikli RE, Beam WC. A 30‐s chair‐stand test as a measure of lower body strength in community‐residing older adults. Res Q Exerc Sport. 1999;70(2):113‐119. [DOI] [PubMed] [Google Scholar]
- 31. Tyson S, Connell L. The psychometric properties and clinical utility of measures of walking and mobility in neurological conditions: a systematic review. Clin Rehabil. 2009;23(11):1018‐1033. [DOI] [PubMed] [Google Scholar]
- 32. Engel‐Hoek L, Knuijt S, Gerven MHJC, et al. The 6‐min mastication test: a unique test to assess endurance of continuous chewing, normal values, reliability, reproducibility and usability in patients with mitochondrial disease. J Oral Rehabil. 2017;44(3):155‐162. [DOI] [PubMed] [Google Scholar]
- 33. Hangartner TN, Warner S, Braillon P, Jankowski L, Shepherd J. The official positions of the international society for clinical densitometry: acquisition of dual‐energy x‐ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J Clin Densitom. 2013;16(4):520‐536. [DOI] [PubMed] [Google Scholar]
- 34. Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis? Part I: review of principles and methods. Clin Nutr. 2004;23(5):1226‐1243. [DOI] [PubMed] [Google Scholar]
- 35. Dey DK, Bosaeus I, Lissner L, Steen B. Body composition estimated by bioelectrical impedance in the Swedish elderly. Development of population‐based prediction equation and reference values of fat‐free mass and body fat for 70‐ and 75‐y olds. Eur J Clin Nutr. 2003;57(8):909‐916. [DOI] [PubMed] [Google Scholar]
- 36. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition ‐ An ESPEN Consensus Statement. Clin Nutr. 2015;34(3):335‐340. [DOI] [PubMed] [Google Scholar]
- 37. Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta‐analysis. Int J Obes. 2010;34(5):791‐799. [DOI] [PubMed] [Google Scholar]
- 38. Ottery F. Patient‐generated subjective global assessment In: McCallum P PC, ed. The Clinical Guide to Oncology Nutrition. The American Dietetic Association: Chicago; 2000:11‐23. [Google Scholar]
- 39. Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12(12):1995‐2004. [DOI] [PubMed] [Google Scholar]
- 40. Schutz Y, Kyle UU, Pichard C. Fat‐free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord. 2002;26(7):953‐960. [DOI] [PubMed] [Google Scholar]
- 41. Legrand D, Adriaensen W, Vaes B, Mathei C, Wallemacq P, Degryse J. The relationship between grip strength and muscle mass (MM), inflammatory biomarkers and physical performance in community‐dwelling very old persons. Arch Gerontol Geriatr. 2013;57(3):345‐351. [DOI] [PubMed] [Google Scholar]
- 42. Aitkens S, Kilmer DD, Wright NC, McCrory MA. Metabolic syndrome in neuromuscular disease. Arch Phys Med Rehabil. 2005;86(5):1030‐1036. [DOI] [PubMed] [Google Scholar]
- 43. WHO . Waist Circumference and Waist‐Hip Ratio Report of a WHO Expert Consultation. 2008.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


