Dear Editor,
The Psoriasis Area and Severity Index (PASI) is a validated, frequently used, psoriasis severity assessment tool. 1 , 2 , 3 In several countries, PASI assessment is compulsory for reimbursement considerations. 2 , 3 PASI calculation requires full body evaluation of the severity of erythema, thickness and scaling of psoriatic plaques in four body regions (head/neck, trunk, upper limbs and lower limbs). Some components of PASI (i.e. plaque thickness, extent of scalp involvement) are difficult to assess by teledermatology, making its incorporation into clinical practice challenging.
We sought to develop and validate a model for estimating total PASI score using only the practical PASI features suitable for assessment in teledermatology (i.e. scores for erythema, scaling and affected areas of the trunk, lower limbs and upper limbs; Tele‐PASI).
Materials and methods
Tele‐PASI model development and statistical analyses
The Tele‐PASI model was developed (for full details see Supplementary Materials 1) using screening PASI data from 4215 patients enrolled in three multicentre, double‐blind, randomised controlled ixekizumab trials (UNCOVER‐1, NCT01474512; UNCOVER‐2, NCT01597245; UNCOVER‐3, NCT01646177). 4 , 5 Of these, 3866 randomised patients (≥18 years) with moderate‐to‐severe plaque psoriasis (≥6 months), defined as ≥10% body surface area, static Physician’s Global Assessment (sPGA) ≥3, and PASI ≥12 were included in the model.
The correlation between scores of thickness and erythema and between scores of thickness and scaling were analysed within each and across the four PASI body regions (head/neck, upper limbs, trunk and lower limbs) (Supplementary Figure S1). Difficult to assess thickness scores were estimated from erythema and scaling scores, and head/neck scores from upper limb and trunk scores (using linear regression). Both were then included in the PASI calculations as surrogates for real measures (Tele‐PASI model: Tables S1 and S2).
From Pearson correlation coefficients and associated r2‐values the respective P‐values were calculated. All data processing and statistical analyses were performed using R version 3.0.1.
External Tele‐PASI model validation
PASI data from baseline up to 12 weeks of treatment from 1691 randomised patients from three additional multicentre, double‐blind, randomised controlled ixekizumab trials (RHBP, NCT02513550; RHBS, NCT02561806; RHBZ, NCT02634801) were used to validate the Tele‐PASI model. 6 , 7 , 8
RESULTS
At screening, thickness significantly correlated with both erythema and scaling in all four body regions. Correlation between the original total PASI score and the total PASI score excluding thickness was very high and almost unchanged when the head/neck components were excluded (Supplementary Figure S2).
In the model development population, very high correlations were observed between original and modelled total PASI scores at baseline (r2 = 0.95, P < 0.0001) (Fig. 1) and at all follow‐up visits, irrespective of treatment (Fig. 2 and Supplementary Figure S3).
Figure 1.
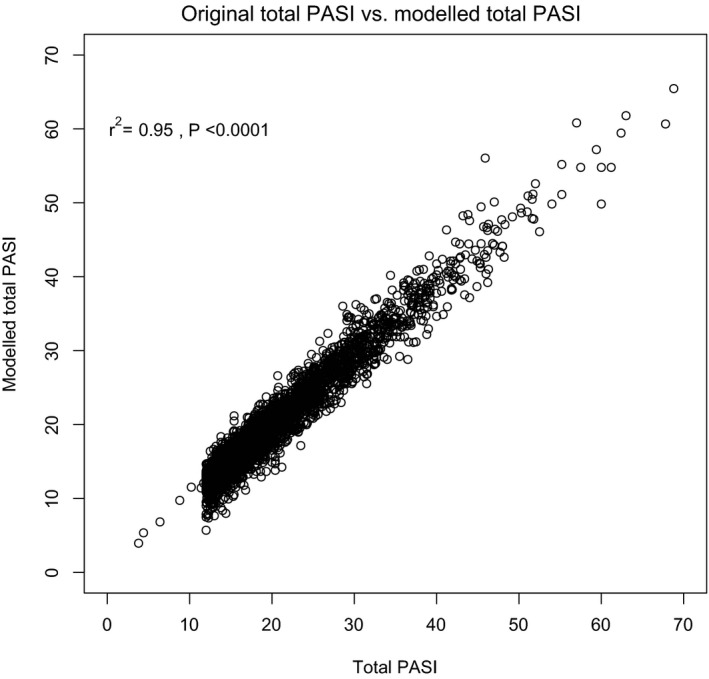
Correlation between original and modelled total PASI scores in the Tele‐PASI model development population.Abbreviations: PASI, Psoriasis Area and Severity Index.
Figure 2.
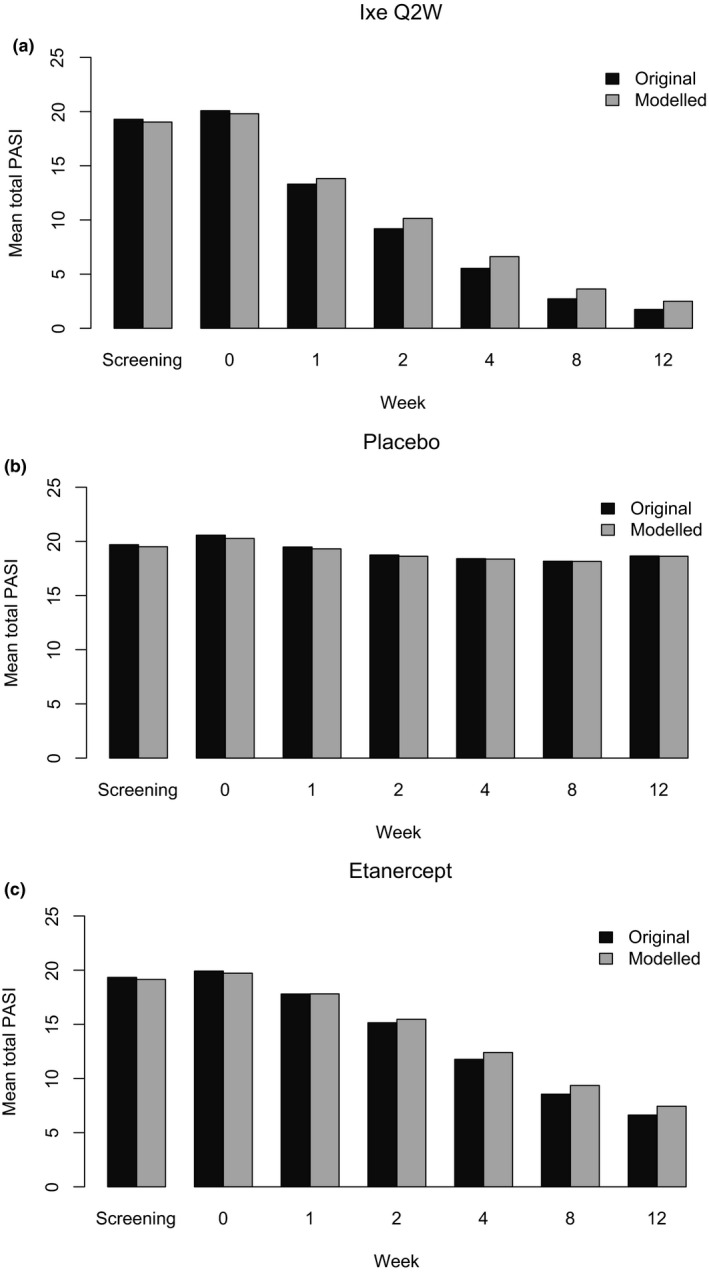
Original and modelled total PASI scores from screening to Week 12 in each treatment group in model development population (r2 range for IXE [Q2W, Q4W], ETN and placebo groups were 0.945‐0.9982; P <0.0001). n range is from screening to Week 12. Only data from the first 12 weeks of treatment were used to validate the Tele‐PASI model (n range for IXE Q2W: n = 1119‐1169; placebo: n = 746‐792; etanercept: n = 701‐740). Abbreviations: ETN, etanercept; IXE Q2W, 80 mg ixekizumab every 2 weeks; n, number of patients; PASI, Psoriasis Area and Severity Index.
Similarly, very high correlations between the original and modelled total PASI scores at baseline and at all follow‐up visits in all treatment groups were observed in the external model validation population (Supplementary Figures S4‐S6).
DISCUSSION
The Tele‐PASI model described here estimates total PASI scores before and during treatment using only scores for erythema, scaling and affected areas of the trunk, lower limbs and upper limbs. During model development, very strong correlations between the original and modelled total PASI scores were observed in all treatment groups at screening, baseline and during treatment. The model performance was confirmed using data from three independent trials including various systemic treatments. The modelled total PASI scores correlated very strongly with the original total PASI scores at baseline and during treatment, suggesting that Tele‐PASI may be an appropriate tool for PASI assessment in teledermatology settings.
The strengths of this study were the use of data from large scale, double‐blind, placebo‐ and active‐comparator controlled trials for model development and validation that included various systemic treatments. Because Tele‐PASI was developed with data from adults with moderate‐to‐severe plaque psoriasis who were enrolled in clinical trials for up to 12 weeks of treatment only and because data were collected during physical examinations and not via photos/live imaging, further real‐world validation, including in patients undergoing longer‐term treatment, is required.
This study has demonstrated that Tele‐PASI accurately estimates total PASI for patients with moderate‐to‐severe psoriasis receiving various systemic treatments. The Tele‐PASI may supplement PASI in clinical practice, especially when face‐to‐face consultations are not possible and evaluation is made in teledermatology settings (see Supplementary Materials 4 ‐ Worksheet − an online spreadsheet that allows calculation of the Tele‐PASI score).
Role of the sponsor
Eli Lilly and Company was involved in the study design, data collection, data analysis and preparation of the manuscript.
Supporting information
Fig S1. Schematic explaining how the Tele‐PASI model was developed and validated.
Fig S2. Correlation studies in the Tele‐PASI model development population based on screening PASI.
Fig S3. Original and modelled total PASI scores from screening to Week 12 in IXE Q4W treatment group in model development population (r2 range for IXE [Q2W, Q4W], ETN, and placebo groups was 0.945−0.9982; P <0.0001).
Fig S4. Original and modelled total PASI scores at baseline and all follow‐up visits in each treatment group in external Tele‐PASI model validation population (RHBP trial).
Fig S5. Original and modelled total PASI scores from baseline to Week 12 in each treatment group in external Tele‐PASI validation population (RHBS trial).
Fig S6. Original and modelled total PASI scores from baseline to Week 12 in each treatment group in external Tele‐PASI validation population (RHBZ trial).
Table S1. Parameters estimate the Tele‐PASI model components from the respective scores obtained at screening in the three UNCOVER trials using linear regressions
Table S2. Example of original PASI and modelled total PASI calculations
Supplementary Material
Method S1. Model Development Methods.
Acknowledgement
The authors thank all the dedicated patients who participated in each of the trials, as well as the investigators and site personnel for their active involvement in all trials described in this study.
Funding support: This study was sponsored by Eli Lilly and Company. Medical writing assistance was provided by Tania Dickson, PhD, CMPP and Serina Stretton, PhD, CMPP of ProScribe – Envision Pharma Group and was funded by Eli Lilly and Company. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Conflicts of interest: HP and YD are current employees and NB is a former employee of Eli Lilly and Company. YD and NB own shares in Eli Lilly and Company. JW and KG are consultants and advisory board members for AbbVie, Celgene, Eli Lilly and Company, Janssen, and Novartis. MG is a speaker/consultant and/or advisory board member for AbbVie, Akros, Amgen, Arcutis Pharmaceuticals, Inc., Boehringer Ingelheim, Celgene, Dermira, Eli Lilly and Company, Galderma, Medimmune, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Roche, Sanofi Genzyme, Sun Pharmaceuticals Industries Ltd., UCB, and Valeant Pharmaceuticals International, Inc.
References
- 1. Puzenat E, Bronsard V, Prey S et al What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J. Eu.r Aca.d Dermato.l Venereol. 2010; 24(Suppl 2): 10–6. [DOI] [PubMed] [Google Scholar]
- 2. Commonwealth of Australia ‐ Department of Health . Severe chronic plaque psoriasis initial PBS authority application form. at URL: https://www.humanservices.gov.au/organisations/health‐professionals/forms/pb112. (Accessed 2 Feb 2018).
- 3. National Institute for Health and Care Excellence. Psoriasis: Assessment and management. at URL: https://www.nice.org.uk/guidance/cg153/ (2012). (Accessed 2 Feb 2018).
- 4. Griffiths CE, Reich K, Lebwohl M et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): Results from two phase 3 randomised trials. Lancet 2015; 386: 541–51. [DOI] [PubMed] [Google Scholar]
- 5. Gordon KB, Blauvelt A, Papp KA et al Phase 3 trials of ixekizumab in moderate‐to‐severe plaque psoriasis. N. Engl. J. Med. 2016; 375: 345–56. [DOI] [PubMed] [Google Scholar]
- 6. Reich K, Augustin M, Thaci D et al A 24‐week multicentre, randomized, open‐label, parallel‐group study comparing the efficacy and safety of ixekizumab vs. fumaric acid esters and methotrexate in patients with moderate‐to‐severe plaque psoriasis naive to systemic treatment. Br. J. Dermatol. 2019; 182: 869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Langley RG, Papp K, Gooderham M et al Efficacy and safety of continuous every 2‐week dosing of ixekizumab over 52 weeks in patients with moderate‐to‐severe plaque psoriasis in a randomized phase 3 trial (IXORA‐P). Br. J. Dermatol. 2018; 178: 1315–1323. [DOI] [PubMed] [Google Scholar]
- 8. Reich K, Pinter A, Lacour JP et al Comparison of ixekizumab with ustekinumab in moderate‐to‐severe psoriasis: 24‐week results from IXORA‐S, a phase III study. Br. J. Dermatol. 2017; 177: 1014–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Schematic explaining how the Tele‐PASI model was developed and validated.
Fig S2. Correlation studies in the Tele‐PASI model development population based on screening PASI.
Fig S3. Original and modelled total PASI scores from screening to Week 12 in IXE Q4W treatment group in model development population (r2 range for IXE [Q2W, Q4W], ETN, and placebo groups was 0.945−0.9982; P <0.0001).
Fig S4. Original and modelled total PASI scores at baseline and all follow‐up visits in each treatment group in external Tele‐PASI model validation population (RHBP trial).
Fig S5. Original and modelled total PASI scores from baseline to Week 12 in each treatment group in external Tele‐PASI validation population (RHBS trial).
Fig S6. Original and modelled total PASI scores from baseline to Week 12 in each treatment group in external Tele‐PASI validation population (RHBZ trial).
Table S1. Parameters estimate the Tele‐PASI model components from the respective scores obtained at screening in the three UNCOVER trials using linear regressions
Table S2. Example of original PASI and modelled total PASI calculations
Supplementary Material
Method S1. Model Development Methods.


