Abstract
Introduction
Periodontal diseases (PD) are complex oral inflammatory diseases initiated by keystone bacteria such as Porphyromonas gingivalis. A vaccine for PD is desirable as clinical treatment involves protracted maintenance strategies aimed to retain dentition. Although prior immunization approaches targeting P. gingivalis have reported variable success in limiting facets of disease such as oral bone loss, it remains that a vaccine for this disease may be attainable.
Aim
To investigate cell‐free protein synthesis (CFPS) as a platform to produce vaccinable targets suitable for efficacy testing in a P. gingivalis‐induced murine oral bone loss model.
Materials and Methods
Recombinantly generated P. gingivalis minor fimbriae protein (Mfa1), RgpA gingipain hemagglutinin domain 1 (HA1), and RgpA gingipain hemagglutinin domain 2 (HA2) were combined in equivalent doses in adjuvants and injected intramuscularly to immunize mice. Serum levels of protein‐specific antibody were measured by ELISA, and oral bone levels were defined by morphometrics.
Results
Recombinantly generated P. gingivalis proteins possessed high fidelity to predicted size and elicited protein‐specific IgG following immunization. Importantly, immunization with the vaccine cocktail protected from P. gingivalis elicited oral bone loss.
Conclusion
These data verify the utility of the CFPS technology to synthesize proteins that have the capacity to serve as novel vaccines.
Keywords: cell‐free protein synthesis, gingipain, immunization, minor fimbriae, oral bone loss, Porphyromonas gingivalis
1. INTRODUCTION
Periodontal diseases (PD) are common human diseases of bacterial origin, with recent estimates in the US supporting 60% of individuals over 40 years of age with measurable oral bone loss (Eke et al., 2012). Since 1990, the numbers of individuals with severe periodontitis have been on the rise, and in 2015, it was estimated 538 million individuals were impacted globally (Kassebaum et al., 2017). Bacteria present in the subgingival plaque initiate non‐resolving chronic inflammation that is a principal driver of the progressive destruction of the soft and hard tissues supporting the teeth. Current treatment strategies do not cure PD; instead, these are aimed to reduce pathogen burden, limit inflammation, and protect existing bone levels. Thus, an effective vaccine against PD would significantly impact PD burden.
The subgingival microflora is a highly diverse community of organisms (Kroes, Lepp, & Relman, 1999; Paster et al., 2001). Despite this diversity, bacteria such as Porphyromonas gingivalis are associated with chronic PD (Hajishengallis, Darveau, & Curtis, 2012; Hong et al., 2015; Socransky, Haffajee, Cugini, Smith, & Kent, 1998; Yost, Duran‐Pinedo, Teles, Krishnan, & Frias‐Lopez, 2015). This organism possesses fimbriae, gingipains (a group of cysteine proteases), and others molecules that impact aspects of disease pathogenesis including attachment of bacteria to host cells and other community microbes, development of inflammation, and microbial‐driven dysbiosis (Bostanci & Belibasakis, 2012; Lamont & Jenkinson, 1998). For example, as compared with wild‐type bacteria, a P. gingivalis major fimbria‐deficient organism were less capable in eliciting oral bone loss in a rat model (Malek et al., 1994). P. gingivalis major and minor fimbriae mutants differentially activate immune cells and pro‐inflammatory cytokine expression (Arjunan, El‐Awady, Dannebaum, Kunde‐Ramamoorthy, & Cutler, 2016; Malek et al., 1994; Takahashi, Davey, Yumoto, Gibson, & Genco, 2006). The arginine‐specific gingipain RgpA is necessary for optimal growth of P. gingivalis and participates in fimbrial maturation (Imamura, 2003; Nm, Veith, Dashper, & Reynolds, 2003; Potempa, Sroka, Imamura, & Travis, 2003).
An inconclusive picture currently exists regarding the role played by elicited antibodies to key organisms such as P. gingivalis and progression of PD. In one study, no correlation was observed with organism‐specific antibody levels and disease severity (Whitney et al., 1992). While in another study, gingipain‐specific antibodies present in sera of individuals with PD served to facilitate bacterial recognition and opsonophagocytic uptake by PMNs (Gibson, Savelli, Van Dyke, & Genco, 2005). Interestingly, purified IgG elicited by immunization facilitated P. gingivalis clearance, as well as reduced infection‐elicited oral bone loss in a murine model (Gibson, Gonzalez, Wong, & Genco, 2004). Thus, strategies aimed at targeting specific molecules integral to critical organisms associated with PD, and ability to elicit antibodies that functionally alter the course of disease may offer a path to aid in control and/or protection from oral tissue destruction.
Vaccination has been examined by many groups to aid in treatment of PD and mixed results are evident. Polak et al. (Polak, Wilensky, Shapira, Weiss, & Houri‐Haddad, 2010) reported that immunization of mice with P. gingivalis or F. nucleatum elicited specific immune responses, but did not provide protection from infection, while Leone et al. (2006) reported that immunization with P. gingivalis exacerbated bone loss. Yet, others have reported that immunization with various molecules of P. gingivalis in pure form can provide protection from the key feature of PD—oral bone loss (Gibson & Genco, 2001; Gonzalez, Tzianabos, Genco, & Gibson, 2003; Han, LaRosa, Kawai, & Taubman, 2014; Miyachi, Ishihara, Kimizuka, & Okuda, 2007; O'Brien‐Simpson et al., 2016; Puth et al., 2017; Wilensky, Potempa, Houri‐Haddad, & Shapira, 2017; Zhu et al., 2013). Reports from our group and others support that RgpA (Genco, Odusanya, Potempa, Mikolajczyk‐Pawlinska, & Travis, 1998; Gibson & Genco, 2001; Miyachi et al., 2007; Wilensky et al., 2017) and fimbriae (Evans et al., 1992; Han et al., 2014) represent potential targets for vaccine development based on their capacity to elicit immune responses that facilitate bacterial uptake by cells, and their potential to provide protection against infection‐elicited oral bone loss (Gibson et al., 2005); however, the level of protection was not complete suggesting that optimization to include focus on the specific domains of these proteins that provide protection or optimization to include additional molecules in the vaccine preparation, or delivery approach (prophylactic vs. therapeutic vaccine) need to be understood.
Cell‐free protein synthesis (CFPS) systems represent a protein production approach to construct proteins of complex structure (Kapoor et al., 2018). The absence of the requirement to maintain cell viability allows for the optimization of the protein synthetic capacity of the cell‐free extract by direct addition of components to manipulate transcription, translation and folding, and provide precise modulation of the protein expression process. Historically, CFPS systems derived from Escherichia coli have been used in small‐scale as experimental tools for exploring the molecular biology of transcription and translation and for in vitro protein production (Legastelois et al., 2017; Swartz, 2006). In the context of biopharmaceutical development of vaccine targets, the novel XpressCFTM CFPS system (SutroVax, Inc.) using an E. coli K12‐derived production strain has been developed to use standard industrial microbial fermentation and process equipment (Xu et al., 2015; Zawada et al., 2011). The system is linearly scalable to manufacturing levels to produce low‐cost vaccine antigens. Further, use of the XpressCF™ protein production system permits the construction of heretofore difficult to produce protein antigens (Kapoor et al., 2018).
We chose a multi‐hit vaccine approach targeting P. gingivalis at the levels of microbial community attachment, and gingipains. P. gingivalis Mfa1 has been shown to mediate the interaction between P. gingivalis and Streptococcus gordonii, an antecedent members of the subgingival bacterial biofilm (Park et al., 2005). The selection of RgpA was based on prior reports from our group and others that this molecule participate in protection from P. gingivalis‐elicited oral bone loss (Gibson & Genco, 2001; Wilensky et al., 2017). Targeting the HA1 domain was based on knowledge that this region participates in microbial coaggregation (Ito, Ishihara, Shoji, Nakayama, & Okuda, 2010) and protects against oral bone loss in a rodent model (Muramatsu, Kokubu, Shibahara, Okuda, & Ishihara, 2011), while HA2 was selected as this molecule participates in haem adsorption (Nakayama et al., 1998) and thus represents a key molecule of P. gingivalis that could influence the physiology of this organism as iron is important for P. gingivalis survival (Smalley & Olczak, 2017).
We report that P. gingivalis minor fimbriae protein (Mfa1), RgpA gingipain hemagglutinin domain 1 (HA1), and RgpA gingipain hemagglutinin domain 2 (HA2) were synthesized, purified, and when used in combination, elicited robust protein‐specific IgG responses. Further, in initial proof‐of‐concept studies, we observed that prophylactic immunization with this protein combination protected mice from P. gingivalis‐elicited oral bone loss. Our findings support that the XpressCF™ CFPS system can generate active vaccine candidates for use in PD.
2. MATERIALS AND METHODS
2.1. Generation of cell‐free extract
Cell‐free extracts containing additional DsbC chaperone were prepared as previously described by Groff et al. (2014). Briefly, E. coli strain SBJY001 (Yin et al., 2012) was transformed with a pACYC plasmid carrying tandem copies of the dsbC gene. Cells were grown, harvested, and homogenized as described by Zawada et al., (2011). Subsequent clarification via centrifugation yielded the extract used for subsequent cell‐free expression reaction.
2.2. Generation of recombinant Mfa1, HA1, HA2, and purification
DNA sequences were designed based on encoding the HA1, HA2, and Mfa1 fimbrilin proteins, codon optimized (DNA 2.0; Menlo Park, CA), synthesized, and cloned into the previously described pYD317 vector (Yin et al., 2012). Cell‐free reactions were performed with the XpressCF™ CFPS system essentially as previously described (Yin et al., 2012; Zimmerman et al., 2014). For expression of Mfa1 fimbrilin, reactions were performed with iodoacetamide (IAM) pre‐treatment, at 25°C, with the addition of oxidized glutathione (2 mM) to create an oxidizing environment for the disulphide bonds. Expression of HA1 and HA2 was performed without IAM treatment of the cell extract and addition of reduced glutathione (8 mM) to maintain a reducing expression environment. After 16 hr of reaction time, the expressed proteins were isolated from the cell‐free reaction mixtures using his‐tag affinity purification on Ni Sepharose resin (GE Lifesciences, Pittsburg, PA) as per the manufacturer's recommendations. Further purification of the HA1, HA2, and Mfa1 fimbrilin protein was achieved via cation exchange chromatography on SP ImpRes resin (GE Lifesciences). Briefly, the Ni Sepharose elution pools were exchanged into sodium citrate (50 mM), NaCl (50 mM), pH 4.5, applied to the column, and eluted via gradient elution to Tris (50 mM), NaCl (1 M), pH 7.5. Similarly, HA2 was further purified via anion exchange chromatography on Q ImpRes (GE Lifesciences). Column equilibration and loading was performed in Tris (50 mM), NaCl (50 mM), pH 7.5, with subsequent gradient elution to Tris (50 mM), NaCl (1 M), pH 7.5.
After polishing chromatography, all proteins were dialysed into Dulbecco's PBS and analysed via SDS–PAGE under reducing and non‐reducing conditions with Coomassie blue staining. Intact mass analysis of each protein was determined via Q‐TOF (Agilent, Santa Clara, CA). In the case of HA1, intact mass analysis conclusively showed that the cysteines were oxidized and had formed the expected disulphide bond.
2.3. Cultivation of P. gingivalis and bacterial purity assessment
Porphyromonas gingivalis strain A7436 was handled as described previously (Huang, Shaik‐Dasthagirisaheb, LaValley, & Gibson, 2015). In brief, log‐phase broth‐grown bacteria were harvested by centrifugation and were suspended to 1 × 1010 CFU/ml in 2% carboxymethylcellulose in pyrogen‐free saline for oral challenge (100 μL/challenge/mouse). Gram‐stain was performed on all P. gingivalis broth cultures to ensure purity.
2.4. Mice, immunizations, and oral challenge
Six‐week‐old female BALB/c mice (Charles River Laboratories, Wilmington, MA) were randomly separated into six groups (n = 8/group), housed in specific pathogen‐free facilities, and received water and food ad libitum. All live animal use was in accordance with National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978), employing institutional IACUC‐approved procedures. Groups included G1) non‐immunized/no oral challenge control, G2) non‐immunized/P. gingivalis oral challenge, G3) Mfa1 + HA1 + HA2 combined immunization in alum/P. gingivalis oral challenge, G4) Mfa1 + HA1 + HA2 combined immunization in monophosphoryl Lipid A (MPL; Sigma‐Aldrich, St. Louis, MO)/P. gingivalis oral challenge, and G5) Mfa1 + HA1 + HA2 combined immunization in injection‐grade saline/P. gingivalis oral challenge (to understand whether molecules alone provide protection in the absence of adjuvant). Baseline serum was obtained from each animal prior to immunization, and the respective groups of mice were immunized by intramuscular injection with Mfa1 + HA1 + HA2 (5 μg of each protein/injection) suspended in alum (Imject, ThermoFisher Sci, Rockford, IL), MPL, or injection‐grade saline. Booster immunizations were delivered 2 and 4 weeks after initial immunization. Two weeks after completion of immunization, serum samples were collected from mice prior to oral challenge with P. gingivalis. Oral challenge was accomplished as previously reported (Gonzalez et al., 2003). In brief, animals received 10‐day oral sulphamethoxazole/trimethoprim (Hi‐Tech Pharmical, Amityville, NY) in drinking water, followed by removal of antibiotics and a three‐day rest. A P. gingivalis slurry (1 × 1010 CFU/ml + 2% carboxymethylcellulose in injection‐grade saline) was gently applied to the gums of mice using a feeding needle fitted to a syringe 3 times over a 1‐week period. Control animals were orally challenged with 2% carboxymethylcellulose alone. After 42‐day rest, animals were sacrificed, terminal sera were collected and stored at −80°C, and the head of each mouse was processed for oral bone loss measurements.
2.5. Detection of Mfa1‐, HA1‐, and HA2‐specific IgG in mouse sera
Antigens (0.5 μg/ml) were adsorbed at 4°C overnight on Maxisorp plates (NUNC, Rochester, NY), and unoccupied sites were blocked with 1% BSA for a minimum of 1 hr. Serial 2‐fold diluted serum samples were added to individual wells (100 μl/well) and incubated for 2 hr at room temperature. Following washing, isotype‐specific antibody conjugated to horseradish peroxidase (1:6000 dilution; Southern Biotech, Birmingham, AL) was added, and following washing, the wells were developed with TMB substrate (Pierce, Rockford, IL), and the reaction stopped with 50 μl H2SO4 (1.0 M). Absorbances were measured at 450 nm minus the absorbance at 570 nm to correct for plate abnormalities. The resulting data for each sample were plotted to obtain a curve of the reciprocal dilution vs. the A450–A570 measurement. The antibody titre was determined as the midpoint of the dilution curve as defined by EC50 calculations using Prism statistical analysis software (GraphPad Software, La Jolla, CA). The mean of the EC50 for each cohort was used as the antibody titre.
2.6. Measurement of oral bone loss
Oral bone levels were determined by morphometric analyses, as done previously (Gibson & Genco, 2001). After sacrifice, soft tissue was removed around the maxillary molars, and the skulls were stained with methylene blue. Prior to initiation of bone measurements, samples were blinded by a researcher not aware of the groupings. Oral bone measurements were determined from obtained digital images using onscreen measurement from the alveolar bone crest (ABC) to the cementum–enamel junction (CEJ) at 14 landmark sites (Baker, Evans, & Roopenian, 1994). Image analysis was performed using ImageJ (Schneider, Rasband, & Eliceiri, 2012) with pixel lengths converted to millimetres, and data from the 7 sites were combined with measurements from all animals in the group to achieve a group level mean length ± SEM. Statistical analysis was used to compare levels between groups.
2.7. Statistical analysis
Data were analysed with Prism statistical analysis software (GraphPad). Comparison between groups was performed as indicated using Kruskal–Wallis non‐parametric ANOVA with Dunns multiple comparison post‐testing. A p < 0.05 was considered significant.
3. RESULTS
3.1. Genome analysis, selection of vaccine targets, and CFPS protein generation
We initially investigated published nucleic acid sequences for P. gingivalis and identified 3 candidate regions, 1 within Mfa1 (minor fimbrilin) gene and 2 within two hemagglutinin domains (HA1 and HA2) of the RgpA gingipain. CFPS was performed to generate each protein, and each purified recombinant protein was found to possess high fidelity to the predicted mass for each protein (Table 1). Further analysis of these proteins was accomplished by SDS–PAGE and Coomassie blue staining under reducing (Figure 1) and non‐reducing conditions (data not shown), supporting the purity of each product.
Table 1.
Q‐TOF analysis of Mfa1, HA1, and HA2
| Protein sample | Observed mass | Theoretical mass | Mass accuracy |
|---|---|---|---|
| Mfa1 | 60019.4 Da | 60018.4 Da | Δ +17 ppm |
| HA1 | 19058.7 Da | 19058.0 Da | Δ +37 ppm |
| HA2 | 48299.4 Da | 48299.6 Da | Δ ‐4 ppm |
Figure 1.
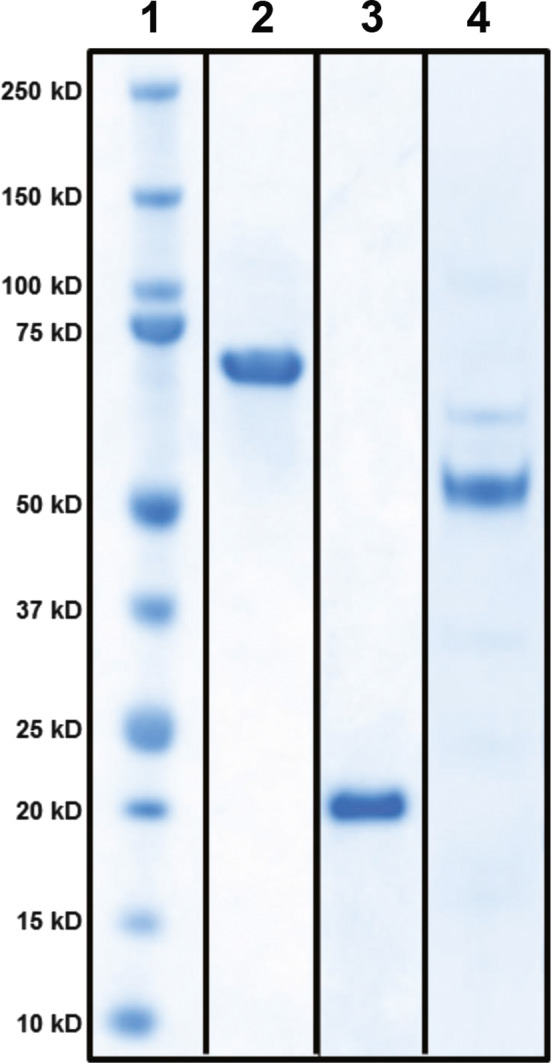
SDS–PAGE analysis of purified proteins generated by cell‐free protein synthesis (CFPS). Denatured proteins were added to wells (3 μg/well), separated on 4%–12% Bis‐Tris gradient gels, and stained with Coomassie blue. Lane 1: molecular mass markers; Lane 2: Mfa1; Lane 3: HA1; Lane 4: HA2
3.2. Immunization and evaluation of protein‐specific IgG
To determine whether the proteins delivered by intramuscular injection elicited protein‐specific IgG antibody responses, and to understand whether different adjuvants (alum vs. MPL) influenced the elicited IgG response for the respective preparations, sera were collected from groups of mice at the completion of the immunization period, and at sacrifice were tested for levels of Mfa1‐, HA1‐, and HA2‐specific IgG by ELISA. Titration curves for each serum sample were converted to EC50 values. As anticipated, sera collected from the non‐immunized groups of mice (G1 and G2) prior to oral challenge possessed low levels of IgG to Mfa1, HA1, and HA2 (Figure 2). For the groups of mice, immunized IM with the combined proteins suspended in alum (G3), MPL (G4), or injection‐grade saline (G5) revealed that all mice receiving the vaccine combination responded with antigen‐specific IgG responses (Figure 2). Comparing postvaccination levels of IgG between groups of animals receiving vaccine revealed that for Mfa1 the MPL adjuvant provided a significant advantage over alum or no adjuvant groups in the developed molecule‐specific IgG response (p < 0.05 for both; Figure 2). No significant differences were observed between groups with regard to postvaccination levels of HA1‐specific IgG levels (Figure 2). Lastly, serum levels of antibodies from the groups of mice that received vaccine with alum and MPL were both significantly enhanced in HA2‐specific IgG as compared to the vaccine without adjuvant (p < 0.05 for both; Figure 2). These data show that all groups of mice that were vaccinated responded with increased molecule‐specific IgG over the unmanipulated control group. MPL was the best adjuvant for developing Mfa1‐specific IgG, while both alum and MPL were similarly effective in boosting IgG levels to HA2.
Figure 2.
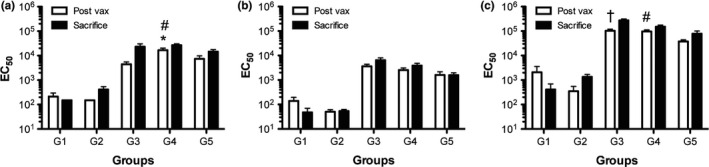
Serum IgG EC 50 values against P. gingivalis Mfa1, HA1, and HA2. Groups of animals served as controls or experimental groups as indicated (G1‐5; methods section), and serum samples were collected from animals immediately prior to oral challenge (Post‐Vax; open bars) or at sacrifice (filled bars), and molecule‐specific IgG EC 50 values were calculated from ELISA data against P. gingivalis (a) Mfa1, (b) HA1, and (c) HA2. Mean ± SEM for group. Kruskal–Wallis non‐parametric ANOVA with Dunns multiple comparisons was used to compare Post‐Vax EC 50 values between the vaccine groups (G3–G5); *P < 0.05 G3 vs. G4, # P < 0.05 G4 vs G5, † P < 0.05 G3 vs. G5
Examination of IgG levels at sacrifice revealed that the group of non‐immunized mice oral challenged with P. gingivalis A7436 possessed only a modest increase in molecule‐specific levels of IgG to Mfa1, HA1, and HA2 that did not approach the levels achieved by vaccination (Figure 2).
3.3. Impact of vaccination on P. gingivalis‐elicited oral bone loss
To understand if the protein combination could effectively limit the extent of P. gingivalis‐elicited oral bone loss, immunized animals were subjected to P. gingivalis oral challenge (Figure 3a). Groups of mice that were not immunized served as controls. In comparison to mock challenged mice (G1; mean ABC‐CEJ distance = 0.1178 mm), animals orally challenged with P. gingivalis A7436 (G2; mean ABC‐CEJ distance = 0.195 mm) developed oral bone loss as evidenced by an increase in mean distance from ABC to CEJ (p < 0.001; Figure 3). Groups of mice that received the combination protein vaccine generated from a heterologous strain of P. gingivalis suspended in either alum (G3) or MPL (G4) were protected from P. gingivalis‐elicited oral bone loss (p < 0.01 for each vs. P. gingivalis oral challenge alone; Figure 3). No differences in the level of protection (ABC to CEJ measurements) were observed between adjuvants, indicating that intramuscular delivery of the vaccine candidate provided similar protective responses (p > 0.05; Figure 3). Interestingly, it was also observed that the group of animals immunized with the combination protein vaccine suspended in saline solution (G5) were also protected from P. gingivalis oral challenge similar to that observed when the proteins were delivered intramuscularly with adjuvant (p > 0.05 vs. alum or MPL adjuvants; Figure 3), and the level of protection was similar regardless of adjuvant employed (approximately 68% protection for all; p > 0.05 for all; Figure 3).
Figure 3.
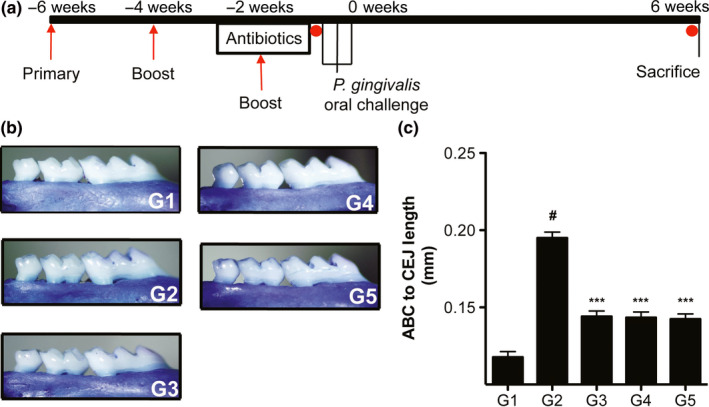
Experimental organization and oral bone loss. (a) BALB/c mice were randomized into groups (G1‐5; outlined in the methods section; n = 7–8 mice/group) and immunized animals received 3 intramuscular injections of combined protein cocktail in respective adjuvant, or in injection‐grade saline at 2‐week intervals (primary and 2 boosts; red arrows). All animals were placed on 10‐day sulphamethoxazole/trimethoprim (Antibiotics) in drinking water, followed by removal of antibiotics 3 days prior to mock oral challenge (G1), or P. gingivalis oral challenge (3 times over a 1‐week period; G2‐5). After completion of oral challenge (0 weeks.), animals were allowed rest for 6 weeks and were sacrificed. Serum samples were collected from animals immediately prior to the initiation of oral challenge and at sacrifice (red circles); (b) Digital micrographs of representative hemimaxilla from each group stained with methylene blue; (c) Average distance between cementum–enamel junction (CEJ) and alveolar bone crest (ABC) in mm ± SEM, # P < 0.001 vs. G1 (unchallenged), *** P < 0.01 vs. G2 (P. gingivalis oral challenge only) using Kruskal–Wallis non‐parametric ANOVA with Dunns multiple comparisons
4. DISCUSSION
Development of an effective vaccine for PD would be especially useful as this disease occurs in a significant portion of the adult population globally and is increasingly more prevalent in individuals as they age. However, PD is a multifactorial disease with factors including the bacterial composition of dental plaque, host genetic make‐up, and environmental factors contributing unique barriers to our fundamental understanding of PD pathogenesis, and thus the potential for targeted vaccine development. In the past, various strategies such as killed whole organism preparations (Giardino, Ebersole, & Holt, 1996; Gibson & Genco, 2001; Persson et al., 1994), purified molecules from PD associated organisms (Gibson & Genco, 2001; Moritz, Cappelli, Lantz, Holt, & Ebersole, 1998; Reynolds et al., 2015), and implementation of recombinantly generated molecules (DeCarlo, Huang, Collyer, Langley, & Katz, 2003; Genco et al., 1998; O'Brien‐Simpson et al., 2016; Wilensky et al., 2017) have met with varying degrees of success. Although it is clear that there are important differences in the immune systems of rodents and humans (Graves, Kang, Andriankaja, Wada, & Rossa, 2012), rodents remain a key tool in the armamentarium of early phase vaccine development and testing and are useful in mechanistic studies aimed at better understanding the pathogenesis of PD (Hajishengallis, Lamont, & Graves, 2015). In this study, we employed the CFPS technology to synthesize specific proteins of P. gingivalis and demonstrate that this platform effectively produces proteins that evoke strong antigen‐specific host IgG responses when delivered intramuscularly, and in proof‐of‐concept application in an accepted model of infection‐driven oral bone loss, the combination vaccine was effective as it significantly prevented P. gingivalis‐elicited oral bone loss. The antigens targeted include Mfa1, HA1, and HA2 which on their own have been shown to provide protection in similar situations; however, as a multi‐component vaccine, our approach is novel, and the data support continued consideration of the multiple‐hit approach (targeting bacterial attachment, and protease functions). Further, these findings are significant as these data support that the CFPS platform is an effective way to generate antigens for recombinant vaccines.
The microbiology of subgingival plaque has been under close inspection recently by molecular analyses, as microbial dysbiosis as a consequence of specific keystone bacteria is more deeply appreciated to be central to the development of PD than de facto presence of specific organisms (Hajishengallis et al., 2012; Mira, Simon‐Soro, & Curtis, 2017); however, the specific factors that underlie the tip from oral health to disease remain enigmatic. Despite these complexities, substantial evidence supports that there is a core group of organisms in subgingival plaque that is disproportionally important in driving microbial dysbiosis and resultant immune dysregulation that characterizes PD. Although possibly not being the specific organism responsible for the disease, organisms such as P. gingivalis remain the principal driver of disease by their keystone position (Hajishengallis et al., 2012), thus continued targeting of P. gingivalis and other highly associated bacteria remains rational for development of a vaccine for PD. Our data support that use of CFPS‐generated proteins from specific regions of P. gingivalis minor fimbriae and RgpA when used in combination provide protection from the oral bone loss elicited by P. gingivalis. Unexpectedly in our studies, use of different adjuvant combinations did not suggest an adjuvant effect or an advantage of one adjuvant over another in the protection of infection‐elicited oral bone loss, despite showing some capacity to slightly modulate IgG responsivity to each specific protein. It is not clear why the three combinations provided similar final antibody levels and similar protection from oral bone loss. Indeed, it appears that the trivalent vaccine without adjuvant was able to elicit robust molecule‐specific antibody in the absence of adjuvant (albeit to slightly lower levels at sacrifice that levels achieved with alum or MPL). It could be that with fewer vaccinations, an adjuvant effect may be clearer. Alternatively, vaccination with this molecular cargo with adjuvant may elicit a concomitant induction of regulatory T‐cell populations (Zitvogel, Apetoh, Ghiringhelli, & Kroemer, 2008). However, as protection from oral bone loss was achieved, thus our data suggest that absence of an adjuvant effect is not be a significant barrier in the context of acute exposure, but may need to be considered in the context of long‐term protection. Further understanding of adjuvant optimization and vaccination strategy (systemic vs. mucosal delivery) as well as vaccine scheduling will be need in the context of development for prophylactic or therapeutic vaccine use in human disease.
Previous studies in non‐human primates and other animal models have reported vaccination targeting P. gingivalis effective in generating specific antibody responses, and mixed results of vaccination have been observed where protection with whole organisms provide protection (Gibson & Genco, 2001; O'Brien‐Simpson et al., 2016; Persson et al., 1994; Roberts et al., 2004), while others suggest no effect or exacerbated inflammation (Ebersole, Brunsvold, Steffensen, Wood, & Holt, 1991; Moritz et al., 1998; Polak et al., 2010). One strategy to maximize the efficacy of a vaccine is to base the composition on those structures/epitopes that elicit a protective response. It is unclear what are the optimal P. gingivalis structures to target, thus several lines of investigation are under exploration and have primarily focused on virulence factors of this organism such as major fimbriae (Evans et al., 1992; Takahashi et al., 2007) and gingipains (Genco et al., 1998; Gibson & Genco, 2001; O'Brien‐Simpson et al., 2016; Rajapakse, O'Brien‐Simpson, Slakeski, Hoffmann, & Reynolds, 2002). These studies have met with a general consensus of providing a measure of protection to P. gingivalis oral infection; however, while the level of protection is significant, it often does not achieve protection to the level of the no challenge control. Thus, understanding the specific epitope structures of these proteins may afford further optimization of these vaccine candidate proteins. Our work and work from other groups are in agreement with the finding that protection is not complete despite the strong protection provided by this multi‐component vaccine. Indeed, independent of vaccine or adjuvant, it is frequently observed that protection is not to the level of the mock challenged group. Therefore, further optimizations of this system are necessary, including regimen, relative proportions of the components, adjuvant combination, as well as to determine whether the elicited protective response to vaccination provides long‐term recall and protection to challenge. Our data show that shortly after completion of vaccination, all vaccine combination provided similar protection. Although our study provides clear evidence that the CFPS‐generated trivalent vaccine is promising as a vaccine candidate, much needs to be understood. How durable is the immunologic memory? What adjuvant maximizes protection and immunologic memory to provide durable protection later in life. How does systemic delivery of this vaccine provide protection at the oral mucosa to limit oral bone loss? Does the vaccine disrupt P. gingivalis colonization or numbers in the subgingival plaque, and how might vaccination impact on the overall microbial community in the subgingival space? Another important element for future consideration is need for prophylactic vs. therapeutic vaccine delivery approach. Here, we used a prophylactic approach; however, a therapeutic approach may be beneficial in the context of PD as identification of individuals for vaccination recently diagnosed with disease would target those most in need to stop progression.
In summary, these studies provide an initial proof‐of‐concept for the CFPS platform in the context of vaccine development targeting the extent of oral infection‐elicited oral bone loss accompanying PD. Our findings support the potential for further targeted vaccine approaches for PD and in developing this technology for continued development for clinical utility.
CONFLICT OF INTEREST
Ms. Elyse Shimomura is an employee at SutroVax. Dr. Fairman is a co‐founder and currently employed by SutroVax. All other authors declare no conflicts of interest.
5.
Clinical Relevance.
Scientific rationale for the study: A vaccine for periodontal disease (PD) could shift disease management. It is unclear why prior approaches to vaccinate against PD have met with mixed results. Cell‐free protein synthesis (CFPS) is a platform to generate proteins with challenging structure and may be suitable for application with vaccine development.
Principle findings: CFPS effectively generated 3 Porphyromonas gingivalis proteins. Immunization with the 3‐protein combination elicited protection from oral bone loss in a murine model.
Practical implications: CFPS‐generated vaccine targets limit P. gingivalis‐elicited oral bone loss and support utility of this technology for the development of vaccine proteins.
Huang N, Shimomura E, Yin G, et al. Immunization with cell‐free‐generated vaccine protects from Porphyromonas gingivalis‐induced alveolar bone loss. J Clin Periodontol. 2019;46:197–205. 10.1111/jcpe.13047
Funding information
This work was supported by SutroVax, Inc., and research start‐up from The University of Florida (FCG).
The copyright line for this article was changed on 28 January 2021 after original online publication.
REFERENCES
- Arjunan, P. , El‐Awady, A. , Dannebaum, R. O. , Kunde‐Ramamoorthy, G. , & Cutler, C. W. (2016). High‐throughput sequencing reveals key genes and immune homeostatic pathways activated in myeloid dendritic cells by Porphyromonas gingivalis 381 and its fimbrial mutants. Mol Oral Microbiol, 31, 78–93. 10.1111/omi.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, P. J. , Evans, R. T. , & Roopenian, D. C. (1994). Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Archives of Oral Biology, 39, 1035–1040. 10.1016/0003-9969(94)90055-8 [DOI] [PubMed] [Google Scholar]
- Bostanci, N. , & Belibasakis, G. N. (2012). Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiology Letters, 333, 1–9. 10.1111/j.1574-6968.2012.02579.x [DOI] [PubMed] [Google Scholar]
- DeCarlo, A. A. , Huang, Y. , Collyer, C. A. , Langley, D. B. , & Katz, J. (2003). Feasibility of an HA2 domain‐based periodontitis vaccine. Infection and Immunity, 71, 562–566. 10.1128/IAI.71.1.562-566.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole, J. L. , Brunsvold, M. , Steffensen, B. , Wood, R. , & Holt, S. C. (1991). Effects of immunization with Porphyromonas gingivalis and Prevotella intermedia on progression of ligature‐induced periodontitis in the nonhuman primate Macaca fascicularis . Infection and Immunity, 59, 3351–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke, P. I. , Dye, B. A. , Wei, L. , Thornton‐Evans, G. O. , Genco, R. J. & Cdc Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washin) (2012) Prevalence of periodontitis in adults in the United States: 2009 and 2010. Journal of Dental Research 91, 914–920. 10.1177/0022034512457373 [DOI] [PubMed] [Google Scholar]
- Evans, R. T. , Klausen, B. , Sojar, H. T. , Bedi, G. S. , Sfintescu, C. , Ramamurthy, N. S. , … Genco, R. J. (1992). Immunization with Porphyromonas (Bacteroides) gingivalis fimbriae protects against periodontal destruction. Infection and Immunity, 60, 2926–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco, C. A. , Odusanya, B. M. , Potempa, J. , Mikolajczyk‐Pawlinska, J. , & Travis, J. (1998). A peptide domain on gingipain R which confers immunity against Porphyromonas gingivalis infection in mice. Infection and Immunity, 66, 4108–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino, A. , Ebersole, J. L. , & Holt, S. C. (1996). Characteristics of systemic antibody responses of nonhuman primates following active immunization with Porphyromonas gingivalis, Prevotella intermedia and Bacteroides fragilis . Oral Microbiology and Immunology, 11, 79–87. 10.1111/j.1399-302X.1996.tb00340.x [DOI] [PubMed] [Google Scholar]
- Gibson, F. C. 3rd , & Genco, C. A. (2001). Prevention of Porphyromonas gingivalis‐induced oral bone loss following immunization with gingipain R1. Infection and Immunity, 69, 7959–7963. 10.1128/IAI.69.12.7959-7963.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, F. C. 3rd , Gonzalez, D. A. , Wong, J. , & Genco, C. A. (2004). Porphyromonas gingivalis‐specific immunoglobulin G prevents P. gingivalis‐elicited oral bone loss in a murine model. Infection and Immunity, 72, 2408–2411. 10.1128/IAI.72.4.2408-2411.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, F. C. 3rd , Savelli, J. , Van Dyke, T. E. , & Genco, C. A. (2005). Gingipain‐specific IgG in the sera of patients with periodontal disease is necessary for opsonophagocytosis of Porphyromonas gingivalis . Journal of Periodontology, 76, 1629–1636. 10.1902/jop.2005.76.10.1629 [DOI] [PubMed] [Google Scholar]
- Gonzalez, D. , Tzianabos, A. O. , Genco, C. A. , & Gibson, F. C. 3rd (2003). Immunization with Porphyromonas gingivalis capsular polysaccharide prevents P. gingivalis‐elicited oral bone loss in a murine model. Infection and Immunity, 71, 2283–2287. 10.1128/IAI.71.4.2283-2287.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves, D. T. , Kang, J. , Andriankaja, O. , Wada, K. , & Rossa, C. Jr (2012). Animal models to study host‐bacteria interactions involved in periodontitis. Front Oral Biol, 15, 117–132. 10.1159/000329675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff, D. , Armstrong, S. , Rivers, P. J. , Zhang, J. , Yang, J. , Green, E. , … Yam, A. Y. (2014). Engineering toward a bacterial “endoplasmic reticulum” for the rapid expression of immunoglobulin proteins. MAbs, 6, 671–678. 10.4161/mabs.28172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis, G. , Darveau, R. P. , & Curtis, M. A. (2012). The keystone‐pathogen hypothesis. Nature Reviews Microbiology, 10, 717–725. 10.1038/nrmicro2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis, G. , Lamont, R. J. , & Graves, D. T. (2015). The enduring importance of animal models in understanding periodontal disease. Virulence, 6, 229–235. 10.4161/21505594.2014.990806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X. , LaRosa, K. B. , Kawai, T. , & Taubman, M. A. (2014). DNA‐based adaptive immunity protect host from infection‐associated periodontal bone resorption via recognition of Porphyromonas gingivalis virulence component. Vaccine, 32, 297–303. 10.1016/j.vaccine.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, B. Y. , Furtado Araujo, M. V. , Strausbaugh, L. D. , Terzi, E. , Ioannidou, E. , & Diaz, P. I. (2015). Microbiome profiles in periodontitis in relation to host and disease characteristics. PLoS ONE, 10, e0127077 10.1371/journal.pone.0127077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, N. , Shaik‐Dasthagirisaheb, Y. B. , LaValley, M. P. , & Gibson, F. C. 3rd (2015). Liver X receptors contribute to periodontal pathogen‐elicited inflammation and oral bone loss. Mol Oral Microbiol, 30, 438–450. 10.1111/omi.12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura, T. (2003). The role of gingipains in the pathogenesis of periodontal disease. Journal of Periodontology, 74, 111–118. 10.1902/jop.2003.74.1.111 [DOI] [PubMed] [Google Scholar]
- Ito, R. , Ishihara, K. , Shoji, M. , Nakayama, K. , & Okuda, K. (2010). Hemagglutinin/Adhesin domains of Porphyromonas gingivalis play key roles in coaggregation with Treponema denticola . FEMS Immunology and Medical Microbiology, 60, 251–260. 10.1111/j.1574-695X.2010.00737.x [DOI] [PubMed] [Google Scholar]
- Kapoor, N. , Vanjak, I. , Rozzelle, J. , Berges, A. , Chan, W. , Yin, G. , … Miura, K. (2018). Malaria derived glycosylphosphatidylinositol anchor enhances anti‐Pfs25 functional antibodies that block Malaria transmission. Biochemistry, 57, 516–519. 10.1021/acs.biochem.7b01099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassebaum, N. J. , Smith, A. G. C. , Bernabe, E. , Fleming, T. D. , Reynolds, A. E. , Vos, T. , … Collaborators, G. B. D. O. H. (2017). Global, regional, and national prevalence, incidence, and disability‐adjusted life years for oral conditions for 195 countries, 1990‐2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. Journal of Dental Research, 96, 380–387. 10.1177/0022034517693566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes, I. , Lepp, P. W. , & Relman, D. A. (1999). Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci U S A, 96, 14547–14552. 10.1073/pnas.96.25.14547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont, R. J. , & Jenkinson, H. F. (1998). Life below the gum line: Pathogenic mechanisms of Porphyromonas gingivalis . Microbiology and Molecular Biology Reviews, 62, 1244–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legastelois, I. , Buffin, S. , Peubez, I. , Mignon, C. , Sodoyer, R. , & Werle, B. (2017). Non‐conventional expression systems for the production of vaccine proteins and immunotherapeutic molecules. Hum Vaccin Immunother, 13, 947–961. 10.1080/21645515.2016.1260795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone, C. W. , Bokhadhoor, H. , Kuo, D. , Desta, T. , Yang, J. , Siqueira, M. F. , … Graves, D. T. (2006). Immunization enhances inflammation and tissue destruction in response to Porphyromonas gingivalis . Infection and Immunity, 74, 2286–2292. 10.1128/IAI.74.4.2286-2292.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek, R. , Fisher, J. G. , Caleca, A. , Stinson, M. , van Oss, C. J. , Lee, J. Y. , … Dyer, D. W. (1994). Inactivation of the Porphyromonas gingivalis fimA gene blocks periodontal damage in gnotobiotic rats. Journal of Bacteriology, 176, 1052–1059. 10.1128/jb.176.4.1052-1059.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira, A. , Simon‐Soro, A. , & Curtis, M. A. (2017). Role of microbial communities in the pathogenesis of periodontal diseases and caries. Journal of Clinical Periodontology, 44(Suppl 18), S23–S38. 10.1111/jcpe.12671 [DOI] [PubMed] [Google Scholar]
- Miyachi, K. , Ishihara, K. , Kimizuka, R. , & Okuda, K. (2007). Arg‐gingipain A DNA vaccine prevents alveolar bone loss in mice. Journal of Dental Research, 86, 446–450. 10.1177/154405910708600511 [DOI] [PubMed] [Google Scholar]
- Moritz, A. J. , Cappelli, D. , Lantz, M. S. , Holt, S. C. , & Ebersole, J. L. (1998). Immunization with Porphyromonas gingivalis cysteine protease: Effects on experimental gingivitis and ligature‐induced periodontitis in Macaca fascicularis. Journal of Periodontology, 69, 686–697. 10.1902/jop.1998.69.6.686 [DOI] [PubMed] [Google Scholar]
- Muramatsu, K. , Kokubu, E. , Shibahara, T. , Okuda, K. , & Ishihara, K. (2011). HGP44 induces protection against Porphyromonas gingivalis‐Induced alveolar bone loss in mice. Clinical and Vaccine Immunology, 18, 888–891. 10.1128/CVI.00556-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, K. , Ratnayake, D. B. , Tsukuba, T. , Kadowaki, T. , Yamamoto, K. , & Fujimura, S. (1998). Haemoglobin receptor protein is intragenically encoded by the cysteine proteinase‐encoding genes and the haemagglutinin‐encoding gene of Porphyromonas gingivalis . Molecular Microbiology, 27, 51–61. 10.1046/j.1365-2958.1998.00656.x [DOI] [PubMed] [Google Scholar]
- Nm, O. B.‐S. , Veith, P. D. , Dashper, S. G. , & Reynolds, E. C. (2003). Porphyromonas gingivalis gingipains: The molecular teeth of a microbial vampire. Current Protein and Peptide Science, 4, 409–426. [DOI] [PubMed] [Google Scholar]
- O'Brien‐Simpson, N. M. , Holden, J. A. , Lenzo, J. C. , Tan, Y. , Brammar, G. C. , Walsh, K. A. , … Reynolds, E. C. (2016). A therapeutic Porphyromonas gingivalis gingipain vaccine induces neutralising IgG1 antibodies that protect against experimental periodontitis. Npj Vaccines, 1, 16022 10.1038/npjvaccines.2016.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, Y. , Simionato, M. R. , Sekiya, K. , Murakami, Y. , James, D. , Chen, W. , … Lamont, R. J. (2005). Short fimbriae of Porphyromonas gingivalis and their role in coadhesion with Streptococcus gordonii. Infection and Immunity, 73, 3983–3989. 10.1128/IAI.73.7.3983-3989.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster, B. J. , Boches, S. K. , Galvin, J. L. , Ericson, R. E. , Lau, C. N. , Levanos, V. A. , … Dewhirst, F. E. (2001). Bacterial diversity in human subgingival plaque. Journal of Bacteriology, 183, 3770–3783. 10.1128/JB.183.12.3770-3783.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson, G. R. , Engel, D. , Whitney, C. , Darveau, R. , Weinberg, A. , Brunsvold, M. , & Page, R. C. (1994). Immunization against Porphyromonas gingivalis inhibits progression of experimental periodontitis in nonhuman primates. Infection and Immunity, 62, 1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak, D. , Wilensky, A. , Shapira, L. , Weiss, E. I. , & Houri‐Haddad, Y. (2010). Vaccination of mice with Porphyromonas gingivalis or Fusobacterium nucleatum modulates the inflammatory response, but fails to prevent experimental periodontitis. Journal of Clinical Periodontology, 37, 812–817. 10.1111/j.1600-051X.2010.01598.x [DOI] [PubMed] [Google Scholar]
- Potempa, J. , Sroka, A. , Imamura, T. , & Travis, J. (2003). Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: Structure, function and assembly of multidomain protein complexes. Current Protein and Peptide Science, 4, 397–407. 10.2174/1389203033487036 [DOI] [PubMed] [Google Scholar]
- Puth, S. , Hong, S. H. , Park, M. J. , Lee, H. H. , Lee, Y. S. , Jeong, K. , … Lee, S. E. (2017). Mucosal immunization with a flagellin‐adjuvanted Hgp44 vaccine enhances protective immune responses in a murine Porphyromonas gingivalis infection model. Hum Vaccin Immunother, 13, 2794–2803. 10.1080/21645515.2017.1327109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse, P. S. , O'Brien‐Simpson, N. M. , Slakeski, N. , Hoffmann, B. , & Reynolds, E. C. (2002). Immunization with the RgpA‐Kgp proteinase‐adhesin complexes of Porphyromonas gingivalis protects against periodontal bone loss in the rat periodontitis model. Infection and Immunity, 70, 2480–2486. 10.1128/IAI.70.5.2480-2486.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, E. C. , O'Brien‐Simpson, N. , Rowe, T. , Nash, A. , McCluskey, J. , Vingadassalom, D. , & Kleanthous, H. (2015). Prospects for treatment of Porphyromonas gingivalis‐mediated disease ‐ immune‐based therapy. J Oral Microbiol, 7, 29125 10.3402/jom.v7.29125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, F. A. , Houston, L. S. , Lukehart, S. A. , Mancl, L. A. , Persson, G. R. , & Page, R. C. (2004). Periodontitis vaccine decreases local prostaglandin E2 levels in a primate model. Infection and Immunity, 72, 1166–1168. 10.1128/IAI.72.2.1166-1168.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, C. A. , Rasband, W. S. , & Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley, J. W. , & Olczak, T. (2017). Heme acquisition mechanisms of Porphyromonas gingivalis ‐ strategies used in a polymicrobial community in a heme‐limited host environment. Mol Oral Microbiol, 32, 1–23. 10.1111/omi.12149 [DOI] [PubMed] [Google Scholar]
- Socransky, S. S. , Haffajee, A. D. , Cugini, M. A. , Smith, C. , & Kent, R. L. Jr (1998). Microbial complexes in subgingival plaque. Journal of Clinical Periodontology, 25, 134–144. 10.1111/j.1600-051X.1998.tb02419.x [DOI] [PubMed] [Google Scholar]
- Swartz, J. (2006). Developing cell‐free biology for industrial applications. Journal of Industrial Microbiology and Biotechnology, 33, 476–485. 10.1007/s10295-006-0127-y [DOI] [PubMed] [Google Scholar]
- Takahashi, Y. , Davey, M. , Yumoto, H. , Gibson, F. C. 3rd , & Genco, C. A. (2006). Fimbria‐dependent activation of pro‐inflammatory molecules in Porphyromonas gingivalis infected human aortic endothelial cells. Cellular Microbiology, 8, 738–757. 10.1111/j.1462-5822.2005.00661.x [DOI] [PubMed] [Google Scholar]
- Takahashi, Y. , Kumada, H. , Hamada, N. , Haishima, Y. , Ozono, S. , Isaka, M. , … Umemoto, T. (2007). Induction of immune responses and prevention of alveolar bone loss by intranasal administration of mice with Porphyromonas gingivalis fimbriae and recombinant cholera toxin B subunit. Oral Microbiology and Immunology, 22, 374–380. 10.1111/j.1399-302X.2007.00373.x [DOI] [PubMed] [Google Scholar]
- Whitney, C. , Ant, J. , Moncla, B. , Johnson, B. , Page, R. C. , & Engel, D. (1992). Serum immunoglobulin G antibody to Porphyromonas gingivalis in rapidly progressive periodontitis: Titer, avidity, and subclass distribution. Infection and Immunity, 60, 2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky, A. , Potempa, J. , Houri‐Haddad, Y. , & Shapira, L. (2017). Vaccination with recombinant RgpA peptide protects against Porphyromonas gingivalis‐induced bone loss. Journal of Periodontal Research, 52, 285–291. 10.1111/jre.12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. , Lee, J. , Tran, C. , Heibeck, T. H. , Wang, W. D. , Yang, J. , … Yin, G. (2015). Production of bispecific antibodies in “knobs‐into‐holes” using a cell‐free expression system. MAbs, 7, 231–242. 10.4161/19420862.2015.989013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, G. , Garces, E. D. , Yang, J. , Zhang, J. , Tran, C. , Steiner, A. R. , … Murray, C. J. (2012). Aglycosylated antibodies and antibody fragments produced in a scalable in vitro transcription‐translation system. MAbs, 4, 217–225. 10.4161/mabs.4.2.19202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost, S. , Duran‐Pinedo, A. E. , Teles, R. , Krishnan, K. , & Frias‐Lopez, J. (2015). Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Medicine, 7, 27 10.1186/s13073-015-0153-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawada, J. F. , Yin, G. , Steiner, A. R. , Yang, J. , Naresh, A. , Roy, S. M. , … Murray, C. J. (2011). Microscale to manufacturing scale‐up of cell‐free cytokine production–a new approach for shortening protein production development timelines. Biotechnology and Bioengineering, 108, 1570–1578. 10.1002/bit.23103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, C. , Yang, J. , Sun, J. , Shi, J. , Gou, J. , & Li, A. (2013). Induction of immune response and prevention of alveolar bone loss with recombinant Porphyromonas gingivalis peptidylarginine deiminase. Archives of Oral Biology, 58, 1777–1783. 10.1016/j.archoralbio.2013.09.006 [DOI] [PubMed] [Google Scholar]
- Zimmerman, E. S. , Heibeck, T. H. , Gill, A. , Li, X. , Murray, C. J. , Madlansacay, M. R. , … Sato, A. K. (2014). Production of site‐specific antibody‐drug conjugates using optimized non‐natural amino acids in a cell‐free expression system. Bioconjugate Chemistry, 25, 351–361. 10.1021/bc400490z [DOI] [PubMed] [Google Scholar]
- Zitvogel, L. , Apetoh, L. , Ghiringhelli, F. , & Kroemer, G. (2008). Immunological aspects of cancer chemotherapy. Nature Reviews Immunology, 8, 59–73. 10.1038/nri2216 [DOI] [PubMed] [Google Scholar]


