Summary
In Amazonia, human activities that occurred hundreds of years ago in the pre‐European era can leave long‐lasting effects on the forests – termed ecological legacies. These legacies include the intentional or nonintentional enrichment or depletion of certain species. The persistence of these legacies through time varies by species, and creates complex long‐term trajectories of post‐disturbance succession that affect ecosystem processes for hundreds of years. Most of our knowledge of Amazonian biodiversity and carbon storage comes from a series of several hundred forest plots, and we only know the disturbance history of four of them. More empirical data are needed to determine the degree to which past human activities and their ecological legacies affect our current understanding of Amazonian forest ecology.
Keywords: biodiversity, biomass, carbon storage, disturbance, forest dynamics, species enrichment or depletion, succession
1.
| Contents | ||
|---|---|---|
| Summary | 1 | |
| I. | Introduction | 1 |
| II. | Ecological legacies on forest composition | 2 |
| III. | Ecological legacies on biomass and carbon dynamics | 3 |
| IV. | Outlook: advancing our knowledge of long‐term ecological legacies | 3 |
| Acknowledgements | 4 | |
| References | 4 |
2. Introduction
The importance of Amazonian rainforests for an array of ecosystem services and functions is well known amongst scientists but perhaps less so amongst policymakers (Levis et al., 2020). The biodiversity of Amazonian forests is immense (ter Steege et al., 2020), but the mechanisms driving the relative abundances and distributions of this diversity remain largely unresolved. Environmental gradients, biotic interactions and dispersal limitation all play a role in structuring diversity patterns in Amazonian forests (e.g. Wright, 2005). An emerging hypothesis is that past disturbances in the landscape, particularly those caused by human activities, have also played a role in shaping the structure, function and diversity patterns observed in modern forests (Levis et al., 2017; McMichael et al., 2017b).
People have lived in Amazonia for over 10 000 yr (Roosevelt, 2013) and have cultivated maize in some regions for over 6000 yr (Brugger et al., 2016; Bush et al., 2016). Besides cultivation, people in the pre‐European era also used fire to clear forests and amend soils, and they domesticated several plant species (e.g. Neves & Petersen, 2006; Piperno, 2011; Clement et al., 2015). Some of these forests have been managed continually by indigenous people for hundreds or even thousands of years, sometimes termed intensive or opportunistic agroforestry (Neves, 2013; Levis et al., 2018). But many areas that were cleared and managed at the time of European arrival c. 500 years ago were abandoned, when a majority of indigenous populations collapsed (Denevan, 2014). Following European colonization, many Jesuit missions were established but were quickly abandoned (Reeve, 1993). The ‘Amazonian rubber boom’ (c. ad 1850–1920) was a subsequent influx of European colonists that later collapsed because establishing rubber plantations was cheaper in Malaysia (Hecht, 2013). It is likely that all of these past waves of colonization and abandonment in the landscape have left ecological legacies on the forests, where trees often have life spans exceeding 150 yr.
Ecological legacy refers to the influence of an event (i.e. disturbance) on an ecosystem and its persistence over a given time period, and is a term that has been widely used in succession studies. The type and intensity of human disturbance (e.g. clear cut versus forest burning) affect the trajectory of the ecological legacy in Amazonian systems on decadal timescales (e.g. Mesquita et al., 2015). The long‐term ecological legacies of past human impacts during the pre‐ and post‐European eras, however, remain more obscure. Here I review recent advances in our understanding of long‐term ecological legacies in Amazonia with a focus on biodiversity and carbon storage, and highlight why assessing past disturbances is crucial for understanding the patterns and dynamics observed in these globally important forests.
3. Ecological legacies on forest composition
Most studies of ecological legacies on Amazonian forest composition have focused on the enrichment and long‐term persistence of useful species. It has been suggested that Bertholettia excelsa (Brazil Nut), Bactris gasipaes (Peach Palm) and other edible plants were enriched in the pre‐European era, and their abundances have remained artificially high ever since (i.e. for hundreds of years) (Fig. 1a; Scoles & Gribel, 2011; Clement et al., 2015; Thomas et al., 2015; Maezumi et al., 2018). In a series of c. 1100 forest plots in Amazonia, there were higher richnesses and abundances of domesticated tree species in locations that were closest to known pre‐European archaeological sites (Levis et al., 2017). Many of these same domesticated species that show a relationship with pre‐European occupation are also some of the most abundant across the basin (ter Steege et al., 2013).
Fig. 1.
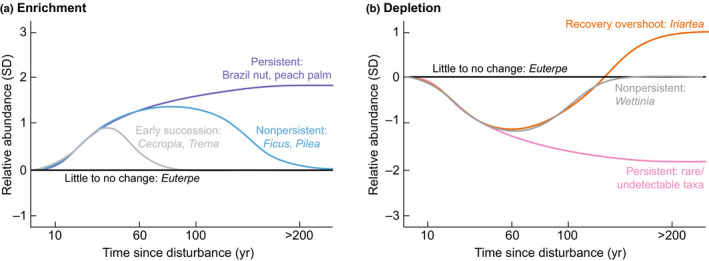
The long‐term effects of human disturbances on Amazonian tree species. (a) The enrichment of taxa can be either intentional or nonintentional, and can be persistent or nonpersistent through time. (b) The depletion of taxa can also be persistent or nonpersistent, although sometimes the recovery of these taxa overshoots the abundances before the disturbance. Some taxa, while useful, show no real change in abundances with low levels of past human disturbance.
Ecological legacies following disturbances may not always be persistent, as is the case with early successional taxa, such as Cecropia or Trema (Fig. 1a). Mid‐ to late‐successional taxa, such as Ficus and Pilea, have longer life spans and can persist for centuries, but eventually decrease in abundance (Åkesson et al., 2020). In Costa Rican forests, the proportion of old‐growth taxa can reach 30–40% within 25–30 ys following a disturbance, but then only reaches 50% at 80 yr following a disturbance (Chazdon et al., 2009). The systems are expected to continue shifting in their composition for at least 200 yr following a disturbance (Foster, 1990; Loughlin et al., 2018). These nonpersistent ecological legacies are often simply part of the long‐term successional process.
Ecological legacies in Amazonia can also include the depletion of species by people (Fig. 1b). The most commonly observed example of species depletion in palaeoecological records is the palm Iriartea deltoidea, which occurs in higher abundances where there is little to no evidence of human activity compared with areas containing past fire and cultivation (Bush & McMichael, 2016; Heijink et al., 2020). Iriartea deltoidea usually recovers c. 100 yr after site abandonment and often reaches abundances higher than before the disturbance (Fig. 1b). Iriartea deltoidea is currently the sixth most common tree species in Amazonia (ter Steege et al., 2020), and it is possible that this rise to dominance occurred as result of recovery from past depletions. It is hard to find examples of persistent depletion, which would require a species to have poor recruitment and limited seed dispersal. These types of species are rare in the landscape (Wills et al., 1997), and therefore almost undetectable using palaeoecological reconstructions.
Palms are disproportionately abundant in Amazonia compared with other tree families, and have varying degrees of responses to human disturbances. Wettinia is a genus of mid‐successional palms that has a similar, nonpersistent, negative response to human disturbance like I. deltoidea. Wettinia, however, does not seem to have the recovery overshoot that has been documented in Iriartea (Fig. 1b; Åkesson et al., 2020). The palm genus Euterpe includes the first and seventh most common tree species in Amazonia (ter Steege et al., 2013). Both of these Euterpe species are useful for their fruit, but their abundances do not seem to shift drastically in response to low levels of human disturbance (Fig. 1; Heijink et al., 2020).
4. Ecological legacies on biomass and carbon dynamics
Amazonia provides a significant input to global carbon and climate models, and is believed to sequester more carbon than it releases (i.e. is a carbon sink; e.g. Aragao et al., 2014). Global climate and carbon models assume that forests are not recovering from past disturbances, although this is intensely debated (Wright, 2013). Over recent decades, the carbon sequestration potential of Amazonia has been declining because increases in tree productivity rates have slowed and mortality rates have increased (Brienen et al., 2015). The effects of short‐term disturbances (e.g. El Niño events) have been studied (Phillips et al., 2009), but very little is known about the longer‐term disturbance histories within the forest plots that are used to estimate Amazonian carbon dynamics.
Old growth forests typically contain high amounts of biomass, but have relatively low productivity and mortality rates (Fig. 2a). Landscape modifications by people lower the biomass but increase the productivity and mortality of the system until the disturbance ceases (Fig. 2b). Of these modifications, fire and deforestation are the most intense, and biomass recovery patterns are known to be linked to disturbance intensity (de Avila et al., 2018). Early successional species transition to mid‐successional species, which have a higher biomass, c. 60 yr after abandonment, and this process can happen for over 100 yr (Fig. 2c; Loughlin et al., 2018). Biomass recovery, however, has been shown to exceed 100% of the pre‐disturbance values until at least 100 yr following an event (Fig. 2d; Poorter et al., 2016). There are no current estimates of how long it takes for the long‐lived, mid‐successional species to die off and for biomass to return to pre‐disturbance values (Fig. 2e). There are also no data yet as to how long‐term succession may be affecting the forest dynamics observed in recent decades.
Fig. 2.
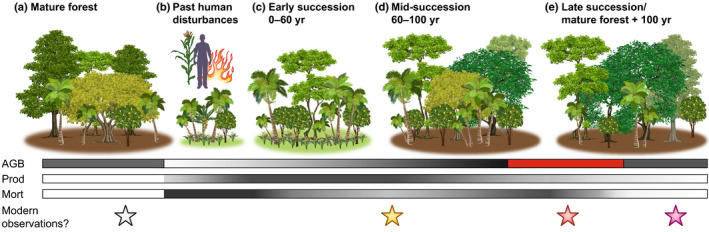
It is unknown where modern observations lie in the context of long‐term successional trajectories. (a) Mature forests have more large trees, fewer understorey trees and few grasses (brown forest floor). (b) Past human disturbances include fire, forest clearance, cultivation, and tree domestication (increased palms and fruit trees). Canopy openings result in a thicker understorey, increased numbers of grasses (green forest floor) and pioneer taxa. (c) Early successional forests retain high numbers of domesticated species, palms and pioneers, and begin accumulating large trees. (d) Mid‐successional forests retain high abundances of domesticates, long‐lived pioneers and large trees, resulting in higher biomass than mature forests (red bar, above‐ground biomass (AGB)). (e) Pioneers die off and mature forests re‐emerge, although they are compositionally different than before the disturbance. Darker shading indicates higher values and lighter shading indicates lower values for changes in AGB, productivity (Prod), and mortality (Mort) through time following a large‐scale disturbance.
It is possible that the decline of the Amazonian carbon sink and slowing down of productivity observed in the last 30 yr (Brienen et al., 2015) reflect biomass and carbon dynamics returning to pre‐disturbance values over the last several hundred years (Fig. 2d,e). Biomass and carbon dynamics are directly linked with species composition (e.g. Phillips et al., 2019), and thus ecological legacies of species composition (Fig. 1) probably translate to legacies on biomass and carbon dynamics (Fig. 2). High abundances of Bertholettia excelsa in southwestern Amazonia, which may be related to past human enrichment (Fig. 1a), play a large role in the overall carbon storage potential of those forests (Selaya et al., 2017). The large changes in palm abundances seen over the last several thousand years (Bush & McMichael, 2016) have also probably affected biomass and carbon dynamics. The forest plots used to measure carbon dynamics in Amazonia are disproportionately located in areas containing high densities of archaeological sites and high probabilities of pre‐European settlement (McMichael et al., 2017b). These plots are thus probably capturing changes in carbon dynamics related to long‐term successional dynamics and ecological legacies.
5. Outlook: advancing our knowledge of long‐term ecological legacies
There are several knowledge gaps and debatable aspects regarding ecological legacies in Amazonian forests. The first concerns the timing and intensity of the disturbance that created the ecological legacy. Most research has focused on linking pre‐European human activities with modern vegetation, but the impacts of the last 400 yr of postcolonial activities are also beginning to be considered (McMichael et al., 2017a; Arienzo et al., 2019). These two eras had different types and intensities of land use, which affect long‐term successional trajectories (Bodin et al., 2020).
The time since the last major disturbance is almost unknown in the forest plots used to study biodiversity and carbon dynamics. The time since the last fire has been published in only four out of the hundreds of surveyed forest plots (Fig. 3; Heijink et al., 2020). Los Amigos in Peru has burned in some areas as recently as 50 yr ago (Figs 2, 3, yellow star), whereas Amacayacu in Colombia has not burned in over 1600 yr (Figs 2, 3, white star). The other two forest plots had burned between 300 and 600 yr ago, and it is unknown whether biomass and composition have returned to pre‐disturbance values (Figs 2, 3, pink and red stars). Interestingly, palm abundances in the modern vegetation and in vegetation reconstructions were significantly lower at Los Amigos, which has had more recent and frequent fire events over the last 4000 yr compared with the other plots (Heijink et al., 2020). The timing of the last major disturbance for the majority of these forest plots remains unknown (Fig. 3).
Fig. 3.
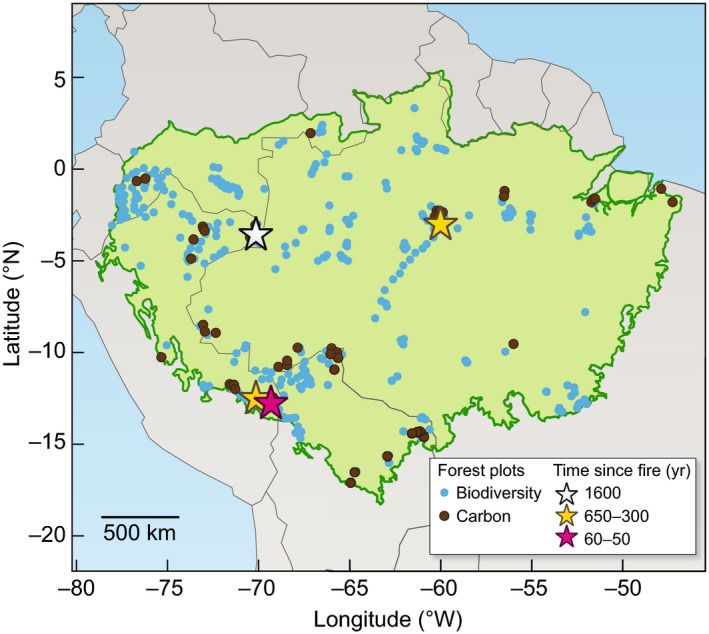
Map showing the distribution of Amazonian forest plots that are used to observe biodiversity (blue circles) and carbon dynamics (brown circles). Stars represent forest plots where there is information on the time since the last fire (see Fig. 2).
The spatial extent of these past human activities and ecological legacies into less well‐studied and less accessible regions of the forest also remains unknown and is highly debated. Some have argued that the extent of site abandonment and subsequent forest regrowth after European arrival was so great that it caused a global decrease in CO2 concentrations (Koch et al., 2019). But these assumptions rely on archaeological datasets, which, like the forest plots, are biased towards the accessible areas in Amazonia (McMichael et al., 2017a). Many soil surveys conducted in randomized and less accessible areas show little to no evidence of past fire or human occupation, or even the slightest bit of past forest opening (Piperno et al., 2019). Despite extensive scanning of hundreds of samples for charcoal in soils collected from a forest plot in the Colombian Amazon, only three were collected that were > 10 mg, or the minimum size required for 14C dating (Heijink et al., 2020). There was no evidence of maize or past forest openings in the 90 phytolith samples analysed from this forest plot, and the most recent fire occurred 1600 yr ago (Figs 2, 3; Heijink et al., 2020). The probability of the modern vegetation reflecting past human activities, or an ecological legacy, at this site is almost zero.
The integration of ecological, palaeoecological, and archaeological data are crucial to understanding the long‐term ecology and ecological legacies in Amazonian forests. Archaeologists and palaeoecologists are beginning to collect complementary datasets (Mayle & Iriarte, 2014; Maezumi et al., 2018; Åkesson et al., 2019). But to fully understand how past human activities affect modern processes, the palaeoecological and archaeological data must also be collected within the series of ecological surveys – the Amazonian forest plots that are used for estimating biodiversity and carbon dynamics. The four plots with past fire and vegetation data tell radically different stories, and filling in the gaps on the continuum of past disturbances is necessary to make links with the patterns found in the modern observational data (Figs 1, 2, 3).
Advancements in techniques of looking into the past are pushing the boundaries of what can be learned from ecological, palaeoecological and archaeological datasets. One example is by extracting dendrochronological, isotopic and genetic information from living trees, and using that information as time capsules of past human and climatic change (Caetano‐Andrade et al., 2020). Another example is by using the chemical and morphological composition of charcoal found within palaeoecological and archaeological archives to infer the temperature (intensity) of past fires and the types of plant material that were burned (Goulart et al., 2017; Gosling et al., 2019). These technical developments, as well as those geared towards improving the taxonomic identification of macro‐ and microfossils, are providing deeper insights into how past disturbances are manifested in modern systems.
Acknowledgements
I would like to thank my dear friends and colleagues, Mark B. Bush and William D. Gosling, for numerous insightful discussions that resulted in this manuscript. I was funded by European Research Council Starting Grant StG 853394 (2019).
References
- Åkesson CM, Matthews‐Bird F, Bitting M, Fennell C‐J, Church WB, Peterson LC, Valencia BG, Bush MB. 2019. 2,100 years of human adaptation to climate change in the High Andes. Nature Ecology & Evolution 4: 66–74. [DOI] [PubMed] [Google Scholar]
- Åkesson CM, McMichael CNH, Raczka MF, Huisman SN, Palmeira M, Vogel J, Neill D, Veizaj J, Bush MB. 2020. Long‐term ecological legacies in western Amazonia. Journal of Ecology. doi: 10.1111/1365-2745.13501. [DOI] [Google Scholar]
- Aragao LE, Poulter B, Barlow JB, Anderson LO, Malhi Y, Saatchi S, Phillips OL, Gloor E. 2014. Environmental change and the carbon balance of Amazonian forests. Biological Reviews 89: 913–931. [DOI] [PubMed] [Google Scholar]
- Arienzo MM, Maezumi SY, Chellman NJ, Iriarte J. 2019. Pre‐Columbian fire management linked to refractory black carbon emissions in the Amazon. Fire 2: 31. [Google Scholar]
- Bodin SC, Molino J‐F, Odonne G, Bremond L. 2020. Unraveling pre‐Columbian occupation patterns in the tropical forests of French Guiana using an anthracological approach. Vegetation History and Archaeobotany 29: 567–580. [Google Scholar]
- Brienen RJW, Phillips OL, Feldpausch TR, Gloor E, Baker TR, Lloyd J, Lopez‐Gonzalez G, Monteagudo‐Mendoza A, Malhi Y, Lewis SL et al 2015. Long‐term decline of the Amazon carbon sink. Nature 519: 344–348. [DOI] [PubMed] [Google Scholar]
- Brugger SO, Gobet E, van Leeuwen JF, Ledru M‐P, Colombaroli D, van der Knaap W, Lombardo U, Escobar‐Torrez K, Finsinger W, Rodrigues L. 2016. Long‐term man–environment interactions in the Bolivian Amazon: 8000 years of vegetation dynamics. Quaternary Science Reviews 132: 114–128. [Google Scholar]
- Bush M, Correa‐Metrio A, McMichael C, Sully S, Shadik C, Valencia B, Guilderson T, Steinitz‐Kannan M, Overpeck J. 2016. A 6900‐year history of landscape modification by humans in lowland Amazonia. Quaternary Science Reviews 141: 52–64. [Google Scholar]
- Bush MB, McMichael CN. 2016. Holocene variability of an Amazonian hyperdominant. Journal of Ecology 104: 1370–1378. [Google Scholar]
- Caetano‐Andrade VL, Clement CR, Weigel D, Trumbore S, Boivin N, Schöngart J, Roberts P. 2020. Tropical trees as time capsules of anthropogenic activity. Trends in Plant Science 25: 369–380. [DOI] [PubMed] [Google Scholar]
- Chazdon RL, Peres CA, Dent D, Sheil D, Lugo AE, Lamb D, Stork NE, Miller SE. 2009. The potential for species conservation in tropical secondary forests. Conservation Biology 23: 1406–1417. [DOI] [PubMed] [Google Scholar]
- Clement CR, Denevan WM, Heckenberger MJ, Junqueira AB, Neves EG, Teixeira WG, Woods WI. 2015. The domestication of Amazonia before European conquest. Proceedings of the Royal Society B: Biological Sciences 282: 20150813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Avila AL, van der Sande MT, Dormann CF, Peña‐Claros M, Poorter L, Mazzei L, Ruschel AR, Silva JN, de Carvalho JO, Bauhus J. 2018. Disturbance intensity is a stronger driver of biomass recovery than remaining tree‐community attributes in a managed Amazonian forest. Journal of Applied Ecology 55: 1647–1657. [Google Scholar]
- Denevan WM. 2014. Estimating Amazonian Indian Numbers in 1492. Journal of Latin American Geography 13: 207–221. [Google Scholar]
- Foster RB. 1990. Long‐term change in the successional forest community of the Rio Manu floodplain In: Gentry AH, ed. Four Neotropical Rainforests. New Haven, CT, USA: Yale University Press, 565–572. [Google Scholar]
- Gosling WD, Cornelissen HL, McMichael CNH. 2019. Reconstructing past fire temperatures from ancient charcoal material. Palaeogeography, Palaeoclimatology, Palaeoecology 520: 128–137. [Google Scholar]
- Goulart AC, Macario KD, Scheel‐Ybert R, Alves EQ, Bachelet C, Pereira BB, Levis C, Junior BHM, Marimon BS, Quesada CA. 2017. Charcoal chronology of the Amazon forest: a record of biodiversity preserved by ancient fires. Quaternary Geochronology 41: 180–186. [Google Scholar]
- Hecht SB. 2013. The scramble for the Amazon and the" lost paradise" of Euclides da Cunha. Chicago, IL, USA: University of Chicago Press. [Google Scholar]
- Heijink BM, McMichael CN, Piperno DR, Duivenvoorden JF, Cárdenas D, Duque Á. 2020. Holocene increases in palm abundances in north‐western Amazonia. Journal of Biogeography 47: 698–711. [Google Scholar]
- Koch A, Brierley C, Maslin MM, Lewis SL. 2019. Earth system impacts of the European arrival and Great Dying in the Americas after 1492. Quaternary Science Reviews 207: 13–36. [Google Scholar]
- Levis C, Costa FRC, Bongers F, Peña‐Claros M, Clement CR, Junqueira AB, Neves EG, Tamanaha EK, Figueiredo FOG, Salomão RP et al 2017. Persistent effects of pre‐Columbian plant domestication on Amazonian forest composition. Science 355: 925–931. [DOI] [PubMed] [Google Scholar]
- Levis C, Flores BM, Mazzochini GG, Manhães AP, Campos‐Silva JV, de Amorim PB, Peroni N, Hirota M, Clement CR. 2020. Help restore Brazil’s governance of globally important ecosystem services. Nature Ecology & Evolution 4: 172–173. [DOI] [PubMed] [Google Scholar]
- Levis C, Flores BM, Moreira PA, Luize BG, Alves RP, Franco‐Moraes J, Lins J, Konings E, Peña‐Claros M, Bongers F. 2018. How people domesticated Amazonian forests. Frontiers in Ecology and Evolution 5: 171. [Google Scholar]
- Loughlin NJ, Gosling WD, Mothes P, Montoya E. 2018. Ecological consequences of post‐Columbian indigenous depopulation in the Andean‐Amazonian corridor. Nature Ecology & Evolution 2: 1233–1236. [DOI] [PubMed] [Google Scholar]
- Maezumi SY, Alves D, Robinson M, de Souza JG, Levis C, Barnett RL, de Oliveira EA, Urrego D, Schaan D, Iriarte J. 2018. The legacy of 4,500 years of polyculture agroforestry in the eastern Amazon. Nature Plants 4: 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayle FE, Iriarte J. 2014. Integrated palaeoecology and archaeology–A powerful approach for understanding pre‐Columbian Amazonia. Journal of Archaeological Science 51: 54–64. [Google Scholar]
- McMichael CNH, Feeley KJ, Dick CW, Piperno DR, Bush MB. 2017a. Comment on “Persistent effects of pre‐Columbian plant domestication on Amazonian forest composition”. Science 358: eaan8347. [DOI] [PubMed] [Google Scholar]
- McMichael CNH, Matthews‐Bird F, Farfan‐Rios W, Feeley KJ. 2017b. Ancient human disturbances may be skewing our understanding of Amazonian forests. Proceedings of the National Academy of Sciences 114: 522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita RdCG, Massoca PEdS, Jakovac CC, Bentos TV, Williamson GB. 2015. Amazon rain forest succession: Stochasticity or land‐use legacy? BioScience 65: 849–861. [Google Scholar]
- Neves EG. 2013. Was agriculture a key productive activity in pre‐colonial Amazonia? The stable productive basis for social equality in the central Amazon In: Brondizio ES, Moran EF, eds. Human‐environment interactions. Dordrecht, the Netherlands: Springer, 371–388. [Google Scholar]
- Neves E, Petersen J. 2006. Political economy and pre‐columbian landscape transformation in Central Amazonia In: Balee W, Erickson CL, eds. Time and complexity in historical ecology: studies in the Neotropical lowlands. New York, NY, USA: Columbia University Press, 279–310. [Google Scholar]
- Phillips OL, Aragão LEOC, Lewis SL, Fisher JB, Lloyd J, López‐González G, Malhi Y, Monteagudo A, Peacock J, Quesada CA. 2009. Drought sensitivity of the Amazon rainforest. Science 323: 1344. [DOI] [PubMed] [Google Scholar]
- Phillips OL, Sullivan MJ, Baker TR, Mendoza AM, Vargas PN, Vásquez R. 2019. Species matter: wood density influences tropical forest biomass at multiple scales. Surveys in geophysics 40: 913–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno DR. 2011. The origins of plant cultivation and domestication in the New World tropics. Current Anthropology 52(S4): S453–S470. [Google Scholar]
- Piperno DR, McMichael CN, Bush MB. 2019. Finding forest management in prehistoric Amazonia. Anthropocene 26: 100211. [Google Scholar]
- Poorter L, Bongers F, Aide TM, Almeyda Zambrano AM, Balvanera P, Becknell JM, Boukili V, Brancalion PHS, Broadbent EN, Chazdon RL et al 2016. Biomass resilience of neotropical secondary forests. Nature 530: 211–214. [DOI] [PubMed] [Google Scholar]
- Reeve M‐E. 1993. Regional interaction in the Western Amazon: the early colonial encounter and the Jesuit years: 1538‐1767. Ethnohistory 41: 106–138. [Google Scholar]
- Roosevelt AC. 2013. The Amazon and the anthropocene: 13,000 years of human influence in a tropical rainforest. Anthropocene 4: 69–87. [Google Scholar]
- Scoles R, Gribel R. 2011. Population structure of Brazil Nut (Bertholletia excelsa, Lecythidaceae) stands in two areas with different occupation histories in the Brazilian Amazon. Human Ecology 39: 455–464. [Google Scholar]
- Selaya NG, Zuidema P, Baraloto C, Vos V, Brienen R, Pitman N, Brown F, Duchelle A, Araujo‐Murakami A, Oliveira Carillo LA et al 2017. Economically important species dominate aboveground carbon storage in forests of southwestern Amazonia. Ecology and Society 22: 1–21. [Google Scholar]
- ter Steege H, Pitman NCA, Sabatier D, Baraloto C, Salomão RP, Guevara JE, Phillips OL, Castilho CV, Magnusson WE, Molino J‐F et al 2013. Hyperdominance in the Amazonian Tree Flora. Science 342: 1243092. [DOI] [PubMed] [Google Scholar]
- ter Steege H, Prado PI, Lima RAFde, Pos E, de Souza Coelho L, de Andrade Lima Filho D, Salomão RP, Amaral IL, de Almeida Matos FD, Castilho CV. 2020. Biased‐corrected richness estimates for the Amazonian tree flora. Scientific Reports 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E, Alcázar Caicedo C, McMichael CH, Corvera R, Loo J. 2015. Uncovering spatial patterns in the natural and human history of Brazil nut (Bertholletia excelsa) across the Amazon Basin. Journal of Biogeography 42: 1367–1382. [Google Scholar]
- Wills C, Condit R, Foster RB, Hubbell SP. 1997. Strong density‐and diversity‐related effects help to maintain tree species diversity in a neotropical forest. Proceedings of the National Academy of Sciences, USA 94: 1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SJ. 2005. Tropical forests in a changing environment. Trends in Ecology & Evolution 20: 553–560. [DOI] [PubMed] [Google Scholar]
- Wright SJ. 2013. The carbon sink in intact tropical forests. Global Change Biology 19: 337–339. [DOI] [PubMed] [Google Scholar]


