Abstract
Background
Glycolic acid (GA) is an effective way of reversing the signs of age and photodamage. GA enhances desquamation of the stratum corneum and induces biological responses that can help restore skin's integrity. GA can, however, cause irritation, especially when its concentration is high, and its pH is low. Thus, most commercially available products for home use contain relatively low GA concentrations and are partially neutralized to a pH around 4.
Aims
The aim of this study was to determine the biological effects and relative efficacy of cosmetic formulations containing GA at concentrations ranging from 8% to 25% at pH 4 in human ex vivo skin explants.
Methods
Human skin explants were topically treated with gel formulations and oil‐in‐water creams containing 8%, 10%, 15%, or 25% GA, adjusted to pH 4, daily for 5 days. The degree of desquamation, their effect on cell proliferation, and their impact upon total collagen levels were determined 24 hours later. Levels of tumor necrosis factor‐alpha (TNF‐α) were measured after days 3 and 6.
Results
All formulations effectively induced desquamation in a concentration‐dependent manner. Total collagen levels were increased at all concentrations, with greatest effects at higher GA concentrations. No effect on TNF‐α expression was observed.
Conclusions
These data suggest that partially neutralized GA formulations retain skin rejuvenating properties without causing irritation and inflammation and that their use can be tailored to individual needs based on the concentration of GA in the formulation.
Keywords: cosmetics, glycolic acid, keratolytic agents, rejuvenation, skin aging
1. INTRODUCTION
Alpha‐hydroxy acids (AHAs) are a class of naturally occurring organic acids derived from fruit and dairy products. 1 They are widely used to treat a variety of skin conditions including photodamage, acne, exfoliating conditions such as ichthyosis, xeroderma, and psoriasis, hyperpigmentation disorders, actinic keratoses, fine wrinkles, lentigines, melasma, and seborrheic keratoses. 1 Glycolic acid (GA), the smallest and most extensively used of the AHAs, has proven clinically effective at improving the appearance of photodamaged skin, with significant reduction in fine lines and wrinkles, smoothing of rough and uneven skin texture, normalization of skin tone, and reduction in hyperpigmentation. 2 , 3 , 4 , 5 , 6 , 7
The anti‐photoaging effects of GA are related to its ability to induce desquamation of the outermost layers of the epidermis. GA reduces cohesion within the stratum corneum by enhancing degradation of the corneodesmosomes responsible for corneocyte adhesion. 8 GA has also been demonstrated to increase epidermal and dermal hyaluronic acid levels, 9 increase keratinocyte and fibroblast proliferation rates, 10 , 11 stimulate collagen production, 2 , 9 , 10 , 11 , 12 and improve the quality of elastic fibers. 2 An inhibitory effect on melanin synthesis has also been reported. 13
The ability of GA to invoke these biological responses is determined by its capacity to penetrate into skin. 1 This itself depends upon the amount of GA in its most biologically active free acid form, its pH and concentration, its contact time with the skin, and the vehicle used to deliver it. 1 Like all acids, however, GA can cause skin irritation and erythema, and these effects are greatest when the concentration of GA is high, and its pH is low. Most commercially available GA‐containing products for the treatment of photoaging at home are therefore partially neutralized or buffered to a pH around 4. It has been suggested that at these pHs, however, AHAs are no more effective at stimulating epidermal turnover than non‐AHA‐containing moisturizing lotions. 14
Despite multiple published studies regarding the clinical benefits and biological effects of GA‐containing chemical peels (reviewed in Ref. [1]), most of these studies focused on one particular concentration of GA. Moreover, there are few studies specifically comparing the effects of partially neutralized formulations on the biological response to GA. In this study, we sought to redress this by examining the ability of gel and oil‐in‐water (o/w) creams containing 8%, 10%, 15%, and 25% GA adjusted to pH 4 to mediate desquamation, induce collagen synthesis, stimulate cell proliferation, and invoke an inflammatory response in human skin explants when applied topically. Based on these results, we propose an algorithm for their rational use in the treatment of photoaging.
2. MATERIALS AND METHODS
2.1. Test products
The effects of the following formulations were assessed: (a) o/w cream containing 8% GA (with 3.23% free GA); (b) gel containing 10% GA (with 4.03% free GA); (c) o/w cream containing 15% GA (with 6.05% free GA); (d) gel containing 15% GA (with 6.05% free GA); and (e) gel containing 25% GA (with 10.1% free GA). All formulations were partially neutralized with ammonium hydroxide to pH 4.0.
2.2. Human skin explants
Human skin explants of an average diameter of 12 mm (±1 mm) were prepared from surgical skin residues of a 58‐year‐old Caucasian woman (Fitzpatrick phototype II) who had undergone brachioplasty. Explants were maintained in BEM culture medium (BIO‐EC's Explants Medium), a proprietary explant culture medium, at 37°C in a humid, 5% CO2 atmosphere. A quarter of the culture medium (0.5 mL/well) was renewed daily. Four explants from the same donor were used per treatment group.
2.3. Ethical approval and informed consent
All human skin explants used in this study were obtained from surgical residues after written informed consent from the donor and in full respect of the Declaration of Helsinki and article L.1245‐2 of the French Public Health Code. 15 The latter does not require any prior authorization by an ethics committee for the use of surgical waste.
2.4. Product application
Test products were evenly applied to the surface of the explant with a small spatula at a final concentration of 2 mg/cm2 each morning on 5 consecutive days. Each evening, the products were removed using a humidified wipe. 24 hours after the final application (day 6), explants were fixed in buffered formalin, dehydrated, and sections prepared using standard techniques. Untreated control batches did not receive any treatment except renewal of the culture media.
2.5. Measurement of stratum corneum thickness
5‐µm skin sections were stained with Masson‐Goldner trichrome stain (RAL Diagnostics). SC thickness was determined from digitized images using Cell^D software (Olympus Life Science). A total of 27 images were analyzed per experimental condition (9 images per explant; 3 explants per condition).
2.6. Determination of number of SC layers
The number of cell layers of the SC was determined from 7 µm cryosections as described previously. 16 Briefly, sections were treated with 0.4 N NaOH, and, following cell swelling, the number of layers of the SC determined manually in 27 sections per condition (9 sections per explant; 3 explants per condition).
2.7. Corneodesmosin immunostaining
Corneodesmosin (CDSN) immunostaining was performed using a polyclonal anti‐CDSN antibody (Sigma‐Aldrich [ref. HPA044730]), diluted 1:200 in PBS containing 0.3% BSA and 0.05% Tween‐20. Briefly, 5‐µm skin sections were incubated for 1 hour at room temperature, enhanced with a streptavidin/biotin system, and revealed using Vector VIP (Vector Laboratories). CDSN was quantified in 9 images per condition by determining the area of the SC positive for CDSN using Cell^D software (Olympus Life Science).
2.8. Determination of epidermal and dermal proliferation indices
Ki‐67 immunostaining was performed using a monoclonal anti‐Ki‐67 (clone 7B11) antibody (Invitrogen) diluted 1:200 in PBS containing 0.3% BSA and 0.05% Tween‐20, as described above. Cell nuclei were counterstained using Mayer's hemalum solution (RAL Diagnostics). Ki‐67‐positive cells were identified in digitized images captured from 3 skin sections using a BX63 microscope (Olympus Life Science) and cellSens imaging software (Olympus Life Science). The Epidermal Proliferation Index (EPI) was determined by dividing the number of Ki‐67‐positive cells in the viable epidermis by the total number of cells in the same region. The Dermal Proliferation Index was determined by dividing the number of Ki‐67–positive cells in the papillary dermis and upper reticular dermis by the total number of cells in the same region.
2.9. Total collagen staining
Total collagen was stained with Picro‐Sirius F3B (RAL Diagnostics). The area of the dermis stained for collagen was determined in 9 images per condition (3 replicates per explant; 3 explants per condition) using Cell^D software (Olympus Life Science).
2.10. Determination of TNF‐α production
The concentration of tumor necrosis factor‐alpha (TNF‐α) released into the culture medium was determined on days 3 (following the 4th application) and 6 (24 hours after the final application) using human TNF‐α ELISA kit (Cayman Chemical), according to the manufacturer's instructions. Absorbance at 412 nm was measured using an Infinite 200 Pro microplate reader and Magellan software (Tecan). Quantification of TNF‐α was performed on culture medium from 4 explants per condition.
2.11. Statistical analysis
For each replicate, donor, and experimental condition, the mean ± standard error (SEM) was calculated. Data were analyzed using Student's t test. A P value <.05 was considered significant.
3. RESULTS
3.1. Effect on the stratum corneum
All products significantly increased the thickness of the SC after 5 days of treatment, such that the normally discrete and tightly packed layers of corneocytes within the SC became more loosely assembled (Figure 1C). These effects were dose‐dependent, with greatest effects, as indicated by a thicker SC, seen in skin treated with higher strength formulations (Figure 1A). Skin treated with 15% and 25% gels presented with flaking of the outermost layers of the SC (Figure 1C). The SC of skin treated with the 15% gel formulation was significantly thicker than that treated with the equivalent cream formulation (+77.0% for 15% gel vs +62.8% for 15% cream; P < .01; Figure 1A).
FIGURE 1.
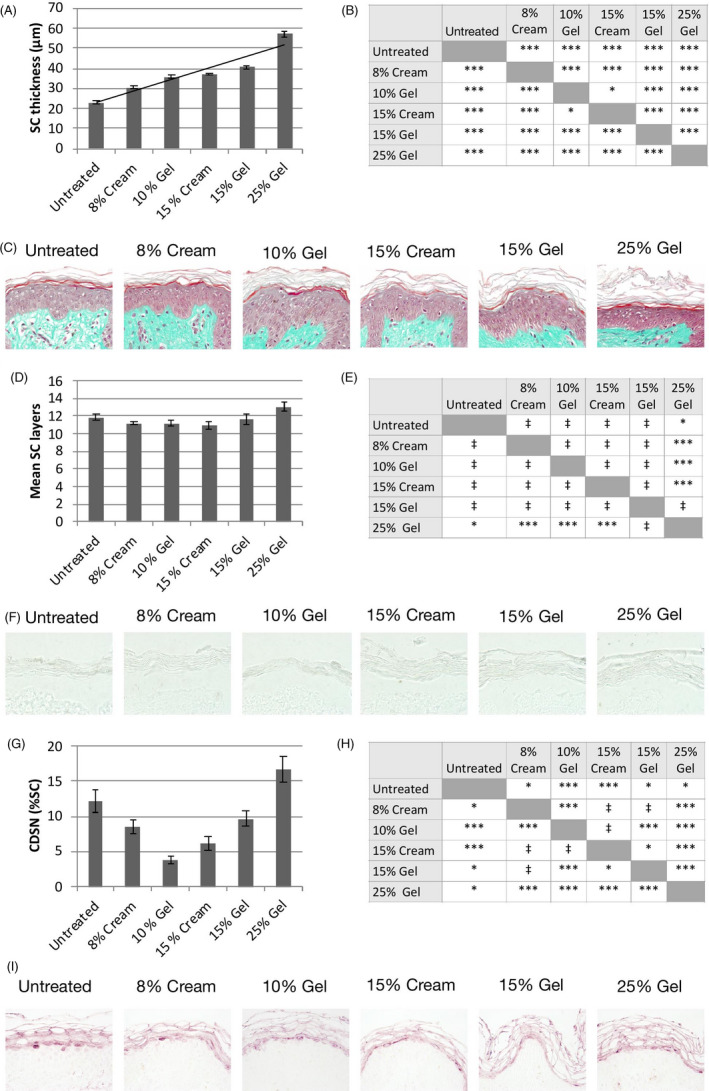
Effect on the stratum corneum. A, Thickness of the stratum corneum (SC) on day 6. Data show mean ± SEM of 27 images. B, Statistical comparisons between treatment groups; ***P < .01; *P < .05. C, Representative skin sections stained with Masson‐Goldner trichrome stain on day 6. D, Number of corneocyte layers on day 6. Data show mean ± SEM of 27 images. E, Statistical comparisons between treatment groups; ***P < .01; *P < .05; ‡ not significant. F, Representative skin sections following treatment with 0.4 N NaOH. G, Corneodesmosin (CDSN) expression levels on day 6. Data show mean ± SEM of 9 images per condition. H, Statistical comparisons between treatment groups; ***P < .01; *P < .05; ‡ not significant. I, Representative skin sections stained with a polyclonal anti‐CDSN antibody on day 6
The mean number of corneocyte layers in skin treated with formulations containing between 8% and 15% GA was unchanged after 5 days of treatment (11.3 ± 2.2 for 8%‐15% GA‐treated skin vs 11.9 ± 1.9 for untreated skin; P not significant; Figure 1D,E). Moreover, no difference between the 15% gel and 15% cream formulations was observed (Figure 1D,E). Treatment with the 25% GA gel, however, significantly increased the mean number of corneocyte layers (13.1 ± 2.5 for 25% GA‐treated skin vs 11.9 ± 1.9 for untreated skin; P < .05; Figure 1D,E).
Levels of CDSN, an extracellular glycoprotein component of corneodesmosomes, were significantly reduced at GA concentrations between 8% and 15%, with CDSN expression lowest in skin treated with 10% gel (Figure 1G,H). CDSN levels in skin treated with the 15% cream were also significantly lower than the equivalent gel formulation (Figure 1G). Conversely, in skin treated with 25% GA gel, CDSN levels were increased by 36.7% relative to untreated skin (P < .05; Figure 1G,H).
3.2. Effect on the viable epidermis
Epidermal proliferation, as measured by Ki67 immunostaining, was increased by 29% and 106% for the 8% and 15% creams, respectively, and by 39% and 295% for the 10% and 15% gels, respectively (Figure 2A). Interestingly, the EPI of skin treated with the 25% gel (+126%) was lower than that of skin treated with the 15% gel (Figure 2A). No significant difference in the EPI between the 15% gel and 15% cream formulations was observed (Figure 2A,B).
FIGURE 2.
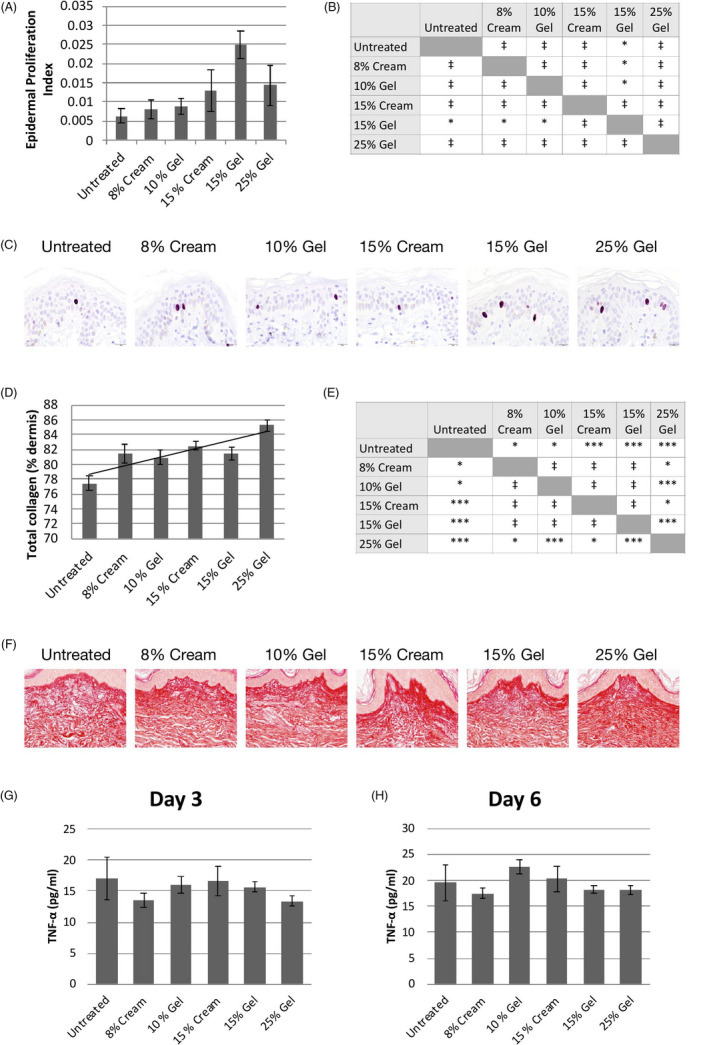
Effect on keratinocyte proliferation, total collagen, and TNF‐α levels. A, Epidermal Proliferation Index on day 6. Data show mean ± SEM of 3 explants. B, Statistical comparisons between treatment groups; *P < .05; ‡ not significant. C, Representative skin sections stained for Ki‐67. D, Total collagen levels on day 6. Data show mean ± SEM of 9 images per condition. A trend line is shown. E, Statistical comparisons between treatment groups; ***P < .01; *P < .05; ‡ not significant. F, Representative skin sections stained with Sirius red on day 6. TNF‐α levels on day 3 (G) and day 6 (H). Data show mean ± SEM of 4 replicates per condition. All comparisons are not significant
3.3. Effect on the dermis
Total collagen levels were significantly increased at all concentrations after 5 days of treatment (Figure 2D,E). These effects were dose‐dependent, with higher total collagen levels observed in explants treated with products with higher GA concentrations (Figure 2D). In skin treated with the 25% gel, for example, total collagen levels were increased by 10.1% (P < .01) with respect to untreated skin. At lower GA concentrations (8%‐15%), total collagen levels were increased by between 5% and 6% (P < .05 at all concentrations). No difference in total collagen levels between the 15% gel and 15% cream formulations was observed (Figure 2D,E).
Fibroblast proliferation rates were unchanged following treatment with all GA formulations (data not shown).
3.4. Effect on TNF‐α levels
TNF‐α levels were not significantly different from those secreted from untreated skin at both day 3 (Figure 2G) and day 6 (Figure 2H). Moreover, there was no difference between the 15% gel and 15% cream formulations at either time point.
4. DISCUSSION
Topical GA is widely utilized in cosmetics and dermatology but exhibits different therapeutic and cosmetic benefits depending on its concentration and pH. As a peeling agent, GA is used at high concentrations and low pHs. Seventy percent GA solutions are commonly used as superficial chemical peeling agents, with a pH ranging from 0.08 to 2.75. 1 At these values, in which the free acid concentration of GA is very high, GA readily disrupts cohesion of the corneocytes of the skin barrier but also causes skin irritation, which is harmful to the skin. On the contrary, GA at low concentrations and higher pHs appears to exhibit anti‐inflammatory effects because of epigenetic modifications of the inflammasome complex, 17 thus benefiting skin. Moreover, in neutralizing these formulations by raising the pH, the formulation itself is nearer the natural physiological pH of skin, which is itself less irritative. Within these parameters, however, the efficacy of GA at inducing desquamation and stimulating collagen production is reduced. Indeed, it has been suggested that when the product pH is 4 or more, the efficacy of AHAs is lost. 14 Considering that these low‐strength, neutralized GA formulations are those that are most commonly used by consumers at home for the treatment of photoaging, it is important to understand their efficacy and their effects on skin biology in order to make better treatment recommendations depending on individual needs.
The results of this study suggest that cosmetic formulations containing GA at concentrations between 8% and 25% and adjusted to pH 4 can effectively cause desquamation and that these effects are concentration dependent. Concurrent with the view that GA disrupts corneodesmosomes within the SC, 18 , 19 levels of the corneodesmosome glycoprotein CDSN were reduced upon treatment with 8% to 15% GA.
A previous study has suggested that at low doses, GA has little effect upon SC thickness. 8 This also appeared to be the case in this study with GA at concentrations up to 15%. At 25%, however, the number of SC layers was increased, suggesting that SC renewal was occurring at this concentration. Notably, CDSN levels were also increased at this concentration. DiNardo et al 20 reported a similar finding in vivo, where a 13% pH 3.8 GA formulation increased SC thickness but lower strength formulations (3.25%, 6.50%, and 9.75%) at the same pH reduced it. 20 They suggested this may be a rebound effect from the initial exfoliation process. 20 It would be interesting to further examine the differential effects of GA concentration on keratinocyte differentiation to better understand this phenomenon.
One of the effects of GA and other AHAs is epidermal thickening due to increased keratinocyte proliferation. 2 , 21 , 22 Stimulation of keratinocyte proliferation was also observed upon GA treatment in this study. Interestingly, the effects of GA on basal keratinocyte proliferation in this study were concentration‐dependent only up to 15%.
GA has also been shown to stimulate fibroblast proliferation in vitro. 10 , 11 In this study, however, it had no effect, suggesting perhaps that GA did not penetrate into the dermis at the GA concentrations we tested. Nevertheless, we demonstrated that GA at these concentrations increased dermal collagen levels, and this effect was largely concentration dependent. Okano et al 23 conducted an elegant study in which they show that keratinocyte‐derived factors indirectly stimulate collagen synthesis in fibroblasts. 23 Our results certainly support this hypothesis and suggest that collagen synthesis occurs even at low GA concentrations that might fail to penetrate into the dermis.
None of the tested products had an effect on TNF‐α, with levels equivalent to those of untreated skin at both days 3 and 6. Since proinflammatory cytokines such as TNF‐α are responsible for the irritancy, burning, erythema, and swelling that occur with use of high concentrations of GA, it suggests that cutaneous acceptability of partially neutralized GA formulations will be high. Confirming this, the cream and gel formulations described here have proven to be well‐tolerated in a series of acceptability tests involving women with moderate skin aging (data on file).
In this study, the 15% GA formulated in gel appeared to have greater efficacy than its equivalent cream. As these gels contain ethanol, its vaporization can leave behind a higher GA concentration in the formulation after application to the skin. This increases the thermodynamic activity of GA in the residual formulation, which enhances its delivery into the SC. 24 Ethanol is also capable of extracting lipids from the SC, thus modifying skin's barrier properties. 24
In a market replete with products containing GA at varying concentrations, the selection of the optimum galenic form and strength of acid appropriate for skin type, age, and degree of photodamage can be confusing for users and dermatologists alike. For this reason we propose the following: (a) topical gels for oily and acne‐prone skin, and creams for dry skin; (b) for subjects with mild or moderate photoaging according to Glogau classification system, 25 the recommendation will be to start with lower concentrations for the first week and, depending on individual tolerance, to increase the concentration over subsequent weeks (Figure 3).
FIGURE 3.
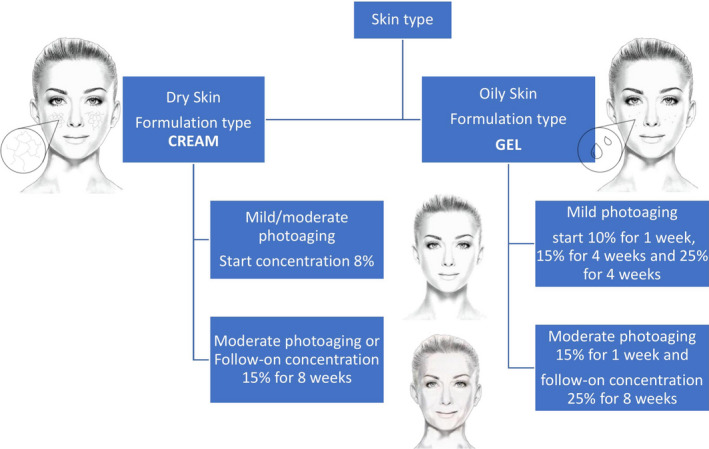
Algorithm for the rational use of 8% to 25% glycolic acid (pH 4) in photodamaged skin
In summary, our histological analysis of ex vivo human skin explants suggests that GA retains its skin rejuvenating properties even when adjusted to pH 4. Moreover, because increasing GA concentrations are associated with more significant effects (summarized in Table 1), the dermatologist can choose and recommend a formulation based upon the degree of photodamage and individual tolerance.
TABLE 1.
Summary of effects of glycolic acid (GA) concentration on the stratum corneum, epidermis, and dermis
| GA concentration | Free GA | Effect on stratum corneum | Effect on viable epidermis | Effect on dermis |
|---|---|---|---|---|
| 8% | 3.23% | Moderate desquamation | Moderate increase in keratinocyte proliferation | Moderate increase in collagen levels |
| 10% | 4.03% | Moderate desquamation | Moderate increase in keratinocyte proliferation | Moderate increase in collagen levels |
| 15% | 6.05% | Significant desquamation | Significant increase in keratinocyte proliferation | Moderate increase in collagen levels |
| 25% | 10.1% | Significant desquamation | Moderate increase in keratinocyte proliferation | Significant increase in collagen levels |
CONFLICTS OF INTEREST
Mridvika Narda, Carles Trullas, and Corinne Granger are employees of ISDIN, the manufacturer of the formulations under study. Anthony Brown and Jaime Piquero‐Casals are paid consultants to ISDIN. Gabriela Fabbrocini has no conflict of interest to declare.
ACKNOWLEDGMENTS
We wish to thank Laurent Peno‐Mazzarino of Laboratoire Bio‐EC, Longjumeau, France, who performed the ex vivo study described here. This study was wholly funded by ISDIN, the manufacturer of the formulations under study.
Narda M, Trullas C, Brown A, Piquero‐Casals J, Granger C, Fabbrocini G. Glycolic acid adjusted to pH 4 stimulates collagen production and epidermal renewal without affecting levels of proinflammatory TNF‐alpha in human skin explants. J Cosmet Dermatol.2021;20:513–521. 10.1111/jocd.13570
REFERENCES
- 1. Fabbrocini G, De Padova MP, Tosti A. Glycolic acid In: Tosti A, Grimes PE, De Padova MP, eds. Color Atlas of Chemical Peels. Springer, Berlin: Heidelberg; 2012:9‐16. [Google Scholar]
- 2. Ditre CM, Griffin TD, Murphy GF, et al. Effects of alpha‐hydroxy acids on photoaged skin: a pilot clinical, histologic, and ultrastructural study. J Am Acad Dermatol. 1996;34(2 Pt 1):187‐195. 10.1016/s0190-9622(96)80110-1 [DOI] [PubMed] [Google Scholar]
- 3. Newman N, Newman A, Moy LS, Babapour R, Harris AG, Moy RL. Clinical improvement of photoaged skin with 50% glycolic acid. A double‐blind vehicle‐controlled study. Dermatol Surg. 1996;22(5):455‐460. 10.1111/j.1524-4725.1996.tb00347.x [DOI] [PubMed] [Google Scholar]
- 4. Thibault PK, Wlodarczyk J, Wenck A. A double‐blind randomized clinical trial on the effectiveness of a daily glycolic acid 5% formulation in the treatment of photoaging. Dermatol Surg. 1998;24(5):573‐578. 10.1111/j.1524-4725.1998.tb04209.x [DOI] [PubMed] [Google Scholar]
- 5. Kubiak M, Mucha P, Debowska R, Rotsztejn H. Evaluation of 70% glycolic peels versus 15% trichloroacetic peels for the treatment of photodamaged facial skin in aging women. Dermatol Surg. 2014;40(8):883‐891. 10.1097/01.DSS.0000452669.84787.bf [DOI] [PubMed] [Google Scholar]
- 6. Oresajo C, Yatskayer M, Hansenne I. Clinical tolerance and efficacy of capryloyl salicylic acid peel compared to a glycolic acid peel in subjects with fine lines/wrinkles and hyperpigmented skin. J Cosmet Dermatol. 2008;7(4):259‐262. 10.1111/j.1473-2165.2008.00403.x [DOI] [PubMed] [Google Scholar]
- 7. Stiller MJ, Bartolone J, Stern R, et al. Topical 8% glycolic acid and 8% L‐lactic acid creams for the treatment of photodamaged skin. A double‐blind vehicle‐controlled clinical trial. Arch Dermatol. 1996;132(6):631‐636. [PubMed] [Google Scholar]
- 8. Fartasch M, Teal J, Menon GK. Mode of action of glycolic acid on human stratum corneum: ultrastructural and functional evaluation of the epidermal barrier. Arch Dermatol Res. 1997;289(7):404‐409. 10.1007/s004030050212 [DOI] [PubMed] [Google Scholar]
- 9. Bernstein EF, Lee J, Brown DB, Yu R, Van Scott E. Glycolic acid treatment increases type I collagen mRNA and hyaluronic acid content of human skin. Dermatol Surg. 2001;27(5):429‐433. 10.1046/j.1524-4725.2001.00234.x [DOI] [PubMed] [Google Scholar]
- 10. Kim SJ, Park JH, Kim DH, Won YH, Maibach HI. Increased in vivo collagen synthesis and in vitro cell proliferative effect of glycolic acid. Dermatol Surg. 1998;24(10):1054‐1058. 10.1111/j.1524-4725.1998.tb04074.x [DOI] [PubMed] [Google Scholar]
- 11. Kim SJ, Won YH. The effect of glycolic acid on cultured human skin fibroblasts: cell proliferative effect and increased collagen synthesis. J Dermatol. 1998;25(2):85‐89. [PubMed] [Google Scholar]
- 12. Moy LS, Howe K, Moy RL. Glycolic acid modulation of collagen production in human skin fibroblast cultures in vitro. Dermatol Surg. 1996;22(5):439‐441. 10.1111/j.1524-4725.1996.tb00344.x [DOI] [PubMed] [Google Scholar]
- 13. Usuki A, Ohashi A, Sato H, Ochiai Y, Ichihashi M, Funasaka Y. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp Dermatol. 2003;12(Suppl 2):43‐50. 10.1034/j.1600-0625.12.s2.7.x [DOI] [PubMed] [Google Scholar]
- 14. Thueson DO, Chan EK, Oechsli LM, Hahn GS. The roles of pH and concentration in lactic acid‐induced stimulation of epidermal turnover. Dermatol Surg. 1998;24(6):641‐645. 10.1111/j.1524-4725.1998.tb04221.x [DOI] [PubMed] [Google Scholar]
- 15. Code de la santé publique ‐ Article L1245‐2. https://www.legifrance.gouv.fr/affichCodeArticle.do;jsessionid=36358885826C9FA5965AFB6605E8A722.tplgfr32s_1?idArticle=LEGIARTI000024325419&cidTexte=LEGITEXT000006072665&dateTexte=20200202. Accessed February 2, 2020.
- 16. Christophers E, Kligman AM. Visualization of the cell layers of the stratum corneum. J Invest Dermatol. 1964;42:407‐409. 10.1038/jid.1964.88 [DOI] [PubMed] [Google Scholar]
- 17. Tang S‐C, Yang J‐H. Dual effects of alpha‐hydroxy acids on the skin. Molecules. 2018;23(4):863 10.3390/molecules23040863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X. A theory for the mechanism of action of the alpha‐hydroxy acids applied to the skin. Med Hypotheses. 1999;53(5):380‐382. 10.1054/mehy.1998.0788 [DOI] [PubMed] [Google Scholar]
- 19. Horikoshi T, Matsumoto M, Usuki A, et al. Effects of glycolic acid on desquamation‐regulating proteinases in human stratum corneum. Exp Dermatol. 2005;14(1):34‐40. 10.1111/j.0906-6705.2005.00224.x [DOI] [PubMed] [Google Scholar]
- 20. DiNardo JC, Grove GL, Moy LS. Clinical and histological effects of glycolic acid at different concentrations and pH levels. Dermatol Surg. 1996;22(5):421‐424. 10.1111/j.1524-4725.1996.tb00341.x [DOI] [PubMed] [Google Scholar]
- 21. Bernstein EF, Underbill CB, Lakkakorpi J, et al. Citric acid increases viable epidermal thickness and glycosaminoglycan content of sun‐damaged skin. Dermatol Surg. 1997;23(8):689‐694. 10.1111/j.1524-4725.1997.tb00391.x [DOI] [PubMed] [Google Scholar]
- 22. Denda S, Denda M, Inoue K, Hibino T. Glycolic acid induces keratinocyte proliferation in a skin equivalent model via TRPV1 activation. J Dermatol Sci. 2010;57(2):108‐113. 10.1016/j.jdermsci.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 23. Okano Y, Abe Y, Masaki H, Santhanam U, Ichihashi M, Funasaka Y. Biological effects of glycolic acid on dermal matrix metabolism mediated by dermal fibroblasts and epidermal keratinocytes. Exp Dermatol. 2003;12(Suppl 2):57‐63. 10.1034/j.1600-0625.12.s2.9.x [DOI] [PubMed] [Google Scholar]
- 24. Williams A. Pharmaceutical solvents as vehicles for topical dosage forms In: Augustijns P, Brewster ME, eds. Biotechnology: Pharmaceutical Aspects. Volume VI: Solvent Systems and Their Selection in Pharmaceutics and Biopharmaceutics. New York, NY: Springer; 2007:405‐426. [Google Scholar]
- 25. Glogau R. Chemical peeling and aging skin. J Geriatr Dermatol. 1994;2:31‐35. [Google Scholar]


