Abstract
Background and Objectives
A previous pre‐clinical study on electromagnetic muscle stimulation (EMMS) suggested that fat cell apoptosis occurs following treatment in a porcine model. While EMMS can induce changes in muscle, the effect on fat tissue is not established. This clinical study sought to assess adipose tissue response to EMMS in comparison to cryolipolysis treatment.
Study Design/Materials and Methods
Study subjects were recruited prior to abdominoplasty to receive body contouring treatments and subsequently to obtain tissue for histological analysis. Non‐invasive abdominal treatments were delivered using a commercially available (n = 6) or prototype (n = 3) EMMS system or a cryolipolysis system (n = 2). Subjects received a single EMMS treatment (100% intensity for 30 minutes) or a single cryolipolysis treatment (−11°C for 35 minutes) to the abdomen. Superficial and deep (i.e., adjacent to muscle layer) subcutaneous adipose tissue was harvested at set timepoints post‐treatment. The presence or absence of an inflammatory response was evaluated using standard hematoxylin and eosin (H&E) staining. As adipocytes that are destined to become apoptotic cannot be distinguished by traditional H&E staining during the early phases of injury, irreversible fat cell injury was assessed using perilipin immunofluorescence.
Results
Following H&E histological analysis at 3, 10, 11, and 17 days post‐treatment, no EMMS‐treated samples showed an inflammatory response in either the superficial or deep subcutaneous adipose tissue. For the cryolipolysis‐treated adipose tissue, however, the H&E staining revealed a marked inflammatory response with an influx of neutrophils, lymphocytes, and macrophages at timepoints consistent with previous histological studies. Further, loss of perilipin staining provided clear visual evidence of irreversible fat cell injury in the cryolipolysis‐treated adipose tissue. In contrast, the electromagnetic muscle stimulation‐treated samples showed persistence of perilipin staining of adipose tissue indicating that all fat cells were viable.
Conclusion
This study failed to demonstrate either fat cell injury or inflammatory response following EMMS treatment. While electromagnetic muscle stimulation may non‐invasively induce muscle changes, this clinical study found no evidence of an impact injurious or otherwise on subcutaneous fat. © 2020 The Authors. Lasers in Surgery and Medicine published by Wiley Periodicals LLC
Keywords: apoptosis, cryolipolysis, EMS, EMMS, electromagnetic muscle stimulation, HIMMS, HIFEM, histology, MMS, muscle toning, muscle strengthening, NMES, non‐invasive body contouring, non‐surgical fat reduction, perilipin, qPCR, TUNEL
INTRODUCTION
There is continued interest on the part of the public in seeking safe and effective aesthetic improvement in the absence of downtime commonly associated with surgical procedures. Non‐invasive body contouring with cryolipolysis is a popular fat reduction procedure that treats undesirable subcutaneous fat with controlled cooling. Cryolipolysis is based upon the greater susceptibility of lipid‐rich adipocytes to cold injury compared with surrounding water‐rich cells [1, 2, 3]. Numerous clinical studies have demonstrated the safety, efficacy, and tolerability of cryolipolysis in multiple body areas including the abdomen, flanks, inner thighs, outer thighs, arms, back, bra fat area, banana roll, and submental area [4, 5, 6, 7, 8, 9, 10, 11].
As patients grow increasingly comfortable with aesthetic procedures and non‐invasive body contouring, so does their appetite for greater refinement in appearance. In addition to reducing unwanted subcutaneous fat, some patients are interested in toning of the underlying muscles, while improving their feeling of well‐being and performance in the gym or on the athletic field.
Recently, electromagnetic muscle stimulation (EMMS) has been developed for aesthetic body contouring. While electromagnetic muscle stimulation has been utilized for decades in physical therapy and urological applications, it is a new technology in the field of aesthetic medicine. Based upon the inherent electrical properties of the skin, fat, and muscle, current flow can be induced directly in the muscle layer, stimulating powerful contractions without dermal discomfort. Clinical studies have been conducted to show safety and efficacy of EMMS for body contouring in the abdomen and buttocks [12, 13, 14, 15, 16]. EMMS has also been used for toning and strengthening thighs. Induction of muscle fiber hypertrophy has been reported following electromagnetic muscle stimulation treatment in a porcine model [17].
It has been hypothesized that EMMS can cause fat cell injury, specifically induction of fat apoptosis in a porcine model after a single treatment [18]. These results, however, have not yet been proven to occur in human subjects. Human histology has not demonstrated a post‐inflammatory response that would normally follow injury to a large number of adipocytes and the subsequent digestion and removal of cellular debris. These histologically observable events would be expected to precede a clinically measurable fat layer reduction.
Fat cell injury can be assessed directly or indirectly. Direct assessment can be achieved by measuring the viability of the adipocyte outer plasma membrane or inner lipid droplet membrane. Perilipin is a key regulator of lipolysis in adipocytes, where it is abundantly expressed as a phosphoprotein associated with the adipocyte lipid droplet [19]. It serves important functions in the regulation of both basal and hormonally stimulated lipolysis [20]. Under basal conditions, perilipin restricts the access of cytosolic lipases to lipid droplets and thus promotes triglyceride storage. In times of energy deficit, perilipin is phosphorylated by protein kinase A and facilitates maximal lipolysis by hormone‐sensitive lipase and adipose triglyceride lipase [21]. Investigations on the fate of adipocytes have pointed out that living and dead adipocytes cannot be distinguished with traditional H&E staining but can be distinguished with immunohistochemistry for perilipin [22]. Thus, perilipin staining has become a standard method of assessing adipocyte viability in research areas such as fat grafting [22, 23, 24]. Nevertheless, hematoxylin and eosin (H&E) staining is a powerful tool to inspect the inflammatory response post‐treatment and can be used as an indirect marker of fat injury.
In summary, while EMMS treatment can induce muscle changes, its effect on fat tissue is not well‐established. This study investigated adipose tissue response to electromagnetic muscle stimulation in comparison to cryolipolysis using H&E and perilipin immunofluorescence staining.
MATERIALS AND METHODS
This was a multicenter, prospective, open label, non‐randomized feasibility study. The protocol was approved by an independent review board (Salus IRB, Austin, TX). Eligible subjects were male or female, at least 18 years of age, with a scheduled surgical excision of abdominal fat and skin.
Exclusion criteria included previous surgical procedures such as liposuction or non‐invasive fat reduction in or near the treatment area in the past 6 months, a known history of cryoglobulinemia, cold urticaria, cold agglutinin disease, paroxysmal cold hemoglobinuria, Raynaud's disease, bleeding disorders, or concurrent medications that could increase the risk of bruising, dermatological conditions such as excessive skin laxity or scars that may interfere with the treatment or evaluation, intrauterine contraceptive device inserted or removed within the past month, cardiac disorder, pulmonary insufficiency, and metal implant or active implanted devices such as a pacemaker, defibrillator, or drug delivery system.
Subjects had a single EMMS treatment visit with one applicator placed on the center of the abdomen. Each EMMS treatment cycle was delivered at 100% intensity for 30 minutes. Six subjects were treated with System A (Emsculpt; BTL Industries, Marlborough, MA) and three were treated with System B (CoolTone Prototype; ZELTIQ Aesthetics, Pleasanton, CA).
Two subjects were treated on the abdomen with a cryolipolysis system (CoolSculpting; ZELTIQ Aesthetics, Pleasanton, CA) using one cycle per treatment area (−11°C for 35 minutes) delivered by a cooled cup vacuum applicator (CoolAdvantage applicator series, ZELTIQ Aesthetics, Pleasanton, CA). A protective gel pad (CoolAdhesive GelPad, ZELTIQ Aesthetics, Pleasanton, CA) was applied to the skin and suction was initiated. With the applicator thus secured to the treatment area, the subject reclined throughout the cryolipolysis procedure. At the end of the treatment cycle, the applicator was removed, and a manual massage of the treatment area was performed for 2 minutes. Safety was monitored by documentation of any adverse events throughout the study, as was clinical assessment of the treatment site.
One treated and one untreated control sample was taken from each study subject. Subcutaneous fat harvested from the treated areas was further subdivided into superficial and deep specimens to assess whether the fat that was immediately adjacent to the stimulated muscle was impacted more than the superficial fat. Harvested tissue was immediately fixed in formalin after surgery and later processed for histological study using standard H&E and perilipin immunofluorescence staining.
Perilipin immunofluorescence optimization was performed on formalin‐fixed paraffin‐embedded 5‐μm tissue sections using a BOND automated immunostainer (Leica Biosystems, Buffalo Grove, IL) and an anti‐perilipin antibody (#ab61682; Abcam, Cambridge, MA) at 1:1000 dilution. Heat‐induced antigen retrieval was performed using a BOND Epitope Retrieval Solution 2 (Leica Biosystems) (ethylenediaminetetraacetic acid solution, pH 9.0) for 20 minutes. Non‐specific background was blocked with Novolink Protein Block (Leica Biosystems). Slides were incubated with primary antibody overnight at 4°C, followed by incubation with Alexa Fluor® 647 Donkey α‐Mouse IgG (H + L) (Invitrogen, #A31571, Lot#1900251, Thermo Fisher Scientific, Waltham, MA) secondary antibody at 1:200 dilution. Slides were mounted with 4′,6‐diamidino‐2‐phenylindole (DAPI) in Fluoro‐Gel II (Thermo Fisher Scientific) for nuclear visualization.
RESULTS
Eleven subjects were enrolled and completed treatment. All subjects were female and n = 6 Hispanic/Latino, n = 2 Asian, n = 2 Caucasian, and n = 1 African American.
The subject ages ranged from 33 to 49 years (mean 41.5 years). The average weight was 171.7 lbs. (range 132–208 lbs.) with mean body mass index 29.1 kg/m2 (range 24.9–34.0).
As shown in Table 1, subjects had abdominoplasty surgery from 3 to 17 days after the EMMS or cryolipolysis procedures.
Table 1.
Treatment Systems and Surgical Timepoints
| Treatment system | Subjects (n) | Subject ID | Age (years) | BMI | Race/ethnicity | Time point (days) |
|---|---|---|---|---|---|---|
| EMMS System A | 6 | OKA‐050 | 40 | 32.6 | Hispanic | 3 |
| OKA‐052 | 49 | 29.8 | Hispanic | 3 | ||
| OKA‐051 | 48 | 30.0 | Hispanic | 10 | ||
| BAC‐021 | 39 | 27.2 | Caucasian | 11 | ||
| OKA‐053 | 33 | 24.9 | Asian | 17 | ||
| OKA‐056 | 46 | 27.6 | Hispanic | 17 | ||
| EMMS System B | 3 | OKA‐047 | 46 | 30.2 | African American | 3 |
| OKA‐048 | 44 | 25.1 | Hispanic | 10 | ||
| OKA‐058 | 42 | 26.6 | Caucasian | 10 | ||
| Cryolipolysis | 2 | BAC‐018 | 33 | 34.0 | Hispanic | 11 |
| OKA‐035 | 37 | 32.6 | Asian | 17 |
BMI, body mass index; EMMS, electromagnetic muscle stimulation.
All histology slides were carefully inspected for any tissue processing artifacts and proper perilipin staining. Histology samples stained with H&E are shown in Figures 1, 2, 3 for EMMS‐treated samples at 3, 11, and 17 days. The EMMS‐treated samples harvested from the superficial and deep subcutaneous fat layers, at all timepoints analyzed, and in both the treated and control sites, were similar with no signs of an inflammatory response.
Fig. 1.
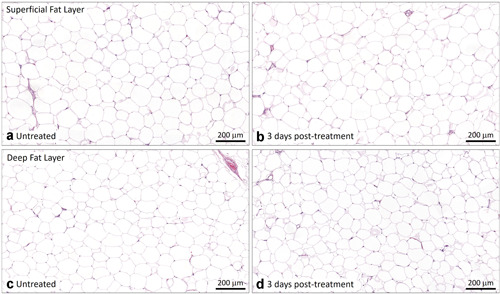
Representative untreated control (a,c) and EMMS‐treated (b,d) tissue from superficial (a,b) and deep (c,d) subcutaneous fat layers, harvested 3 days post‐treatment, subject OKA‐050. H&E stain, Scale bar = 200 μm. EMMS, electromagnetic muscle stimulation; H&E, hematoxylin and eosin.
Fig. 2.
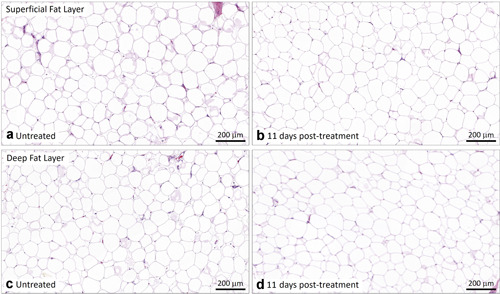
Representative untreated control (a,c) and EMMS‐treated (b,d) tissue from superficial (a,b) and deep (c,d) subcutaneous fat layers, harvested 11 days post‐treatment, subject BAC‐021. H&E stain, Scale bar = 200 μm. EMMS, electromagnetic muscle stimulation; H&E, hematoxylin and eosin.
Fig. 3.
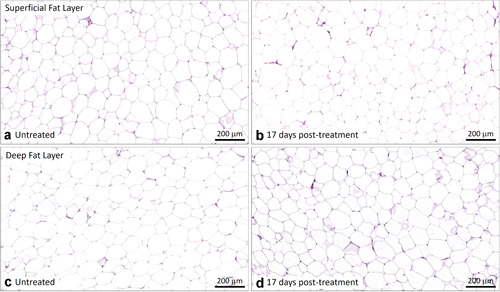
Representative untreated control (a,c) and EMMS‐treated (b,d) tissue from superficial (a,b) and deep (c,d) subcutaneous fat layers, harvested 17 days post‐treatment, subject OKA‐053. H&E stain, Scale bar = 200 μm. EMMS, electromagnetic muscle stimulation; H&E, hematoxylin and eosin.
Similarly, there was no loss of perilipin staining in the EMMS‐treated samples, indicating viable adipocytes throughout the superficial and deep fat layers and the absence of any disruptive effect on perilipin. Specifically, Figures 4, 5, 6 show untreated control samples alongside EMMS‐treated samples at 3, 11, and 17 days, respectively. All these adipocyte specimens have the usual bright yellow immunofluorescent stain marking the periphery of the adipocyte lipid droplet membrane. Nuclear stain marker is shown in blue.
Fig. 4.
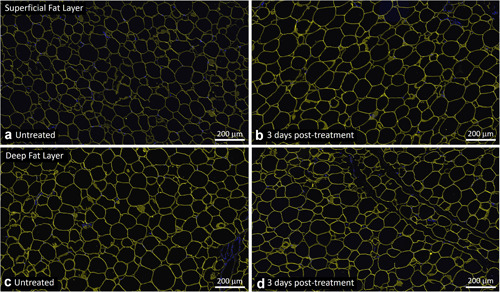
Representative untreated control (a,c) and EMMS‐treated (b,d) tissue from superficial (a,b) and deep (c,d) subcutaneous fat layers, harvested 3 days post‐treatment, subject OKA‐050. Perilipin immunofluorescence stain (TRITC, yellow) and nuclear stain (DAPI, blue), Scale bar = 200 μm. DAPI, 4′,6‐diamidino‐2‐phenylindole; EMMS, electromagnetic muscle stimulation.
Fig. 5.
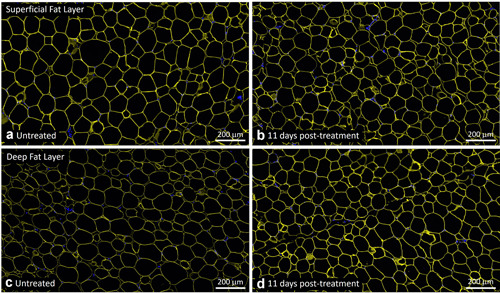
Representative untreated control (a,c) and EMMS‐treated (b,d) tissue from superficial (a,b) and deep (c,d) subcutaneous fat layers, harvested 11 days post‐treatment, subject BAC‐021. Perilipin immunofluorescence stain (TRITC, yellow) and nuclear stain (DAPI, blue), Scale bar = 200 μm. DAPI, 4′,6‐diamidino‐2‐phenylindole; EMMS, electromagnetic muscle stimulation.
Fig. 6.
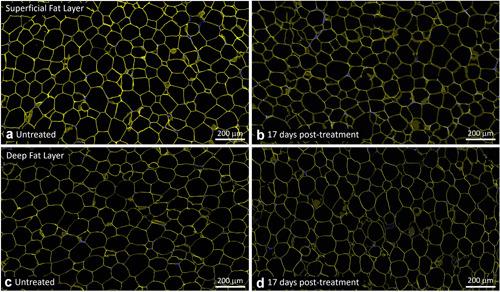
Representative untreated control (a,c) and EMMS‐treated (b,d) tissue from superficial (a,b) and deep (c,d) subcutaneous fat layers, harvested 17 days post‐treatment, subject OKA‐053. Perilipin immunofluorescence stain (TRITC, yellow) and nuclear stain (DAPI, blue), Scale bar = 200 μm. DAPI, 4′,6‐diamidino‐2‐phenylindole; EMMS, electromagnetic muscle stimulation.
Figures 7 and 8 compare cryolipolysis‐treated tissue to untreated controls and EMMS‐treated samples stained with H&E. The cryolipolysis‐treated tissue exhibits an inflammatory response in the fat layer, which is consistent with previously published histology data [1, 2, 3] and is characteristic of injured fat. The control and EMMS‐treated samples, however, showed no inflammatory response and look very similar. Figures 9 and 10 confirm the H&E observations. In contrast, the cryolipolysis‐treated samples show loss of perilipin within the subcutaneous fat, indicative of irreversible fat cell injury. The EMMS‐treated samples, however, look comparable to the untreated controls and exhibit no signs of adipocyte injury.
Fig. 7.
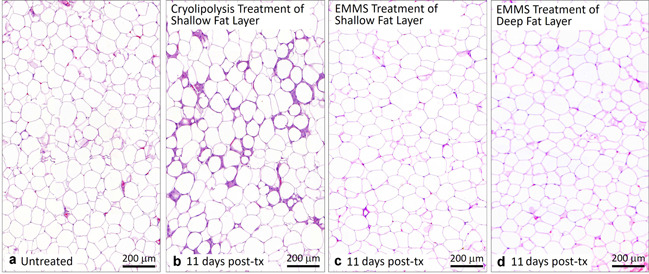
Comparison of (a) untreated control, (b) cryolipolysis, and EMMS‐treated tissue (c) superficial and (d) deep fat, harvested 11 days post‐treatment. H&E stain, Scale bar = 200 μm. DAPI, 4′,6‐diamidino‐2‐phenylindole; EMMS, electromagnetic muscle stimulation; H&E, hematoxylin and eosin.
Fig. 8.
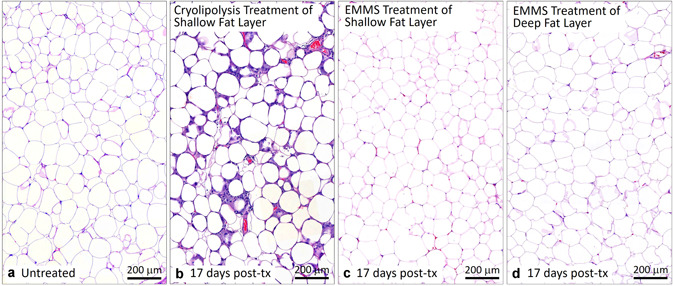
Comparison of (a) untreated control, (b) cryolipolysis, and EMMS‐treated tissue (c) superficial and (d) deep fat, harvested 17 days post‐treatment. H&E stain, Scale bar = 200 μm. EMMS, electromagnetic muscle stimulation; H&E, hematoxylin and eosin.
Fig. 9.
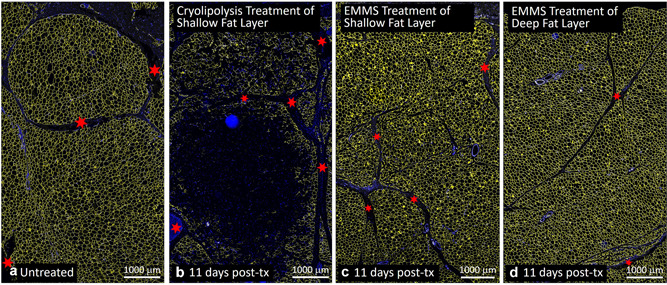
Comparison of (a) untreated control, (b) cryolipolysis, and EMMS‐treated (c) superficial and (d) deep adipose tissue, harvested 11 days post‐treatment. Perilipin immunofluorescence staining (TRITC) shown in yellow. Nuclear stain (DAPI) shown in blue. Scale bar = 1000 μm. Red stars highlight fiber septae or connective tissue structures. DAPI, 4′,6‐diamidino‐2‐phenylindole; EMMS, electromagnetic muscle stimulation.
Fig. 10.
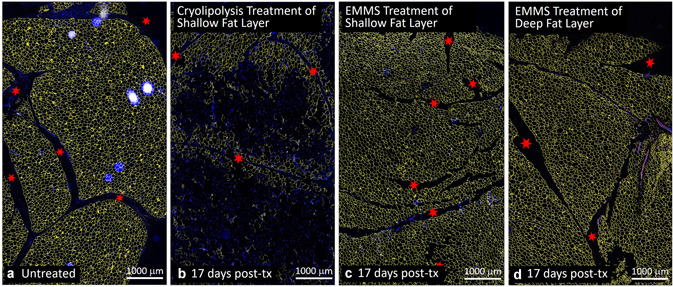
Comparison of (a) untreated control, (b) cryolipolysis (17 days post‐treatment), and EMMS‐treated (c) superficial and (d) deep adipose tissue (17 days post‐treatment). Perilipin immunofluorescence staining (TRITC) shown in yellow. Nuclear stain (DAPI) shown in blue. Scale bar = 1000 μm. Red stars highlight fiber septae or connective tissue structures. DAPI, 4′,6‐diamidino‐2‐phenylindole; EMMS, electromagnetic muscle stimulation.
DISCUSSION
This is the first study to compare adipocyte viability after EMMS and cryolipolysis in human adipose tissue. There are numerous clinical studies on EMMS and cryolipolysis showing clinical safety and efficacy, but there is little published fundamental research exploring the microscopic effect on subcutaneous adipose tissue following clinical treatment.
This feasibility study is limited by the small sample sizes and differing post‐treatment timepoints. The tissue specimens were obtained from abdominoplasty surgeries and timepoints vary somewhat based upon surgical schedule and study subject availability for experimental device treatment. Pre‐abdominoplasty study subjects are difficult to recruit and the clinical studies require significant resources, thus the resultant small sample sizes limit the feasibility study data from being generalized to the entire population.
In this study, two different EMMS systems were used. Histology results were similar between the two and the sample sizes are too small to statistically differentiate between the two systems. Thus, the data are pooled for both EMMS systems here.
The assessment of fat cell injury and loss is based on two characteristic features that are present in the case of irreversible fat injury: the first is loss of cell viability and the second is an inflammatory response due to fat cell death. The two major modes of cell death, which might occur following energy‐based device treatments, are necrosis and apoptosis. Although there are wide morphological differences between necrosis and apoptosis, both present cell membrane alterations indicative of injury.
During necrosis, the ultimate breakdown of the plasma membrane causes cytoplasmic contents to be released extracellularly, and in vivo, necrotic cell death is often associated with extensive tissue damage and an intense inflammatory response. In contrast, during apoptosis, the plasma membrane initially maintains its integrity; later, however, cells undergo morphological changes, such as cytoplasm shrinkage, and fragmentation into smaller bodies. In the case of adipocytes, the cell membrane and the lipid droplet membrane have an active metabolic function in homeostatic and stimulated conditions such as lipogenesis and lipolysis [25].
Accordingly, irreversible injury can be detected by the loss of key membrane‐associated proteins, such as perilipin. Although an often‐accepted dogma is that apoptotic cells do not cause inflammation, there is evidence that apoptotic adipocytes are phagocytosed and digested by macrophages [26, 27, 28]. Since adipocytes can be tens of times larger than macrophages and since the apoptotic rate may be much higher than the regularly encountered magnitudes in other cells/tissues, adipocytes are too large and too abundant to be digested by conventional phagocytic processes by resident phagocytes. Consequently, inflammatory cells need to be recruited to the zone of injury for proper digestion and clearance of adipocytes. Uptake and complete degradation of adipocytes requires a high number of macrophages resulting in many foam cells, which are clearly visible histologically with standard microscopy [29, 30].
Both EMMS systems showed similar histological results when comparing the treated tissue and the untreated controls. The EMMS treatment did not produce loss of adipocyte viability, as indicated by persistence of perilipin immunofluorescence staining, or an inflammatory response as evaluated by H&E staining, as shown in Figures 1, 2, 3, 4, 5, 6. The cryolipolysis‐treated tissue, in contrast, showed both an inflammatory response in the subcutaneous fat and loss of perilipin, indicative of irreversible adipocyte injury, as shown in Figures 7, 8, 9, 10.
While this study found no fat cell injury following EMMS treatment, previously published studies have suggested fat layer reduction. Clinical studies have demonstrated fat layer reduction as measured by computed tomography, magnetic resonance imaging, and ultrasound [12, 13, 14, 15, 16]. It is possible that the fat layer is reduced by metabolic changes that are not yet understood following EMMS treatment or due to synergistic changes in diet and/or physical activity, which cannot be observed histologically. But the current study results show that the fat cells are not injured following EMMS treatment and did not result in the cellular and inflammatory response typically seen following cryolipolysis or other energy‐based device procedures, such as high‐intensity focused ultrasound or non‐thermal focused ultrasound or laser heating.
Prior to the clinical study discussed here, we reviewed a previously reported pre‐clinical study that found evidence of apoptosis in a porcine model [18]. We did carefully replicate the published study methods for the porcine study and failed to reproduce the results for apoptosis following EMMS treatment. Both fluorometric and colorimetric terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining and quantification was used along with assessment of RNA markers via quantitative polymerase chain reaction analysis in the replication study and all methods failed to replicate the published results for apoptosis in a porcine model. Those pre‐clinical study replication results are consistent with the clinical data reported here, showing no effect of EMMS treatment on the adipose tissue layer.
There is much left to explore in the field of fat metabolism and adipocyte response to injury. While this study found consistent lack of fat cell inflammatory response and loss of perilipin in response to EMMS treatment, the study size was small. In a larger population, adipose tissue metabolism will vary from patient to patient and in response to degree of external injury. Additional fundamental research is warranted to further explore fat injury and metabolism.
EMMS is an exciting new energy‐based device treatment that utilizes electromagnetic energy to induce powerful contractions in the targeted muscle layer. This procedure appears to produce clinically meaningful changes in the muscle layer leading to toning, strengthening, and firming and, anecdotally, improved balance. It may be an important new procedure to complement a variety of non‐invasive body contouring procedures, such as cryolipolysis, radiofrequency heating, 1060 nm laser heating, high‐intensity focused ultrasound, and non‐thermal focused ultrasound. This procedure is relatively new to the world of aesthetic medicine and more basic science research should be done to explore the effect on the underlying muscle, fat, and skin layers.
CONCLUSION
A fundamental viability study was conducted to explore fat cell injury following cryolipolysis and EMMS for non‐invasive body contouring in human subjects. The EMMS treatment failed to produce either fat cell injury or an inflammatory response, whereas the cryolipolysis treatment induced an inflammatory response in the H&E stained adipose tissue and loss of perilipin in the immunofluorescence stained tissue, indicative of irreversible fat cell injury.
ACKNOWLEDGMENTS
This clinical study was sponsored by ZELTIQ Aesthetics, exclusive distributor of CoolTone and manufacturer of CoolSculpting. ZELTIQ Aesthetics is an affiliate of Allergan, plc.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: Christopher Zachary and Jay Burns are Advisory Board members for Allergan, plc. Linda Pham and Joel Jimenez Lozano are employees of Allergan, plc. There are no other relevant commercial disclosures.
REFERENCES
- 1. Manstein D, Laubach H, Watanabe K, Farinelli W, Zurakowski D, Anderson RR. Selective cryolysis: A novel method of non‐invasive fat removal. Lasers Surg Med 2008;40(9):595–604. [DOI] [PubMed] [Google Scholar]
- 2. Zelickson B, Egbert BM, Preciado J, et al. Cryolipolysis for noninvasive fat cell destruction: Initial results from a pig model. Dermatol Surg 2009;35(10):1462–1470. [DOI] [PubMed] [Google Scholar]
- 3. Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med 2009;41(10):703–708. [DOI] [PubMed] [Google Scholar]
- 4. Zelickson BD, Burns AJ, Kilmer SL. Cryolipolysis for safe and effective inner thigh fat reduction. Lasers Surg Med 2015;47(2):120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stevens WG, Bachelor EP. Cryolipolysis conformable‐surface applicator for nonsurgical fat reduction in lateral thighs. Aesthet Surg J 2015;35(1):66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J 2013;33(6):835–846. [DOI] [PubMed] [Google Scholar]
- 7. Bernstein EF, Bloom JD. Safety and efficacy of bilateral submental cryolipolysis with quantified 3‐dimensional imaging of fat reduction and skin tightening. JAMA Facial Plast Surg 2017;19(5):350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kilmer SL, Burns AJ, Zelickson BD. Safety and efficacy of cryolipolysis for non‐invasive reduction of submental fat. Lasers Surg Med 2016;48(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carruthers JD, Humphrey S, Rivers JK. Cryolipolysis for reduction of arm fat: Safety and efficacy of a prototype CoolCup applicator with flat contour. Dermatol Surg 2017;43(7):940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rivers JK, Ulmer M, Vestvik B, Santos S. A customized approach for arm fat reduction using cryolipolysis. Lasers Surg Med 2018;50(7):732–737. [DOI] [PubMed] [Google Scholar]
- 11. Friedmann DP. Cryolipolysis for noninvasive contouring of the periumbilical abdomen with a nonvacuum conformable‐surface applicator. Dermatol Surg 2019;45(9):1185–1190. [DOI] [PubMed] [Google Scholar]
- 12. Katz B, Bard R, Goldfarb R, Shiloh A, Kenolova D. Ultrasound assessment of subcutaneous abdominal fat thickness after treatments with a high‐intensity focused electromagnetic field device: A multicenter study. Dermatol Surg 2019;45(12):1542–1548. [DOI] [PubMed] [Google Scholar]
- 13. Jacob C, Kinney B, Busso M, et al. High intensity focused electro‐magnetic technology (HIFEM) for non‐invasive buttock lifting and toning of gluteal muscles: A multi‐center efficacy and safety study. J Drugs Dermatol 2018;17(11):1229–1232. [PubMed] [Google Scholar]
- 14. Kinney BM, Lozanova P. High intensity focused electromagnetic therapy evaluated by magnetic resonance imaging: Safety and efficacy study of a dual tissue effect based non‐invasive abdominal body shaping. Lasers Surg Med 2019;51(1):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacob CI, Paskova K. Safety and efficacy of a novel high‐intensity focused electromagnetic technology device for noninvasive abdominal body shaping. J Cosmet Dermatol 2018;17(5):783–787. [DOI] [PubMed] [Google Scholar]
- 16. Kinney BM, Kent DE. Long‐term follow‐up on patients with HIFEM‐induced abdominal tissue changes: MRI and CT assisted quantification of muscle growth and fat reduction. Lasers Surg Med 2019;51(S30):S20. [Google Scholar]
- 17. Duncan D, Dinev I. Noninvasive induction of muscle fiber hypertrophy and hyperplasia: Effects of high‐intensity focused electromagnetic field evaluated in an in‐vivo porcine model: A pilot study. Aesthet Surg J 2020;40(5):568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weiss RA, Bernardy J. Induction of fat apoptosis by a non‐thermal device: Mechanism of action of non‐invasive high‐intensity electromagnetic technology in a porcine model. Lasers Surg Med 2019;51(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brasaemle DL, Subramanian V, Garcia A, Marcinkiewicz A, Rothenberg A. Perilipin A and the control of triacylglycerol metabolism. Mol Cell Biochem 2009;326(1–2):15–21. [DOI] [PubMed] [Google Scholar]
- 20. Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life 2004;56(7):379–385. [DOI] [PubMed] [Google Scholar]
- 21. Brasaemle DL. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J Lipid Res 2007;48(12):2547–2559. [DOI] [PubMed] [Google Scholar]
- 22. Eto H, Kato H, Suga H, et al. The fate of adipocytes after nonvascularized fat grafting: Evidence of early death and replacement of adipocytes. Plast Reconstr Surg 2012;129(5):1081–1092. [DOI] [PubMed] [Google Scholar]
- 23. Suga H, Eto H, Aoi N, et al. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg 2010;126(6):1911–1923. [DOI] [PubMed] [Google Scholar]
- 24. Mashiko T, Yoshimura K. How does fat survive and remodel after grafting? Clin Plast Surg 2015;42(2):181–190. [DOI] [PubMed] [Google Scholar]
- 25. Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol 2015;208(5):501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gregory CD, Devitt A. The macrophage and the apoptotic cell: An innate immune interaction viewed simplistically? Immunology 2004;113(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fischer‐Posovszky P, Wang QA, Asterholm IW, Rutkowski JM, Scherer PE. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology 2011;152(8):3074–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46(11):2347–2355. [DOI] [PubMed] [Google Scholar]
- 29. Haka AS, Barbosa‐Lorenzi VC, Lee HJ, et al. Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J Lipid Res 2016;57(6):980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hao X, Zhang T, Yang Y, et al. Morphological features of cell death and tissue remolding of fat grafts. Ann Plast Surg 2015;74(6):722–727. [DOI] [PubMed] [Google Scholar]


