Abstract
The ligands that comprise the Transforming Growth Factor β superfamily highly govern the development of the embryonic growth plate. Members of this superfamily activate canonical TGFβ and/or BMP (Bone Morphogenetic Protein) signaling pathways. How these pathways interact with one another is an area of active investigation. These two signaling pathways have been described to negatively regulate one another through crosstalk involving Smad proteins, the primary intracellular effectors of canonical signaling. More recently, a mechanism for regulation of the BMP pathway through TGFβ and BMP receptor interactions has been described. Here in this review, we demonstrate examples of how TGFβ is a gatekeeper of BMP action in the developing growth plate at both the receptor and transcriptional levels.
Keywords: Growth plate, cartilage, TGF β, BMP, Receptor interaction
1. Introduction
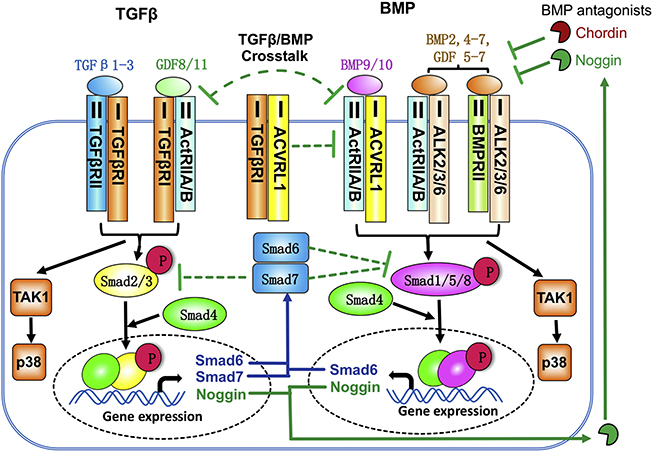
BMP pathways control nearly every aspect of chondrogenesis [1–6]. Thus, understanding how BMP and TGFβ pathways intersect is fundamental to understanding the mechanisms controlling cartilage formation and maintenance. The Transforming Growth Factor beta (TGFβ) superfamily is comprised of approximately 30 secreted ligands, 7 type I receptors, 5 Type II receptors, and 8 SMAD proteins [7]. In brief, BMP and TGFβ signaling pathways are activated upon ligand binding to their cognate TGFβ/BMP Type I and II kinase receptors [2,7,8]. Ligand binding enables formation of complexes of Type I and II Serine/Threonine kinase receptors on the cell surface and subsequent Type I receptor phosphorylation and activation (Fig. 1). Through phosphorylation, the type I receptor kinase activates R-Smads2/3 for TGFβ and R-Smadsi/5/8 for BMP. Whether TGFβ or BMP signaling is activated is determined by the identity of the type I receptor, which exhibits specificity for either Smads 2/3 or Smads 1/5/8. These effectors complex with Smad4 and translocate to the nucleus where they bind directly or indirectly to DNA to modulate expression of target genes [9–11]. This form of activation is known as the canonical signaling pathway. In addition, TGFβs/BMPs signal through a variety of noncanonical or Smad-independent avenues, utilizing MAP kinases, TAK1, RhoA, and mTOR pathways [8,12–17].
Figure. 1.
Crosstalk between TGFβ and BMP signaling. TGFB ligands bind and activate the type II TGF-B receptor and the type I receptor TGFβRI (ALK5), triggering canonical and noncanonical pathways. In the canonical pathway, transcription factors Smad2 and Smad3 are phosphorylated and associate with Smad4. This complex enters the nucleus and regulates gene expression. BMP ligands activate Smadsi, 5 and 8, which regulate a distinct set of genes. BMP ligands bind to type I BMP receptors ACVRLi (ALKi), ACVRi (ALK2), BMPRiA (ALK3), and BMPRiB (ALK6). BMPs 9/i0 are the only ligands that activate ALKi. However, the BMP type II receptors ACTRIIA and aCtRIIB can associate with either TGFβRI or with type I BMP receptors ALKi/2/3/6 to transduce either a TGFB or a BMP pathway. In addition, TGFβRI can complex with ALKi. The competition for ACTRIIA/B and binding of TGFβRI/ALKi creates a negative crosstalk between TGFβ and BMP signaling at the receptor level. Moreover, TGFβ signaling can inhitit BMP signaling by increasing Smad6, Smad7 and Noggin expression. Smad6 and Smad7 inhibit the phosphorylation and activation of Smadi/5/8. Noggin is a BMP antagonist that blocks the binding of BMPs(2,4–7) and GDFs(5–7) to receptors, but it can not antagonize BMP9 and BMP10.
2. Growth plate cartilage formation
The skeleton is composed primarily of cartilage and bone. Throughout the axial and appendicular skeleton, with the exception of the skull, the skeleton is formed from a hyaline cartilage template. During development, mesenchymal stem cells from sclerotome, paraxial mesoderm, and neural crest, condense and undergo chondrogenesis to form a cartilage model (anlagen), which is later replaced by mineralized bone [18,19]. Differentiating chondrocytes are organized into growth plates comprised of different layers, including resting, columnar, prehypertrophic, and hypertrophic zones. The pool of resting chondrocytes at the distal ends of developing long bones supplies cells for building growth plate cartilage. Resting chondrocytes differentiate into columnar chondrocytes, which have a high proliferative rate and stack together into columns. Columnar cells further differentiate into prehypertrophic chondrocytes, which cease proliferation and enlarge. These cells further differentiate into hypertrophic chondrocytes, which have a larger cell size and eventually undergo apoptosis or transdifferentiate into osteoblasts [20,21]. Growth plate cartilage supports bone elongation and provides an essential platform for the generation of articular joints [11,18,22,23]. TGFβs/BMPs play critical roles in regulating chondrocyte differentiation from early to terminal stages, including condensation, proliferation and terminal differentiation [1–6,11,24–28].
Both TGFjS and BMP signaling pathways play essential roles for growth plate development, regulating multiple cellular behaviors such as proliferation, self-renewal, migration, mesenchymal condensation, chondrogenic cell commitment, terminal differentiation, maintenance, senescence, and apoptosis [4–6,25,27,29,30]. Through mouse genetic studies, mutations in several TGFβ/BMP family members, receptors, extracellular modulators, and intracellular transducers, have been reported that cause developmental defects of the cartilaginous skeleton. In this review, we focus on inhibitory crosstalk between BMP and TGFβ pathways via a balance of intracellular effectors (inhibitory Smads 6 and 7) and cell membrane mediated antagonism via TGFβ/BMP-receptor interactions (TGFβBRI and ACVRL1). Considering that modulation of TGFβ and BMP signaling is emerging as a promising therapeutic strategy for joint repair, improving bone mass and quality [1,3,14], these new insights into TGFβ/BMP signaling in cartilage may provide new prospects for generating novel therapies against cartilage diseases.
3. Overview of TGFβ/BMP proteins and receptors
Ligands that activate TGFβ pathways include TGFβs (1, 2, and 3), Activin (A and B), Nodals, GDFs (1, 8, 9, 10 and 11), and Mullerian inhibiting hormone. The bone morphogenetic protein (BMP) subfamily of ligands consists of BMPs 2, 4–10, and the growth and differentiation factors (GDFs 5, 6 and 7) [1,8,10,24,31–33]. (Fig. 1).
Seven type I receptors have been described [9,31,34]. The BMP subfamily proteins bind ACVRL1 (ALK1), ACVR1 (ALK2), BMPR1A (ALK3), and BMPR1B (ALK6) and signal via the Smads1/5/8 (BMP signaling) [2,10]. The TGFβ subfamily ligands bind to ACVR1B (ALK4), TGFβRI (ALk5), ACVR1C (ALK7) and signal through Smads2/3 (TGFβ signaling) [9,31,35,36]. A closer analysis of receptor structure has revealed that receptors ACVRL1 and ACVR1 are structurally more similar to each other than to BMPRiA and BMPRiB, while BMPRiA and BMPRiB are highly similar to each other [37–39]. The majority of BMP ligands activate multiple receptors, but with varied affinities. BMPs 9 and 10 are among the most specific, in that they bind only to ACVRLi and ACVRi at physiologically relevant concentrations. BMPs 2, 4, and 7 bind and signal through BMPRiA, BMPRiB, and ACVRi, but not ACVRLi [40–43]. The TGFβ receptor TGFβRI, on the other hand, is activated by TGFβs i-3, and GDFs 8-ii; ACVRiB and ACVRiC, are activated by Activins, GDFs 8 and ii [9,31,35,36]. Distinct BMP/ TGFβ ligands thus exert distinct effects via activation of combinations of type I receptors in a concentration- dependent manner, and for this reason, pathway activation is dependent on the levels expression and ratios of both ligands and receptors [44].
There are five type II receptors: TGFβRII, ACTRIIA, ACTRIIB, BMPRII, and MISRII [2,8,10,31,36]. TGFβs i-3 trigger complex formation betweeen TGFβRII and TGFβRI to activate Smad 2/3 signaling. However, TGFβRII/TGFβRI complexes can also interact with ACVRLi and ACVRi in some cell types, enabling activation of BMP Smads 1,5,8 by TGFβ [45,46]. BMPRII complexes with ACVRi, BMPRiA and BMPRiB to activate BMP signaling. ACTRIIA and ACTRIIB are unique in that they can complex with type I TGFβ/activin receptors TGFβRI, ACVRiB and ACVRiC to activate TGFβ signaling, or with BMP receptors ACVRLi, ACVRi, BMPRiA and BMPRiB to activate BMP signaling [10,29].
4. The function of TGFβ pathways in the growth plate
4.1. TGFβ subfamily proteins
In vitro data demonstrate that TGFβs play important roles in early chondrogenesis to induce mesenchymal cell condensation [47–51]. However, the in vivo roles of TGFβs in chondrogenesis and cartilage development are unclear. TGFβs i, 2 and 3 are expressed in mesenchymal condensations. Levels of expression of all of these ligands are reduced at later stages in cartilage [52,53]. In the perichondrium, TGFβ3 is expressed at higher levels than other TGFβs [52,53]. In appendicular growth plates, TGFβ1 and TGFβ3 are expressed mainly in the proliferative and hypertrophic zones, whereas TGFβ2 is expressed in all zones, but at its highest levels in the hypertrophic zone [54–57].
The expression of TGFβi and 3 in cartilage suggests they may have a role there. However, knockouts for either TGFβi or 3 do not exhibit phenotypes that support an essential role in cartilage. Tgfbi null mice that survive to birth do not exhibit any skeletal defects, but instead die from diffuse inflammation [58]. Similarly, while loss of TGFβ3 leads to perinatal lethality and defects in palate formation, defects in chondrogenesis are not observed [59,60]. However, mice lacking TGFβ2 present with generalized chondrodysplasia that appears to have an onset at late gestation stages [61]. The evidence thus suggests that TGFβ2 may be the predominant TGFβ ligand impacting chondrogenesis in vivo. However, the chondrocyte-autonomous function of TGFβ2 is still not known, and a cartilage specific knock-out of Tgffi2 would be essential to understand its role in cartilage development. Furthermore, cartilage-specific deletion of TGFβs i-3 alone or in combination in adults might reveal functions for these ligands in articular cartilage.
In addition, the TGFβ subfamily proteins GDF 1, 10 and 11 are expressed in both condensed cartilage and mature growth plate cartilage [62,63]. Among them, GDF10 is expressed at the highest level; GDF 1 and 11 are detected at low levels. GDF11 was found to inhibit chondrogenesis in the developing chick limb in ex vivo assays [64]. However, little is known of the roles of Gdf 1, 10 and 11 in growth plate chondrocytes in vivo.
4.2. TGFβRI function in the growth plate
The TGFβ receptor TGFβRI (ALK5) is the only receptor known to be activated by both TGFβs and GDFs. Conditional ablation of TGFβRI using Dermo1-Cre, which is expressed in skeletal progenitor cells prior to condensation, results in cartilage malformation and short limbs [65]. In these mice, progenitor cells condense and chondrocytes proliferate and differentiate, but ectopic cartilaginous tissues protrude into the perichondrium. These protrusions are related to the abnormally thin perichondrial layer and to increased chondrocyte proliferation in the protruded cartilage. However, because Dermo1-Cre is expressed in many mesodermal-derived tissues, whether TGFβRI had a direct function in chondrocytes could not be ascertained. Recently, a cartilage specifically knockout of TGFβRI using Col2-Cre was generated to address this unknown [29].
Loss of TGFβRI (TgfbriCo12) in committed chondrocytes leads to a lethal chondrodysplasia. The precise cause of death is unknown, but mutants exhibit major defects in both axial and appendicular skeletal elements. Interestingly, these defects are distinct from those seen in Tgfb2~/~ mice. At E13.5 and E14.5, there are no patterning defects in TgfbriCo12 mice, but mutants have smaller vertebrae and occipital bones. At E16.5, mutants develop abnormal spinal curvature and kinks in the ribs, shortened sternebrae, and shorter limbs. At E18.5, mutants have bent scapulae, kinks at the distal ends of humeri, dislocation of elbow joints, and smaller condyles and entheses of tibiae. The axial phenotype appears to be similar to that in TgfbriDermo1 mice [65]. Col2Cre is expressed in the sclerotome before the specification of separate lineages for vertebral cartilage and intervertebral discs. Since both Col2Cre and Dermo1Cre target this tissue [66,67],, the axial defects in TgfbriCo12 and TgfbriDermo1 mice most likely arise through the same mechanism.
TgfbriCo12 mice also develop severe defects in appendicular growth plates at E16.5. TGFβRI expression is low in the growth plate until E16.5. Expression is strong at E16.5 and E18.5 in the columnar zone but is absent from the hypertrophic zone [29]. The appendicular growth plate phenotype of TgfbriCo12 is correlated with increased proliferation of chondrocytes in columnar and resting zones [29], suggesting that loss of TGFβRI leads to shorter limbs by premature conversion of slowly dividing resting cells into rapidly proliferating columnar cells and depletion of the resting zone. The above findings demonstrate an essential function for TGFβRI, but raise questions regarding the identity of the ligands that mediate the effects of TGFβRI in the growth plate. If TGFβs 1–3 are the ligands that activate TGFβRI in cartilage, the same defects seen in TGFβRI mutants should be observed in the mice lacking these ligands or TGFβRII in cartilage (Tgfbr2Cot2), because TGFβRII is the type II receptor for TGFβs 1–3. However, similar defects were not seen in mice lacking any of the TGFβ ligands, or in Tgfbr2Co12 mice [58–61,68].
Conditional deletion of TGFβRII in committed CoZ2ai-expressing chondrocytes did not lead to obvious defects in appendicular elements [68]. In axial elements, Tgfbr2Co12 mice develop defective segmentation and malformation of intervertebral discs [68]. Although TgfbriCo12 mice exhibit smaller vertebral bodies, they do not exhibit defective segmentation [29]. Other differences between TgfbriCo12 and Tgfbr2Co12 mice can be seen in the condyles of appendicular elements. In TgfbriCo12 mice, the posterior tibia condyle is smaller than the anterior tibia condyle. These findings establish TGFβRI as a gene that regulates the differential development of condyles. In Tgfbr2Prx1 mice, the tibial medial condyle and deltoid tuberosity do not form [69]. However, Tgfbr2Co12 mice do not show such phenotypes [68] suggesting that the defects in Tgfbr2Prx1 mice reflect a role for TGFβRII in condensing cells prior to their commitment to chondrocytes. Furthermore, the condyle defects in Tgfbri0012 mice are distinct from those in Tgfbr2Prx1 mice [29]. This suggests that TGFβRI regulates posterior and anterior condyle formation in cartilage, but not through TGFβRII mediated TGFβ signaling. Scx+;Sox9+ cells in the TGFβRI-expressing regions of the condyles give rise to ligaments and the ligamentous junction [70]. Future studies would thus be of interest to elucidate the role of TGFβRI in the differential development of condyles, ligaments, and associated structures, and to understand the basis for the differing condylar phenotypes in TgfbriCo12 and Tgfbr2Co12 mice. In summary, given that the same Col2-Cre alleles was used in both studies, these phenotypic differences suggest that TGFβRI and TGFβRII function to some extent in independent signaling complexes.
The in vivo observations discussed above suggest that TGFβRI can transduce its effects independently of TGFβRII in growth plate cartilage. However, there is a possibility that TGFβRI transduces TGFβ signaling independently of TGFβRII. Prior evidence for this came from a study showing that in cranial neural crest cells, TGFβs activate non-canonical TGFβ signaling through TAK1/JNK in the absence of TGFβRII [71]. However, this mechanism cannot account for the differences between TgfbriCo12 and Tgfbr2Co12 mice because analysis of the growth plate cartilage showed that loss of TGFβRI does not change pTAKi activation [29]. Additional genetic evidence that TGFβRI does not mediate its effects via this noncanonical pathway is discussed below.
4.3. TGFβ signaling mediators
The above findings raise the possiblity that TGFβRI utilizes ligands other than TGFβs 1–3 to mediate its effects in the growth plate. GDF1, GDF10, and GDF11 can activate TGFβ signaling through TGFβRI in complexes containing ACTRIIA or ACTRIIB [72–74]. At present, there is no direct evidence for a role for these ligands in the growth plate in vivo.
TGFβs 1–3, GDFs 1, 10, 11, and other cytokines in the TGFβ/activin sub-family act through canonical and non-canonical pathways, and canonical signals are transduced via the transcriptional regulators Smad2 and Smad3. Once activated, Smads 2/3 complex with Smad4, translocate into the nucleus, and recruit coactivators and repressors to regulate the expression of target genes. Thus, examination of the phenotypes of mice lacking Smads 2 and 3 would reveal a potential role for multiple TGFβ/activin subfamily ligands.
Smad2, Smad3 and Smad4 are co-expressed throughout the growth plate [25,75–78]. Mice with cartilage-specific loss of Smad2 (Smad2Col2), global loss of Smad3 (Smad.3or both (Smad2CoX2; Smad3~/~) develop a subtle cartilage defect at E18.5 and survive after birth [25]. The defect in growth plate cartilage in single Smad2Co12 or Smad3−/− mutants and in double Smad2/3 mutant mice is thus very different from the perinatal lethal chondrodyspalsia of TgfbriCo12 mice [29]. There are no condyle formation defects in Smad2, Smad3 and double Smad2/3 mutant mice, as are found in TgfbriCo1 mice. There are no apparent defects in columnar zones in Smad2, Smad3 and Smad2/3 mutant mice, but there is a two-fold increase in cell proliferation in this region in Tgfbri0012 mice. There is an increase in the length of the hypertrophic zone in Smad2, Smad3 and double Smad2/3 mutant mice, but not in Tgfbri001 mice [25,29]. Overall, the far more subtle growth plate defects in Smad2/3 mutants compared to TgJbriCol2mice suggest that the main role of TGFΒRI in growth plate is not to transduce canonical TGFβ signaling via Smad2/3.
There are numerous non-canonical mechanisms for transduction of TGFβ signals, including various MAPK, Rho-like GTPase and phosphatidylinositol-3-kinase (PI3K)/AKT pathways [15–17,79]. There is solid evidence that these pathways are important for chondrogenesis, but the extent to which TGFβs mediate their effects through these pathways in cartilage in vivo is unknown [11]. The most extensively studied noncanonical pathways are those mediated by TGFβ activating kinase 1 (TAK1), a member of the MAPKKK family. TAK1 is activated by type I BMP and TGFβ receptors, and subsequently activates several MAP kinases (MAPKs), including p38, JNK, and ERK. TGFβ receptors also activate PI3K and its downstream target AKT. If TGFβRI transduces its effects through these non-canonical pathways, genetic deletion of TAK1 or AKT in cartilage will lead to similar defects as in TgfbrI mutant mice. One of the main defects in TgfbriCo12 mice is a significant increase of cell proliferation in the columnar zone. However, this defect is not seen in mutant mice with deletion of TAK1 [80], JNKs [81], ERK1 [82] or AKT1 [83]. Furthermore, as discussed above, TgfbriCo12mice do not exhibit obvious changes in levels of activation of TAK1 [29]. These findings suggest that altered signaling through these noncanoical pathways is not responsible for the effects of TGFβRI in the growth plate.
TGFβRI is unique as a type I receptor in that it not only activates canonical TGFβ pathways, but it also enables TGFβs to activate BMP signaling through a mechanism involving association of the BMP receptor ACVRL1 with TGFβRI/TGFβRII complexes [45,84]. TGFβ signaling through ACVRL1/TGFβRI/TGFβRn complexes increases BMP signaling in vascular cells [45]. According to this model, loss of TGFβRI should lead to decreased BMP signaling. Surprisingly, there is dramatically increased BMP signaling in TgfbriCo12 growth plates as evidenced by increased pSmad1/5 levels. This suggests that a major function for TGFβRI is to block BMP signaling in the growth plate. Genetic evidence for this is provided by the observation that simultaneous loss of TGFβRI and the BMP receptor ACVRL1 restores BMP signaling to normal and rescues the cell proliferation defect seen in TgfbriCo12 mutant growth plates [29]. This suggests that the main role of TGFβRI in growth plate is not to transduce TGFβ signaling but rather to block BMP signaling. These studies thus identify a novel form of crosstalk and provide genetic evidence for a pathological role for ACVRL1 in the growth plate.
5. The function of BMP pathways in the growth plate
5.1. BMP subfamily proteins
There are more than 15 structurally related BMPs. They are categorized into subgroups based on amino acid or nucleotide similarity, including BMP2/4, BMP5/6/7/8, BMP9/BMP10, and BMP12/13/14 (GDF5/6/7) [85]. In developing limb cartilage, BMP 2, 4, and 7 are expressed in the perichondrium, whereas BMP6 is detected in prehypertrophic and hypertrophic chondrocytes [86–89]. In addition, BMP7 is expressed in chick sternal prehypertrophic and mouse metatarsal proliferating chondrocytes [89,90].
BMPs 2, 4, 5, 6, and 7 promote chondrogenesis [91,92]. In addition, BMPs have a strong effect on chondrocyte proliferation and matrix synthesis. In growth plate chondrocytes BMPs 2, 4 and 5 upregulate cell proliferation and matrix production [93,94]. Little is known about chondrocyte-intrinsic roles for BMP ligands, but chondrocyte-specific deletion of Bmp2 and Bmp4 showed that BMP2, but not BMP4, is a main player for chondrocyte proliferation and maturation during endochondral bone development [95].
BMP9 is among the most osteogenic BMPs, promoting osteoblastic differentiation of mesenchymal stem cells (MSCs) both in vitro and in vivo [96–98]. Its role in growth plate cartilage is not clear. BMP9 is not expressed in cartilage, but is expressed in the liver and the protein is detected in the circulation. BMP9 protein can be detected in the growth plate, indicating that circulating BMP9 penetrates cartilage [29]. In addition, in vitro assays demonstrated that loss of TGFβRI in chondrocytes increases BMP9 activity through ACVRL1, indicating BMP9 activity is negatively regulated by TGFβRI [29]. However, little is known about the role of BMP9 in cartilage development.
GDF5, 6 and 7 activate BMP signaling in vitro [99], but they do not have robust osteogenic activity compared with other BMPs [97,98]. Studies of GDF5 and GDF6 knockout mice and humans bearing mutations in these genes show that they have variety of defects in joint and cartilage formation [24]. GDF5 stimulates mesenchymal condensation and cartilage formation, and organizes joint formation and segmentation events across developing skeletal structures [100,101]. GDF5 promotes cartilage growth in vivo; transgenic mice expressing Gdf5 under the control of a Coliia2 promoter show extensive cartilage overgrowth and complete absence of joints [102]. GDF6 is not critical for early chondrogenesis, but it promotes cell proliferation in surface cartilage [103]. Moreover, Gdfs/Gdfô double knock-out mice develop a more severe skeletal phenotype [103]. Interestingly, mice lacking GDF7 exhibited a defect in hypertophic phase duration opposite to that reported for other GDF5 mutants [104]. While the basis for the differing phenotypes remains unclear, GDF5, 6, and 7 mutant phenotypes confirm an important role for GDF molecules in cartilage growth and joint development.
5.2. BMP Receptors
There are fewer BMP receptors than ligands, and therefore studies of BMP receptor knockout mice could provide a broader picture of the role of BMP signaling. The results suggest that BMP receptors promote chondrocyte proliferation and differentiation. Loss of type 1 receptors ACVRL1, ACVR1, BMPR1A, or BMPR1B leads to no or mild cartilage phenotypes during embryonic development, but combining deletion of two of these type I receptors in cartilage leads to more profound chondrodysplasia. Loss of BMPR1A in chondrocytes leads to decreased cell proliferation, delayed hypertrophy in growth plate cartilage and shortened limbs [6]; global loss of BMPRiB leads to delayed hypertrophy of metacarpal/metatarsal chondrocytes [6]. However, BMPR1A/BMPR1B double mutants display a very severe chondrodysplasia and a lack of endochondral ossification [6]. Loss of ACVRi in cartilage impacts development of axial skeletal elements, but has little consequence in appendicular growth plate cartilage development at embryonic stages [4]. However, loss of both ACVRi and BMPRiA in chondrocytes or loss of ACVRi in chondrocytes on a global BMPRiB knockout background leads to a generalized chondrodysplasia that is more severe than each single mutation alone [4]. This suggests that ACVRi, BMPRiA and BMPRiB have some redundant roles. Recently, it was shown that depletion of ACVRLi in cartilage does not affect embryonic cartilage development [29]. However, since ACVRi is structurally related to ACVRLi, it is possible that ACVRLi and ACVRi have overlapping functions in cartilage such that ACVRi can compensate for the loss of ACVRLi. A combined deletion of ACVRLi and ACVRi in cartilage may help to further understand their roles.
6. TGFβ limits BMP signaling
6.1. TGFβ limits BMP signaling through extracellular and intracellular mechanisms
BMP signaling pathways regulate multiple aspects of endochondral bone formation. The intensity and duration of BMP signaling in the growth plate is regulated extracellularly and intracellularly. One example of extracellular regulation occurs via binding of antagonists, such as noggin, follistatin and chordin, to BMPs, thereby preventing them from binding to or enabling formation of BMP receptor complexes [2,105–109]. Extracellular regulation by the BMP antagonist noggin is required, as noggin-deficient mice exhibit massively enlarged growth plates and joint fusions [105,110]. TGFβ1 induces noggin expression in the growth plate, although not as potently as the BMPs [111]. Noggin functions to limit BMP signaling during the recruitment of progenitor cells into cartilage elements. At later stages Noggin is essential to insulate articular cartilage from BMP signaling that would otherwise promote hypertrophy in this tissue [110].
Activation of both TGFβ and BMP signaling pathways can lead to intracellular antagonism via crosstalk between the R-Smad mediators of TGF-P and BMP signaling. In vivo data demonstrated that <Smad3 can repress Smadi/5/8 activation to prevent chondrocyte hypertrophy [112]. In accordance, there is an increase in the level of pSmadi/5/8 activity with the loss of Smad3. Smad3−/− chondrocytes were more responsive to BMP2, exhibiting increased pSmadi/5/8 levels, and BMP-responsive luciferase reporter activity [112]. In vitro assays using MDA-MB-231 breast cancer cell lines showed that TGFβ can also inhibit BMP responses by inducing the formation of pSmad3-pSmadi/5 complexes, which bind to BMP-responsive elements and mediate TGFβ-induced transcriptional repression [113]. In ATDC5 chondrocytic cells, TGFβ suppresses BMP signaling and chondrocyte hypertrophy via SnoN, a transcriptional corepressor [114]. SnoN is induced by TGFβ signaling in maturing chondrocytes and suppresses the BMP-Smad signaling pathway to inhibit hypertrophic maturation of chondrocytes [114].
Intracellular regulation also occurs, in part, through the actions of inhibitory Smads (I-Smads) 6 and 7. Smad7 can inhibit multiple pathways, including TGFβ/activin and BMP signaling, while Smad6 has been known to inhibit BMP signaling [U5-U7]. I-Smads block the phosphorylation of R-Smads by forming stable associations with activated type I receptors [118–120]. In addition, I-Smads can recruit E3 ubiquitin ligases to type I receptors, leading to ubiquitination and subsequent degradation of these receptors [121–123]. I-Smads can also bind to Smad1, thereby interfering with Smad1-Smad4 complex formation [123–125], Furthermore, I-Smads directly regulate transcription of TGFβ family signaling in the nucleus [126–128]. The expression of I-Smads is directly induced by TGFβ and BMP signaling, thus forming a negative feedback loop [129–131].
In vitro and in vivo studies reveal that (I-Smads 6 and 7 regulate BMP-mediated effects in chondrocytes. Both gain of function and loss of function studies in mice show that they limit BMP signaling in cartilage and are required for proper skeletal development [116,117]. In particular, Smad6, induced by TGFβ1 and BMPs [132] is required for inhibition of endochondral bone formation; Smad6~/~ mice have abnormal growth plates exhibiting an expanded hypertrophic zone and enhanced expression of Ihh, a factor shown by Seki et al. to be a direct target of canonical BMP pathways [133]. I-Smad7 inhibits both BMP and TGFβ signaling. Smad7 is induced by TGFβ ligands [129,130]. Studies in which Smad7 was overexpressed in chondrocytes demonstrated that Smad7 impacts chondrogenesis by inhibiting BMP signaling [134]. Smad7 is required for both axial and appendicular skeletal development in mice [117]. Loss of Smad7 in chondrocytes resulted in cell cycle impairments and defects in terminal maturation. This phenotype was attributed to upregulation of both BMP and TGFβ signaling.
6.2. TGFβ limits BMP signaling at the receptor level
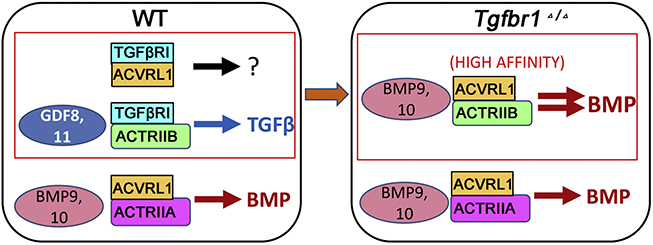
As discussed above, loss of the TGFβ type I receptor TGFβRI leads to enhanced BMP signaling in the growth plate [29]. Biochemical and genetic studies indicate that this crosstalk occurs at the level of receptor complex formation. Genetic evidence for receptor-level crosstalk came from the finding that growth plate defects in TgfbriCo12 mice were rescued by depletion of ACVRL1 in the Tgfbri mutant background, indicating that TGFβRI blocks BMP signaling transduced by ACVRL1. Evidence that this rescue is due to alterations in receptor complex formation came from co-immunoprecipitation assays, which confirmed that ACVRL1 complexes with ACTRIIA but not with ACTRIIB in normal chondrocytes. However, loss of TGFβRI increases ACVRL1/ACTRIIB complex formation in cartilage. This is potentially signficiant because BMP9, a ligand that preferentially binds to ACVRL1, is detected in growth plate cartilage, and ACTRIIB has about 300-fold higher affinity for BMP9 than to ACTRIIA [135]. In accordance, co- immunoprecipitation assays showed that TGFβRI associates with ACTRIIB but not with ACTRIIA in normal chondrocytes. These results suggest that TGFβRI blocks ACVRL1/ACTRIIB complex formation, thereby preventing formation of these high affinity BMP9-binding complexes (Fig. 2). It is not clear why TGFβRI is required to inhibit ACVRL1-mediated BMP signaling in cartilage; one possibility is that because BMP9 is in circulation and cannot be inhibited by Noggin [2,136,137], this alternative method is utilized.
Figure 2.
Proposed model for ACVRL1/TGFβRI interaction. In WT chondrocytes, a significant proportion of ACVRLi and ACTRIIB receptors are contained in complexes with TGFβRI, and TGFβRI blocks ACVRLi complexing with ACTRIIB. In TGFβRI mutant cells, loss of TGFβRI releases both ACTRIIB and ACVRLi receptors, enabling formation of ACVRLi/ACTRIIB complexes that have high binding affinity with BMP9.
7. Summary and Perspectives
Genetic models have provided a better understanding of the physiological functions of TGFβ and BMP signaling network components and their crosstalk during cartilage development. However, many questions remain regarding the relative importance of various pathways downstream of TGFβ and BMP signaling, the mechanisms and consequences of many types of TGFβ/BMP ligands competing for a more limited set of receptors, the mechanisms determining type I and type II receptor interactions, and how TGFβ/BMP-regulated canonical and noncanonical pathways intersect. It is clear that the absolute and relative output of TGFβ/BMP signaling, and crosstalk between these signaling pathways, determines the final cellular response. Not every signaling component, however, is well understood with regard to in vivo functions at different developmental stages. For example, the functions of ACTRIIA and ACTRIIB in mediating TGFβ and BMP actions in cartilage development are still not clear. Given that ACTRIIA/B can transduce both TGFβ and BMP signals that have fundamentally different and usually opposing effects in cartilage, understanding the extent to which these receptors utilize TGFβ/BMP pathways in vivo is an important goal for the future. Similarly, it will be of interest to assess the importance of genetic and physical interactions between ACVR1L and TGFβRI in multiple skeletal tissues, and whether TGFβRI can limit the signaling of similar BMP type I receptors, like ACVR1.
Highlights.
TGFβ and BMP signaling negatively regulate one another during cartilage development TGFβ inhibits the BMP pathway through receptor interactions and transcriptional regulation TGFβRI blocks BMP signaling through restricting ability of ACVRL1 to complex with ACTRIIB
Acknowledgements
This work was supported by the National Institutes of Health [R01 Grants AR073793].
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Thielen NGM, van der Kraan PM, van Caam APM, TGFβ/BMP Signaling Pathway in Cartilage Homeostasis, Cells. 8 (2019). 10.3390/cells8090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lowery JW, Rosen V, The BMP Pathway and Its Inhibitors in the Skeleton, Physiological Reviews. 98 (2018) 2431–2452. 10.1152/physrev.00028.2017. [DOI] [PubMed] [Google Scholar]
- [3].Salazar VS, Gamer LW, Rosen V, BMP signalling in skeletal development, disease and repair, Nat Rev Endocrinol 12 (2016) 203–221. 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- [4].Rigueur D, Brugger S, Anbarchian T, Kim JK, Lee Y, Lyons KM, The type I BMP receptor ACVR1/ALK2 is required for chondrogenesis during development, J Bone Miner Res. 30 (2015) 733–41. 10.1002/jbmr.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Retting KN, Song B, Yoon BS, Lyons KM, BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation, Development. 136 (2009) 1093–1104. 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM, Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo, Proc. Natl. Acad. Sci. U.S.A. 102 (2005) 5062–5067. 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yadin D, Knaus P, Mueller TD, Structural insights into BMP receptors: Specificity, activation and inhibition, Cytokine Growth Factor Rev. 27 (2016) 13–34. 10.1016/j.cytogfr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- [8].Tzavlaki K, Moustakas A , TGF-P Signaling, Biomolecules. 10 (2020). 10.3390/biom10030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Derynck R, Budi EH, Specificity, versatility, and control of TGF-P family signaling, Sci Signal 12 (2019). 10.1126/scisignal.aav5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nickel J, Mueller TD, Specification of BMP Signaling, Cells. 8 (2019). 10.3390/cells8121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang W, Rigueur D, Lyons KM, TGFß signaling in cartilage development and maintenance, Birth Defects Res C Embryo Today. 102 (2014) 37–51. 10.1002/bdrc.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Finnson KW, Almadani Y, Philip A, Non-canonical (non-SMAD2/3) TGF-ß signaling in fibrosis: Mechanisms and targets, Semin. Cell Dev. Biol. 101 (2020) 115–122. 10.1016/j.semcdb.2019.11.013. [DOI] [PubMed] [Google Scholar]
- [13].Ahmadi A, Najafi M, Farhood B, Mortezaee K, Transforming growth factor-ß signaling: Tumorigenesis and targeting for cancer therapy, J. Cell. Physiol. 234 (2019) 12173–12187. 10.1002/jcp.27955. [DOI] [PubMed] [Google Scholar]
- [14].Wu M, Chen G, Li Y-P, TGF-ß and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease, Bone Res 4 (2016) 16009 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mu Y, Gudey SK, Landström M, Non-Smad signaling pathways, Cell Tissue Res. 347 (2012) 11–20. 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- [16].Zhang YE, Non-Smad pathways in TGF-beta signaling, Cell Res. 19 (2009) 128–139. 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moustakas A, Heldin C-H, Non-Smad TGF-beta signals J Cell. Sci. 118 (2005) 3573–3584. 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- [18].Long F, Ornitz DM, Development of the endochondral skeleton, Cold Spring Harb Perspect Biol. 5 (2013) a008334 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pitsillides AA, Beier F, Cartilage biology in osteoarthritis--lessons from developmental biology, Nat Rev Rheumatol 7 (2011) 654–663. 10.1038/nrrheum.2011.129. [DOI] [PubMed] [Google Scholar]
- [20].Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B, Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice, PLoS Genet. 10 (2014) e1004820 10.1371/journal.pgen.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lui JC, Yue S, Lee A, Kikani B, Temnycky A, Barnes KM, Baron J, Persistent Sox9 expression in hypertrophic chondrocytes suppresses transdifferentiation into osteoblasts, Bone. 125 (2019) 169–177. 10.1016/j.bone.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Karsenty G, Kronenberg HM, Settembre C, Genetic control of bone formation, Annu. Rev. Cell Dev. Biol. 25 (2009) 629–648. 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- [23].Kronenberg HM, Developmental regulation of the growth plate, Nature. 423 (2003) 332–336. 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- [24].Lyons KM, Rosen V, BMPs, TGFß, and border security at the interzone, Curr. Top. Dev. Biol. 133 (2019) 153–170. 10.1016/bs.ctdb.2019.02.001. [DOI] [PubMed] [Google Scholar]
- [25].Wang W, Song B, Anbarchian T, Shirazyan A, Sadik JE, Lyons KM, Smad2 and Smad3 Regulate Chondrocyte Proliferation and Differentiation in the Growth Plate, PLoS Genet. 12 (2016) e1006352 10.1371/journal.pgen.1006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].van der Kraan PM, Blaney Davidson EN, Blom A, van den Berg WB, TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis, Osteoarthritis and Cartilage. 17 (2009) 1539–1545. 10.1016/jjoca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- [27].Li T-F, TGF-b signaling in chondrocytes, Frontiers in Bioscience. 10 (2005) 681 10.2741/1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Serra R, Chang C, TGF-? signaling in human skeletal and patterning disorders, Birth Defects Research Part C: Embryo Today: Reviews. 69 (2003) 333–351. 10.1002/bdrc.10023. [DOI] [PubMed] [Google Scholar]
- [29].Wang W, Chun H, Baek J, Sadik JE, Shirazyan A, Razavi P, Lopez N, Lyons KM, The TGFβ type I receptor TGFβRI functions as an inhibitor of BMP signaling in cartilage, Proceedings of the National Academy of Sciences. (2019) 201902927 10.1073/pnas.1902927116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL, Expression of a Truncated, Kinase-Defective TGF-P Type II Receptor in Mouse Skeletal Tissue Promotes Terminal Chondrocyte Differentiation and Osteoarthritis, The Journal of Cell Biology. 139 (1997) 541–552. 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hinck AP, Structural studies of the TGF-Ps and their receptors - insights into evolution of the TGF-P superfamily, FEBS Lett. 586 (2012) 1860–1870. 10.1016/j.febslet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- [32].Guo X, Wang X-F, Signaling cross-talk between TGF-beta/BMP and other pathways, Cell Res. 19 (2009) 71–88. 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gordon KJ, Blobe GC, Role of transforming growth factor-beta superfamily signaling pathways in human disease, Biochim. Biophys. Acta. 1782 (2008) 197–228. 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- [34].Gomez-Puerto MC, Iyengar PV, García de Vinuesa A, Ten Dijke P, Sanchez- Duffhues G, Bone morphogenetic protein receptor signal transduction in human disease, J. Pathol. 247 (2019) 9–20. 10.1002/path.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Massagué J, TGFβ signalling in context, Nat. Rev. Mol. Cell Biol. 13 (2012) 616–630. 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Weiss A, Attisano L, The TGFbeta superfamily signaling pathway, Wiley Interdiscip Rev Dev Biol. 2 (2013) 47–63. 10.1002/wdev.86. [DOI] [PubMed] [Google Scholar]
- [37].Mohedas AH, Xing X, Armstrong KA, Bullock AN, Cuny GD, Yu PB, Development of an ALK2-biased BMP type I receptor kinase inhibitor, ACS Chem. Biol. 8 (2013) 1291–1302. 10.1021/cb300655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Macias D, Gañan Y, Sampath TK, Piedra ME, Ros MA, Hurle JM, Role of BMP-2 and OP-1 (BMP-7) in programmed cell death and skeletogenesis during chick limb development, Development. 124 (1997) 1109–1117. [DOI] [PubMed] [Google Scholar]
- [39].Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM, TGF-p/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation, Bone Res 3 (2015) 15005 10.1038/boneres.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mahlawat P, Ilangovan U, Biswas T, Sun L-Z, Hinck AP, Structure of the Alk1 Extracellular Domain and Characterization of Its Bone Morphogenetic Protein (BMP) Binding Properties, Biochemistry. 51 (2012) 6328–6341. 10.1021/bi300942x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Luo J, Tang M, Huang J, He B-C, Gao J-L, Chen L, Zuo G-W, Zhang W, Luo Q, Shi Q, Zhang B-Q, Bi Y, Luo X, Jiang W, Su Y, Shen J, Kim SH, Huang E, Gao Y, Zhou J-Z, Yang K, Luu HH, Pan X, Haydon RC, Deng Z-L, He T-C, TGFβ/BMP Type I Receptors ALK1 and ALK2 Are Essential for BMP9-induced Osteogenic Signaling in Mesenchymal Stem Cells, Journal of Biological Chemistry. 285 (2010) 29588–29598. 10.1074/jbc.M110.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].David L, Mallet C, Mazerbourg S, Feige J-J, Bailly S, Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells, Blood. 109 (2007) 1953–1961. 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- [43].Mitrofan C-G, Appleby SL, Nash GB, Mallat Z, Chilvers ER, Upton PD, Morrell NW, Bone morphogenetic protein 9 (BMP9) and BMP10 enhance tumor necrosis factor-a-induced monocyte recruitment to the vascular endothelium mainly via activin receptor-like kinase 2, Journal of Biological Chemistry. 292 (2017) 13714–13726. 10.1074/jbc.M117.778506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Antebi YE, Linton JM, Klumpe H, Bintu B, Gong M, Su C, McCardell R, Elowitz MB, Combinatorial Signal Perception in the BMP Pathway, Cell. 170 (2017) 1184–1196.e24. 10.1016/j.cell.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P, Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling, Mol. Cell. 12 (2003) 817–828. 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- [46].Daly AC, Randall RA, Hill CS, Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth, Mol. Cell. Biol. 28 (2008) 6889–6902. 10.1128/MCB.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ruiz M, Maumus M, Fonteneau G, Pers Y-M, Ferreira R, Dagneaux L, Delfour C, Houard X, Berenbaum F, Rannou F, Jorgensen C, Noël D, TGFβi is involved in the chondrogenic differentiation of mesenchymal stem cells and is dysregulated in osteoarthritis, Osteoarthr. Cartil 27 (2019) 493–503. 10.1016/jjoca.2018.11.005. [DOI] [PubMed] [Google Scholar]
- [48].Sasaki H, Rothrauff BB, Alexander PG, Lin H, Gottardi R, Fu FH, Tuan RS, In Vitro Repair of Meniscal Radial Tear With Hydrogels Seeded With Adipose Stem Cells and TGF-P3, Am J Sports Med 46 (2018) 2402–2413. 10.1177/0363546518782973. [DOI] [PubMed] [Google Scholar]
- [49].Chen MJ, Whiteley JP, Please CP, Schwab A, Ehlicke F, Waters SL, Byrne HM, Inducing chondrogenesis in MSC/chondrocyte co-cultures using exogenous TGF-P: a mathematical model, J. Theor. Biol. 439 (2018) 1–13. 10.1016/jjtbi.2017.11.024. [DOI] [PubMed] [Google Scholar]
- [50].Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS, Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk, J. Biol. Chem. 278 (2003) 41227–41236. 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- [51].Barry F, Boynton RE, Liu B, Murphy JM, Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components, Exp. Cell Res. 268 (2001) 189–200. 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- [52].Pelton RW, Dickinson ME, Moses HL, Hogan BL, In situ hybridization analysis of TGF beta 3 RNA expression during mouse development: comparative studies with TGF beta 1 and beta 2, Development. 110 (1990) 609–620. [DOI] [PubMed] [Google Scholar]
- [53].Pelton RW, Saxena B, Jones M, Moses HL, Gold LI, Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development, J. Cell Biol. 115 (1991) 1091–1105. 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Horner A, Kemp P, Summers C, Bord S, Bishop NJ, Kelsall AW, Coleman N, Compston JE, Expression and distribution of transforming growth factor-beta isoforms and their signaling receptors in growing human bone, Bone. 23 (1998) 95–102. 10.1016/s8756-3282(98)00080-5. [DOI] [PubMed] [Google Scholar]
- [55].Millan FA, Denhez F, Kondaiah P, Akhurst RJ, Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo, Development. 111 (1991) 131–143. [DOI] [PubMed] [Google Scholar]
- [56].Sandberg M, Vuorio T, Hirvonen H, Alitalo K, Vuorio E, Enhanced expression of TGF-beta and c-fos mRNAs in the growth plates of developing human long bones, Development. 102 (1988) 461–470. [DOI] [PubMed] [Google Scholar]
- [57].Thorp BH, Anderson I, Jakowlew SB, Transforming growth factor-beta 1, -beta 2 and - beta 3 in cartilage and bone cells during endochondral ossification in the chick, Development. 114 (1992) 907–911. [DOI] [PubMed] [Google Scholar]
- [58].Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease, Nature. 359 (1992) 693–699. 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J, Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction, Nat. Genet. 11 (1995) 415–421. 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- [60].Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T, Transforming growth factor-beta 3 is required for secondary palate fusion, Nat. Genet. 11 (1995) 409–414. 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T, TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes, Development. 124 (1997) 2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cameron TL, Belluoccio D, Farlie PG, Brachvogel B, Bateman JF, Global comparative transcriptome analysis of cartilage formation in vivo, BMC Dev. Biol. 9 (2009) 20 10.1186/1471-213X-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Garrison P, Yue S, Hanson J, Baron J, Lui JC, Spatial regulation of bone morphogenetic proteins (BMPs) in postnatal articular and growth plate cartilage, PLoS ONE. 12 (2017) e0176752 10.1371/journal.pone.0176752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gamer LW, Cox KA, Small C, Rosen V, Gdf11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb, Dev. Biol. 229 (2001) 407–420. 10.1006/dbio.2000.9981. [DOI] [PubMed] [Google Scholar]
- [65].Matsunobu T, Torigoe K, Ishikawa M, de Vega S, Kulkarni AB, Iwamoto Y, Yamada Y, Critical roles of the TGF-beta type I receptor ALK5 in perichondrial formation and function, cartilage integrity, and osteoblast differentiation during growth plate development, Dev. Biol. 332 (2009) 325–338. 10.1016/j.ydbio.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR, Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice, Genesis. 26 (2000) 145–146. [PubMed] [Google Scholar]
- [67].Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM, Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth, Development. 130 (2003) 3063–3074. 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- [68].Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R, Conditional deletion of the TGF-P type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones, Developmental Biology. 276 (2004) 124–142. 10.1016/j.ydbio.2004.08.027. [DOI] [PubMed] [Google Scholar]
- [69].Blitz E, Sharir A, Akiyama H, Zelzer E, Tendon-bone attachment unit is formed modularly by a distinct pool of Scx - and Sox9 -positive progenitors, Development. 140 (2013) 2680–2690. 10.1242/dev.093906. [DOI] [PubMed] [Google Scholar]
- [70].Sugimoto Y, Takimoto A, Akiyama H, Kist R, Scherer G, Nakamura T, Hiraki Y, Shukunami C, Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament, Development. 140 (2013) 2280–2288. 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- [71].Iwata J, Hacia JG, Suzuki A, Sanchez-Lara PA, Urata M, Chai Y, Modulation of noncanonical TGF-P signaling prevents cleft palate in Tgfbr2 mutant mice, J. Clin. Invest. 122 (2012) 873–885. 10.1172/JCI61498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L, Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis, Mol. Cell. Biol. 23 (2003) 7230–7242. 10.1128/mcb.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Li S, Nie EH, Yin Y, Benowitz LI, Tung S, Vinters HV, Bahjat FR, Stenzel- Poore MP, Kawaguchi R, Coppola G, Carmichael ST, GDF10 is a signal for axonal sprouting and functional recovery after stroke, Nat. Neurosci. 18 (2015) 1737–1745. 10.1038/nn.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Andersson O, Reissmann E, Ibanez CF, Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis, EMBO Rep. 7 (2006) 831–837. 10.1038/sj.embor.7400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yang X, Chen L, Xu X, Li C, Huang C, Deng CX, TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage, J. Cell Biol. 153 (2001) 35–46. 10.1083/jcb.153.L35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Billiar RB, St Clair JB, Zachos NC, Burch MG, Albrecht ED, Pepe GJ, Localization and developmental expression of the activin signal transduction proteins Smads 2, 3, and 4 in the baboon fetal ovary, Biol. Reprod. 70 (2004) 586–592. 10.1095/biolreprod.103.018598. [DOI] [PubMed] [Google Scholar]
- [77].Sakou T, Onishi T, Yamamoto T, Nagamine T, k Sampath T, Ten Dijke P, Localization of Smads, the TGF-beta family intracellular signaling components during endochondral ossification, J. Bone Miner. Res. 14 (1999) 1145–1152. 10.1359/jbmr.1999.14.7.1145. [DOI] [PubMed] [Google Scholar]
- [78].Minina E, Schneider S, Rosowski M, Lauster R, Vortkamp A, Expression of Fgf and Tgfbeta signaling related genes during embryonic endochondral ossification, Gene Expr. Patterns. 6 (2005) 102–109. 10.1016/j.modgep.2005.04.012. [DOI] [PubMed] [Google Scholar]
- [79].Yeganeh B, Mukherjee S, Moir LM, Kumawat K, Kashani HH, Bagchi RA, Baarsma HA, Gosens R, Ghavami S, Novel non-canonical TGF-P signaling networks: emerging roles in airway smooth muscle phenotype and function, Pulm Pharmacol Ther. 26 (2013) 50–63. 10.1016/j.pupt.2012.07.006. [DOI] [PubMed] [Google Scholar]
- [80].Gunnell LM, Jonason JH, Loiselle AE, Kohn A, Schwarz EM, Hilton MJ, O’Keefe RJ, TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways, J. Bone Miner. Res. 25 (2010) 1784–1797. 10.1002/jbmr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Beier F, Loeser RF, Biology and pathology of Rho GTPase, PI-3 kinase-Akt, and MAP kinase signaling pathways in chondrocytes, J. Cell. Biochem. 110 (2010) 573–580. 10.1002/jcb.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pagès G, Guérin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouysségur J, Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice, Science. 286 (1999) 1374–1377. 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- [83].Fukai A, Kawamura N, Saito T, Oshima Y, Ikeda T, Kugimiya F, Higashikawa A, Yano F, Ogata N, Nakamura K, Chung U-I, Kawaguchi H, Akt1 in murine chondrocytes controls cartilage calcification during endochondral ossification under physiologic and pathologic conditions, Arthritis Rheum. 62 (2010) 826–836. 10.1002/art.27296. [DOI] [PubMed] [Google Scholar]
- [84].Pardali E, Goumans M-J, ten Dijke P, Signaling by members of the TGF-beta family in vascular morphogenesis and disease, Trends Cell Biol. 20 (2010) 556–567. 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- [85].Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, Idowu O, Li M, Shen C, Hu A, Haydon RC, Kang R, Mok J, Lee MJ, Luu HL, Shi LL, Bone Morphogenetic Protein (BMP) signaling in development and human diseases, Genes Dis 1 (2014) 87–105. 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lyons KM, Pelton RW, Hogan BL, Patterns of expression of murine Vgr-1 and BMP- 2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development., Genes & Development. 3 (1989) 1657–1668. 10.1101/gad.3.11.1657. [DOI] [PubMed] [Google Scholar]
- [87].Lyons KM, Pelton RW, Hogan BL, Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A), Development. 109 (1990) 833–844. [DOI] [PubMed] [Google Scholar]
- [88].Jones CM, Lyons KM, Hogan BL, Involvement of Bone Morphogenetic Protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse, Development. 111 (1991)531–542. [DOI] [PubMed] [Google Scholar]
- [89].Grimsrud CD, Romano PR, D’souza M, Puzas JE, Reynolds PR, Rosier RN, O’keefe RJ, BMP-6 Is an Autocrine Stimulator of Chondrocyte Differentiation, Journal of Bone and Mineral Research. 14 (1999) 475–482. 10.1359/jbmr.1999.14.4.475. [DOI] [PubMed] [Google Scholar]
- [90].Haaijman A, Karperien M, Lanske B, Hendriks J, Lowik CWGM, Bronckers ALJJ, Burger EH, Inhibition of terminal chondrocyte differentiation by bone morphogenetic protein 7 (OP-1) in vitro depends on the periarticular region but is independent of parathyroid hormone-related peptide, Bone. 25 (1999) 397–404. 10.1016/S8756-3282(99)00189-1. [DOI] [PubMed] [Google Scholar]
- [91].Pizette S, Niswander L, BMPs Are Required at Two Steps of Limb Chondrogenesis: Formation of Prechondrogenic Condensations and Their Differentiation into Chondrocytes, Developmental Biology. 219 (2000) 237–249. 10.1006/dbio.2000.9610. [DOI] [PubMed] [Google Scholar]
- [92].Chimal-Monroy J, Rodriguez-Leon J, Montero JA, Gañan Y, Macias D, Merino R, Hurle JM, Analysis of the molecular cascade responsible for mesodermal limb chondrogenesis: sox genes and BMP signaling, Developmental Biology. 257 (2003) 292–301. 10.1016/S0012-1606(03)00066-6. [DOI] [PubMed] [Google Scholar]
- [93].Erickson DM, Harris SE, Dean DD, Harris MA, Wozney JM, Boyan BD, Schwartz Z, Recombinant bone morphogenetic protein (BMP)-2 regulates costochondral growth plate chondrocytes and induces expression of BMP-2 and BMP-4 in a cell maturation-dependent manner, Journal of Orthopaedic Research. 15 (1997) 371–380. 10.1002/jor.1100150309. [DOI] [PubMed] [Google Scholar]
- [94].Mailhot G, Yang M, Mason-Savas A, MacKay CA, Leav I, Odgren PR, BMP-5 expression increases during chondrocyte differentiation in vivo and in vitro and promotes proliferation and cartilage matrix synthesis in primary chondrocyte cultures, Journal of Cellular Physiology. 214 (2008) 56–64. 10.1002/jcp.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shu B, Zhang M, Xie R, Wang M, Jin H, Hou W, Tang D, Harris SE, Mishina Y, O’Keefe RJ, Hilton MJ, Wang Y, Chen D, BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development, Journal of Cell Science. 124 (2011) 3428–3440. 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, He T-C, Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery, Gene Therapy. 11 (2004) 1312–1320. 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- [97].Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He T-C, OSTEOGENIC ACTIVITY OF THE FOURTEEN TYPES OF HUMAN BONE MORPHOGENETIC PROTEINS (BMPS):, The Journal of Bone and Joint Surgery-American Volume. 85 (2003) 1544–1552. 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- [98].Luu HH, Song W-X, Luo X, Manning D, Luo J, Deng Z-L, Sharff KA, Montag AG, Haydon RC, He T-C, Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells, Journal of Orthopaedic Research. 25 (2007) 665–677. 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- [99].Lorda-Diez CI, Montero JA, Choe S, Garcia-Porrero JA, Hurle JM, Ligand- and Stage-Dependent Divergent Functions of BMP Signaling in the Differentiation of Embryonic Skeletogenic Progenitors In Vitro: BMP SIGNALING AND EMBRYONIC SKELETOGENIC DIFFERENTIATION, Journal of Bone and Mineral Research. 29 (2014) 735–748. 10.1002/jbmr.2077. [DOI] [PubMed] [Google Scholar]
- [100].Baur ST, Mai JJ, Dymecki SM, Combinatorial signaling through BMP receptor IB and GDF5: shaping of the distal mouse limb and the genetics of distal limb diversity, Development. 127 (2000) 605–619.10631181 [Google Scholar]
- [101].Storm EE, Kingsley DM, GDF5 Coordinates Bone and Joint Formation during Digit Development, Developmental Biology. 209 (1999) 11–27. 10.1006/dbio.1999.9241. [DOI] [PubMed] [Google Scholar]
- [102].Tsumaki N, Nakase T, Miyaji T, Kakiuchi M, Kimura T, Ochi T, Yoshikawa H, Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis, J. Bone Miner. Res. 17 (2002) 898–906. 10.1359/jbmr.2002.17.5.898. [DOI] [PubMed] [Google Scholar]
- [103].Settle SH, Rountree RB, Sinha A, Thacker A, Higgins K, Kingsley DM, Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes, Dev. Biol. 254 (2003) 116–130. 10.1016/s0012-1606(02)00022-2. [DOI] [PubMed] [Google Scholar]
- [104].Mikic B, Ferreira MP, Battaglia TC, Hunziker EB, Accelerated hypertrophic chondrocyte kinetics in GDF-7 deficient murine tibial growth plates, J. Orthop. Res. 26 (2008) 986–990. 10.1002/jor.20574. [DOI] [PubMed] [Google Scholar]
- [105].Brunet LJ, McMahon JA, McMahon AP, Harland RM, Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton, Science. 280 (1998) 1455–1457. 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- [106].Pathi S, Rutenberg JB, Johnson RL, Vortkamp A, Interaction of Ihh and BMP/Noggin signaling during cartilage differentiation, Dev. Biol. 209 (1999) 239–253. 10.1006/dbio.1998.9181. [DOI] [PubMed] [Google Scholar]
- [107].Gazzerro E, Gangji V, Canalis E, Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts, J. Clin. Invest. 102 (1998) 2106–2114. 10.1172/JCI3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Stafford DA, Monica SD, Harland RM, Follistatin interacts with Noggin in the development of the axial skeleton, Mech. Dev. 131 (2014) 78–85. 10.1016/j.mod.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhang D, Ferguson CM, O’Keefe RJ, Puzas JE, Rosier RN, Reynolds PR, A role for the BMP antagonist chordin in endochondral ossification, J. Bone Miner. Res. 17 (2002) 293–300. 10.1359/jbmr.2002.17.2.293. [DOI] [PubMed] [Google Scholar]
- [110].Ray A, Singh PNP, Sohaskey ML, Harland RM, Bandyopadhyay A, Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation, Development. 142 (2015) 1169–1179. 10.1242/dev.110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Spagnoli A, O’Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K, Moses HL, TGF-beta signaling is essential for joint morphogenesis, J. Cell Biol. 177 (2007) 1105–1117. 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Li T-F, Darowish M, Zuscik MJ, Chen D, Schwarz EM, Rosier RN, Drissi H, O’Keefe RJ, Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation, J. Bone Miner. Res. 21 (2006) 4–16. 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gronroos E, Kingston IJ, Ramachandran A, Randall RA, Vizân P, Hill CS, Transforming growth factor β inhibits bone morphogenetic protein-induced transcription through novel phosphorylated Smad1/5-Smad3 complexes, Mol. Cell. Biol. 32 (2012) 2904–2916. 10.1128/MCB.00231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kawamura I, Maeda S, Imamura K, Setoguchi T, Yokouchi M, Ishidou Y, Komiya S, SnoN suppresses maturation of chondrocytes by mediating signal cross-talk between transforming growth factor-P and bone morphogenetic protein pathways, J. Biol. Chem. 287 (2012) 29101–29113. 10.1074/jbc.M112.349415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Miyazawa K, Miyazono K, Regulation of TGF-P Family Signaling by Inhibitory Smads, Cold Spring Harbor Perspectives in Biology. 9 (2017) a022095 10.1101/cshperspect.a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Estrada KD, Retting KN, Chin AM, Lyons KM, Smad6 is essential to limit BMP signaling during cartilage development, Journal of Bone and Mineral Research. 26 (2011) 2498–2510. 10.1002/jbmr.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Estrada KD, Wang W, Retting KN, Chien CT, Elkhoury FF, Heuchel R, Lyons KM, Smad7 regulates terminal maturation of chondrocytes in the growth plate, Developmental Biology. 382 (2013) 375–384. 10.1016/j.ydbio.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Mochizuki T, Miyazaki H, Hara T, Furuya T, Imamura T, Watabe T, Miyazono K, Roles for the MH2 domain of Smad7 in the specific inhibition of transforming growth factor-beta superfamily signaling, J. Biol. Chem. 279 (2004) 31568–31574. 10.1074/jbc.M313977200. [DOI] [PubMed] [Google Scholar]
- [119].Kamiya Y, Miyazono K, Miyazawa K, Smad7 inhibits transforming growth factor-beta family type i receptors through two distinct modes of interaction, J. Biol. Chem. 285 (2010) 30804–30813. 10.1074/jbc.M110.166140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hanyu A, Ishidou Y, Ebisawa T, Shimanuki T, Imamura T, Miyazono K, The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling, J. Cell Biol. 155 (2001) 1017–1027. 10.1083/jcb.200106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL, Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation, Mol. Cell. 6 (2000) 1365–1375. 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- [122].Suzuki C, Murakami G, Fukuchi M, Shimanuki T, Shikauchi Y, Imamura T, Miyazono K, Smurf1 regulates the inhibitory activity of Smad7 by targeting Smad7 to the plasma membrane, J. Biol. Chem. 277 (2002) 39919–39925. 10.1074/jbc.M201901200. [DOI] [PubMed] [Google Scholar]
- [123].Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T, Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads, Mol. Biol. Cell. 14 (2003) 2809–2817. 10.1091/mbc.e02-07-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hata A, Lagna G, Massagué J, Hemmati-Brivanlou A, Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor, Genes Dev. 12 (1998) 186–197. 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Yan X, Liao H, Cheng M, Shi X, Lin X, Feng X-H, Chen Y-G, Smad7 Protein Interacts with Receptor-regulated Smads (R-Smads) to Inhibit Transforming Growth Factor-P (TGF-P)/Smad Signaling, J. Biol. Chem. 291 (2016) 382–392. 10.1074/jbc.M115.694281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng X-H, Meng A, Chen Y-G, Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation, Mol. Cell. Biol. 27 (2007) 4488–4499. 10.1128/MCB.01636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Pulaski L, Landstrom M, Heldin CH, Souchelnytskyi S, Phosphorylation of Smad7 at Ser-249 does not interfere with its inhibitory role in transforming growth factor-beta- dependent signaling but affects Smad7-dependent transcriptional activation, J. Biol. Chem. 276 (2001) 14344–14349. 10.1074/jbc.M011019200. [DOI] [PubMed] [Google Scholar]
- [128].Bai S, Cao X, A nuclear antagonistic mechanism of inhibitory Smads in transforming growth factor-beta signaling, J. Biol. Chem. 277 (2002) 4176–4182. 10.1074/jbc.M105105200. [DOI] [PubMed] [Google Scholar]
- [129].Denissova NG, Pouponnot C, Long J, He D, Liu F, Transforming growth factor beta - inducible independent binding of SMAD to the Smad7 promoter, Proc. Natl. Acad. Sci. U.S.A. 97 (2000) 6397–6402. 10.1073/pnas.090099297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Nagarajan RP, Zhang J, Li W, Chen Y, Regulation of Smad7 promoter by direct association with Smad3 and Smad4, J. Biol. Chem. 274 (1999) 33412–33418. 10.1074/jbc.274.47.33412. [DOI] [PubMed] [Google Scholar]
- [131].Benchabane H, Wrana JL, GATA- and Smad1-dependent enhancers in the Smad7 gene differentially interpret bone morphogenetic protein concentrations, Mol. Cell. Biol. 23 (2003) 6646–6661. 10.1128/mcb.23.18.6646-6661.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Jung SM, Lee J-H, Park J, Oh YS, Lee SK, Park JS, Lee YS, Kim JH, Lee JY, Bae Y-S, Koo S-H, Kim S-J, Park SH, Smad6 inhibits non-canonical TGF-pi signalling by recruiting the deubiquitinase A20 to TRAF6, Nat Commun. 4 (2013) 2562 10.1038/ncomms3562. [DOI] [PubMed] [Google Scholar]
- [133].Seki K, Hata A, Indian Hedgehog Gene Is a Target of the Bone Morphogenetic Protein Signaling Pathway, Journal of Biological Chemistry. 279 (2004) 18544–18549. 10.1074/jbc.M311592200. [DOI] [PubMed] [Google Scholar]
- [134].Iwai T, Murai J, Yoshikawa H, Tsumaki N, Smad7 Inhibits chondrocyte differentiation at multiple steps during endochondral bone formation and down-regulates p38 MAPK pathways, J. Biol. Chem. 283 (2008) 27154–27164. 10.1074/jbc.M801175200. [DOI] [PubMed] [Google Scholar]
- [135].Townson SA, Martinez-Hackert E, Greppi C, Lowden P, Sako D, Liu J, Ucran JA, Liharska K, Underwood KW, Seehra J, Kumar R, Grinberg AV, Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex, J. Biol. Chem. 287 (2012) 27313–27325. 10.1074/jbc.M112.377960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wang Y, Hong S, Li M, Zhang J, Bi Y, He Y, Liu X, Nan G, Su Y, Zhu G, Li R, Zhang W, Wang J, Zhang H, Kong Y, Shui W, Wu N, He Y, Chen X, Luu HH, Haydon RC, Shi LL, He T-C, Qin J, Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells, J. Orthop. Res. 31 (2013) 1796–1803. 10.1002/jor.22427. [DOI] [PubMed] [Google Scholar]
- [137].Cheng A, Gustafson AR, Schaner Tooley CE, Zhang M, BMP-9 dependent pathways required for the chondrogenic differentiation of pluripotent stem cells, Differentiation. 92 (2016) 298–305. 10.1016/j.diff.2016.03.005. [DOI] [PubMed] [Google Scholar]