Abstract
The novelty of new human coronavirus COVID-19/SARS-CoV-2 and the lack of effective drugs and vaccines gave rise to a wide variety of strategies employed to fight this worldwide pandemic. Many of these strategies rely on the repositioning of existing drugs that could shorten the time and reduce the cost compared to de novo drug discovery. In this study, we presented a new network-based algorithm for drug repositioning, called SAveRUNNER (Searching off-lAbel dRUg aNd NEtwoRk), which predicts drug–disease associations by quantifying the interplay between the drug targets and the disease-specific proteins in the human interactome via a novel network-based similarity measure that prioritizes associations between drugs and diseases locating in the same network neighborhoods. Specifically, we applied SAveRUNNER on a panel of 14 selected diseases with a consolidated knowledge about their disease-causing genes and that have been found to be related to COVID-19 for genetic similarity (i.e., SARS), comorbidity (e.g., cardiovascular diseases), or for their association to drugs tentatively repurposed to treat COVID-19 (e.g., malaria, HIV, rheumatoid arthritis). Focusing specifically on SARS subnetwork, we identified 282 repurposable drugs, including some the most rumored off-label drugs for COVID-19 treatments (e.g., chloroquine, hydroxychloroquine, tocilizumab, heparin), as well as a new combination therapy of 5 drugs (hydroxychloroquine, chloroquine, lopinavir, ritonavir, remdesivir), actually used in clinical practice. Furthermore, to maximize the efficiency of putative downstream validation experiments, we prioritized 24 potential anti-SARS-CoV repurposable drugs based on their network-based similarity values. These top-ranked drugs include ACE-inhibitors, monoclonal antibodies (e.g., anti-IFNγ, anti-TNFα, anti-IL12, anti-IL1β, anti-IL6), and thrombin inhibitors. Finally, our findings were in-silico validated by performing a gene set enrichment analysis, which confirmed that most of the network-predicted repurposable drugs may have a potential treatment effect against human coronavirus infections.
Author summary
The global pandemic caused by the new coronavirus SARS-CoV-2 (COVID-19) leads a compelling need to find new therapeutic options devoted to fight the disease progression in the short term and to prevent it from happening in the future. The strategy of reusing an ‘old drug’ for new therapeutic purposes (known as drug repurposing) appears as a powerful solution for emerging diseases, such as COVID-19, since it allows to shorten the time and reduce the cost compared to de novo drug discovery. In this context, we propose SAveRUNNER (Searching off-lAbel dRUg aNd NEtwoRk), a new network-medicine-based algorithm for drug repurposing, with the aim to offer a promising framework to efficiently screen potential novel medical indications for various drugs, which are already approved and used in clinical practice, against the new human coronavirus (2019-nCoV/SARS-CoV-2). Our computational analysis predicts several repurposable drugs, including some of the most rumored off-label drugs for COVID-19 treatments as well as new promising candidates worthy of further exploration.
This is a PLOS Computational Biology Software paper.
Introduction
The novel coronavirus disease 2019 (COVID-19) is caused by an enveloped positive-strand RNA virus, named SARS-CoV-2, which affects the respiratory system and whose genome has been likened to the previously identified SARS-CoV strain responsible for SARS outbreak in 2003 [1]. Unfortunately, the world population is completely immune-naïve and therefore vulnerable against this new coronavirus, which has rapidly spread becoming a global pandemic with high morbidity and mortality [2–4]. While COVID-19 lockdowns are easing across the world, main concerns come from its arrival in Africa where the countries are unprepared to counteract the storm’s outburst of the new coronavirus and 1.2 billion people are at tremendous risk [5]. For example, Kenya has only 200 intensive care beds for its entire population of 50 million, whereas United States has 34 beds for every 100,000 people. Countries, like Mali, have only a few ventilators for millions of people, health facilities are overcrowded and understaffed. Yet, in many countries throughout Africa, people live together in close quarters, often without access to clean running water that makes social distancing and frequent handwashing, the only possible prevention strategy, all but impossible [5]. Given that, the COVID-19 pandemic demands the rapid identification of repurposable drug candidates to fight the disease progression in the short term and to prevent it from happening in the future.
Drug repurposing is a recent drug development strategy used to identify novel uses for drugs approved by the US Food and Drug Administration (FDA) outside the scope of their original medical indication [6]. It aims at establishing whether an ‘old drug’ can be reused for new therapeutic purposes representing a faster and cheaper alternative to de novo drug discovery process, which generally takes 12–15 years and 2–3 billion dollars (from production to approval, passing through the various phases of preclinical and clinical trials) [6]. Thus far, several therapeutic agents have been evaluated for the treatment of COVID-19, but none have yet been shown to be efficacious [7, 8]. Currently, the most promising therapeutic candidate, made available under an emergency-use authorization by the FDA, is remdesivir. It is an inhibitor of the viral RNA-dependent RNA Polymerase with proven ability to inhibit SARS-CoV-2 in vitro [9]. In fact, according to recent preliminary results of randomized clinical trials, remdesivir has been shown to be superior to placebo in shortening the time to recovery in adults hospitalized with COVID-19 and evidence of lower respiratory tract infection [10]. However, despite the use of remdesivir, the mortality remains high, indicating that treatment with an antiviral drug alone is not likely to be sufficient. To continue to improve patient outcomes in COVID-19, combinations of antiviral agents or antiviral agents in combination with other therapeutic approaches should be evaluated by future strategies.
Very promising insights comes from the new emerging field of network medicine [11, 12], which applies tools and concepts from network theory to elucidate the relation between perturbations on the molecular level and phenotypic disease manifestations. The basic premise of this exercise is that the human interactome (i.e., the cellular network of all physical molecular interactions) can be interpreted as a map and diseases as local perturbations [13]. Yet, the molecular determinants of a given disease (disease genes) are not to be randomly scattered, but co-localize and agglomerate in specific regions (disease modules) of the interactome and perturbations in these disease modules may contribute to the pathobiological phenotype [12]. From a network medicine perspective, also the action of drugs can be interpreted as a local perturbation of the interactome and thus, for a drug to be on-target effective against a specific disease or to cause off-target adverse effects, its target proteins should be within or in the immediate vicinity of the corresponding disease module. Network-based approaches marrying this philosophy can aid in identifying the specific interactome neighborhood that is perturbed in a certain disease and/or for the effect of a certain drug and guide the search for therapeutic targets, identify comorbidities, as well as rapidly detect drug repurposing candidates [14–18].
Here, we presented SAveRUNNER (Searching off-lAbel dRUg aNd NEtwoRk), a new network-medicine-based algorithm for drug repurposing. It constructs a bipartite drug-disease network by quantifying the interplay between the drug targets and the disease-specific proteins in the human interactome via a novel network-based similarity measure that prioritizes associations between drugs and diseases locating in the same network neighborhoods. SAveRUNNER yielded a high accuracy in the identification of well-known drug indications, thus revealing itself as a powerful tool to rapidly highlight potential novel medical indications for various drugs, which are already approved and used in clinical practice, against the new human coronavirus (2019-nCoV/SARS-CoV-2).
Results
Identification of predicted drug-disease associations
SAveRUNNER algorithm requires in input a list of drug targets and a list of disease genes to evaluate the extent to which a given drug can be repositioned to treat a given disease.
In the present study, disease-associated genes were downloaded from Phenopedia [19], which provides a disease-centered view of genetic association studies; whereas drug-target associations were obtained from DrugBank [20], which collects huge amount of drug-related data, recently enabling the discovery and repurposing of a relevant number of existing drugs to treat rare and newly identified diseases [6, 14] (Fig 1A).
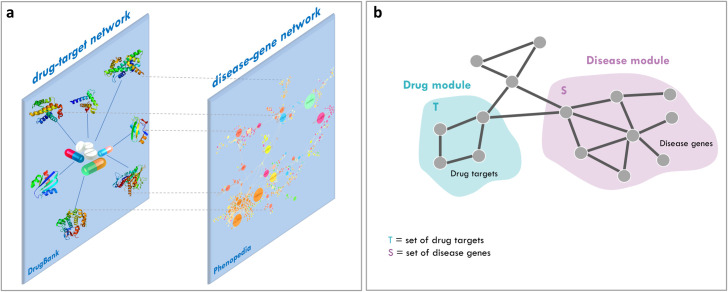
Fig 1. Schematic representation of SAveRUNNER inputs and working hypothesis.
(A) Inputs. SAveRUNNER takes as input the list of drug targets downloaded from DrugBank database and the list of disease genes downloaded from Phenopedia database. These lists can be represented as networks: (i) a drug–target network, within which nodes are drugs and target proteins, linked if the protein is a known target of the drug; and (ii) a disease-gene network, within which nodes are diseases and genes, linked if the gene has been associated to the disease. (B) Working hypothesis. Potential candidate repurposable drugs for a given disease should have target proteins (drug module T) within or in the immediate vicinity of the disease module S.
We tested the performance of SAveRUNNER on a panel of 14 diseases that have been found to be related to COVID-19 for genetic similarity, comorbidity, or for their association to drugs with the potential to shorten the recovery time for seriously ill COVID-19 patients. Thus, we included Severe Acute Respiratory Syndrome (SARS) since it is caused by the coronavirus with the highest nucleotide sequence identity with SARS-CoV-2 [16, 21]. Unlike COVID-19, for which there is still a partial knowledge of its associated disease genes, SARS has been widely studied and a reliable list of the its molecular determinants is available in the most common databases of human genetic associations [19]. Yet, we comprehended cardiovascular diseases, diabetes and hypertension, whose comorbidity in COVID-19 patients is well documented [22, 23]. In addition, we included mosquito-borne infectious disease (i.e., malaria), other viral infections (i.e., HIV and Ebola), and immune disorders (i.e., rheumatoid arthritis), since drugs that have been authorized for their treatment (i.e., chloroquine/hydroxychloroquine, lopinavir/ritonavir, remdesivir, and tocilizumab, respectively) are being investigated worldwide for their potential to treat coronavirus disease (COVID-19) [7, 9, 24–30].
For what concerns drug-target associations, we assembled target information for a total of 1875 FDA-approved drugs. In addition, we considered in our input list of drug targets also the combination of remdesivir with other four antiviral agents, i.e. hydroxycloriquine, chloriquine, lopinavir, and ritonavir (referred as “5-cocktail”), as well as the combination of two of them, i.e. lopinavir and ritonavir (referred as “kaletra”), whose antiviral action against coronavirus infections has been demonstrated both in vitro and in vivo studies [31, 32]. In fact, despite significant progress in the COVID-19 management pointing to remdesivir as the most promising therapeutic candidate, the mortality remains high, indicating that treatment with an antiviral drug alone is not likely to be sufficient and combinations of antiviral agents should be evaluated by future strategies to improve patient outcomes in COVID-19 [10].
The complete lists of the analyzed diseases and drugs are provided in S1 Table.
The rationale behind SAveRUNNER algorithm lies on the hypothesis that, for a drug to be effective against a specific disease, its associated targets (drug module) and the disease-specific associated genes (disease module) should be nearby in the human interactome [14] (Fig 1B). To quantify the vicinity between drug and disease modules, SAveRUNNER implements a novel network similarity measure:
where p is the network proximity measure defined in [14]:
that represents the average shortest path length between drug targets t in the drug module T and the nearest disease genes s in the disease module S; QC is the quality cluster score; m is max(p); c and d are the steepness and the midpoint of f(p), respectively (see Design and Implementation).
The novelty of our approach resides in having implemented a procedure to prioritize the predicted off-label drug indications for a given disease (see Design and Implementation). This prioritization procedure exploits a clustering analysis to reward associations between drugs and diseases belonging to the same network cluster, based on the assumption that if a drug and a disease group together is more likely that the drug can be effectively repurposed for that disease. In this sense, we say that drugs and disease that are members of the same group (cluster), are more similar to each other than to members of other groups (clusters).
SAveRUNNER algorithm releases as output a weighted bipartite drug-disease network, in which one set of nodes corresponds to drugs and the other one corresponds to diseases. A link between a drug and a disease occurs if the corresponding drug targets and disease genes are nearby in the interactome (see Design and Implementation) and the weight of their interaction corresponds to the new defined network-based similarity measure. In this study, the final drug-disease network was composed of a total of 1682 nodes (i.e., 14 diseases associated to 1668 drugs) and 7177 edges.
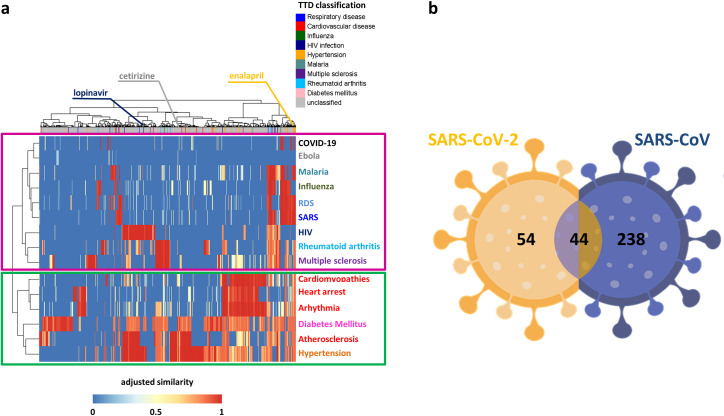
This drug-disease network was naturally rendered as a matrix reporting the 14 diseases on the rows, and the 1668 drugs on the columns (Fig 2), labeled with their original medical indication according to the Therapeutic Target Database (TTD). Each matrix cell was colored according to the corresponding similarity value of a given drug-disease pair: shades of red denote drug-associated targets more proximal (high similarity) to the disease-associated genes in the human interactome, whereas shades of blue denote drug targets more distal (low similarity) to the disease genes (Fig 2).
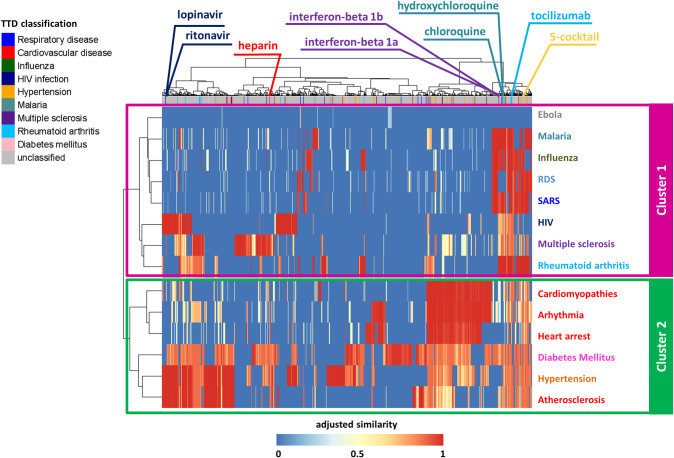
Fig 2. Dendrogram and heatmap of the drug-disease network.
The network adjusted similarity values are clustered according to rows (diseases) and columns (drugs) by a complete linkage hierarchical clustering algorithm and by using the Euclidean distance as distance metric. Heatmap color key denotes the adjusted and normalized network similarity between drug targets and disease genes in the human interactome, increasing from blue (less similar) to red (more similar). Drugs are colored according to the Therapeutic Target Database (TTD) indications listed in legend. Unclassified tag was assigned to those drugs for which a known therapeutic indication was not available in TTD or their indication does not fall in our analyzed disorders.
For elucidating drugs/diseases relatedness in terms of network similarity, we computed a hierarchical biclustering on the drug-disease similarity matrix. This analysis pointed out two main disease clusters: one including SARS, RDS, multiple sclerosis, rheumatoid arthritis, malaria, and viral infection diseases (magenta box in Fig 2); the other one including cardiovascular diseases and their risk factors, i.e., diabetes mellitus and hypertension (green box in Fig 2).
As proof of validity of SAveRUNNER, among the predicted drug-disease associations, we found several already known associations. For example, tocilizumab, interferon-beta 1a/b, chloroquine/ hydroxychloroquine, lopinavir/ritonavir, resulted to be more proximal to the diseases for which they were approved (i.e. rheumatoid arthritis, multiple sclerosis, malaria, and HIV infection, respectively).
SARS-CoV-host interactome
In total, we found 41 host proteins associated with SARS-CoV and their specific subnetwork within the human interactome is shown in Fig 3Aa.
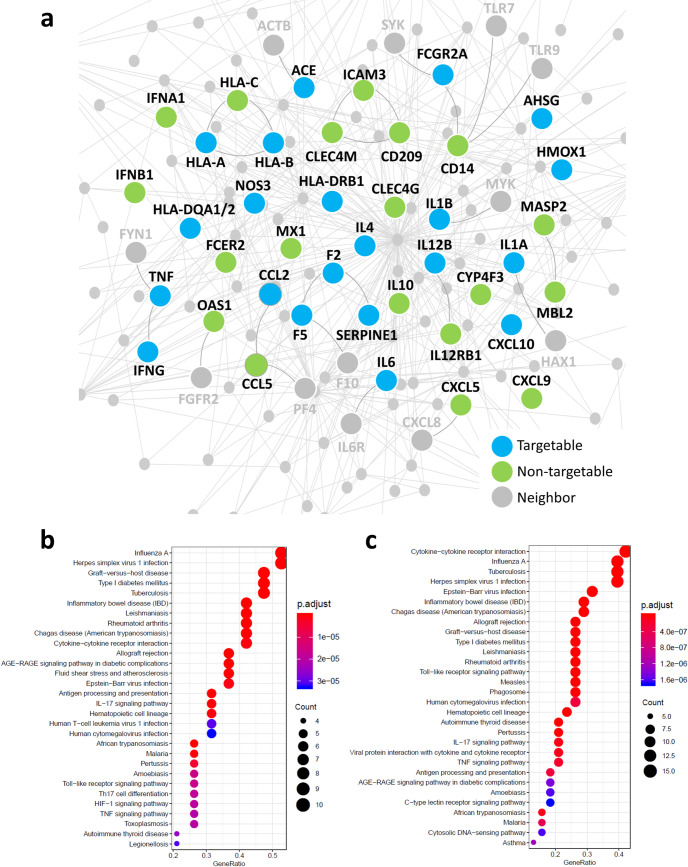
Fig 3. SARS-CoV-host interactome.
(A) The SARS-CoV-associated disease genes subnetwork in the human interactome. Light blue nodes represent SARS-CoV-associated proteins that can be directly targeted by at least one FDA-approved drugs (targetable); green nodes represent SARS-CoV-associated proteins that do not have any known ligands and then cannot be directly targeted by drugs (non-targetable); grey nodes represent interaction partners of SARS-CoV-associated proteins in the human interactome (neighbor). (B-C) KEGG human pathway enrichment analysis for SARS-CoV-associated disease genes. The dot plots of the top 30 enriched KEGG pathways (p-value ≤ 0.05) obtained for the 21 targetable SARS-CoV-associated disease genes (B) and for the total 41 SARS-CoV-associated disease genes (C). The y-axis reports the annotation categories (KEGG pathways) and the x-axis reports the gene ratio (i.e., the number of genes found enriched in each category over the number of total genes associated to that category). The color of the dots represents the adjusted p-values (FDR), whereas the size of the dots represents the number of genes found enriched in each category.
By mapping the known drug–target network into the SARS-CoV-host interactome, we revealed that 21 out of 41 (51%, light blue nodes in Fig 3A) SARS-CoV-associated disease genes are druggable cellular targets, i.e. can be directly targeted by at least one FDA-approved drug. For example, F2, TNF, FCGR2A, ACE, are the most targetable proteins. The KEGG pathway enrichment analysis revealed that this 21 targetable SARS-CoV-associated disease genes are involved in multiple significant (adjusted p-value ≤ 0.05) viral infection-related pathways, including TNF signaling pathway, Toll-like receptor signaling pathway, Cytokine-cytokine receptor interaction, IL-17 signaling pathway, Epstein-Barr virus infection, Inflammatory bowel disease, and Influenza (Fig 3B), mirroring the enrichment results found for the total 41 SARS-CoV-associated disease genes (Fig 3C).
The high druggability of SARS-CoV-host interactome and the relevance of the pathways in which the 21 druggable SARS-CoV-associated disease genes are involved strongly supports a drug repurposing strategy for potential treatment of COVID-19, by specifically targeting cellular proteins associated with SARS.
The remaining 20 non-targetable SARS-CoV-associated disease genes include proteins (e.g., CD14, IFNA1, IFNB1, IL4, IL10, CCL5) having key roles in the immune-inflammatory response to viral infection as well. This strongly supports a network-based view of drug action, according to which most disease phenotypes are difficult to reverse through the use of a single “magic bullet”, that is, an intervention that affects a single node in the network and encourages to inspect all the molecules that are situated in the neighborhood of the targetable proteins, leading to also detect possible side-effects [12].
SARS-CoV drug-disease network
In total, SAveRUNNER identified 282 repurposable drugs that were associated with the SARS-CoV infection (S2 Table). Fig 4A illustrates a sketch of SARS drug-disease network in which SARS is connected to the other analyzed diseases via the high-confidence predicted drugs (with their original medical indications) that could be repurposed for potential SARS fighting. This network is composed of two independent sets of nodes: one set corresponds to 13 diseases (i.e., SARS and the other 12 diseases sharing at least one drug with SARS) represented as red circles and whose size scales with their degree; the other one corresponds to the 66 FDA-approved non-SARS drugs for which known therapeutic indications were available from TTD or to the drugs combination proposed as new potential medical indication, colored accordingly. Two nodes of the two independent sets were connected whether drug targets and disease genes in the human interactome were more proximal than random expectation (z-score proximity ≤-1.65).
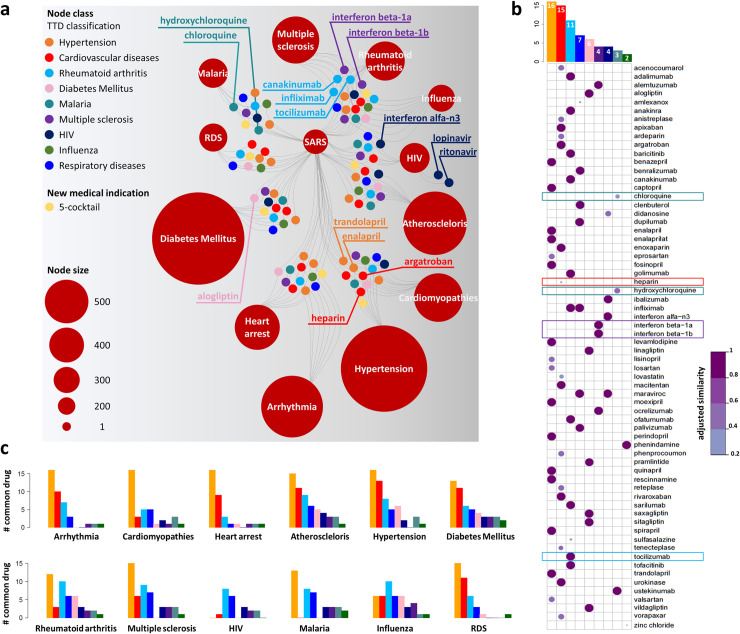
Fig 4. The predicted SARS drug-disease network.
(A) Schematic representation of the SARS predicted drug-disease network. This sketch shows the high-confidence predicted drug-disease associations connecting SARS and other analyzed diseases (red circles) with the 66 FDA-approved non-SARS drugs or the new proposed medical indication (i.e., 5-cocktail). Drugs are colored according to TTD classification reported in the legend, or according to the new proposed medical indication (i.e., 5-cocktail). The node size scales indicate the degree (connectivity) of nodes in the predicted drug-disease network. Labeled drug nodes represent either drugs more proximal to SARS or drugs being currently explored as COVID-19 treatment. (B) Similarity plot. Network-predicted repurposable drugs for SARS (along rows) with their TTD classification (along columns). In the plot, circles are scaled and colored according to the adjusted similarity measure, increasing from light purple (low similarity) to dark purple (high similarity). The barplot placed on the top reports the total number of candidate repurposable drugs for SARS grouped and colored according to the TTD classification reported in the legend (Node class). (C) Common drugs between SARS and other diseases. For each analyzed disease, the barplot reports the total number of drugs shared with SARS grouped and colored according to the TTD classification reported in the legend (Node class).
Looking at Fig 4A, it clearly emerges that there exist several candidate repurposable drugs for treatment of SARS. Among them, we pointed out the 5-cocktail (adjusted similarity = 0.89, z-score proximity = -1.80) that appeared as a promising candidate for targeting SARS showing a quite high network similarity.
In total, among the candidate repurposable drugs for SARS with an already known medical indication, we found 16 drugs indicated for hypertension treatment, including enalapril which is also the top potential anti-SARS-CoV repurposable drug (adjusted similarity = 0.99, z-score proximity = -11.84); 14 drugs for cardiovascular diseases, including heparin (adjusted similarity = 0.19, z-score proximity = -2.61); 11 drugs for rheumatoid arthritis, including tocilizumab (adjusted similarity = 0.99, z-score proximity = -3.22); 7 drugs for respiratory diseases; 6 drugs for diabetes mellitus; 4 drugs for multiple sclerosis, including interferon beta-1a (adjusted similarity = 0.99, z-score proximity = -4.79) and interferon beta-1b (adjusted similarity = 0.99, z-score proximity = -8.13); 4 drugs for HIV infection; 3 drugs for malaria, including chloroquine (adjusted similarity = 0.35, z-score proximity = -2.28) and hydroxycloriquine (adjusted similarity = 0.51, z-score proximity = -4.63); and 2 drugs for influenza (Fig 4B).
By analyzing the distribution of all the known medical indications associated to the drugs shared between SARS infection and each analyzed disease, hypertension- and cardiac-associated drugs appeared as highly frequent drugs, followed by rheumatoid arthritis- and respiratory disease-associated ones (Fig 4C). This findings appear in accordance with the fact that patients with SARS-CoV-2 infection also showed potential hypertension and cardiac injuries, including arrhythmia and myocardial dysfunction [22, 23, 33, 34]. This could be owed to the disruption of the subtle balance between angiotensin-converting enzyme 1 (ACE1) and angiotensin-converting enzyme 2 (ACE2), identified as functional receptor of both SARS-CoV/SARS-CoV-2 for host cell entry [35] and normally devoted to present various cardiovascular protective effects [33].
SARS-CoV-2 host interactome
In this study, we focused on SARS disease, which is caused by the coronavirus having the higher genetic similarity with SARS-CoV-2 [16, 21] and for which the specific disease genes are well-known. Conversely, given the novelty of the new coronavirus COVID-19 in the spectrum of human disease, the knowledge of the COVID-19-associated genes is far from completeness. However, a study has been recently published, where the authors identified 332 human proteins interacting with 26 SARS-CoV-2 proteins by using affinity purification mass spectrometry [36]. Although the authors verified that these proteins were preferentially highly expressed in lung tissue (typical environment where the virus causes a major damage), this study has been carried out on human HEK293T kidney cells that does not represent the primary physiological site of infection.
For a more generalizable understanding of human coronaviruses infection, we completed our analysis by applying SAveRUNNER on these the 332 human proteins preliminarily associated to SARS-CoV-2. The obtained results are encouraging and appears in accordance with the analysis carried out on SARS-CoV. Indeed, by performing a hierarchical clustering on the network of predicted drug-disease associations, we found COVID-19 in the same cluster of SARS, RDS, multiple sclerosis, rheumatoid arthritis, malaria, and viral infection diseases (Fig 5A). This is in accordance with recent studies that are attempting to repurpose, for COVID-19 treatment, drugs approved to treat other viral infections such as influenza, malaria, HIV, and Ebola or immune-related disorders, such as rheumatoid arthritis and multiple sclerosis [9, 24–26, 28, 29, 37].
Fig 5. Candidate repurposable drugs for SARS-CoV-2.
(A) Heatmap and dendrogram of SARS-CoV-2 drug-disease network. The network adjusted similarity values are clustered according to rows (diseases) and columns (drugs) by a complete linkage hierarchical clustering algorithm and by using the Euclidean distance as distance metric. Heatmap color key denotes the adjusted similarity between drug targets and disease genes in the human interactome, increasing from blue (less similar) to red (more similar). Drugs are colored according to the Therapeutic Target Database (TTD) indications listed in legend. Unclassified tag was assigned to those drugs for which a known therapeutic indication was not available in TTD or their indication does not fall in our analyzed disorders. (B) SARS-CoV-2 versus SARS-CoV. Venn diagram detailing the number of common and specific candidate repurposable drugs predicted by SAveRUNNER for SARS-CoV-2 and SARS-CoV infections.
The list of network-predicted drugs potentially able to treat SARS-CoV-2 infection contains a total of 98 drugs, including 54 (i.e., 55%) COVID-19 specific and 44 (i.e., 45%) in common with the 282 candidate repurposable drugs found for SARS-CoV (S2 Table and Fig 5B). The high number of repurposing candidates shared between SARS-CoV-2 an SARS-CoV is also reflected in the result of the greedy clustering algorithm implemented by SAveRUNNER, which places COVID-19 and SARS together in the cluster with the highest quality cluster score.
In a recent study [18], the authors exploited the new knowledge of SARS-CoV-2 host interactome [36] and integrated several network-based drug repurposing strategies to prioritize 81 promising repurposing candidates against COVID-19. In particular, they combined three predictive approaches exploiting the same drug-targets interactions from DrugBank and the same PPI network: 1) proximity-based methods that allowed to measure the distance between the viral protein targets and both (i) the targets of approved drugs and (ii) the differentially expressed genes induced by each drug; 2) diffusion-based methods to rank drugs based on the network similarity of their targets to COVID-19 protein targets; 3) machine learning methods relying on artificial intelligence network. These pipelines offered altogether twelve ranked lists that were merged using a rank aggregation algorithm in order to obtain a final list of 81 prioritized repurposable drugs.
The overlap between these 81 repurposable drugs and the 98 ones predicted by SAveRUNNER is of 5 drugs, i.e. isoniazid, lopinavir, romidepsin, sulfinpyrazone, tadalafil.
GSEA validation
In order to validate the repurposable drugs predicted by SAveRUNNER for SARS-CoV and SARS-CoV-2, we performed a gene set enrichment analysis (GSEA) as in [16], which investigated whether a drug could counteract the gene expression perturbations caused by a disease, i.e. if the drug could up-regulate genes down-regulated by the disease or viceversa.
In particular, we collected two gene expression datasets of hosts infected SARS-CoV available through the GEO public repository: transcriptome data of SARS-CoV-infected samples from patient’s peripheral blood (GSE1739) and Calu-3 cells (GSE33267). We computed the differentially expressed genes for each dataset, and we used them as SARS gene signatures. For what concerns COVID-19, we collected one dataset composed of 120 differentially expressed genes in SARS-CoV-2-infected A549 cells reported in [38] and we used it as COVID-19 gene signature.
The gene expression data of drug-treated human cell lines from the Connectivity Map (CMAP) database [39] were exploited to obtain drug signatures and thus to calculate a GSEA score for each drug as an indication of in-silico validation. We selected drugs with a score > 0 in order to focus on those drugs able to have a potential treatment effect on genes that are hallmark for that phenotype. The assigned GSEA score corresponds to the number of datasets in which the specific drug satisfies this criterion, ranging from 0 to 2 for SARS and from 0 to 1 for COVID-19.
For SARS, this analysis validated a total of 61 out the 282 candidate drugs to be repositioned against SARS infection, including 26 with a GSEA score of 2 and 35 with a GSEA score equal to 1 (S2 Table). For COVID-19, the GSEA analysis revealed that 24 out of 98 candidate drugs to be repositioned for COVID-19 treatment achieved the highest GSEA score. In particular, 14 of them, encompassing lopinavir, were included in the 54 specific-SARS-CoV-2 predicted drugs (S2 Table and Fig 5B).
Prediction of disease comorbidity
In order to predict potential comorbidity patterns among all the diseases analyzed in this study, for each disease module corresponding to the 15 analyzed disorders, we computed the non-Euclidean separation distance, which measures the modules’ overlap [40]:
where p(A, B) is the network proximity defined as:
and d(a, b) is the shortest distance between disease gene a of module A and disease gene b of module B. A positive value for the separation measure indicates that two disease modules are topologically well-separated in the human interactome, whereas a negative value for the separation measure indicates that two disease modules are located in the same network neighborhood and thus overlap. To evaluate the significance of the network separation measure across two disease modules (A, B), we built a reference distance distribution corresponding to the expected distance between two randomly selected groups of proteins of the same size and degree distribution as the original two sets of disease genes in the human interactome. The random selection was repeated 1,000 times in order to build the reference distance distribution. The module separation measure across each pair of lists of disease genes was z-score-normalized by using the mean and the standard deviation of the reference distribution. Subsequently, the p-value for the given z statistic was calculated. A p-value < 0.05 indicates that the separation in the human interactome of the two lists of disease genes is larger (or lower) than expected by chance.
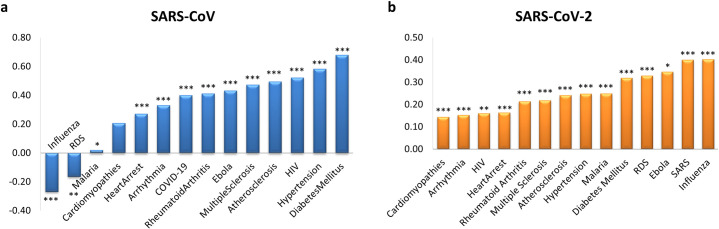
We found that SARS-CoV disease module directly overlapped in a statistically significant way with RDS and Influenza disease modules (s < 0, p-value < 0.05) and appeared to be closest to Malaria (smallest s, p-value < 0.05) (Fig 6A and S3 Table). On the other hand, by using SARS-CoV-2 disease genes, we found that the COVID-19 disease module did not directly overlap with any disease module here analyzed (Fig 6B and S3 Table). However, among the closest diseases (smallest s, p-value < 0.05), we found cardiovascular diseases (i.e., cardiomyopathies, arrhythmia, heart arrest), whose comorbidity in COVID-19 patients has been already discussed [22, 23].
Fig 6. Disease comorbidity.
The bar plots show the values of the non-Euclidean separation distance computed for the SARS-CoV (a) and SARS-CoV-2 (b) disease module with respect to all the other disease modules analyzed in this study. We applied a degree-preserving randomization procedure to assess the statistical significance of each separation value, and we calculated all p-values by applying a two-tailed z test. The stars flag levels of significance for three of the most commonly used levels: p-value < 0.05 is flagged with one star (*); p-value < 0.01 is flagged with two stars (**); and p-value < 0.001 is flagged with three stars (***).
Comparison with other methods
In the last few years, several computational network-based methods have been proposed to predict direct drug–disease associations for drug repositioning [6, 41–45]. Among them, the MBiRW algorithm adopts adopted an effective mechanism to measure similarity for drugs and diseases and applied a Bi-Random walk (BiRW) algorithm to predict potential new indications for existing drugs [45]. This methodology has been shown to outperform other well-known network-based prediction methods [15, 46, 47] in correctly predicting true drug–disease associations. These captivating results prompted us to implement a BiRW-based algorithm (see Design and Implementation) against which we compared the performance of SAveRUNNER.
The effectiveness of the drug–disease predictions provided by BiRW and SAveRUNNER were evaluated and compared in terms of Receiver Operating Characteristic (ROC) probability curves with their corresponding Area Under the Curve (AUC) (see Design and Implementation). In particular, we found that SAveRUNNER yielded over 70% accuracy (AUC = 0.73) for identifying well-known drug-disease relationships and overcame the one obtained by the BiRW-based algorithm (AUC = 0.59). In other words, there is 73% chance that SAveRUNNER algorithm will be able to distinguish between positive class (known drug-disease associations) and negative class (unknown drug-disease associations) against the 59% of the BiRW-based algorithm.
Discussion
Ongoing clinical trials for the management of COVID-19
Thus far, no proven effective therapies for the novel coronavirus disease COVID-19 exist and the majority of available data are based on expert opinions and anecdotal experiences [48]. Old and new agents have been proposed and explored for treatment of COVID-19 [49], but clinical trials are still underway. Currently, 324 clinical trials specific to COVID-19 are present on ClinicalTrials.gov (recruitment status: active, not recruiting, completed, terminated). We will next discuss some of the most popular ongoing clinical trials for the management of the COVID-19 pandemic [7, 9, 27, 50–58].
Remdesevir is a novel nucleotide analogue currently under evaluation in clinical trials for Ebola infection and it has shown an excellent activity against early coronavirus infections (SARS, MERS) both in vitro and in animal models. Recently, Grein J et al. [55] provided remdesivir on a compassionate-use basis to patients hospitalized with COVID-19. In this cohort of patients, clinical improvement was observed in 36 of 53 patients (68%). Although remdesivir has not yet been approved by US Food and Drug Administration, a preliminary report published on May 22, shows that it was superior to placebo in shortening the time to recovery in adults with COVID-19 and evidence of lower respiratory tract infection [10]. On October 22, remdesivir becomes the first COVID-19 treatment to receive FDA approval [56]. It is indicated for use in adult and pediatric patients aged 12 years and over weighing at least 40 kilograms with COVID-19 severe enough to require hospitalization [59]. The approval of remdesivir was supported by the agency’s analysis of data from three randomized, controlled clinical trials that included patients hospitalized with mild-to-severe COVID-19. This approval does not include the entire population that had been authorized to use remdesivir under an emergency-use authorization originally issued on May 1, 2020. Pediatric patients weighing between 3.5 and 40 kg remain covered under a revised emergency-use authorization, where clinical trials assessing the safety and efficacy of remdesivir in this pediatric patient population are ongoing.
Chloroquine is an old and widely used anti-malarial drug and it is also efficacious as an anti-inflammatory agent for rheumatologic disease. Earlier studies have demonstrated a potential antiviral effect of chloroquine that may depend by several mechanisms such as the change of cell membrane pH, which is necessary for viral fusion and the interference with glycosylation of viral proteins. A recent study has demonstrated in vitro efficacy of chloroquine and remdesivir in inhibiting replication of SARS-CoV-2 [9]. Moreover, emerging reports from China suggest that chloroquine has shown a superiority in reducing both the severity and the duration of clinical disease without significant adverse events in almost one hundred patients [26, 50, 51]. In light of this results, an expert consensus group in China has recommended chloroquine for COVID-19 treatment [26]. Hydroxychloroquine (brand name plaquenil) is an analogue of chloroquine, which was demonstrated to be much less toxic than chloroquine in animals and with similar in vitro efficacy on SARS-CoV-2 [25, 27]. However, the effectiveness of chloroquine and hydroxychloroquine remains controversial, with clinical evidence showing contrasting results. Some clinical trials reported therapeutic benefits of the drugs, while others trials showed the opposite, and others ones are still ongoing [60]. While there is growing studies to test therapeutic effect, there is also concern for toxicity and adverse effects of these medications, and thus FDA revoked the emergency-use authorization that allowed for chloroquine phosphate and hydroxychloroquine to be used to treat certain hospitalized patients with COVID-19 when a clinical trial was unavailable [61]. These results prompted the need for larger controlled clinical trials to offer guidance on effective and safe dosing of those drugs.
Lopinavir/ritonavir (brand name kaletra) is a well-known protease inhibitor, which has been widely used for many years for the treatment of HIV infection. Compared to remdesivir, lopinavir/ritonavir has the advantage that it is widely available and has an established toxicity and drug-drug interactions profile. Its antiviral action against coronavirus infections has been previously demonstrated both in vitro and in vivo (animal and human data) studies conducted on SARS [31, 32]. Recently Cao et al. [7] conducted a randomized, controlled, open-label trial involving hospitalized adult patients with confirmed SARS-CoV-2 infection. A total of 199 patients with laboratory-confirmed SARS-CoV-2 infection underwent randomization; 99 were assigned to the lopinavir/ritonavir group, and 100 to the standard-care group. Unfortunately, the trial results were disappointing and no benefit was observed with lopinavir/ritonavir treatment in hospitalized adult patients with severe COVID-19. On October 15, interim results of the World Health Organization’s solidarity trial suggested no benefit on hospitalization duration, initiation of ventilation, or mortality for repurposed antiviral drugs for the treatment of COVID-19, including hydroxychloroquine, lopinavir/ritonavir and interferon-β1a [62]. Nevertheless, clinical evidence about the effectiveness of lopinavir/ritonavir is still limited and controversial. It is currently under investigation within other randomized clinical trials and the results of such trials will provide convincing positive or negative findings on this therapy.
In absence of proven antivirals for COVID-19, adjunctive therapies of support represent the cornerstone of care. In particular: anticytokine drugs, corticosteroids, low molecular weight heparin (LMWH), and immunoglobulin therapy. Monoclonal antibodies directed against key inflammatory cytokines or other aspects of the innate immune response represent another potential class of adjunctive therapies for COVID-19.
One of the most promising monoclonal antibodies under investigation for the management of COVID-19 is tocilizumab (TCZ). Specifically, TCZ is an anti-human IL-6 receptor monoclonal antibody that inhibits signal transduction by binding sIL- 6R and mIL-6R. Currently, TCZ is licensed for the treatment of adult patients with moderately to severely active rheumatoid arthritis, but several studies in China showed a possible correlation of massive inflammation and severe lung damage on the rapid evolution of fatal pneumonia. Indeed, in COVID-19 patients, significant differences in IL-6 plasmatic levels were observed at different stage of disease with a higher expression in severe cases [37]. Despite the lack of clinical trials on TCZ efficacy and safety for COVID-19 treatment, “off-label” TCZ has been used as potential treatment strategy in severe and critical COVID-19 patients. Currently, in Italy a multicenter study on the efficacy and tolerability of tocilizumab in the treatment of patients with COVID-19 pneumonia is ongoing [57]. Moreover, Hoffmann-La Roche promoted a clinical trial to test efficacy, safety, pharmacodynamics, and pharmacokinetics of TCZ compared with a matching placebo in combination with standard of care in hospitalized patients with severe COVID-19 pneumonia and it is currently in Phase 3 [58].
Another remarkable candidate for the treatment of COVID-19 is LMWH, with the aim to improve the coagulation dysfunction of COVID-19 [63, 64]. Really, during the course of SARS-CoV-2 infection, an increased incidence of acute pulmonary embolism episodes was detected. These were often COVID-19 patients with risk factors for embolism, increased D-dimer and deterioration of general conditions and respiratory failure [63, 65]. Recently, Tang N. et al. [52] showed that anticoagulant therapy mainly with LMWH appears to be associated with better prognosis in severe COVID-19 patients meeting criteria of the sepsis-induced coagulopathy score or with markedly elevated D-dimer. However, its efficacy remains to be validated in large clinical trials. Interestingly, LMWH seems exert anti-inflammatory effects by reducing IL-6 and increasing lymphocyte %, so that the potential of LMWH could be to mitigate cytokine storm in severe COVID-19 patients.
To complete the clinical landscape of potential adjunctive therapy for COVID-19, we mention also the use of convalescent plasma or hyperimmune immunoglobulins obtained from recovered patients [66]. The rationale for this treatment is that antibodies from recovered patients may help the immune clearance of SARS-CoV-2.
In conclusion, several drugs demonstrate in vitro activity against SARS-CoV-2. Of these, several repurposed agents used to treat a variety of other diseases (malaria, HIV, rheumatoid arthritis) have been proposed as possible cure options for COVID-19. Lopinavir/ritonavir and chloroquine or hydroxychloroquine are the treatments with the most clinical evidence, either positive or negative, in the treatment of COVID-19. Currently, randomized clinical trials have not proven that any of these drugs are undoubtedly effective and safe.
In-silico drug predictions for the management of COVID-19
In this study, we developed a novel network-based algorithm for drug repurposing, called SAveRUNNER, with the aim to offer a promising framework to efficiently screen potential novel indications for currently marketed drugs against COVID-19.
Our findings, in accordance with several recent works [14, 16, 67], suggested that the discovery of efficacious repurposable drugs (or drug combinations) could benefit from the exploration of the relationship between drug targets and the disease genes in the human interactome. The novelty of SAveRUNNER relies on the definition of a new network-based similarity measure, which quantifies the vicinity between drug and disease modules and considers the drug-disease network modular structure to reward predicted associations between drugs and diseases that are located in the same network neighborhoods.
Focusing on SARS disease, which is caused by the coronavirus having the higher genetic similarity with SARS-CoV-2 [16, 21] and for which the specific disease genes are known, we identified 282 candidate repurposable drugs. Among them, we recovered some of the most rumored off-label drugs, like chloroquine, hydroxycloriquine, tocilizumab, and heparin. While lopinavir/ritonavir and remdesivir were not found to be associated with SARS, their combination together with chloroquine and hydroxycloriquine (here referred as 5-cocktail) was predicted as a promising anti-SARS-CoV repurposable drug.
Although all the 282 repurposable drugs predicted by SAveRUNNER warrant to be explored, a prioritization of these drugs according to the decreasing value of their network similarity value with SARS may offer the possibility to maximize the efficiency of subsequent experimental screening and clinical trial validation. This does not mean that drugs located at a lower-ranking have no potential efficacy or must be a priori excluded from further exploration.
Thus, we found that the 95th percentile of the similarity values distribution includes ACE-inhibitors (i.e., enalapril, trandolapril, fosinopril, benazepril, cilazapril, zofenopril, spirapril, rescinnamine, quinapril), thrombin inhibitors (i.e., thrombomodulin alfa, bivalirudin, dabigatran etexilate, argatroban, ximelagatran), and several monoclonal antibodies like: anti-TNFα (i.e., adalimumab, golimumab, infliximab, certolizumab pegol), anti-IFNγ (i.e., emapalumab), anti-IL1β (i.e., canakinumab), and anti-IL6 (i.e., siltuximab).
These findings are in accordance with the current therapeutic avenues tentatively proposed for fighting COVID-19, even if, for some of them, the effectiveness in treating COVID-19 remains controversial. Indeed, several recent studies hypothesized that COVID-19 patients receiving ACE-inhibitors may be subject to poorer outcomes [68, 69], whereas other investigators argued that the usage of ACE-inhibitors could be beneficial in COVID-19 infection [70]. On the contrary, the potential efficacy of the other top-ranked predicted off-label drugs seems to be less uncertain. Indeed, several evidences suggested that COVID-19 may predispose patients to arterial and venous thrombotic disease and then common antithrombotic medications, including the already mentioned heparin or direct thrombin inhibitors such as dabigatran, have been proposed as potential adjunctive therapy against COVID-19 [71]. Yet, monoclonal antibodies targeting key inflammatory cytokines or other aspects of the innate immune response are increasingly recognized as another promising class of anti-COVID-19 drugs. In particular, the class of anti-TNFα antibodies, mostly used for the treatment of inflammatory rheumatic diseases, could be able to challenge COVID-19 by two main actions: the classical TNFα inhibition and a down-regulation of ACE2 expression resulting in decreased binding sites for SARS-CoV-2 [72, 73]. Thus, anti-TNFα antibodies, and adalimumab in particular thanks to its excellent safety profile [74], could inhibit the basic mechanisms of COVID-19, and could be potentially useful in managing/preventing COVID-19-driven pneumonia[75]. As proof of this, a study evaluating adalimumab injection in COVID-19 patients has recently been registered in order to assess the role of this antibody in treating COVID-19 patients with severe pneumonia [76].
In-silico validation of SAveRUNNER predictions
As indication of in-silico validation of the 282 candidate repurposable drugs predicted by SAveRUNNER, we performed a GSEA analysis that allowed to investigate whether these repositioning candidates have potential treatment effect against SARS-CoV infection. The GSEA analysis validated a total of 61 out the 282 anti-SARS repurposable drugs. Among the drugs achieving highest GSEA scores, we found some of the ACE-inhibitors above-mentioned (i.e., enalapril, benazepril, quinapril, fosinopril, rescinnamine) as well as ACE-inhibitors with multiple targets (i.e., perindopril, captopril, moexipril, ramipril), and chloroquine.
In addition, this analysis came up other interesting but less obvious drugs falling far outside antiviral use such as ruxolitinib, lovastatin, and a group of H1-antihistamines (i.e., loratadine, fexofenadine, levocetirizine, desloratadine, clemastine, ketotifen, diphenhydramine, cetirizine).
Ruxolitinib is a potent and selective Janus-Associated Kinase (JAK) inhibitor approved for treatment of myelofibrosis, but thanks to its powerful anti-inflammatory effect, it could be likely to be effective against the consequences of the elevated levels of cytokines typically observed in patients with COVID-19 [77, 78].
Lovastatin is widely prescribed to reduce the levels cholesterol in the blood and prevent cardiovascular diseases. Experimental evidence suggested that statin-induced reduction of cholesterol in the plasma membrane results in lower viral titers and failure to internalize the virus [79].
H1-antihistamines are mostly used to treat allergic reactions, but they potentially could have beneficial anti-inflammatory effects on immune dysregulation during COVID 19 infection. Indeed, histamine is a biologically active substance that potentiates the inflammatory and immune responses of the body, regulates physiological function in the gut, and acts as a neurotransmitter.
Notably, among the repurposable drugs against SARS-CoV and SARS-CoV-2 both predicted by SAveRUNNER and validated by GSEA analysis, we found 10 common off-label drugs, encompassing ACE inhibitors (i.e., enalapril, and quinapril) and antihistamines (i.e., cetirizine and fexofenadine). Moreover, among repurposable drugs validated by the GSEA analysis as potential treatment effect against SARS-CoV-2 infection, we found the protease inhibitor lopinavir.
Drugs’ likely mechanism-of-action in SARS
Moving beyond the “one drug, one target” vision prompted us to explore all the molecules that are situated in the interactome neighborhood of the drugs targetable proteins, which may be altered by the drug activity as well as may cause side-effects (Fig 7).
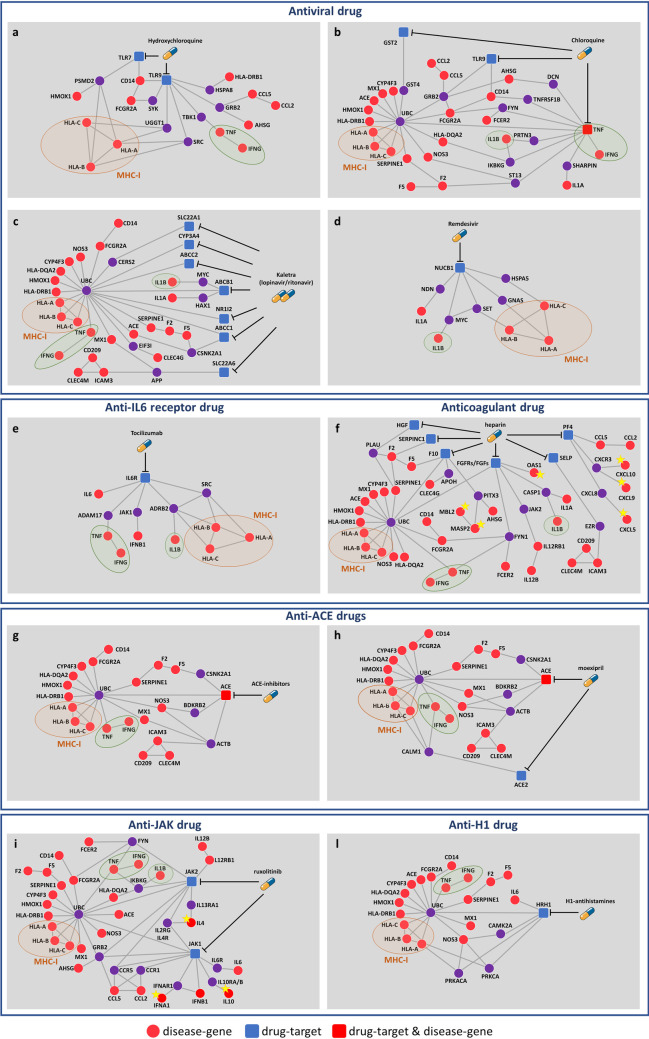
Fig 7. Mechanism-of-action of anti-SARS-CoV repurposable drugs.
The subnetworks show the inferred mechanism-of-action for: antiviral drugs (A-D), tocilizumab (E), heparin (F), ACE-inhibitors (G-H), ruxolitinib (I), and H1-antistamines (L). Each subnetwork was designed to point out the shortest paths from drug targets and SARS disease genes in the human interactome. In each subnetwork, disease genes specifically targeted by each drug are marked with a yellow star; major histocompatibility complex of class I (MCH-I) and pro-inflammatory cytokines shared by all drug networks are marked with an orange and a green circle, respectively. Legend: red circles refer to SARS disease genes, blue squares refer to drug targets, red squares refer to SARS disease genes that are also drug targets, violet circles refer to the first nearest neighbors (that are not disease genes) of the drug targets in the human interactome. Anti-ACE drugs of panel (G) refer to enalapril, trandolapril, fosinopril, benazepril, cilazapril, zofenopril, spirapril, rescinnamine, and quinapril. H1-anistamines of panel (L) refer to fexofenadine, levocetirizine, desloratadine, clemastine, cetirizine.
We interestingly observe that all drug subnetworks include genes involved in the immune-inflammatory response and share individual-specific genetic factors (i.e., HLA-A, HLA-B, HLA-C), as well as some crucial pro-inflammatory cytokines (i.e., IFN-γ, IL-1β, TNF). The HLA genes encodes for the proteins of the major histocompatibility complex of class I (MHC-I), that is directly involved in the antigen presentation process. The cytokines IFN-γ, IL-1β, and TNF have a critical role in promoting inflammation [80, 81] and several studies demonstrated as their suppression may lead to therapeutic effects in many inflammatory diseases, including viral infections [75, 82–84]. An overwhelming production of these pro-inflammatory cytokines indeed contributes to a hyper-inflammatory condition, denoted as cytokine storm, which destroys the normal regulation of the immune response and may induce pathological inflammatory disorders [84–86].
Some drugs are characterized by highly complex interaction networks, whereas others, like the case of remdesivir, seem to have a poorly connected substructure. However, it is worth noting that the observed network complexity does not scale with the potential drug effectiveness. The lack of interactions may be likely ascribable both to the current incompleteness of the human interactome and to the shortage of drug-target information. As in the case of remdesivir, which being designed to target specifically virus proteins, little is known about its human targetable proteins, leading to a less wired subgraph.
This is not the case of the heparin subnetwork (Fig 7F), where the highly complexity in its interaction network perfectly matches its versatile role of anticoagulant, anti-inflammatory, and antiviral drug turning out in a strong multifactorial impact in the new human coronavirus [54]. In fact, the heparin subnetwork includes: disease genes associated to the complement and coagulation cascades pathway (MASP2, MBL2), confirming its primary anticoagulant function; pro-inflammatory cytokines (IL12B and its receptor IL12RB1) and chemokines (CXCL5, CXCL9, CXCL10), supporting its anti-inflammatory function; all the disease genes of the antiviral drugs subnetworks (Fig 7A–7D), linking to its antiviral action.
Yet, the networks of JAK inhibitors such as ruxolitinib (Fig 7I) and of the H1-antihistamines (Fig 7L) strongly confirm their anti-inflammatory effect, showing common and specific key players of the inflammatory response. In particular, ruxolitinib subnetwork includes several pro-inflammatory cytokines (IFN-α, IFN-γ, IL-1β, IL-6, IL-12, TNF-α), chemokines (CCL2, CCL5), and other important specific interleukines (IL-10, IL-4); whereas the H1-antihistamines network shows how the drug may control the level of the interleukin IL-6, directly linked to the histamine receptor H1 (HRH1).
Design and implementation
Human protein–protein interactome
The human protein–protein interactome was downloaded from the Supplementary Data of [14], where the authors merged their inhouse systematic human protein–protein interactome and 15 commonly used databases with several types of experimental evidences (e.g., binary PPIs from three-dimensional protein structures; literature-curated PPIs identified by affinity purification followed by mass spectrometry, Y2H, and/ or literature-derived low-throughput experiments; signaling networks from literature-derived low-throughput experiments; kinase-substrate interactions from literature-derived low-throughput and high-throughput experiments). This updated version of the human interactome is composed of 217,160 protein–protein interactions (edges or links) connecting 15,970 unique proteins (nodes).
Disease-gene associations
Disease-associated genes were downloaded from Phenopedia [19], which provides a disease-centered view of genetic association studies collecting by the online Human Genome Epidemiology (HuGE) encyclopedia [87]. The updated version of Phenopedia (released 27-04-2020) collects gene associations for 3,255 diseases. Among them, we selected a panel of 14 diseases of interest with their associated genes (S1 Table).
Drug-target interactions and drug medical indications
Drug-target interactions were acquired from DrugBank [20], which is a comprehensive, freely accessible, online database containing information on drugs and drug targets. The updated version of DrugBank (version 5.1.6, released 22-04-2020) contains 13,563 drug entries including 2,627 approved small molecule drugs, 1,373 approved biologics (proteins, peptides, vaccines, and allergenics), 131 nutraceuticals, and over 6,370 experimental drugs. For our analysis, we selected a total of 1,875 FDA-approved drugs with at least one annotated target. The target Uniprot IDs were mapped to Entrez gene IDs by using BioMart–Ensembl tool (https://www.ensembl.org/). Note that, for some drugs of interest for which no targets were found in DrugBank, we integrated drug-target interactions available from Therapeutic Target Database (TTD) [88] and Pharmacogenomics Knowledgebase (PharmGKB) [89] database. In particular, for remdesivir (an anti-virus drug designed to target specifically virus proteins), we extracted its human target information from TTD.
The known drug medical indications were obtained from TTD [88], whose last version was released on 11 Nov 2019.
SAveRUNNER algorithm
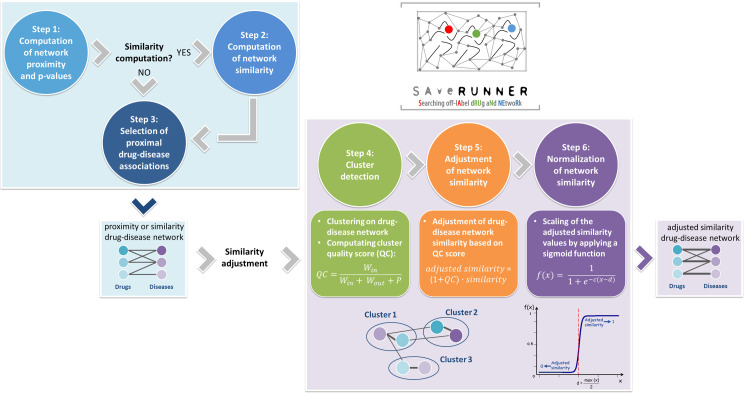
SAveRUNNER (Searching off-lAbel dRUg aNd NEtwoRk) is a network-based algorithm for drug repurposing that, taking as input a list of drug targets and disease genes, constructs a drug-disease network with predicted drug-disease associations by performing the steps that will be next discussed (Fig 8).
Fig 8. SAveRUNNER algorithm.
SAveRUNNER encompasses six steps: (1–3) compute a weighted bipartite drug-disease network, where nodes are both drugs and diseases, edges are proximal drug-disease associations (z-score proximity ≤ selected threshold), and weights are either the proximity or similarity measure; (4–6) compute the normalized adjusted similarity measure to correct the weights of the drug-disease network and to prioritize the predicted drug-disease associations. Legend: QC is the quality cluster score; Win is the total weight of edges within each cluster; Wout is the total weight of edges connecting each cluster to the rest of network; P is the node density within each cluster; c and d parameters are the sigmoid steepness and midpoint, respectively.
Step 1: Computation of network proximity
In order to investigate the extent to which disease and drug modules are close in the human interactome, SAveRUNNER implements the network-based proximity measure defined as [14]:
which is the average shortest path length between drug targets t in the drug module T and the nearest disease genes s in the disease module S (Fig 1B). The observe network proximity was z-score normalized by applying a degree-preserving randomization procedure, expecting a (z ≤ -1.65) for proximal drug and disease modules. To calculate the z-score proximity between the two modules T and S, SAveRUNNER builds a reference distance distribution corresponding to the expected distance between two randomly selected groups of proteins with the same size and degree distribution of the original sets of disease proteins and drug targets in the human interactome. This procedure is repeated 1,000 times and the z statistics is computed by using the mean and the standard deviation of the reference distance distribution. This randomization procedure allows to preserve the biological meaning of the network connections between any pair of nodes [14, 18].
Step 2: Computation of network similarity
The network proximity measure is then translated in a similarity measure assuming values in the range [0–1]:
where null similarity means that the corresponding disease and drug modules are very distal in the human interactome (i.e., p is maximum); whereas maximum similarity means that the corresponding disease and drug modules are very proximal in the human interactome (i.e., p equal to zero).
Step 3: Selection of proximal drug-disease associations
In order to prioritize drug-disease associations, a threshold on the proximity z-score is set. It means that, given a disease A and a drug b, if their z-score proximity is smaller of the chosen threshold the probability that the off-label drug b would be effective for this disease A is greater than expected by chance. In our analysis, predicted drug-disease associations with a z-score ≤ -1.65 were selected.
Step 4: Cluster detection
Next, SAveRUNNER performs a cluster analysis to detect groups of drugs and diseases in such a way that members in the same group (cluster) are more similar to each other than to those in other groups (clusters). To do that, SAveRUNNER exploits a cluster detection algorithm based on the greedy optimization of the network modularity [90]. Specifically, modularity measures the strength of network division into clusters, i.e. networks with high modularity have dense connections between the nodes within clusters but sparse connections between nodes in different clusters. The greedy clustering algorithm was adopted in view of its good performance and high speed in detecting community structure from very large networks [90–92]. The quality of each cluster is evaluated by SAveRUNNER by computing the following quality cluster (QC) score:
where Win denotes the total weight of edges within the cluster, Wout denotes the total weight of edges connecting this cluster to the rest of network, and P is a penalty term which considers the cluster size variances. Specifically, the penalty term P is the fraction of nodes within each cluster. Since each data point has been classified by the greedy clustering algorithm, the term P penalizes those clusters that are too large and could be poorly defined.
Step 5: Adjustment of network similarity
The quality cluster score serves to reward associations between drugs and diseases belonging to the same cluster, based on the assumption that if a drug and a disease group together is more likely that the drug can be effectively repurposed for that disease. In this sense, we say that drug and the disease that are members of the same cluster tend to be “more similar” and this translates into the following adjustment for the similarity:
| (1) |
Thus, whether two nodes fall in the same cluster their similarity value increases by a factor proportional to the QC score of the cluster which they belong; otherwise whether two nodes do not fall in the same cluster QC is set to zero and their similarity value does not change.
Step 6: Normalization of network similarity
To bound the similarity measure defined in Eq 1 to values that monotonically increase from 0 to 1, SAveRUNNER performed a normalization procedure by applying the following sigmoid function:
where x is the adjusted similarity measure (Eq 1), d is the sigmoid midpoint (i.e., the value at which the function approaches to 0.5), c is the sigmoid steepness. We set and c = 10.
At the end of this step, SAveRUNNER offers a list of predicted/prioritized associations between drugs and diseases as a weighted bipartite drug-disease network, in which one set of nodes corresponds to drugs and the other one corresponds to diseases. A link between a drug and a disease occurs if the corresponding drug targets and disease genes were more proximal than random expectation (z-score proximity ≤ -1.65) and the weight of their interaction corresponds to the adjusted and normalized similarity value.
Performance evaluation
The effectiveness of predicted drug–disease associations provided by the SAveRUNNER algorithm was evaluated in terms of the Receiver Operating Characteristic (ROC) probability curve analysis. The drug–disease associations were ranked according to increasing z-score proximity values and a “real association” was assigned according to TTD information: 1 if the predicted drug-disease association is known, 0 otherwise. For a specified z-score threshold, the true positive rate (i.e., sensitivity) was calculated as the fraction of known associations that are correctly predicted, while the false positive rate (i.e., 1-specificity) was computed as the fraction of unknown associations that are predicted. The ROC probability curve was drawn based on these measures at different thresholds and the corresponding Area Under the Curve (AUC) was computed. Higher the AUC, better the algorithm is at distinguishing between two classes (i.e., known drug-disease associations versus unknown drug-disease associations).
Bi-Random walk-based algorithm
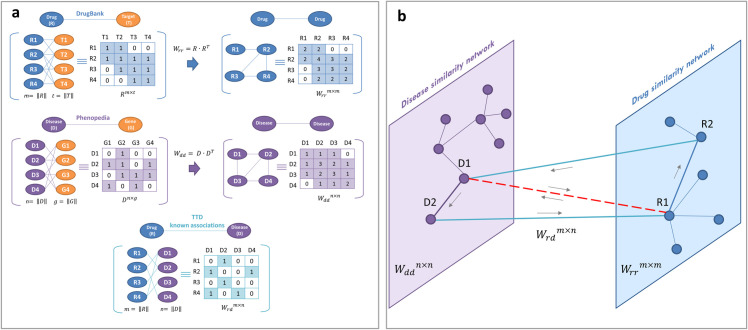
Inspired by an already existing algorithm of drug repurposing called MBiRW [45], we exploited a Bi-Random walk-based (BiRW) approach to infer potential reuse for existing drugs. We started from the known drug–disease associations available on the TTD [19] and iteratively we added new associations integrating information retrieved from DrugBank [20], for what concerns targets of already approved drugs, and from Phenopedia [19], for what concerns disease genes. Denoting with m the total number of the approved drugs and n the total number of diseases, the known drug-disease associations were translated into a binary matrix by assigning 1 if the given drug (matrix row) is associated to a given disease (matrix column), and 0 otherwise. Next, a weighted adjacency matrix was computed to model drug similarity, where weights are the number of common targets between any pair of drugs. Further, a weighted adjacency matrix was computed to model disease similarity, where weights are the number of common disease genes between any pair of diseases (Fig 9A).
Fig 9. BiRW-based algorithm.
Schematic representation of the construction of input matrices (A) and predicted drug-disease associations network (B).
In analogy with [45], the matrices Wrr and Wdd were adjusted based on the known drug–disease associations. The underlying hypothesis is that drug (disease) pairs, whose similarity value is greater of what expected by chance, are more likely to share common diseases (drugs).
From the adjusted matrix Wrr, a weighted drug similarity network was built, in which nodes are the approved drugs and an edge occurs between two nodes if they share at least one target gene, with a weight given by the corresponding element of the matrix Wrr. Likewise, from the adjusted matrix Wdd, a weighted disease similarity network was built, in which nodes are the diseases, and an edge occurs between two diseases if they share at least one disease gene, with a weight given by the corresponding element of the matrix Wdd.
Next, new drug–disease associations were predicted through an iterative random walk process on drug and disease similarity networks, simultaneously. Denoting with the parameters l and r the walks in the drug network (left walks) and in the disease network (right walks), the bi-random walk can be described by the following equations:
where A0 denotes the drug-disease association at t = 0 (matrix Wrd), while and represent the predicted drug–disease associations at iteration t starting from left or right, respectively. The hypothesis behind is that an association between a drug R1 and a disease D1 can be added following these two avenues (Fig 9B):
a random walker starts from a random vertex R1 of the drug similarity network and in each step walks to one of the neighboring vertices (for instance R2, with a known or previously predicted association with D1) with a probability proportional to the weight of the edge traversed (i.e., the corresponding element of Wrr)
a random walker starts from a random vertex D1 of the disease similarity network and in each step walks to one of the neighboring vertices (for instance D2, with a known or previously predicted association with R1) with a probability proportional to the weight of the edge traversed (i.e., the corresponding element of Wdd)
In both cases, the existence of a known association between a drug R1 and a disease D1 has been considered with a probability (1−α). Then, in each step of the iteration process, the predicted drug–disease associations matrix At is given by the mean between and .
Finally, a bipartite drug-disease network was constructed consisting of two sets of nodes: one set corresponding to all disease, the other set corresponding to all approved drugs. An edge between a drug and a disease occurs if an association is already known or has been predicted among them.
Tenfold cross-validation
The performance of BiRW-based algorithm in predicting new drug–disease associations was evaluated through a tenfold cross validation [45]. This is a technique to investigate the predictive power of an algorithm by partitioning the original sample into a training and test set. In particular, the tenfold cross-validation consists of randomly partitioning the original sample (in our case the drug-disease associations) into 10 equal size subsamples. Of the 10 subsamples, a single subsample is retained as test set and the remaining 9 subsamples are used as training set. The cross-validation process is then repeated for 10 iterations so that each of the 10 subsamples is used exactly once as the test set.
The results of each iteration were evaluated in terms of ROC probability curves. In particular, each time, the predicted drug-disease associations were ranked according to the similarity score estimated by the BiRW-based algorithm (i.e., the corresponding element of matrix At) and the N top-ranked drug-disease associations were selected to be evaluated by the ROC curve analysis, where N is the length of the test set. It’s worth to stress that, in each cross-validation trial, we didn’t use the information about the known drug–disease associations for the test set that were put to zero at first iteration of the bi-random walk process. Then, the ROC curve is constructed for different values of a specified threshold, where a true drug–disease association was considered as correctly predicted if the estimated similarity score of this association was higher than the specified threshold. The results of the ROC curve analysis obtained for each iteration were averaged to obtain a mean ROC curve and the corresponding Area Under the Curve (AUC) was calculated. Higher the AUC, better the algorithm is at distinguishing between two classes (i.e., known drug-disease associations versus unknown drug-disease associations).
Functional enrichment analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis aiming to evaluate functional pathways of SARS-CoV-associated disease genes was performed by using R statistical software and the package clusterProfiler [93]. P-values were adjusted with the false discovery rate (FDR) method and a threshold equal to 0.05 was set to identify functional annotations significantly enriched amongst the selected gene lists.
Gene set enrichment analysis
In order to test whether the predicted repurposable drugs for SARS-CoV and the ones predicted for SARS-CoV-2 can counteract the gene expression perturbations caused by the virus, (i.e., whether they down-regulate genes up-regulated by the virus or vice versa), we performed a gene set enrichment analysis (GSEA) as in [16]. We first collected two gene expression datasets of hosts infected SARS-CoV available through the GEO public repository. In particular: transcriptome data of SARS-CoV-infected samples from patient’s peripheral blood (GSE1739) and Calu-3 cells (GSE33267). To define differentially expressed genes, we selected adjusted p-values less than 0.05 and 0.01 for GSE1739 and GSE33267 dataset, respectively. These differentially expressed genes were used as SARS gene signatures. We then collected one dataset of 120 differentially expressed genes in SARS-CoV-2-infected A549 cells reported in [38] and we used it as COVID-19 gene signature.
The gene expression data of drug-treated human cell lines from the Connectivity Map (CMAP) database [39] were used as drug signatures. For each drug that was in both CMAP database and in our disease-drug network, CMAP computed a score to evaluate the treatment effects of various drugs on genes that are hallmarks for the coronavirus infection. Selected repurposing candidates with a score > 0 were considered to have potential treatment effect and the number of SARS/COVID-19 signature datasets was used as the final GSEA score that ranges from 0 to N, being N the total number of signature datasets used (i.e., N = 2 for SARS and N = 1 for COVID-19).
Availability and future directions
All relevant data are within the manuscript and its Supporting Information files; SAveRUNNER code is available upon request.
Our analysis provided a ranked list of existing drugs to be repositioned based on their expected efficacy against human coronavirus. This does not mean that drugs that did not appear in our list must be excluded from further consideration. As the input data improves, we will update our results and the currently highly ranked drugs could move to a lower ranking, as well as other incoming FDA-approved drugs that remain to be tested could appear even in a higher ranking.
Supporting information
The first sheet reports the diseases analyzed in our study with the corresponding number of disease-causing genes obtained from Phenopedia database. The second sheet reports the FDA-approved drugs obtained from DrugBank and processed in our analysis with the corresponding number of target proteins.
(XLSX)
The first sheet reports the bipartite SARS-drug subnetwork released by SAveRUNNER; the second sheet reports the bipartite COVID-19-drug subnetwork released by SAveRUNNER; the third sheet reports the lists of specific and common candidate repurposable drugs for COVID-19 and SARS.
(XLSX)
These values are plotted as bar graphs in Fig 6.
(XLSX)
Acknowledgments
First of all, we would like to thank the health care workers that in this dramatic moment are on the front lines giving everything to fight this novel coronavirus pandemic. We would like to sincerely thank Dr. Gianpiero D’Offizi for his precious advice on drugs and drugs combination to include in our analysis and for his important help in the interpretation of our results.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
PP was financially supported by PRIN 2017 - Settore ERC LS2 (Codice Progetto 20178L3P38) and LF was financially supported by Sapienza University of Rome grant entitled “Network medicine-based machine learning and graph theory algorithms for precision oncology” (n. RM1181642AFA34C2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020. April;5(4):536–44. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang X, Wu C, Li X, Song Y, Yao X, Wu X, et al. On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev [Internet]. [cited 2020 Mar 19]; Available from: https://academic.oup.com/nsr/advance-article/doi/10.1093/nsr/nwaa036/5775463. [DOI] [PMC free article] [PubMed]
- 3.Raoult D, Zumla A, Locatelli F, Ippolito G, Kroemer G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress [Internet]. 2020. March 2 [cited 2020 Apr 6]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7064018/ 10.15698/cst2020.04.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus COVID-19 (2019-nCoV) [Internet]. [cited 2020 Mar 19]. Available from: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6.
- 5.El-Sadr WM, Justman J. Africa in the Path of Covid-19. N Engl J Med. 2020. April 17;0(0):null. 10.1056/NEJMp2008193 [DOI] [PubMed] [Google Scholar]
- 6.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019. January;18(1):41–58. 10.1038/nrd.2018.168 [DOI] [PubMed] [Google Scholar]
- 7.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020. March 18;0(0):null. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open. 2020. April 1;3(4):e208857–e208857. 10.1001/jamanetworkopen.2020.8857 [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020. March;30(3):269–71. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19—Preliminary Report. N Engl J Med. 2020. May 22;0(0):null. [DOI] [PubMed] [Google Scholar]
- 11.Silverman EK, Schmidt HHHW, Anastasiadou E, Altucci L, Angelini M, Badimon L, Balligand JL, Benincasa G, Capasso G, Conte F, Di Costanzo A, Farina L, Fiscon G, Gatto L, Gentili M, Loscalzo J, Marchese C, Napoli C, Paci P, Petti M, Quackenbush J, Tieri P, Viggiano D, Vilahur G, Glass K, Baumbach J. Molecular networks in Network Medicine: Development and applications. Wiley Interdiscip Rev Syst Biol Med. 2020. November;12(6):e1489 10.1002/wsbm.1489 Epub 2020 Apr 19. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barabási A-L, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011. January;12(1):56–68. 10.1038/nrg2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldera M, Buphamalai P, Müller F, Menche J. Interactome-based approaches to human disease. Curr Opin Syst Biol. 2017. June 1;3:88–94. [Google Scholar]
- 14.Cheng F, Desai RJ, Handy DE, Wang R, Schneeweiss S, Barabási A-L, et al. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat Commun. 2018. 12;9(1):2691 10.1038/s41467-018-05116-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng F, Liu C, Jiang J, Lu W, Li W, Liu G, et al. Prediction of Drug-Target Interactions and Drug Repositioning via Network-Based Inference. PLOS Comput Biol. 2012. May 10;8(5):e1002503 10.1371/journal.pcbi.1002503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020. March 16;6(1):1–18. 10.1038/s41421-019-0132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang J, Zhang P, Wang Q, Zhou Y, Chiang C-W, Chen R, et al. Network-based Translation of GWAS Findings to Pathobiology and Drug Repurposing for Alzheimer’s Disease. medRxiv. 2020. January 18;2020.01.15.20017160. [Google Scholar]
- 18.Gysi DM, Valle ÍD, Zitnik M, Ameli A, Gan X, Varol O, et al. Network Medicine Framework for Identifying Drug Repurposing Opportunities for COVID-19. ArXiv200407229 Cs Q-Bio Stat [Internet]. 2020. April 15 [cited 2020 May 17]; Available from: http://arxiv.org/abs/2004.07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu W, Clyne M, Khoury MJ, Gwinn M. Phenopedia and Genopedia: disease-centered and gene-centered views of the evolving knowledge of human genetic associations. Bioinformatics. 2010. January 1;26(1):145–6. 10.1093/bioinformatics/btp618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018. January 4;46(Database issue):D1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020. February 22;395(10224):565–74. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020. February 15;395(10223):507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020. February 7; 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.COVID-19: Finding the Right Fit [Internet]. DrugBank Blog. 2020. [cited 2020 Mar 18]. Available from: https://blog.drugbankplus.com/data-driven-approaches-to-identify-potential-covid-19-therapies/ [Google Scholar]
- 25.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020. March 18;6(1):1–4. 10.1038/s41421-019-0132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–3. 10.5582/bst.2020.01047 [DOI] [PubMed] [Google Scholar]
- 27.Colson P, Rolain J-M, Lagier J-C, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020. March 4;105932 10.1016/j.ijantimicag.2020.105932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarty MF, DiNicolantonio JJ. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog Cardiovasc Dis [Internet]. 2020. February 12 [cited 2020 Mar 18]; Available from: http://www.sciencedirect.com/science/article/pii/S0033062020300372. 10.1016/j.pcad.2020.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hensley LE, Fritz EA, Jahrling PB, Karp C, Huggins JW, Geisbert TW. Interferon-β 1a and SARS Coronavirus Replication. Emerg Infect Dis. 2004. February;10(2):317–9. 10.3201/eid1002.030482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahin AR, Erdogan A, Mutlu Agaoglu P, Dineri Y, Cakirci AY, Senel ME, et al. 2019. Novel Coronavirus (COVID-19) Outbreak: A Review of the Current Literature. EURASIAN J Med Oncol. 2020;4(1):1–7. [Google Scholar]
- 31.Jin Y-H, Cai L, Cheng Z-S, Cheng H, Deng T, Fan Y-P, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020. February 6;7(1):4 10.1186/s40779-020-0233-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004. March;59(3):252–6. 10.1136/thorax.2003.012658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J, Huang Z, Lin L, Lv J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc [Internet]. 2020. April 7 [cited 2020 Apr 7];9(7). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020. February 28; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020. March 4; 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020. April 30;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet [Internet]. 2020. March 16 [cited 2020 Mar 18];0(0). Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30628-0/abstract. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanco-Melo D, Nilsson-Payant BE, Liu W-C, Møller R, Panis M, Sachs D, et al. SARS-CoV-2 launches a unique transcriptional signature from in vitro, ex vivo, and in vivo systems. bioRxiv. 2020. March 24;2020.03.24.004655. [Google Scholar]
- 39.Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell. 2017. November 30;171(6):1437–1452.e17. 10.1016/j.cell.2017.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menche J, Sharma A, Kitsak M, Ghiassian SD, Vidal M, Loscalzo J, et al. Uncovering disease-disease relationships through the incomplete interactome. Science [Internet]. 2015. February 20 [cited 2019 Nov 22];347(6224). Available from: https://science.sciencemag.org/content/347/6224/1257601. 10.1126/science.1257601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alaimo S, Pulvirenti A. Network-Based Drug Repositioning: Approaches, Resources, and Research Directions In: Vanhaelen Q, editor. Computational Methods for Drug Repurposing [Internet]. New York, NY: Springer New York; 2019. [cited 2019 Apr 30]. p. 97–113. (Methods in Molecular Biology). Available from: 10.1007/978-1-4939-8955-3_6. [DOI] [PubMed] [Google Scholar]
- 42.Lotfi Shahreza M, Ghadiri N, Mousavi SR, Varshosaz J, Green JR. A review of network-based approaches to drug repositioning. Brief Bioinform. 2017;19(5):878–892. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Zheng S, Chen B, Butte AJ, Swamidass SJ, Lu Z. A survey of current trends in computational drug repositioning. Brief Bioinform. 2016. January;17(1):2–12. 10.1093/bib/bbv020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue H, Li J, Xie H, Wang Y. Review of Drug Repositioning Approaches and Resources. Int J Biol Sci. 2018. July 13;14(10):1232–44. 10.7150/ijbs.24612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo H, Wang J, Li M, Luo J, Peng X, Wu F-X, et al. Drug repositioning based on comprehensive similarity measures and Bi-Random walk algorithm. Bioinformatics. 2016;32(17):2664–2671. 10.1093/bioinformatics/btw228 [DOI] [PubMed] [Google Scholar]
- 46.Wang W, Yang S, Li J. Drug target predictions based on heterogeneous graph inference. Pac Symp Biocomput Pac Symp Biocomput. 2013;53–64. [PMC free article] [PubMed] [Google Scholar]
- 47.Martínez V, Navarro C, Cano C, Fajardo W, Blanco A. DrugNet: network-based drug-disease prioritization by integrating heterogeneous data. Artif Intell Med. 2015. January;63(1):41–9. 10.1016/j.artmed.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 48.Nicastri E, Petrosillo N, Bartoli TA, Lepore L, Mondi A, Palmieri F, et al. National Institute for the Infectious Diseases “L. Spallanzani”, IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep [Internet]. 2020. March 16 [cited 2020 Apr 18];12(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7097833/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020. April 13; 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- 50.Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020. May 1;177:104762 10.1016/j.antiviral.2020.104762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colson P, Rolain J-M, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020. March 1;55(3):105923 10.1016/j.ijantimicag.2020.105923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost JTH. 2020. March 27; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents [Internet]. 2020. March 29 [cited 2020 May 14]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7118634/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost JTH. 2020. April 2; 10.1111/jth.14821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020. April 10;0(0):null. 10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.FDA Approves First Treatment for COVID-19 [Internet]. FDA. FDA; 2020 [cited 2020 Nov 2]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19.
- 57.Piccirillo MC, Ascierto P, Atripaldi L, Cascella M, Costantini M, Dolci G, et al. TOCIVID-19—A multicenter study on the efficacy and tolerability of tocilizumab in the treatment of patients with COVID-19 pneumonia. Study protocol. Contemp Clin Trials. 2020. October 6;98:106165 10.1016/j.cct.2020.106165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann-La Roche. A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia [Internet]. clinicaltrials.gov; 2020 Sep [cited 2020 Oct 29]. Report No.: NCT04320615. Available from: https://clinicaltrials.gov/ct2/show/NCT04320615.
- 59.Drugs@FDA: FDA-Approved Drugs [Internet]. [cited 2020 Nov 2]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=214787.
- 60.Babayeva M, Loewy Z. Repurposing Drugs for COVID-19: Pharmacokinetics and Pharmacogenomics of Chloroquine and Hydroxychloroquine. Pharmacogenomics Pers Med. 2020;13:531–42. 10.2147/PGPM.S275964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Commissioner O of the. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine [Internet]. FDA. FDA; 2020 [cited 2020 Nov 2]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and.
- 62.Consortium WS trial, Pan H, Peto R, Karim QA, Alejandria M, Henao-Restrepo AM, et al. Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results. medRxiv. 2020. October 15;2020.10.15.20209817. 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hippensteel JA, LaRiviere WB, Colbert JF, Langouët-Astrié CJ, Schmidt EP. Heparin as a therapy for COVID-19: current evidence and future possibilities. Am J Physiol-Lung Cell Mol Physiol. 2020. June 10;319(2):L211–7. 10.1152/ajplung.00199.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gozzo L, Viale P, Longo L, Vitale DC, Drago F. The Potential Role of Heparin in Patients With COVID-19: Beyond the Anticoagulant Effect. A Review. Front Pharmacol [Internet]. 2020. [cited 2020 Oct 29];11 Available from: https://www.frontiersin.org/articles/10.3389/fphar.2020.01307/full. 10.3389/fphar.2020.01307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020. 26;323(20):2052–9. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020. April 1;20(4):398–400. 10.1016/S1473-3099(20)30141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng F, Kovács IA, Barabási A-L. Network-based prediction of drug combinations. Nat Commun. 2019. March 13;10(1):1–11. 10.1038/s41467-018-07882-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rico-Mesa JS, White A, Anderson AS. Outcomes in Patients with COVID-19 Infection Taking ACEI/ARB. Curr Cardiol Rep [Internet]. 2020. [cited 2020 May 11];22(5). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7154066/ 10.1007/s11886-020-01291-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020. April 1;8(4):e21 10.1016/S2213-2600(20)30116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun ML, Yang JM, Sun YP, Su GH. [Inhibitors of RAS Might Be a Good Choice for the Therapy of COVID-19 Pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi Zhonghua Jiehe He Huxi Zazhi Chin J Tuberc Respir Dis. 2020. February 16;43(0):E014. [DOI] [PubMed] [Google Scholar]
- 71.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up. J Am Coll Cardiol [Internet]. 2020. April 17 [cited 2020 May 26]; Available from: https://www.onlinejacc.org/content/early/2020/04/15/j.jacc.2020.04.031. 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang W, Ye L, Ye L, Li B, Gao B, Zeng Y, et al. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007. September;128(1–2):1–8. 10.1016/j.virusres.2007.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garg M, Royce SG, Tikellis C, Shallue C, Batu D, Velkoska E, et al. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut. 2020. May;69(5):841–51. 10.1136/gutjnl-2019-318512 [DOI] [PubMed] [Google Scholar]
- 74.Abbass M, Cepek J, Parker CE, Nguyen TM, MacDonald JK, Feagan BG, et al. Adalimumab for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2019. 14;2019(11). 10.1002/14651858.CD012878.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. The Lancet. 2020. May 2;395(10234):1407–9. 10.1016/S0140-6736(20)30858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chinese Clinical Trial Register (ChiCTR)—The world health organization international clinical trials registered organization registered platform [Internet]. [cited 2020 May 13]. Available from: http://www.chictr.org.cn/showprojen.aspx?proj=49889.
- 77.Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020. April 1;20(4):400–2. 10.1016/S1473-3099(20)30132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020. 15;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bifulco M, Gazzerro P. Statins in coronavirus outbreak: It’s time for experimental and clinical studies. Pharmacol Res. 2020. June;156:104803 10.1016/j.phrs.2020.104803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang S, Brown HM, Hwang S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune Netw [Internet]. 2018. October 17 [cited 2020 May 7];18(5). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6215902/ 10.4110/in.2018.18.e33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kany S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease. Int J Mol Sci [Internet]. 2019. November 28 [cited 2020 May 7];20(23). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6929211/ 10.3390/ijms20236008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conti P, Ronconi G, Caraffa A, Gallenga C, Ross R, Frydas I, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020. 14;34(2). [DOI] [PubMed] [Google Scholar]
- 83.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol Orlando Fla. 2020. May;214:108393 10.1016/j.clim.2020.108393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cameron MJ, Bermejo-Martin JF, Danesh A, Muller MP, Kelvin DJ. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res. 2008. April 1;133(1):13–9. 10.1016/j.virusres.2007.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020. April 1;10(2):102–8. 10.1016/j.jpha.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the Eye of the Cytokine Storm. Microbiol Mol Biol Rev MMBR. 2012. March;76(1):16–32. 10.1128/MMBR.05015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu W, Gwinn M, Clyne M, Yesupriya A, Khoury MJ. A navigator for human genome epidemiology. Nat Genet. 2008. February;40(2):124–5. 10.1038/ng0208-124 [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Zhang S, Li F, Zhou Y, Zhang Y, Wang Z, et al. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020. January 8;48(D1):D1031–41. 10.1093/nar/gkz981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012. October;92(4):414–7. 10.1038/clpt.2012.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clauset A, Newman MEJ, Moore C. Finding community structure in very large networks. Phys Rev E. 2004. December 6;70(6):066111 10.1103/PhysRevE.70.066111 [DOI] [PubMed] [Google Scholar]
- 91.Vieira V da F, Xavier CR, Ebecken NFF, Evsukoff AG. Performance Evaluation of Modularity Based Community Detection Algorithms in Large Scale Networks [Internet]. Vol. 2014, Mathematical Problems in Engineering. Hindawi; 2014. [cited 2020 Nov 5]. p. e502809 Available from: https://www.hindawi.com/journals/mpe/2014/502809/ [Google Scholar]
- 92.Harenberg S, Bello G, Gjeltema L, Ranshous S, Harlalka J, Seay R, et al. Community detection in large-scale networks: a survey and empirical evaluation. WIREs Comput Stat. 2014;6(6):426–39. [Google Scholar]
- 93.Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R Package for Comparing Biological Themes Among Gene Clusters. OMICS J Integr Biol. 2012. May;16(5):284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The first sheet reports the diseases analyzed in our study with the corresponding number of disease-causing genes obtained from Phenopedia database. The second sheet reports the FDA-approved drugs obtained from DrugBank and processed in our analysis with the corresponding number of target proteins.
(XLSX)
The first sheet reports the bipartite SARS-drug subnetwork released by SAveRUNNER; the second sheet reports the bipartite COVID-19-drug subnetwork released by SAveRUNNER; the third sheet reports the lists of specific and common candidate repurposable drugs for COVID-19 and SARS.
(XLSX)
These values are plotted as bar graphs in Fig 6.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.