Abstract
Paragonimiasis is caused by zoonotic trematodes of Paragonimus spp., found in Asia, the Americas and Africa, particularly in tropical regions. These parasites have a complex, multi-host life cycle, with mammalian definitive hosts and larval stages cycling through two intermediate hosts (snails and freshwater decapod crustaceans). In Africa, paragonimiasis is particularly neglected, and remains the only human parasitic disease without a fully characterised life cycle. However paragonimiasis has potentially significant impacts on public health in Africa, and prevalence has likely been underestimated through under-reporting and misdiagnosis as tuberculosis due to a similar clinical presentation. We identified the need to synthesise current knowledge and map endemic foci for African Paragonimus spp. together with Poikilorchis congolensis, a rare, taxonomically distant trematode with a similar distribution and morphology. We present the first systematic review of the literature relating to African paragonimiasis, combined with mapping of all reported occurrences of Paragonimus spp. throughout Africa, from the 1910s to the present. In human surveys, numerous reports of significant recent transmission in Southeast Nigeria were uncovered, with high prevalence and intensity of infection. Overall prevalence was significantly higher for P. uterobilateralis compared to P. africanus across studies. The potential endemicity of P. africanus in Côte d’Ivoire is also reported. In freshwater crab intermediate hosts, differences in prevalence and intensity of either P. uterobilateralis or P. africanus were evident across genera and species, suggesting differences in susceptibility. Mapping showed temporal stability of endemic foci, with the majority of known occurrences of Paragonimus found in the rainforest zone of West and Central Africa, but with several outliers elsewhere on the continent. This suggests substantial under sampling and localised infection where potential host distributions overlap. Our review highlights the urgent need for increased sampling in active disease foci in Africa, particularly using molecular analysis to fully characterise Paragonimus species and their hosts.
Author summary
Paragonimiasis is a lung disease caused by food-borne zoonotic trematodes of Paragonimus spp., multi-host parasites whose complex life cycle includes primary (snail), secondary (freshwater decapod crustacea), and mammalian definitive hosts. In Africa, paragonimiasis is a particularly neglected disease, where prevalence has likely been underestimated through under reporting and misdiagnosis as tuberculosis; and it remains the only human parasite without a fully known life-cycle. We present the first systematic review of paragonimiasis in Africa, including 143 publications. In human studies, we uncovered substantial recent transmission in Southeast Nigeria, and recent transmission also in Côte d’Ivoire and Cameroon in endemic foci; significantly higher prevalence of P. uterobilateralis than P. africanus; and evidence supporting the existence of P. africanus in Côte d’Ivoire. In freshwater crab intermediate hosts, prevalence and intensity of either P. uterobilateralis or P. africanus varied across genera and species, suggesting differences in susceptibility. Mapping revealed evidence of temporal stability at endemic foci in rainforest regions; and widespread outliers. Numerous reports of significant recent transmission, particularly in Southeast Nigeria should be heeded by the international NTD research community. Increased studies are urgently needed to ascertain the real distribution and diversity of Paragonimus species in Africa.
Introduction
Paragonimiasis is caused by food-borne zoonotic trematodes of Paragonimus spp., multi-host parasites whose complex life cycle includes a primary (or first intermediate) snail host; a secondary (or second intermediate) decapod crustacean host, and a mammalian definitive host. Human paragonimiasis has a global distribution in the Americas, Africa, and Asia, particularly in tropical regions [1,2]. An estimated 23 million people are infected worldwide, with the greatest disease burden in Asia [1–3]. The taxonomy of Paragonimus is problematic and in need of revision, but currently there are over 50 recognised species, 7 of which cause disease in humans [1,2]. Paragonimiasis in humans is generally caused by the consumption of incompletely cooked freshwater crabs that harbour the infective metacercarial larval stages of the parasite; but it can also be acquired by eating uncooked paratenic hosts, (such as cases from eating wild boar in Japan) which are infected with juvenile worms in development stasis [1,4]. Paragonimus is a euryxenous parasite with numerous mammalian reservoir hosts, including for example mongooses, civets, primates and domestic dogs and cats in Africa [5–8]. The parasite also develops in a broad range of molluscan first intermediate hosts, such as Rissooidea and Cerithioidea in Asia [9], and numerous crustacean second intermediate hosts, mostly species of brachyuran crab found in freshwater habitats, some of which are semi-terrestrial [10]. In terms of the clinical picture, paragonimiasis is primarily a disease of the lungs, where adult worms lodge in lung tissue, although ectopic infections are relatively common in some Asian species [2]. The main clinical symptoms include persistent cough, hemoptysis, and abdominal or chest pain [3]. With often overlapping symptoms, there is the potential for diagnostic confusion with tuberculosis (TB); [3,11].
In Africa, paragonimiasis-like lung infections were first reported in the 1920s [12,13], and subsequently recorded only occasionally in the following 3 decades [14–18]. The first descriptions of African species of Paragonimus were published in 1965, for Paragonimus africanus [7] and Paragonimus uterobilateralis [8]. Paragonimiasis in Africa came to widespread attention with an outbreak in Southeast Nigeria in the late 1960s, stemming from food shortages and concomitant consumption of freshwater crabs as a result of the Nigerian Civil war of 1967–1970 [19–21]. In more recent times, paragonimiasis in Africa has received remarkably little attention from the international research community, despite several recent studies reporting significant transmission within Nigeria in particular [22–27]; and also Cameroon [28,29]. Paragonimiasis in Africa remains the only human parasite without a fully characterised life cycle, with the first intermediate snail hosts of the African species of Paragonimus unconfirmed, and the miracidia, rediae and cercarial life stages undescribed. Suspected host snail species co-occurring in known crab host habitats include: Achatinidae in Cameroon [30,31]; Potadoma freethii in Cameroon [7] and Nigeria [26,32]; Melania in Nigeria [33]; and P. sanctipauli, Afropomus balanoidea [34], and Homorus (Striosubulina) striateila (Achatinidae) in Liberia [35]. The latter studies in Liberia attempted experimental infections, but the candidate Paragonimus-like cercariae failed to infect known freshwater crab hosts (Liberonautes) collected at the disease foci [34,36]. The second intermediate hosts of Paragonimus in Africa are better known, with 8 species of freshwater crabs in 2 genera, Sudanonautes and Liberonautes, implicated as secondary hosts [10]. However, current taxonomic revisions involving host species may alter the generic assignments of some taxa.
Four species of Paragonimus have been recorded from Africa to date: P. uterobilateralis, P. africanus, P. gondwanensis, and P. kerberti [7,8,37,38]. All species descriptions are based on morphology only, none have yet been characterised with molecular data, and are therefore currently distinguished by the morphological characters of egg and metacercariae. Only three sequences of one species have been published on GenBank to date, 5.8S, 28S rRNA and ITS2 from P. africanus [29,39]. Given that the two species P. gondwanensis and P. kerberti were described as recently as 2014 and 2015 without molecular data [37,38], and that morphological plasticity is well known in Paragonimus [1], they should be regarded as unconfirmed. Aka et al. [40] suggested there may be undescribed species of Paragonimus in West and Central Africa, but the lack of molecular data is hampering the ability to answer this question, as well as the study of the life cycle and distribution of these parasites more generally.
Another trematode has been reported from Paragonimus endemic regions: Poikilorchis congolensis (also known as Achillurbainia congolensis). This trematode is the monotypic species of Poikilorchis and assigned to the family Achillubainiidae Dolfus, 1939, which has only one other species, Achillurbainia nouveli Dolfus, 1939 [41]. While P. congolensis is taxonomically distant, early publications show diagnostic confusion with Paragonimus, with similar egg morphology and overlapping endemism. However, symptomology of a retroauricular or neck cyst [41] never reported in paragonimiasis, and egg negative sputum, appear consistent with infection of P. congolensis only, not Paragonimus [42,43].
Very few attempts at spatial characterisation of paragonimiasis in Africa have been made since the mapping of freshwater crab hosts in endemic foci in Nigeria and Cameroon by Voelker & Sachs [44], despite the need to map endemic foci noted as early as 1972 by Nwokolo [20]. Mapping is essential for highly focal diseases, for example recent studies illustrate the temporal stability of endemic foci in schistosomiasis [45]. Foci of paragonimiasis may be similarly stable over time, given the likelihood of the relatively long persistence of mature metacercariae in freshwater crab hosts, and of adult flukes in the lungs of mammalian definitive hosts [1,4]. Although recent reviews have discussed the need to address the extensive knowledge gaps of paragonimiasis in Africa and update the true public health significance of the disease [46–49], no systematic review or meta-analysis has been performed to date. Consequently, the aims of the current study are: to undertake a systematic review of paragonimiasis in Africa (including reports of the distantly-related trematode, Poikilorchis congolensis with diagnostic overlap with Paragonimus); to synthesise current knowledge and to map endemic foci. The specific aims of the present study are: 1. to ascertain the level of recent disease transmission; 2. to map reported confirmed occurrences of both Paragonimus and the freshwater crab intermediate hosts; and 3. to ultimately establish both the historical and recent distribution of this most neglected tropical disease throughout Africa.
Materials and methods
Ethics statement
The data included in this study were anonymised aggregated data from previously published studies only.
Study protocol
A systematic review was undertaken according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and checklist [50,S1 File]. The a priori review question was as follows:
What is the evidence, extent and distribution of recent/ongoing transmission of paragonimiasis in Africa?
Eligibility criteria
Peer reviewed publications on African Paragonimus spp./paragonimiasis were included in all languages (included Dutch, English, French, German, Italian, and Japanese).
Information sources
Online databases were searched through the Natural History Museum (NHM) London library, including: Google Scholar, African Journals Online, PubMed, ScienceDirect, and Web of Science. Publications not openly accessible were requested through interlibrary loans, and ones unavailable by those means were sourced directly in some instances by contacting authors/researchers in the field (David Aka, David Blair, and Chris Appleton). All citations were comprehensively checked, and any additional publications included. References of all papers were also systematically checked and added if they had not previously been identified through database searches.
Period
The review was carried out from November 2018-June 2020 by authors MER and JW. The date of last search was the 20th June, 2020.
Search strategies
Search terms included: paragonimiasis, Paragonimus, Paragonimus africanus/uterobilateralis/gondwanensis/kerberti, Africa, for all databases. Additional searches including these terms and the following were made in combination searches: all country names for West and Central Africa (Nigeria, Cameroon, Côte d’Ivoire, Gabon, Equatorial Guinea, Ghana, Liberia, Guinea, Senegal, Central African Republic, Democratic Republic of the Congo/DRC, Republic of the Congo, Central African Republic, Benin, Togo, Sierra Leone, Senegal, Gambia and Burkina Faso); names of endemic regions, (Cross River, Imo River); prior country names to ensure historical records were found (Haute-Volta, Zaire); relevant French terms (paragonimoses and distomatoses), and intermediate host crab genera names: Sudanonautes, Liberonautes, Potamonautes and Potamonemus.
Following initial investigations, an additional review was undertaken for Poikilorchis congolensis (also known as Achillurbainia congolensis), given the confusion in identification with Paragonimus evident in the literature. Cases reporting a retroauricular or neck cyst and egg-negative sputum were assessed to be consistent with infection of P. congolensis only, not Paragonimus, as evident in the literature [41–43]. Search terms were as above, minus the crab genera names and replacing Paragonimus spp. with Poikilorchis/congolensis.
Study selection
Criteria for inclusion were publications on African paragonimiasis only, those addressing Asian or American paragonimiasis/Paragonimus species were excluded. All original research, including non-survey based works, for example case reports, experimental studies or questionnaire-only based studies were included. Any publications that did not contain new data, i.e., analysing the findings of an earlier study, were excluded. All papers meeting selection criteria were read in full by MER and JW, apart those in German, read solely by JW. Other papers not in English were translated in Google Translate. The sole paper in Japanese, which also had relevant sections in English, was checked by a native speaker. Reviews were excluded, but those on paragonimiasis in Africa were read in full and references checked against the main publication list and any new references added [46–49,51,52].
Data collection process
Authors MER and JW extracted data from the publications and cross checked a proportion of the other’s data records. A database was created in Microsoft Excel 365 (S1–S5 Tables). Alongside key publication details, data recorded included the following: country; year of study; category (e.g., case report, survey, and for latter, scope of survey = humans/freshwater crabs/freshwater snails or combinations of); Paragonimus species reported; a brief description of the study and key findings; what the paragonimiasis infection was described from/how it was diagnosed, e.g., eggs in sputum (S1 Table). Where present, clinical findings were also recorded, e.g., symptoms observed, and details for any other diseases screened for (e.g., TB, and soil transmitted helminths). Other data recorded included: prevalence measurements for any hosts surveyed; egg size measurements where reported; and parasite counts for analysing intensity of infection (egg or metacercariae numbers per host or adult worm counts for wild-caught mammals), as overall averages and individual counts where available. Additional tables for prevalence, intensity and egg-size measurement data were created to allow for the analysis and recording of individual measurements (S2–S4 Tables). In many cases study period was not reported in the paper, here it was estimated as 2 years prior to publication. In analyses of dates, decade was used to mitigate potential estimation bias around sampling period. For human cases, the period or year when symptoms first appeared was noted (if recorded). If locality maps or photographs of collected larval stages were included, this was recorded. Additional data were sourced directly from authors if required for analysis (see Acknowledgements). All publications, both included and excluded from analysis, are listed in S1 Table.
Methodological assessment
Identification
Identification of Paragonimus in the literature was generally undertaken by microscopy, i.e., the microscopic identification of eggs or metacercariae. As only 4 studies used molecular analysis and/or serology [29,39,53,54], here identification was therefore accepted on microscopy only. This criterion was applied even if the result was contrary to the diagnosis in the paper, e.g., a positive case based on serology results or symptoms only was recorded here as unconfirmed. This assessment was made for consistency and to ensure that only current infections were identified. In cases where Paragonimus eggs were isolated from stool only, any available photographs were assessed, and evidence of positive identification of other parasite species checked to rule out the possibility of misidentification of another trematode.
Assessment of surveys
In human surveys, publications were excluded from further analysis if the study population was selected from people pre-determined as likely infection candidates, for example hospital surveys of pulmonary clinic patients. A publication was also excluded if the total study population was not given. In some papers, study populations were identified and subsequently only those individuals presenting symptoms were recruited for the survey. These publications were included as the original study population total was also given. Both cross-sectional (community-wide) and school-based surveys were included. For mammal surveys also, studies were excluded if total study population was not provided.
Data analysis and summary statistics
All data were analysed in R, version 3.5.3 (2019-03-11) "Great Truth". The majority of the literature in the systematic review was highly heterogeneous and a qualitative review was considered the best approach for analysis. A subset of publications was also selected for a comparison of prevalence and intensity across studies, separately for each host category. Averages and standard deviation values were computed in R, using the tidyverse package [55]. Weighted means were computed, for example, egg measurement averages in a given study weighted by total eggs measured; average intensity, weighted by total number of infected hosts (per host-category) per study, and in prevalence, by total surveyed for the study (Table 1). The statistical significance of differences across studies between groups, for example in prevalence and intensity of infected hosts were tested with a Wilcoxon rank sum test, which does not assume a normal distribution, given the high heterogeneity and variance among studies. All statistical tests were conducted with the significance level α = 0.05 for rejecting null hypotheses.
Table 1. Weighted averages: Prevalence and intensity of infection, by host category (human, other mammal or crab).
| Countries | Species | Cat. | Host | Prev. | SD | Inf. | N | Refs | Int. | SD | Inf. | N | Refs | Mammals* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C, EG | P. africanus | H | 2.9 | 3.2 | 263 | 8978 | 8 | |||||||
| L, N | P. uterobilateralis | 12.4 | 6.8 | 1670 | 13443 | 12 | 108.5 | 84.9 | 1716 | 10477 | 9 | |||
| C, G, N | Paragonimus sp. | 8.7 | 7.8 | 992 | 11415 | 11 | 10.8 | 53.0 | 693 | 4700 | 4 | |||
| C | P. africanus | C | S. africanus | 42.9 | 28.0 | 103 | 240 | 4 | 54.6 | 85.7 | 106 | 214 | 2 | |
| C | P. africanus | S. aubryi | 6.3 | - | 1 | 16 | 1 | 3.8 | - | 1 | 16 | 1 | ||
| C | P. africanus | S. pelli | 1.5 | - | 1 | 66 | 1 | 24.3 | - | 1 | 66 | 1 | ||
| C | P. kerberti | S. africanus | 32.9 | - | 162 | 493 | 1 | |||||||
| L | P. uterobilateralis | L. chaperi | 2.3 | - | 1 | 43 | 1 | 2.0 | - | 1 | 40 | 1 | ||
| L | P. uterobilateralis | L. latidactylus | 24.4 | 36.1 | 87 | 357 | 3 | 117.1 | 286.5 | 85 | 191 | 3 | ||
| L | P. uterobilateralis | L. nanoides | 0.5 | - | 4 | 763 | 1 | 1.0 | - | 4 | 763 | 1 | ||
| N | P. uterobilateralis | S. sp. | 5.5 | 2.1 | 235 | 4249 | 2 | |||||||
| C, G, N | P. uterobilateralis | S. africanus | 5.9 | 41.7 | 156 | 2634 | 5 | 6.0 | 9.8 | 34 | 189 | 2 | ||
| C, G, N | P. uterobilateralis | S. aubryi | 64.8 | 42.8 | 57 | 88 | 3 | 3.5 | 0.5 | 12 | 43 | 2 | ||
| C | P. uterobilateralis | S. pelli | 3.0 | - | 2 | 66 | 1 | 0.8 | - | 2 | 66 | 1 | ||
| Co | Paragonimus sp. | L. latidactylus | 20.0 | - | 6 | 30 | 1 | |||||||
| C, N | Paragonimus sp. | S. africanus | 10.4 | 20.2 | 342 | 3304 | 4 | 17.5 | - | 94 | 207 | 1 | ||
| C | Paragonimus sp. | S. aubryi | 10.0 | - | 5 | 50 | 1 | 2.0 | - | 5 | 59 | 1 | ||
| C | Paragonimus sp. | S. granulatus | 8.1 | - | 3 | 37 | 1 | 4.0 | - | 3 | 37 | 1 | ||
| C | Paragonimus sp. | S. pelli | 0.0 | - | 0 | 11 | 1 | |||||||
| C | P. africanus | M | Feliformia | 58.8 | 33.8 | 10 | 17 | 3 | Civettictis civetta, Crossarchus obscurus, Nandinia binotata | |||||
| C, N | P. africanus | Primates | 39.1 | 13.0 | 25 | 64 | 3 | 96.7 | - | 21 | 49 | 1 | Cercocebus torquatus (& Int.), Mandrillus leucophaeus, Perodicticus potto | |
| L | P. uterobilateralis | domestic | 33.3 | 7.9 | 3 | 9 | 1 | dogs | ||||||
| L | P. uterobilateralis | Eulipotyphla | 12.5 | - | 1 | 8 | 1 | Crocidura flavescens | ||||||
| L, N | P. uterobilateralis | Feliformia | 77.8 | 29.1 | 35 | 45 | 4 | 17.0 | - | 2 | 5 | 1 | Civettictis civetta (& Int.), Crossarchus obscurus | |
| L | P. uterobilateralis | Rodentia | 16.7 | - | 1 | 6 | 1 | Malacomys edwarsi | ||||||
| C, Co, Gh, N | Paragonimus sp. | domestic | 0.5 | 7.9 | 2 | 387 | 4 | dogs, pigs, (cats) | ||||||
| N | Paragonimus sp. | Eulipotyphla | 0.0 | - | 0 | 24 | 1 | (Crocidura flavescens) | ||||||
| C, Co, G, N | Paragonimus sp. | Feliformia | 9.5 | 34.5 | 8 | 84 | 5 | Atilax paludinosus, Civettictis civetta, Crossarchus obscurus, Ichneumia albicauda, (Nandinia binotata), (Genette maculata) | ||||||
| C, T | Paragonimus sp. | Primates | 14.1 | 7.4 | 47 | 333 | 4 | 47.0 | - | 23 | 161 | 1 | Papio anubis (& Int.) (Cercocebus galeritus), (Cercopithecus aethiops), (Cercopithecus mona), (Galagoides demidovii) | |
| C, G, N | Paragonimus sp. | Rodentia | 0.0 | 0.0 | 0 | 55 | 3 | (Atherurus africanus), (Cricetomys emini), (Hystrix cristat), (Malacomys edwarsi), (Cricetomys gambianus) |
Key: Countries: C = Cameroon, G = Gabon, L = Liberia, EG = Equatorial Guinea, Co = Côte d’Ivoire, Gh = Ghana, N = Nigeria, T = Tanzania, Cat. = Host Category: H: Human, C: Freshwater Crab, M: Mammal, Inf. = total infected, Int. = intensity, Host = Host species (or category for mammals), Prev. = prevalence, SD = standard deviation, N = total surveyed, left of bar: totals for prevalence, right of bar, totals for intensity data, Refs = total number of publications, Mammals* = species of mammal surveyed, those with individuals positive for infection in bold, those not infected, in brackets.
Prevalence
Prevalence levels were recorded for all hosts: human/other mammals, and intermediate freshwater crab hosts. Prevalence for crabs was calculated by species, excluding Callinectes marginatus, regarded as an unconfirmed host [56;NC, pers. comm.]. Prevalence differences by demographics (sex and age) were also examined. For sex, both overall total and proportion of female/male patients positive were recorded, and whether any difference was significant, if reported. For age data, the defined age categories, and proportion of prevalence per category were recorded (S2 Table).
Intensity
Range and average for intensity of infection (egg/metacercariae count per host) were recorded, and the average over the study calculated if not reported (S3 Table). Generally, eggs were recorded per 5 ml of sputum (e/5g), or eggs per gram of stool (epg). In one case where egg counts were reported for 1ml sputum samples, this was recorded and counts adjusted to per 5ml to allow for comparability. In another publication, sputum samples of 1-5ml were collected, and this study was excluded from analysis. The sputum and stool sample preparation methods for human and mammal surveys, details on crab dissection and screening for infection, and egg/metacercariae counts per host were recorded. If multiple samples were collected per host and the average reported, this was recorded also. If intensity was reported only by level (in 5ml sputum), these totals were not included as direct comparison was not possible with average egg count totals. Egg count measurements from stool (in addition to sputum) as reported in 3 studies were excluded for consistency. No experimental infections were included in the subset analysis for intensity or prevalence, but measurements of eggs isolated from experimental hosts were included in egg measurement analysis and the host details recorded (S4 Table).
Mapping
Data collection
Occurrence data (each unique sampling event; site and timepoint) were collected and assessed for mapping (S5 Table). All occurrences were recorded individually from each publication meeting criteria for inclusion in the study, e.g., a case report confirmed with evidence of eggs in sputum. No experimental studies were included, unless field collected data were also present. Locality data were also extracted from Table 2 of Cumberlidge et al., 2018 [46] and validated against the literature. Occurrence data collected included: year/s study was undertaken; country; region (verbatim) site name; verbatim site coordinates (if present); site type (village, school, hospital, crab collection site: river/stream or crab market, mammal survey site: forest); Paragonimus species, life stage, host species/taxonomy, totals of host surveyed and number infected per site, if recorded. Contextual notes on the occurrence, the locality and the infection in question were also made. Absence data for Paragonimus spp. were recorded if present, e.g., if a crab survey did not find infected crabs at a given site. Additional spatial data sources included river and waterway shapefiles, downloaded from DIVA-GIS [57]; and crab intermediate host occurrences. These geolocated occurrence records for the freshwater crab second intermediate hosts in West Africa are based on empirically collected data from field research carried out by NC from 1980 to 1984 in Nigeria and Cameroon, and from 1988 to 1989 in Liberia (S6 Table).
Table 2. Egg measurements in μm across studies were reported, for human host, most isolated from sputum samples, some from stool, mammals, from stool or isolated from adult worms.
| species | stage | host | Len. wm | Len. av | Len. sd | Wid. wm | Wid. av | Wid. sd | Lmin wm | Lmin av | Lmax wm | Lmax av | Wmin wm | Wmin av | Wmax wm | Wmax av | tot meas | Refs wm | Refs all |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. africanus | egg | H | 91.8 | 91.7 | 3.4 | 49.7 | 49.8 | 1.9 | 75.0 | 77.1 | 112.6 | 107.5 | 42.3 | 43.9 | 42.3 | 57.3 | 1220 | 6 | 7 |
| P. africanus | egg | M | 92.9 | 91.8 | 3.0 | 49.4 | 49.0 | 1.7 | 77.3 | 77.1 | 105.3 | 106.9 | 41.9 | 42.8 | 41.9 | 56.9 | 1252 | 5 | 6 |
| P. africanus | meta | C | 564.0 | 558.3 | 228.8 | 420.0 | 387.0 | 540.0 | 499.3 | 90 | 2 | 3 | |||||||
| P. uterobilateralis | egg | H | 68.8 | 70.0 | 4.3 | 40.4 | 41.2 | 2.2 | 53.0 | 57.1 | 86.2 | 82.7 | 34.5 | 35.7 | 34.5 | 47.3 | 2779 | 5 | 8 |
| P. uterobilateralis | egg | M | 69.4 | 68.9 | 3.5 | 42.7 | 41.9 | 1.9 | 62.3 | 62.0 | 75.7 | 75.2 | 39.3 | 38.6 | 39.3 | 44.9 | 380 | 4 | 5 |
| P. uterobilateralis | meta | C | 701.6 | 734.4 | 119.4 | 304.1 | 314.5 | 30.4 | 449.8 | 550.0 | 929.7 | 953.3 | 244.0 | 244.0 | 244.0 | 396.0 | 128 | 3 | 4 |
| Paragonimus sp. | egg | H | 95.7 | 92.2 | 13.8 | 58.8 | 56.5 | 13.5 | 78.8 | 78.0 | 109.5 | 103.8 | 48.0 | 45.4 | 48.0 | 62.4 | 155 | 6 | 13 |
| Paragonimus sp. | egg | M | 91.7 | 89.8 | 7.5 | 54.9 | 53.0 | 5.3 | 73.5 | 71.4 | 106.1 | 101.8 | 44.4 | 43.1 | 44.4 | 60.5 | 317 | 3 | 7 |
| Paragonimus sp. | meta | C | 468.3 | 558.0 | 144.2 | 166 | 2 | 2 | |||||||||||
| Poikilorchis congolensis | egg | H | 65.0 | 7.4 | 43.3 | 43.3 | 8.2 | 6 |
Key: Len. wm = weighted mean length (weighted by total number of eggs measured), Len. av = overall/average length (all studies, non-weighted, including studies where total of eggs measured were not reported), Len. sd = standard deviation of average length, Wid. wm = weighted mean width, Wid. av = average width across studies, W sd = standard deviation of average width, W sd = standard deviation of width, Lmin wm = weighted mean of minimum recorded length, L max av = average of minimum length, L max wm = weighted mean of maximum length, Wmin wm = weighted mean of minimum recorded width, Wmin av = average of minimum width, Wmax wm = weighted mean of maximum recorded width, Wmax av = average of maximum width, Tot. Meas. = total number of eggs measured for weighted mean estimates, Refs wm = total number of references for weighted mean estimates, Refs all = total number of references for overall averages.
Inclusion criteria
Records with a confirmed life stage of Paragonimus spp. (e.g., eggs in sputum or metacercariae in crabs) were mapped, in the absence of other diagnostic markers for the disease. Six publications not in the systematic review were included in mapping, either where locality data were more comprehensive than a duplicate publication that was included in the review [58]; or where localities were referenced in another publication, but the primary text was unavailable [59–62]. If the same occurrence was referenced in separate papers, this was recorded. Any occurrences additional to the main data in a study (separate surveys, case reports or personal communications) if not reported in a separate paper, were recorded, with the publication in which they were included/cited as the relevant reference, e.g., Richard Bradbury pers. comm. in Morter et al. [63].
Georeferencing
Coordinates included in a publication were recorded and transformed to decimal degrees. Non-georeferenced localities were located with Google Maps or the place-names database geonames.org, and the data source noted. If the locality name was not found, gazetteers were checked at the NHM library, and paper maps were scanned and checked for site names and geographical details. In some cases localities could not be georeferenced. Where localities or endemic zones were plotted on site maps in a publication, they were mapped using the GDAL georeferencer for QGIS, Version 3.10, Coruña (QGIS.org, 2020), and collated. In Voelker & Sachs [44], many crab occurrences were included in 4 published maps, all georeferenced using GDAL, and site names and locations on maps cross-referenced with Google Maps. Post georeferencing, current region and district names were recorded as well as verbatim ones given in publication, by a spatial join in R to records from GADM version 3.6, the database of GADM database of Global Administrative Areas accessed through the GADM portal at https://gadm.org/.
Uncertainty assessment
Many records, historical and recent, had a high degree of coordinate uncertainty. Contextual notes were made, and transformed to a category to attempt to assess uncertainty in a consistent and semi-structured way. For example, a case report where the patient lived in an endemic disease focus and had never lived elsewhere would be assessed as most likely a case of local transmission and a low uncertainty; vice versa for a patient who had also travelled to different regions and therefore could have obtained the infection elsewhere. Uncertainty was also assessed by site category. Hospitals were recorded as high uncertainty as patients in endemic regions may have travelled considerable distances to the nearest clinic or hospital, far from the site of transmission [19]. If the village/town that a patient was from was not recorded, the hospital locality was used. Schools were recorded as low uncertainty, with the balance of probability that the transmission site was local and that school children were unlikely to have lived previously in other endemic regions. For crab collection sites, markets were assessed as low uncertainty for those in rainforest zones where crabs are generally sold live and fished locally, within a 20 km radius for example (NC, pers. comm.). In urban markets however, crabs may be transported, sometimes even across borders [64], and these were recorded as high uncertainty. Direct crab collection sites, i.e., crab fishing sites in rivers/streams, were recorded as low uncertainty, as this represents direct sampling of the crab habitat; crabs do not move far along a watercourse, reproduce by direct development and have a small home range, although limited seasonal movements may occur with high water conditions (NC, pers. comm.). Mammal survey sites included forest regions, generally nearby settlements, or within settlements themselves for domestic animals. Both hospitals and crab markets were classified as ‘indirect’ sites, potentially representing a large catchment area, particularly hospitals. These low uncertainty localities were marked out separately on maps, e.g., Paragonimus life stages isolated from crab hosts or non-human mammal hosts (excluding domestic animals).
Results
Study selection
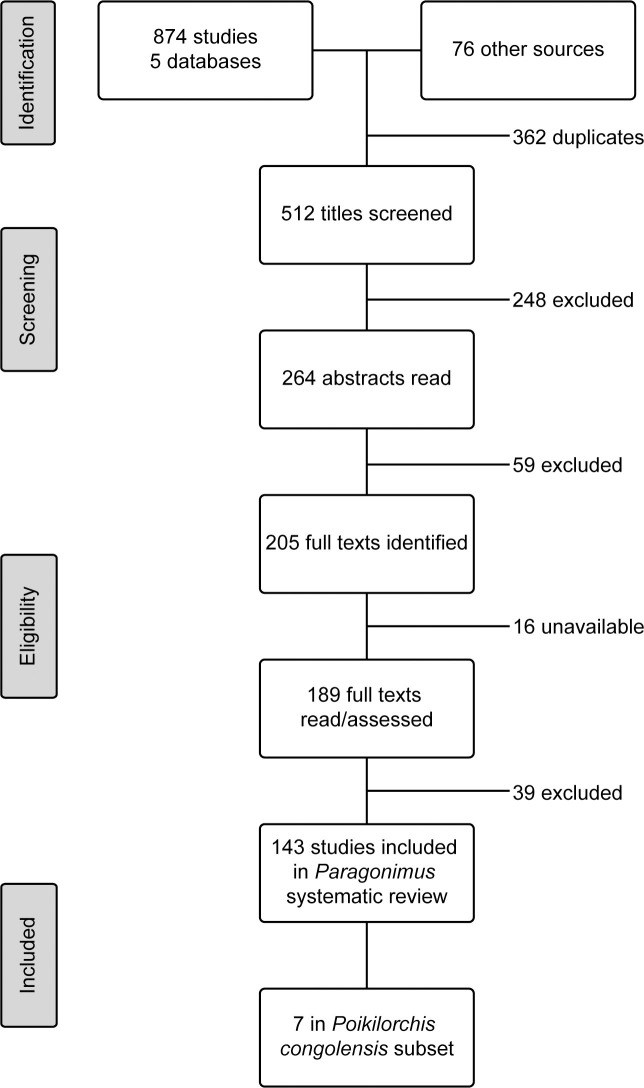
A total of 874 studies were initially recovered from databases, 169, Google scholar; 170, Pubmed; 64, Africa Journals Online; 362, Science Direct; 33, Web of Science; and an additional 76 from other sources, reference lists, and scanning journal holdings in NHM library (Fig 1). After removal of duplicates, 512 titles were screened, of which 205 full texts were identified, where 16 were unavailable and 39 were excluded with reasons given (S1 Table). This resulted in a final total of 143 studies selected for the systematic review, with all databases above represented apart from Science Direct (and all 76 publications from other sources, i.e. reference lists as above), and 7 for the subset review of Poikilorchis congolensis.
Fig 1. Flow diagram for the selection and assessment of studies for the systematic review of paragonimiasis and Paragonimus spp. and the subset of Poikilorchis congolensis, in Sub-Saharan Africa.
General findings/Study characteristics
Of the 143 scientific publications selected, 131 were peer-reviewed publications from 72 journals, and the remainder consisted of 3 book chapters, 2 thesis copies, 3 conference papers, 3 publications from grey literature (government reports/publications), and 1 manuscript in preparation. One journal was particularly strongly represented with 25 publications (Tropical Medicine and Parasitology, formerly Tropenmedizin und Parasitologie). For Poikilorchis congolensis, 7 publications were selected, all published in peer-reviewed journals.
Species
The numbers of studies by species were as follows; Paragonimus uterobilateralis (44), P. africanus (15), both P. africanus and P. uterobilateralis (8), and 1 each for P. kerberti and P. gondwanensis. For 69 studies, the species was recorded here as Paragonimus sp. because the species was either not stated or incorrectly recorded as P. westermani. Two surveys found no evidence of Paragonimus, but were included as absence data [63,65]. The remaining 3 publications were recorded as Trematoda as were solely observations of microcercous cercariae in snails, regarded as unconfirmed [30,31,36; S1 Table]. Positive molecular identification was not a suitable criterion for exclusion having been utilised in only 2 studies [29,39]. A study that reported parasite eggs morphologically consistent with Paragonimus, but with PCR amplification unsuccessful was therefore considered a confirmed case for this review [66]. This author also retrospectively evaluated an earlier survey with an assessment of “Paragonimus-like” infections as positive for Paragonimus based on the available evidence [67], we concurred with the assessment. For Poikilorchis congolensis, 1 case was diagnosed as caused by either Poikilorchis or Paragonimus [68]. With symptoms of a retroauricular cyst containing Poikilorchis eggs and absence of eggs in sputum, consistent with Poikilorchis only; the case was assessed to be the latter (the study authors made a similar observation). Some early publications mistakenly noted Poikilorchis cases as Paragonimus, but these were corrected in later publications for the same reason (Poikilorchis symptoms present only), and the same assessment was made here.
In total, the publications reported ca. 6400 cases of infection with Paragonimus spp. (freshwater crab hosts and human and mammalian hosts together across studies), and ca. 4800 cases in humans only. For Poikilorchis congolensis, 7 publications recorded 11 cases in total. 11 cases based solely on serology, and 3 on symptoms only were recorded as unconfirmed and excluded from totals. Mixed infections were reported from crabs in one study, also from Cameroon [44]. No reports of mixed infection in humans were found. One study from Southwest Cameroon reported eggs of P. africanus and P. uterobilateralis from patients, it was not recorded if any patient was expelling both species [69].
Locations
The majority of studies were from 4 countries: Nigeria, 53; Cameroon, 27; Liberia, 14; and Côte d’Ivoire, 11 (Fig 2). Paragonimus africanus was recorded from Cameroon, DRC, Equatorial Guinea, Gabon and Nigeria; P. uterobilateralis from Cameroon, Gabon, Guinea, Liberia and Nigeria; P. gondwanensis and P. kerberti from Cameroon; and Paragonimus sp. (where species was not specified) from Benin, Côte d’Ivoire, Gambia, Ghana, Libya, Republic of Congo, Senegal, South Africa and Tanzania, in addition to previously mentioned countries Cameroon, DRC, Equatorial Guinea, Gabon, and Nigeria.
Fig 2. Total number of studies by decade and country.
Publications of experimental studies (1970s and 1980s) or case reports where the origin of infection could not be ascertained (1940s, 1960s) are shown in grey (country = NA). For a longitudinal study, the final decade only is shown (87).
Survey period
The systematic review included all available publications since Paragonimus-like lung infections were first reported in Africa, from 1920 to the present. The total number of studies by decade were as follows: 1910s (1), 1920s (1), 1930s (3), 1940s (3), 1950s (2), 1960s (8), 1970s (34), 1980s (27), 1990s (21), 2000s (16), and 2010s (26) (and an additional publication assessed data from 1970s-2000s). Publications increased substantially in the 1970s after the outbreak of paragonimiasis in Nigeria during the Biafran war, also known as the Nigerian Civil War [19; Fig 2]. The majority of recent publications were from Nigeria (Fig 2). For Poikilorchis congolensis, apart from one relatively recent case report in the United Kingdom from a Nigerian patient [70], the remaining 6 publications dated from 1943 to 1975.
Study type
Literature by study type included: 31 case reports (sole human cases); 3 vet case reports (for sole domestic cat/dog cases); 11 case reviews (multiple cases, 3 also including surveys of crab intermediate hosts and 2 including clinical studies); 10 experimental studies; 8 clinical studies (including 3 drug treatment studies); 3 general biology studies, and 3 species descriptions. There were 70 surveys in total: 27 solely human surveys (several also including clinical studies/drug treatment trials); 4 solely livestock surveys; 8 solely non-livestock mammal surveys; 3 solely snail surveys; 8 solely crab surveys; and the remainder of 20 various combinations of above survey categories. Experimental studies were carried out in the 1970s and 1980s (Fig 2), generally with mandrills, Mandrillus leucophaeus or rhesus macaques, Macaca mulatta as the experimental host [71]. An additional 3 surveys were questionnaire-based only, not included in further data analysis. For P. congolensis, apart from the species description [41], all publications were case reports or reviews (S1 Table).
Subset analysis
Prevalence and intensity of infection in humans
Prevalence estimates were included in 42 publications, of which 31 met our criteria for further analysis (S1 Table). In this subset, the study population size range was 127–3600 (average 1091). The total number of studies by country: Nigeria (18), Cameroon (10), Liberia (1), Equatorial Guinea (1), and Gabon (1). Between 3–10 studies were carried out per decade since the 1970s (none from 1960s), and 1 survey also from the 1950s [18]. The majority of studies were cross sectional; both schoolchildren and adults were surveyed, 7 surveyed school-aged populations only. In 10 studies other hosts were also surveyed e.g., crabs. Most studies surveyed populations within a given area, especially within known endemic regions, but many compared prevalence by locality within the surveyed region.
The overall prevalence in the subset (Paragonimus uterobilateralis and P. africanus or unspecified) ranged between 0–27.0% (average: 8.4%). Average prevalence by decade, for both species combined: 1950s, 4% from 1 study only, 1960s no data, 1970s, 5.2% (5 studies), 1980s 8% (3 studies), 1990s 7.6% (8 studies), 2000s, 7.7% (6 studies), and 2010s, 12.3% (8 studies). In total, 11 publications reported a study prevalence over 10%, 1 from Cameroon [72], and the remainder from Nigeria, and 6 from recent surveys, post 2000 [22–24,26,33,73–77].
Average prevalence across studies was significantly higher overall in P. uterobilateralis: at 12.4%, range 0–27% (1670/13,443, 12 studies), than P. africanus: at 2.9%, range 0.4–9.5% (263/8978, 8 studies, P = 0.009, Table 1). The average prevalence for surveys where the species was not specified (Paragonimus sp.) was 8.7%, range 0.2–20.8% (992/11415, 7 studies from Nigeria, 3 from Cameroon). By country, prevalence of P. uterobilateralis for studies in Nigeria only at 12.5% (11 studies) was significantly higher than prevalence of P. africanus for studies in Cameroon only at 3.4% (7 studies, P = 0.008).
For the intensity of infection subset (Tables 1 and S3), 14 studies reported intensity measures in human surveys. The overall intensity of infection for P. uterobilateralis was 108.5 e/5g, Table 1, 9 studies, all in Nigeria apart from one in Liberia, [78]; for Paragonimus sp. where the species was not specified, 10.8 e/5g (4 studies). No intensity of infection data for human surveys was available for P. africanus. Range was recorded for 5 studies (Nigeria only), overall, 1–903 e/5g. In the three studies where both sputum (e/5g) and stool (epg) counts were reported, stool showed lower egg counts overall [23,78,79]. In studies where intensity was assessed by category, 3 had standard categories (low at <50 e/5g, medium at 51–100 e/5g, and high at 101+ e/5g); where the overall averages were 43.3% for low intensity, 30.6% for medium and 25.9% for high intensity [33,73,80].
Prevalence and intensity in other mammals
Mammal studies often involved sporadic or opportunistic sampling and very small sample sizes, with few systematic surveys [but see 39,66,67,81; S2 Table]. In total, 27 publications included data from mammals, of which 16 included viable prevalence data (publications excluded if the total surveyed was not given). Surveys were generally carried out in forest near settlements [40] or in case of domestic animals, at settlements themselves [5,81]. Sampling generally involved collection of scats/faecal samples, but in some cases, sacrifice of mammal hosts and resulting autopsies [34,71]. Mammals surveyed included: primates (cercopithecids, lorids), Feliformia (viverrids, herpestids); domestic animals (cats, dogs and pigs); Eulipotyphla (Crocidura) and Rodentia (Atherurus and assorted Muridae). The overall prevalences in all non-human mammal hosts were as follows: P. africanus, range 20–100%, average 43.2% (35/81, 5 studies), P. uterobilateralis, range 12.5–100%, average 35.7% (46/228, 4 studies), and Paragonimus sp., range 14–100%, average 15.5% (56/362, 6 studies). Prevalences were as follows, in Feliformia: P. africanus, range 25–100%, average 58.8% (10/17, (Civetticis civetta, Crossarchus obscurus, Nandinia binotata, 3 studies), P. uterobilateralis, range 40–100%, average 77.8% (35/45, Civetticis civetta, Crossarchus obscurus, 4 studies, and Paragonimus sp., range 20–100%, average 25.8%, Civetticis civetta, Crossarchus obscurus, Ichneumia albicauda, Atilax paludinosus, 8/31, 3 studies); and in primates, P. africanus, range, 20–50%, average 39.1% (25/64, Cercocebus torquatus, Mandrillus leucophaeus, Perodicticus potto, 3 studies), and Paragonimus sp., range 14–14.6%, average 14.4% (47/327, Papio anubis, 2 studies, Table 1). Intensity was reported in 3 studies only, 96.7 epg of P. africanus in Cercocebus torquatus [39], 17 epg of P. uterobilateralis in Civetticis civetta [82], and Paragonimus sp. 47 epg in Papio anubis [66; Table 1].
Prevalence and intensity in crabs
Several systematic crab surveys undertaken near known foci of infection were reported in the literature. Considering parasitological screening methods, generally tissue was homogenised and washed, and resulting sediment and supernatant screened for metacercariae. In 1 study, only the haemocoel was screened [83]. The overall prevalences for all genera/species pooled in freshwater crabs were as follows: Paragonimus africanus: range 0–88% (average 19.9%), (103/333 crabs surveyed total, 4 studies), P. uterobilateralis: range 0–100% (average 25.1%) (542/8300 crabs surveyed total, 14 studies). Prevalence and intensity of Paragonimus spp. varied by genera and species of crab (Table 1). For the most common crab host, Sudanonautes africanus, prevalence pooled across studies for P. africanus was significantly higher at 42.9% (103/240, 4 studies) than for P. uterobilateralis at 5.9% (P = 0.031, 156/2634, 5 studies). Overall intensity of infection in S. africanus across studies was also significantly higher for P. africanus at 54.6 metacercariae per host (106 crabs total, 2 studies) than P. uterobilateralis at 6 (P = 0.002, 34 crabs total, 2 studies; Table 1). Prevalence for P. kerberti in S. africanus was high at 32.9% (493 crabs in total, 1 study). In S. pelli, prevalence was low for both P. africanus at 1.5% (1/66, 1 study), and P. uterobilateralis at 3% (2/66, 1 study), although data were only available from 1 study which included co-infections [44].
Liberonautes has been reported as a host only for P. uterobilateralis to date. Liberonautes latidactylus had both high prevalence of P. uterobilateralis (average 24.4%, 87/357, 4 studies) and intensity of infection (117.1, 85 crabs total, 3 studies, Table 1). Other species of Liberonautes where data were available showed low prevalence (and intensity) of infection, L. chaperi had a prevalence of 2.3% (1/4l, 1 study), and L. nanoides, 0.5% (4/753, 1 study; Table 1).
Egg size measurements
In total, 37 studies included egg size measurements for Paragonimus spp., and 6 for Poikilorchis congolensis (Tables 2 and S4). The overall averages and ranges for length and width were as follows (all hosts combined, weighted averages): Paragonimus africanus: length: average 91μm (+/-3.1 SD), range min and max averages, 76.4–107.5μm; width: average 49.5μm (+/-3.1 SD), range min and max averages, 43.6–59.1μm (from 2472 eggs, 9 studies); Paragonimus uterobilateralis: length: average 69.8μm (+/-4.1 SD), range min and max averages, 58.2–79.9μm; width: average, 41.1μm(+/-2.1 SD), range min and max averages, 36.2–46.1μm (from 3159 eggs, 9 studies); Paragonimus sp.: length: average: 88.2μm (+/-12.2SD), range min and max averages, 73.4–101.1μm; width: average, 52.7μm (+/-12 SD); range min and max averages, 43.1–60.1μm (from 472 eggs, 7 studies total, Fig 3). No data were available for either P. gondwanensis or P. kerberti. For Poikilorchis congolensis: average length was 65μm (+/-7.4 SD), average width 43.3μm (+/-8.2 SD) (non-weighted average, Table 2); and range was 60–63× 40μm for all eggs apart from one measurement, at 60 × 80μm [68, S4 Table]. Measurements were similar by host, i.e., eggs isolated from humans or other mammals showed similar values (Table 2). Metacercariae measurement sample sizes were very small with high standard deviations, but show in contrast to egg measurements, P. uterobilateralis metacercariae are generally larger sizes: P. africanus 564μm (+/-228.8 SD, from Sudanonautes spp., 3 studies), P. uterobilateralis, 701.6 (+/-119.4 SD, Sudanonautes and Liberonautes, 4 studies), and Paragonimus sp. (species not specified), 468.3 (+/-144.2 SD, Sudanonautes, Liberonautes, 2 studies, Table 2).
Fig 3. Average Paragonimus spp. and Poikilorchis congolensis egg length and width measurements in μm reported in the literature, combined data from 37 studies in total.
Data from P. gondwanensis and P. kerberti data were not available therefore not shown.
From the prevalence subset data, general qualitative observations were made for any prevalence trends evident by demographics (age and sex) for human surveys. Additional observations were made on clinical symptoms, drug treatments and other diseases surveyed where reported in any publication. These observations are summarised in the following sections.
General epidemiology
Most studies in the human prevalence subset analysis examined prevalence differences by age (21 studies) and sex (19 studies). Age categories were generally in 5- or 10-year divisions (e.g., 5–10, 11–15, 16–20 year olds), but varied by study population and publication (sometimes totals were not given or reported as region sub-totals). Because of this high heterogeneity, overall findings were aggregated to 10 year periods, and summarised as general observations (S2 Table). Highest prevalences were evident overall in younger age groups, where 10–20 year olds showed highest prevalence in 12 studies in total, including a longitudinal study which reported highest prevalences in this age category for 3 consecutive years [33]; followed by 1–10 year olds in 7 studies. For the remaining 2 studies, one categorised broad age ranges, reporting highest prevalence in 15–44 year olds [75], and the other reported highest prevalence in the 40+ age category [24]. Another study also reported second highest prevalence in 55–59 year olds [84]. Apart from these studies, a sharp decline in prevalence was evident for older age groups. Often prevalence levels between age categories were similar in the younger age groups, e.g., 5-10- and 11-20-year olds showing similar prevalences [22]. Other surveys not in the subset noted a majority of cases in patients younger than 20 years [85]. Examining differences in prevalence of infection by sex, 6 studies had higher prevalence in females than males, 10 studies, vice versa, and in 2, results were equivocal. Only 8 studies in total had significant differences, 3 with higher prevalence in females (all studies in Nigeria), and 5 with higher prevalence in males (4 studies in Nigeria, 1 in Cameroon). Unlike age, patterns of sex and prevalence of infection varied by study, and findings appeared to be largely study dependent, rather than indicating sex as a demographic factor affecting infection risk.
Clinical Findings
Clinical presentation of paragonimiasis was consistent across studies. Clinical signs and symptoms were mentioned in the majority of studies, and directly analysed or described in 18 publications, including 2 primate surveys [39,66]. The recorded symptoms in order of most to least common were: cough, hemoptysis, chest pain, abdominal pain, headache, fever, dyspnoea, fatigue, uritcaria, eosinophilia, and diarrhoea [23,28,29,33,80,86–91]. Hemoptysis was very common, with 1 study also reporting that periods of egg excretion in sputum correlated with hemoptysis [92]. Another study found no evidence of hemoptysis in any paragonimiasis case, although this was based on a small sample size [28]. In terms of unusual symptoms, 5 studies reported symptoms of epilepsy/convulsions/hemiplegia or similar in study participants positive for Paragonimius infection [12,18,29,90,91]. Six studies also detailed radiological findings, such as pulmonary “shadows”; lesions and cavitations [85, 86,88,91–93]. Two studies noted that it was difficult to distinguish the radiological patterning of paragonimiasis from early TB infection [85,86], although the latter study noted evidence of subcutaneous tissue wasting in study participants with TB only.
Chemotherapies
Several publications studied effects of drug treatment, including the following chemotherapies: Niclofolan [94,95]; Tricloabendazole [96], Bithionol [94], Menichlopholan [97]; and Praziquantel [69,79,98,99], with the study population treated ranging from 6 to 360. Early treatments included emetine and chloroquine, and appeared to have had low efficacy, required repeat treatments and had significant side effects [11,16,18,94]. Later treatments included Bithionol, Menichlopholan and Niclofolan, with higher success rates, but still evidence of side effects [94,97]. Praziquantel was first trialled in 1980 [98] and found to be highly efficacious with few side-effects [69], and was used exclusively from the 1980s onwards for treatment [76,86,100].
Other diseases surveyed
Nine studies surveyed and reported on TB infections, 3 with study subjects selected from those positive for pulmonary symptoms, and 3 with randomly selected study population (not showing symptoms). Studies that also screened for TB used the Ziehl Nielsen stain for Acid Fast Bacilli (AFB) for diagnosis. Two studies reported higher prevalence of TB than paragonimiasis, 19% vs 1.5% [101], and 16.9% vs 2.7% for [102]. The remaining studies had either higher prevalence of paragonimiasis than TB [21,27,86,87,103], or only paragonimiasis with no TB infections present [88,89]. In 3 studies, co-infections of TB and paragonimiasis were evident. In Sachs et al. [104] 1 case of coinfection in 3 paragonimiasis-positive patients was found (although only 2 were tested for TB), 3 cases in 100 for Nwokolo [21], and in Ibanga et al. [27] prevalences of TB only at 1.8%, and paragonimiasis at 9.4% including 10 patients with co-infection (0.9% of the total study population of 1100). There were also reports of patients with clinical histories of treatment for TB despite being tested as negative, and identified as positive for paragonimiasis in the present study [85]. Ten human surveys and 2 mammal surveys also screened for other parasites, generally soil transmitted helminths. In these studies, Ascaris lumbricoides, Tricuris trichiura, Necator americana and Strongyloides stercoralis (in decreasing order) were common; other parasites included Schistosoma mansoni, Enterobius vermicularis, Entamoeba histolytica and Taenia saginata [7,28,29,66,67,72,88,89,98,105–107].
Mapping
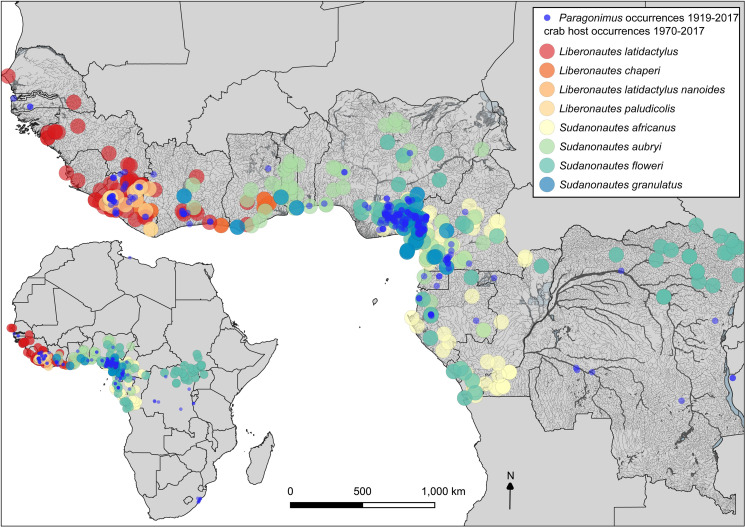
For the mapping dataset, 132 publications were included (field surveys with viable occurrences/those including field-data), and for 120 publications at least 1 locality could be georeferenced (S5 Table). In total, 390 localities were selected for mapping (5 localities being Poikilorchis congolensis occurrences). Crab host genera show a wide distribution in West and Central Africa (Sudanonautes from Côte d’Ivoire to South Sudan, and Liberonautes from Senegal to Ghana, with occurrences spanning 1970–2017 (S1 Fig). The distribution of some of the freshwater crab host species (Sudanonautes floweri) extends beyond the known foci of paragonimiasis in the rainforests into the drier woodland and savannas of central and northern Nigeria, but only 2 outliers of Paragonimus reported from this region (Fig 4).
Fig 4. Freshwater crab host occurrences by species.
1970–2017, Africa wide, and West and Central African distributions. Crab occurrence data combines the dataset from author NC (S6 Table), and records from literature via systematic review (S5 Table). All river and waterway shapefile data sourced from DIVA GIS (downloaded from http://www.diva-gis.org/).
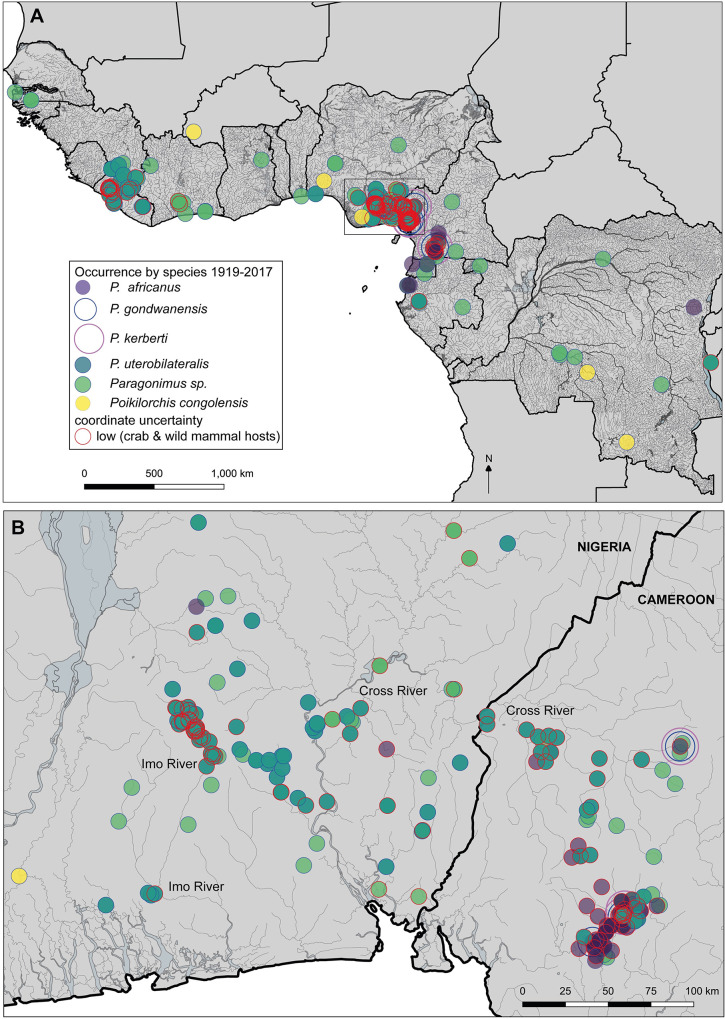
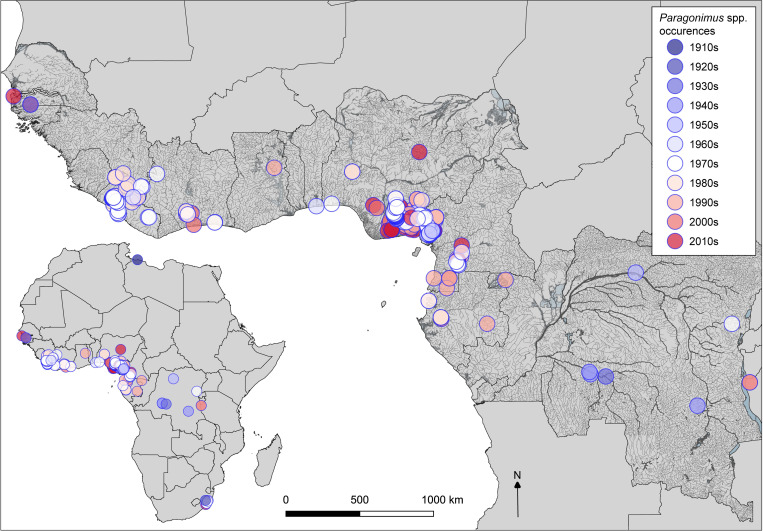
Examining Paragonimus distribution by species, P. africanus was reported mainly in Cameroon, but also in Nigeria in 2 studies [39,108], with 1 occurrence from the Cross River Basin, the other at Enugu, northwest of the endemic zone from a hospital patient [108]. In addition, sole reports were evident for DRC (no supporting data) [109]; Gabon (based on egg morphology) [110]; and Equatorial Guinea (also based on egg morphology, Fig 5) [111]. Paragonimus uterobilateralis in contrast was reported from Guinea to Gabon. Occurrences in outlier foci of KwaZulu-Natal in South Africa and Tanzania were reported as Paragonimus sp., although in Tanzania the infections, in olive baboons, Papio anubis were suspected to be Paragonimus uterobilateralis based on egg morphology [66]. Coordinate uncertainty was high for most localities, and those with low uncertainty occurrences (from non-human hosts, either crabs or wild mammals), were all evident within the rainforest zone (Fig 5). There was evidence of temporal stability with occurrences spanning several decades at endemic foci at Southeast Nigeria and Southwest Cameroon (Fig 6). These main endemic regions also show the close association of these foci with the Cross and Imo River systems in the River basin/Gulf of Guinea region (Fig 5).
Fig 5. Occurrences by species: Paragonimus spp. and Poikilorchis congolensis distribution from confirmed occurrences in the literature, 1910s- present (1919–2017).
(A), West and Central Africa, (B), close-up to the main endemic region, the southeast Nigeria/Southwest Cameroon focus in the Cross River Basin, showing the Imo River and Cross River systems. Localities with low coordinate uncertainty (e.g. from intermediate crab hosts) are shown with red outlines.
Fig 6. Paragonimus occurrences by decade, 1910s- 2010s (1919–2017).
Main endemic foci in Southeast Nigeria, Southwest Cameroon show evidence of ongoing infection spanning decades. Another key focus of infection in Liberia has not been surveyed post the 1980s as shown. No recent case occurrences were evident in the literature for DRC and Republic of Congo, as shown.
Discussion
Summary of key findings
In total, 143 papers were reviewed, where significant ongoing transmission of paragonimiasis with high prevalence and intensity of infection was uncovered, particularly for Southeast Nigeria. Across studies, we found higher prevalence overall for P. uterobilateralis than P. africanus in human surveys; variable prevalence and intensity of Paragonimus spp. infection in different crab hosts; and the potential endemicity of P. africanus in Côte d’Ivoire. In human surveys, the majority of the disease burden was consistently higher in younger age groups (<20 years). This review also suggests paragonimiasis is a wildlife disease of importance in primates and Feliformia (viverrids and herpestids) in Africa. Mapping showed the close association of Paragonimus distribution with rainforest zones, but also outliers indicating localised transmission at locations with suitable ecology for all hosts. Temporal stability in endemic foci was evident. The mapping also illustrates extensive data gaps and under-sampling, and more widespread transmission than previously reported in Cumberlidge et al. [46].
Infection in humans
We found numerous reports of significant recent transmission (1995–2016), with notably high prevalence and intensity in Southeast Nigeria in 15 studies, including 10 publications reporting prevalences over 10% [22–24,26,33,73–77]; Table 1). Recent transmission was also evident for Côte d’Ivoire (2006–2017) [40,53,112,113]; and Cameroon (1993–2014) [28,29,89,90,102]. Recent high prevalence in Nigeria has been attributed to widespread eating of uncooked freshwater crabs due to a lack of both current knowledge and health campaigns on the risks [24,114]. In rural communities in Nigeria, ongoing poverty may contribute to dependence on crabs as a food source; and inadequate sanitation in endemic regions is likely also to increase local transmission [23]. Prevalence in fact may be more widespread with under-reporting due to a lack of training of health workers to identify paragonimiasis [25,115,116]; TB surveys omitting differential diagnoses for paragonimiasis [83,117]; and reliance on microscopy for diagnosis (where low intensity cases may go undetected) rather than the more sensitive serology methods [29,117,118]. Serology is in fact a ubiquitous diagnostic for Paragonimus in Asia [2]. One relatively recent report showed evidence of co-infections of TB and paragonimiasis in Nigeria [27], supporting under-reporting by misdiagnosis as TB as a distinct possibility. Overall, these prevalence figures are closely associated with endemic foci, and given the highly focal nature of the disease should not be extrapolated nationwide [1]. Further, widespread praziquantel usage in many of the countries as chemotherapy for schistosomiasis may also be impacting paragonimiasis prevalence, although this has not been documented. However, the prevalence and intensity levels seen in these recent surveys in Africa are very troubling and would be cause for considerable attention in other less neglected tropical diseases [1].
Another unexpected result was the significantly higher overall prevalence of P. uterobilateralis at 12.4% than P. africanus at 2.9% (P = 0.009). For 11 surveys where the species identity was not reported, overall prevalence was 8.7%. As most of these studies took place in Nigeria, the majority were likely to be P. uterobilateralis (Fig 5), which fits the trend of higher prevalence of this species. This difference also reflects higher endemicity in Nigeria with overall prevalence of 12% (for P. uterobilateralis), vs P. africanus in Cameroon at 3.9%. Reasons for higher prevalence in P. uterobilateralis are unclear. Paragonimus uterobilateralis is much more broadly distributed, with a wider crab host range (Figs 4 and S1), but P. africanus may be present in other foci, and there may be inaccuracies in species identification in some studies. Molecular analyses are required to ascertain true distribution.
In other mammals, prevalence was variable and sampling generally sporadic with very small sample sizes, but prevalence was higher than in humans. Infections were primarily reported in Feliformia (viverrids and herpestids); primates (cercopithedids and lorids), and less commonly in domestic animals (cats, dogs and pigs). In Bakuza [66], olive baboons were observed eating freshwater crabs at Lake Tanganyika, identified as Potamonautes (although not screened for infection). The infected baboons showed evidence of advanced emaciation and high intensity of infection [119], suggesting a substantial worm burden. Friant et al. [39] found higher coughing frequency in infected Cercocebus torquatus in Nigeria, and suggested the primates may maintain local transmission as sylvatic hosts. The importance of paragonimiasis as a wildlife disease and the role of sylvatic hosts/degree of zoonotic transmission has not yet been fully realised. Further studies on the disease in mammals are needed using non-invasive faecal sampling [46].
Considering clinical findings and general epidemiology, a clear-cut association of higher prevalence with younger age groups was evident apart from two studies [24,84], where the timing of onset of infection would fit with those infected as children during the Biafran war. Five studies reported epilepsy/convulsive episodes/hemiplegia in paragonimiasis-positive patients, suggesting the possibility of extrapulmonary cerebral localisation [12,18,29,90,91]. In one of these studies, 2 cases were in fact assessed to be cerebral paragonimiasis [18]. Extrapulmonary (and cerebral) paragonimiasis has been frequently reported in Asian paragonimiasis [1,2], but appears to be rare in Paragonimus infections in Africa [48]. Nevertheless, recent reports of high rates of epilepsy in endemic regions [84] and incidences in paragonimiasis positive patients [29] requires further study.
Infection in crabs
Both prevalence and intensity varied within genera according to Paragonimus species transmitted (Table 1). The most abundant host species Sudanonautes africanus had significantly higher prevalence (P = 0.031) and intensity (P = 0.002) of P. africanus than P. uterobilateralis infections overall. In Liberonautes, L. latidactylis showed high prevalence and intensity of P. uterobilateralis infections and in contrast L. nanoides very low levels of infection (Table 1) [120]. This presumably reflects susceptibility differences, which in turn would suggest crab species host range is a critical factor in Paragonimus distribution, although the key species have a wide range (Fig 4). Freshwater crabs are found throughout the range of Paragonimus in West and Central Africa, and it appears that the trematode is not host specific, developing in a number of crab species depending on which are found in the focus of disease [10]. The different species of Paragonimus may also adapt locally more to one host or another, resulting in local-scale differences in susceptibility. Data from co-infected Sudanonautes africanus/pelli in Mejek, Cameroon showed higher intensity of P. africanus within crabs in localities where it was more prevalent overall, likewise for P. uterobilateralis [44]. It is not clear what is driving the predominance of either species of Paragonimus in this region where they overlap, potentially an interaction of factors at a local scale. It would be worthwhile to investigate these processes further in mixed infection foci.
Another notable finding in the literature is evidence of up to 100% prevalence and high intensity in crabs within endemic foci, e.g., the Lower Bakossi, Cameroon [44]. This phenomenon has also been recorded at some foci for Asian Paragonimus [2]. We hypothesise that evidence of highly concentrated prevalence and intensity in crabs in endemic foci suggests accumulation of the parasites in the crab over time. This would fit with a food-borne transmission pathway in the crabs, whereby the infected freshwater/semi-terrestrial snail host is eaten. Indeed, there is evidence that Sudanonautes crabs in Nigeria feed on freshwater molluscs [121]. Alternatively, cercariae may leave an infected snail, either actively homing to a crab host, or adopting a sit-and-wait approach. For example, if snail hosts are freshwater-dwelling, once leaving the snail cercariae could disperse and detect freshwater crabs within waterbodies, or if the snail host is semi-aquatic, by crawling on damp vegetation. Waterborne cercariae are a common life history strategy in trematodes (e.g., Opisthorchis), enabling wide dispersal. The exact path of transmission at this point is not even known in the better studied Asian Paragonimus species however [1].
The role of crabs in epidemiology overall is key, as metacercariae can persist in crabs for years, and intensity of infection can determine infectivity [4]. There are however, few recent studies of Paragonimus infection in crabs [28,83,122], the last systematic studies being in the early 1990s [120,123–125]. Recent studies have suggested that parasite intensity has declined in crab hosts [28], but there are insufficient recent data to compare with the surveys of Voelker & Sachs for example [44]. Factors such as deforestation, hunting, pollution and waterway alterations are likely negatively impacting intermediate and definitive hosts of Paragonimus. Mammals in particular may have declined locally in areas under intensive hunting pressure [126]. However prevalences in humans do not show a decline and further investigation is needed to understand the overall picture.
Egg/Metacercariae morphology and species distribution
Trends in egg size should be regarded with caution with potential misidentification, given overlap in egg size morphology; and that egg size can be affected by fixation/preparation method; and potentially by host [127]. For the identification of African Paragonimus species however, egg (or metacercariae) morphology is generally the only method or data available. For Côte d’Ivoire, egg measurements of Paragonimus show a large size range (Fig 3, Tables 2 and S4); [128]. The variation may indicate the presence of both P. africanus and P. uterobilateralis in Côte d’Ivoire. Further, metacercariae with morphology consistent with P. africanus (i.e., thick-walled cysts) were evident from studies where the metacercariae were isolated from Liberonautes/Potamonautes (see Fig 5 in [40]). Both Sudanonautes and Liberonautes crab hosts are present in Côte d’Ivoire, the only country where they overlap and where Paragonimus has been found, apart from one livestock report from Ghana [81]. Paragonimus africanus has only previously been reported from Cameroon, Nigeria, DRC [109], Gabon [110] and Equatorial Guinea (Fig 5); [111]. Potentially P. africanus is present in Côte d’Ivoire, but alternatively it may be an undescribed species [40,129,130]. Other possibilities include a hybrid of P. africanus and P. uterobilateralis, or even a species complex (as may be the case for P. westermani in Asia) [1,2]. Overall Côte d’Ivoire presents a complex disease landscape. Here Paragonimus infections have also been reported in the brackish-water swimming crab Callinectes [53,64,112] but may be the same unidentified trematode in Traoré et al. [56]; (see Fig 35 in [131]; Fig 2 in [112]). Clearly molecular studies are required to clarify Paragonimus taxonomy in this region [40].
Unlike Paragonimus, Poikilorchis congolensis egg size showed little variation, almost uniformly 60–63 × 40um (Table 2). Potentially it also has a crab intermediate host, and there is evidence of overlapping distribution with paragonimiasis [132], but very little is known of general biology. Oyediran et al. [42] observed that the characteristic retroauricular cysts where eggs are effectively trapped could represent an evolutionary dead-end for the parasite in humans, although Schuster et al. [70] also found evidence of eggs in stool. Work is needed to ascertain the definitive host. If there are many questions for Paragonimus in Africa, there are many more for Poikilorchis. With benign symptoms and limited public health impact underreporting may be high, particularly given the main endemic region appears to be DRC; but it does seem to be a rare parasite. It is however certainly worthy of separate investigation.
Distribution mapping
Mapping revealed few, but widespread outliers. Most occurrences were closely associated with endemic regions in rainforest zones [133] particularly low-uncertainty ones (Fig 5), illustrating the key importance of rainforest cover. However outliers also suggest localised transmission where ecology is favourable, such as cases in South Senegal and Gambia, although crabs have yet to be surveyed in this region. The main endemic foci in Nigeria and Cameroon are closely associated with the Imo and Cross River catchments (Fig 5). Endemic zones essentially represent regions of overlapping host distribution, with suitable ecological conditions for all hosts. Further studies on the ecology of the catchments are required to understand the disease landscape in this region. Although freshwater crab intermediate hosts are present in the Niger River catchment and Southwest Nigeria (Fig 4; S1 Fig), only 1 study has found infected crabs in these areas [134]. Crab hosts are also present in northern Nigeria, but only one report of Paragonimus (in livestock) evident [135]. Here limiting factors may be snail host distribution; or given that these regions are outside of the main rainforest zone, mammal host abundances may be low. Endemic foci also show temporal stability (Fig 6), similar to Asian Paragonimus species, e.g., at Sin Ho in Vietnam [2].
Paragonimus uterobilateralis showed a more widespread distribution overall, P. africanus in contrast more geographically contained, within tightly clustered endemic foci (Fig 5). Liberia showed more dispersed occurrences, due potentially to its more extensive inland forest [133]. Gaps were evident, with no records between Nigeria and Côte d’Ivoire, apart from Ghana [81], but in a domestic pig, and a case in Benin, but potentially acquired via market crabs from Nigeria [64]. These outlier cases should be confirmed with further studies. Likely ecological factors are in play with the markedly different ecology of the Dahomey gap in the region, with much drier conditions and forests fragmented with savannah [136]. However, this could be partly due to sampling gaps, illustrated overall in the mapping (Figs 4–6). DRC for example has the most extensive rainforest in Africa, but sparse occurrences have been recorded, spanning an immense area. Moving further east, evidence of Paragonimus in primates in Tanzania [66] suggests potential for contiguous distribution from the Central African forests at least to Lake Tanganyika. Additionally, there is a focus in KwaZulu-Natal, South Africa [47]. Here potential freshwater crab hosts belong to Potamonautes [137], but no studies have screened these or any potential intermediate hosts for Paragonimus (Chris Appleton, pers. comm.). Little is known of the distribution of Paragonimus outside of the recognised endemic regions. Paragonimus spp. could be distributed far more widely than the known foci in West and Central Africa.
First intermediate host
One of the more intriguing aspects of paragonimiasis in Africa is that the first intermediate snail host is yet to be identified [46]. Studies investigating snail hosts so far include the following as potential species: Afropomus balanoidea [34], Homorus (Striosubulina) striateila [35,36], Potadoma freethii [26,31,32], Potadoma sanctipauli [34], Achatinidae [30], and Achatina achatina (Roger Moyou-Somo pers. comm.). Some of these studies may have actually identified microcercous cercariae of Paragonimus in molluscs [30,35], but morphological and molecular data in combination are required for confirmation. As Asian Paragonimus has a wide host range within Rissooidea and Cerithioidea [9], African species may also infect a range of snails, contrary to the suggestion of Cumberlidge et al. [46] of a specific snail host range. The authors also proposed the first intermediate host snail may be amphibious/semi-terrestrial rather than fully aquatic, inhabiting the moist conditions of the tropical forest floor and picking up miracidia from a scat, in turn consumed by a semi-terrestrial freshwater crab, thereby infecting the second intermediate host. Transmission may even occur in both freshwater and semi-terrestrial snail species, especially given a likely wide snail host range.
Limitations of study
Overall, reported prevalences are potentially underestimates, partly because they are based on microscopy only which lacks sensitivity. Improved diagnostics will likely lead to evidence of greater numbers of cases. However, the high prevalences reported in recent surveys also reflects a sampling bias toward high endemicity areas. Intensity in crabs is complicated by age (size) of crabs, with studies showing higher intensity in larger crabs [44,108,138], but there were insufficient individual measurements for further analysis. Existing bias within studies includes possibility of species misidentification; therefore higher prevalence of P. uterobilateralis would need to be confirmed with longitudinal molecular studies. In terms of potential bias in our methods, we analysed P. kerberti and P. gondwanensis as separate species, although they should be regarded as currently unconfirmed. Another potential bias is our estimate of study period where not reported, and temporal inaccuracy when infections were acquired when onset of symptoms was not clear in case reports. This was mitigated by assessing time period by decade only. All these factors may affect the quantitative data presented here, which may result in cumulative potential bias in our analysis. Further, our quantitative analysis does not represent a meta-analysis, not feasible given the array of non-comparable methods and data gaps, and the quantitative findings should duly be regarded as requiring confirmation in additional studies. As a general point, conflict and political instability may have impacted survey coverage. Paragonimiasis is most likely present in Sierra Leone, although to date no surveys have been carried out to our knowledge; likewise in the DRC, all occurrences obtained from historical case reports only; and no recent surveys have been done in Liberia. These inherent sampling gaps reflect the state of the published literature, and could have been mitigated by expanding searches into ‘grey’ literature such as national Ministries of Health records, potentially useful particularly for countries like DRC where paragonimiasis surveys have not been published. A further study limitation was use of Google Translate for a limited number of works, which may have limited our understanding of some non-English publications. Overall however, the risk of bias at the study selection stage was low. Our search included all languages and some of the contributions included here were challenging to obtain. Undoubtedly some publications with medical implications have remained overlooked until now. Here we present a near-comprehensive literature review of all publications to date on African Paragonimus that considerably expands and updates the recent study by Cumberlidge et al. [46].
Conclusions and future directions
Our systematic review has strengthened the existing body of knowledge on paragonimiasis in West and Central Africa. In terms of future work, molecular studies are urgently required to address major knowledge gaps in general biology. Utilising molecular methods such as metabarcoding and environmental DNA sampling could also help narrow the field of potential first intermediate hosts, which could then be intensively examined with dissection as prevalences may be low in the snail host, as seen in schistosomiasis [139]. Metabarcoding of infected crabs could also provide an approach to investigate potential first intermediate hosts via identification of diet items of the host crab species, this being a potential transmission pathway. In terms of mapping and spatial analysis, bayesian spatial modelling could illuminate potential Paragonimus distribution outside key endemic zones. Extensive human surveys in endemic foci, aided by better diagnostics, are also needed to establish the true extent of human infection, and treatment and control need greater consideration. As paragonimiasis is a foodborne disease, much could be done to promote safer eating practices. In the wake of the COVID-19 crisis, there is a renewed focus on zoonotic transmission and the One Health approach. Paragonimiasis-endemic regions, such as Cameroon, Nigeria and Côte d’Ivoire have a public health landscape of risk of infection with multiple NTDs as well as infectious diseases like malaria, TB and others. To achieve goals on reducing transmission of the NTDs, particularly in a One Health context, there is a need to understand all these diseases collectively. This is a call to action for the international NTD community to commit programmes of research into paragonimiasis in Sub-Saharan Africa.
Supporting information
Data compiled from the literature (S5 Table) and an the occurrence dataset from NC (S6 Table).
(TIF)
Full list of all publications where the full text was assessed, including those excluded from the final list with reasons given, is provided.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Includes all georeferenced records of Paragonimus spp., and absence data (records where infection surveyed for but not present).
(XLSX)
(XLSX)
(DOC)
Acknowledgments
The authors are immensely grateful to the following people for their assistance: Rosie Jones, John Rose, Angela Thresher, Ben Nathan, Helen Pethers and Nial Briggs at the NHM library for assistance with interlibrary loans and scanning maps; authors David Aka, David Blair and Chris Appleton for sending publications; Chiho Ikebe for translation of Yamamoto et al. (1979), and authors Agathe Nkouawa, Richard Bradbury, Fabrice Zobel Cheuyem Lekeumo, Basil Okeahialam, Saffiatoe Darboe and Roger Moyou-Somo for sending additional data from published works.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Blair D. Paragonimiasis In: Toledo R, Fried B. (eds.) Digenetic Trematodes. Advances in Experimental Medicine and Biology. Springer Nature Switzerland AG, Cham, Switzerland, 2019; 1154:105–138. 10.1007/978-3-030-18616-6_5 [DOI] [PubMed] [Google Scholar]
- 2.Yoshida A, Doanh PN, Maruyama H. Paragonimus and paragonimiasis in Asia: An update. Acta tropica. 2019. November 1;199:105074 10.1016/j.actatropica.2019.105074 [DOI] [PubMed] [Google Scholar]
- 3.WHO. Control of foodborne trematode infections. Report of a WHO study group. World Health Organ. Technical Report Series. 1995; 849:1–157. . [PubMed]
- 4.Yokogawa S, Cort WW, Yokogawa M. Paragonimus and paragonimiasis. Experimental Parasitology. 1960; 10(2):139–205. 10.1016/S0065-308X(08)60364-4 [DOI] [PubMed] [Google Scholar]
- 5.Sachs R, Cumberlidge N. The dog as natural reservoir host for Paragonimus uterobilateralis in Liberia, West Africa. Annals of Tropical Medicine and Parasitology. 1990a; 84(1):101–102. 10.1080/00034983.1990.11812440 [DOI] [PubMed] [Google Scholar]
- 6.Voelker J, Sachs R, Volkmer KJ, Braband H. Zur Epidemiologie der Paragonimiaais bei Mensch und Tier in Nigeria, Westafrika. Veterinär-medizinische Nachrichten. 1975; 1/2:158–172. [Google Scholar]
- 7.Vogel H, Crewe W. Beobachtungen über die Lungenegel-Infektion in Kamerun (Westafrika). Zeitschrift für Tropenmedizin und Parasitologie. 1965; 16(2):109–125. . [PubMed] [Google Scholar]
- 8.Voelker J, Vogel H. Zwei neue Paragonimus-Arten aus West-Africa: Paragonimus africanus und Paragonimus uterobilateralis (Troglotrematidae, Trematoda). Zeitschrift für Tropenmedizin und Parasitologie. 1965; 16:125–147. [PubMed] [Google Scholar]
- 9.Blair D, Davis GM, Wu B. Evolutionary relationships between trematodes and snails emphasizing schistosomes and paragonimids. Parasitology. 2001; 123(7):229–243. 10.1017/s003118200100837x . [DOI] [PubMed] [Google Scholar]
- 10.Cumberlidge N. The freshwater crabs of West Africa: family Potamonautidae. Faune et Flore tropicales 35, 1999. IRD, Paris.
- 11.Nwokolo C. Paragonimiasis in Eastern Africa. Journal of Tropical Medicine and Hygiene. 1964; 67(1):1–4. [PubMed] [Google Scholar]
- 12.Onorato R. La paragonimiasi in Tripolitania. Archivio Italiano di Scienze Mediche Coloniali 1920; 1:1–13. [Google Scholar]
- 13.Mönnig, H. O. Check list of the worm parasites of domesticated animals in South Africa. Pretoria: Government Printer and Stationery Office. 13th and 14th Reports of the Director of Veterinary Education and Research, 1928; 2:801–840.
- 14.Libert CEMJ. A Case of Paragonimiasis. West African Medical Journal. 1932; 5(3):51. [Google Scholar]
- 15.van Hoof L. Case of Paragonimus infection in Belgian Congo. Annales de la Société Belge de Médecine Tropicale. 1933; 13:473–478. [Google Scholar]
- 16.Bertrand J. Distomatose pulmonaire ou hémoptysie pulmonaire à Paragonimus. Annales de la Société Belge de Médecine Tropicale. 1947; 27:1–4. [Google Scholar]
- 17.Cannon D. A. Eggs of Paragonimus in sputum of patient in the Cameroons. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1949; 42(4):322. [Google Scholar]
- 18.Zahra A. Paragonimiasis in the Southern Cameroons: a Preliminary Report. West African Medical Journal. 1952; 1(2):75–82. [Google Scholar]
- 19.Nwokolo C. Endemic paragonimiasis in Africa. Bulletin of the World Health Organization. 1974; 50(6):569–571. . [PMC free article] [PubMed] [Google Scholar]
- 20.Nwokolo C. Outbreak of paragonimiasis in Eastern Nigeria. The Lancet. 1972a; 299(7740):32–33. 10.1016/s0140-6736(72)90017-7 . [DOI] [PubMed] [Google Scholar]
- 21.Nwokolo C. Endemic paragonimiasis in Eastern Nigeria. Clinical features and epidemiology of the recent outbreak following the Nigerian civil war. Tropical and Geographical Medicine. 1972b; 24(2):138–47. . [PubMed] [Google Scholar]
- 22.Iboh CI. Investigation of Crab-eating Influence on Paragonimiasis Infection in Six Communities of Abayong from Cross River State Nigeria. South Asian Journal of Parasitology. 2018; 1(1):1–9. 10.9734/SAJP/2018/41742 [DOI] [Google Scholar]
- 23.Eze JC. Epidemiological Pattern of Presentation of Paragonimus Infection in the Human Host in South East Nigeria and Their Correlative Sonographic Findings, 2015; University of Nigeria, Thesis.
- 24.Uttah EC, Etim SE, Ibe DC. Familial and occupational clustering of paragonimiasis in a riverine community in Eastern Nigeria. Transnational Journal of Science and Technology. 2013; 3(1):25–35. [Google Scholar]
- 25.Eke RA, Udochi I, Nwosu M, Enwereji EE, Emerole CV. Paragonimiasis Reemergence in Nigeria: Predisposing Factors and Recommendations for Early Intervention and Everlasting Eradication. ISRN Infectious Diseases. 2013; 2013(257810): 4 pages. 10.5402/2013/257810 [DOI] [Google Scholar]
- 26.Asor JE, Ibanga ES, Arene FI. The Epidemiology of Pulmonary Paragonimiasis in Cross River Basin in Nigeria: Update on infection prevalence and distribution of the snail and crab intermediate hosts. Mary Slessor Journal of Medicine. 2003a; 3(1):5–12. 10.4314/msjm.v3i1.10988 [DOI] [Google Scholar]
- 27.Ibanga ES, Arene FOI, Asor JE. Association of pulmonary paragonimiasis with active pulmonary tuberculosis in rural Yakurr community in Cross River Basin, Nigeria. Mary Slessor Journal of Medicine. 2003; 3(1):19–23. [Google Scholar]
- 28.Moyou-Somo R, Mfouapong-Ewane HB, Nkoa T, Etaluka-Mungo B, Kum-Kan W, Kefie-Arrey C. A new focus of pleuro-pulmonary paragonimiasis in Manjo Health District, Littoral Region of Cameroon. International Journal of Tropical Disease and Health. 2014; 4(9):963–972. 10.9734/IJTDH/2014/11252 [DOI] [Google Scholar]
- 29.Nkouawa A, Okamoto M, Mabou AK, Eding E, Yamasaki H, Sako Y, et al. Paragonimiasis in Cameroon: molecular identification, serodiagnosis and clinical manifestations. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009; 103(3):255–261. 10.1016/j.trstmh.2008.09.014 . [DOI] [PubMed] [Google Scholar]
- 30.Moyou-Somo R, Guemgne-Simo G. Paragonimiasis in southwest Cameroon: isolation of microcercous cercariae from land snails caught in a Paragonimus africanus endemic zone. Wilderness and Environmental Medicine. 1995; 6(1):44–47. 10.1016/S1080-6032(13)80008-7 [DOI] [Google Scholar]
- 31.Moyou-Somo R, Enyong PA, Kouamouo J. Isolation of Paragonimus africanus cercariae from a wild caught mollusc Potadoma freethi. XI Intentional Congress of Tropical Medicine and Malaria, Canada: Calgary. 1984; pp. 208.
- 32.Arene FO, Ibanga ES, Asor JE. Freshwater snail and crab intermediate hosts of Paragonimus species in two rural communities in Cross River Basin Nigeria. Global Journal of Pure and Applied Sciences. 1999; 5:184–188. [Google Scholar]
- 33.Udonsi JK. Endemic Paragonimus infection in the Upper Igwun Basin, Nigeria: a preliminary report on a renewed outbreak. Annals of Tropical Medicine and Parasitology. 1987; 81(1):57–62. 10.1080/00034983.1987.11812091 . [DOI] [PubMed] [Google Scholar]
- 34.Voelker J. Morphologisch-taxonomische Untersuchungen über Paragonimus uterobilateralis (Trematoda, Troglotrematidae) sowie Beobachtungen über den Lebenszyklus und die Verbreitung des Parasiten in Liberia.Tropenmedizin und Parasitologie. 1973; 24:4–20. [PubMed] [Google Scholar]
- 35.Sachs R, Cumberlidge N. Isolation of microcercous cercariae from land snails caught in an endemic focus of P. uterobilateralis in Liberia, West Africa. Tropenmedizin und Parasitologie. 1989; 40(1):69–72. . [PubMed] [Google Scholar]
- 36.Sachs R, Cumberlidge N. Notes on the ecology of Homorus striatellus (Rang, 1831), snail host of microcercous cercariae in Liberia, West Africa. Zeitschrift für Angewandte Zoologie. 1991a;78(1):45–53. [Google Scholar]
- 37.Bayssade-Dufour C, Chermette R, Šundić D, Radujković BM. Paragonimus gondwanensis n. sp. (Digenea, Paragonimidae), parasite of mammals (humans and carnivores) in Cameroon. Ecologica Montenegrina. 2014; 1(4):256–267. 10.37828/em.2014.1.34 [DOI] [Google Scholar]
- 38.Bayssade-Dufour C, Chermette R, Šundić D, Radujković BM. Paragonimus kerberti n.sp. (Digenea, Paragonimidae), parasite of carnivores in Cameroon. Ecologica Montenegrina. 2015; 2(3):71–277. [Google Scholar]
- 39.Friant S, Brown K, Saari MT, Segel NH, Slezak J, Goldberg TL. Lung fluke (Paragonimus africanus) infects Nigerian red-capped mangabeys and causes respiratory disease. International Journal for Parasitology: Parasites and Wildlife. 2015; 4(3):329–332. 10.1016/j.ijppaw.2015.08.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aka NA, Sanogho Y, Yapo-Kouadio CG, Dou GSP, Zika KD, Adoubryn KD. Persistance du foyer de distomatose pulmonaire a Lakota (Côte d’Ivoire): cas du village de Gbahiri. Bio-Africa Revue. 2018; 17):54–59. [Google Scholar]
- 41.Fain A, Vandepitte J. A new trematode: Poikilorchis congolensis, n.g., n.s., living in subcutaneous retrouricular cysts in man from the Belgian Congo. Nature. 1957; 179:740 10.1038/179740a0 . [DOI] [PubMed] [Google Scholar]
- 42.Oyediran ABOO Fajemisin AA, Abioye AA Lagundoye SB, Olugbile AOB. Infection of the mastoid bone with a Paragonimus-like trematode. American journal of tropical medicine and hygiene. 1975; 24(2):268–273. 10.4269/ajtmh.1975.24.268 . [DOI] [PubMed] [Google Scholar]
- 43.van Nieuwenhuyse E, Gatti F. A case of chronic mastoid infestation by a rare parasite (Poikilorchis congolensis). Acta OtoLaryngologica. 1968; 66(5):444–448. 10.3109/00016486809126309 . [DOI] [PubMed] [Google Scholar]
- 44.Voelker J, Sachs R. Über die Verbreitung von Lungenegeln, Paragonimus africanus und P. uterobilateralis, in WestKamerun und OstNigeria auf Grund von Untersuchungen an Süsswasserkrabben auf Befall mit Metacercarien. Tropenmedezin und Parasitologie. 1977a; 28(1):29–133. . [PubMed] [Google Scholar]
- 45.Kittur N, King CH, Campbell CH Jr, Kinung’hi S, Mwinzi PN, Karanja DM, et al. Persistent hotspots in schistosomiasis consortium for operational Research and evaluation studies for gaining and sustaining control of schistosomiasis after four years of mass drug administration of praziquantel. American Journal of Tropical Medicine and Hygiene. 2019; 101(3):617–627. 10.4269/ajtmh.19-0193 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cumberlidge N, Rollinson D, Vercruysse J, Tchuem Tchuenté L-A, Webster B, Clark PF. Paragonimus and paragonimiasis in West and Central Africa: unresolved questions. Parasitology. 2018; 145(13):1749–1757. 10.1017/S0031182018001439 . [DOI] [PubMed] [Google Scholar]
- 47.Appleton CC. Paragonimiasis in KwaZulu-Natal province, South Africa. Journal of Helminthology. 2014; 88(1):123–128. 10.1017/S0022149X12000831 . [DOI] [PubMed] [Google Scholar]
- 48.Mazigo HD, Morona D, Kweka EJ, Waihenya R, Mnyone L, Heukelbach J. Epilepsy and tropical parasitic infections in Sub-Saharan Africa: a review. Tanzania Journal of Health Research. 2013; 15(2): 102–19. 10.4314/thrb.v15i2.5 . [DOI] [PubMed] [Google Scholar]
- 49.Aka NA, Adoubryn K, Rondelaud D, Dreyfuss G. Human paragonimiasis in Africa. Annals of African Medicine. 2008a; 7(4):153–162. 10.4103/1596-3519.55660 . [DOI] [PubMed] [Google Scholar]
- 50.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine. 2009; 6(7):e1000097 10.1371/journal.pmed.1000097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pozio E. Current status of food-borne parasitic zoonoses in Mediterranean and African regions. Southeast Asian Journal of Tropical and Medical Public Health. 1991; 2285–87. . [PubMed] [Google Scholar]
- 52.Goldsmid JM. Ecological and cultural aspects of human trematodiasis (excluding schistosomiasis) in Africa. The Central African Journal of Medicine. 1975; 21(3):49–53. . [PubMed] [Google Scholar]
- 53.Aka NA, Assoumou A, Adoubryn KD., Domoua K, Kouadio F, Moyou-Somo R, et al. documents sur la séroépidémiologie de la paragonimose humaine au centre anti-tuberculeux de Divo, République de Côte d’Ivoire (Afrique de l’Ouest). Parasite. 2008c; 15(2):157–161. 10.1051/parasite/2008152157 . [DOI] [PubMed] [Google Scholar]
- 54.Malvy D, Ezzedine KH, Receveur MC, Pistone T, Mercie P, Longy-Boursier M. Extra-pulmonary paragonimiasis with unusual arthritis and cutaneous features among a tourist returning from Gabon. Travel Medicine and Infectious Disease. 2006; 4(6):340–342. 10.1016/j.tmaid.2006.01.003 . [DOI] [PubMed] [Google Scholar]
- 55.Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al. Welcome to the Tidyverse. Journal of Open Source Software. 2019;4(43):1686 10.21105/joss.01686 [DOI] [Google Scholar]
- 56.Traoré SG, Koussemon M, Odermatt P, Aka ND, Adoubryn KD, Assoumou A, Dreyfuss G, Bonfoh B. Risque de contraction de trématodoses alimentaires avec la consommation des crustacés vendus sur les marchés d’Abidjan. Revue Africaine de Santé et de Productions Animales. 2010; 120:514–520. [Google Scholar]
- 57.Hijmans RJ, Guarino L, Cruz M, Rojas E. Computer tools for spatial analysis of plant genetic resources data: 1. DIVA-GIS. Plant Genetic Resources Newsletter. 2001; 127:15–19. [Google Scholar]
- 58.Sachs R, Distribution of Paragonimus uterobilateralis metacercariae in Liberonautes latidactylus, Annual Report 1985, Liberia Research Unit of the Tropical Institute Hamburg 1985c:4–47.
- 59.Chukwu O, Degiri A, Uzoigwe RN, Chukwe ID, Ogo IN, Ojemudia IT, et al. Polyparasitic infections in school children of Equatorial Guinea. International Journal of Natural and Applied Sciences. 2009; 5(1). 10.4314/ijonas.v5i1.49948 [DOI] [Google Scholar]
- 60.N’Dzossi AAC. Observations clinique et épidémiologique du premier cas de paragonimose humaine au Congo-Brazzaville (Doctoral dissertation). University of Bordeaux. 2000.
- 61.Bosse, D. Endémicité et parasitoses autochtones en Afrique noire: à propos d’un foyer insolite de distomatose pulmonaire en Côte d’Ivoire. (Doctoral dissertation). University of Montpellier, 1984, 112 p.
- 62.Sam-Abbenyi A. Paragonimose endemique dans le Lower Mundani, arrondissement de Fontem, une etude preliminaire. Bulletin de liaison et de documentation de l’OCEAC. 1982; 52:42–44. [Google Scholar]
- 63.Morter R, Adetifa I, Antonio M, Touray F, De Jong BC, Gower CM, Gehre F. Examining human paragonimiasis as a differential diagnosis to tuberculosis in the Gambia. BMC Research Notes. 2018; 11:31 10.1186/s13104-018-3134-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aka NA, Allabi AC, Dreyfuss G, Kinde-Gazard D, Tawo L, Rondelaud D, et al. Observations épidémiologiques sur le premier cas de paragonimose humaine et les hôtes intermédiaires potentiels de Paragonimus sp. au Bénin. Bulletin de la Société de Pathologie Exotique et de ses filiales. 1999; 92(3):191–194. . [PubMed] [Google Scholar]
- 65.Traoré SG, Odermatt P, Bonfoh B, Utzinger J, Adoubryn KD, Assoumou A, et al. No Paragonimus in high-risk groups in Côte d’Ivoire, but considerable prevalence of helminths and intestinal protozoon infections. Parasites & Vectors. 2011;4(1):96 10.1186/1756-3305-4-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bakuza JS. Epidemiology of Schistosoma mansoni infection in sympatric humans and non-human primates in the Gombe ecosystem Tanzania. 2012; University of Glasgow. PhD thesis. http://theses.gla.ac.uk/3652/
- 67.Müller-Graf CDM, Collins DA, Woolhouse MEJ. Intestinal parasite burden in five troops of olive baboons (Papio cynocephalus anubis) in Gombe Stream National Park, Tanzania. Parasitology. 1996; 112(5): 489–497. 10.1017/s0031182000076952 . [DOI] [PubMed] [Google Scholar]
- 68.Loubiere R, Colette J, Plouvier S, Baudoin C, Sellin B, Prost A. Un Premier Cas De Distomatose En Haute-Volta. Bulletin de la Société de Pathologie Exotique et de ses Filiales. 1976; 68:(5)471–474. [PubMed] [Google Scholar]
- 69.Sam-Abbenyi A. Endemic pulmonary paragonimiasis in Lower Mundani (Fontem district of southwest Cameroon). Results of treatment with praziquantel. Bulletin de la Société de Pathologie Exotique et de ses filiales. 1985; 78(3):334–341. . [PubMed] [Google Scholar]
- 70.Schuster H, Agada FO, Anderson AR, Jackson RS, Blair D, McGann H, Kelly G. Otitis media and a neck lump—Current diagnostic challenges for Paragonimus-like trematode infections. Journal of Infection. 2007; 54(2):e103–6. 10.1016/j.jinf.2006.05.011 . [DOI] [PubMed] [Google Scholar]
- 71.Voelker J, Sachs R. Affen als natürliche und experimentelle Endwirte afrikanischer Lungenegel (Paragonimus africanus und P. uterobilateralis) Tropenmedizin und Parasitologie. 1977; 28:137–144. . [PubMed] [Google Scholar]
- 72.Timsit D. La paragonimose pulmonaire au Cameroun. Médecine Tropicale. 1978; 38:287–297. [PubMed] [Google Scholar]
- 73.Uttah EC. Paragonimiasis and Renewed Crab-Eating Behavior in Six Communities from Two Ethnocultural Clusters in Southeastern Nigeria. International Scholarly Research Notices, 2012; 2013:5 p. 10.5402/2013/569485. [DOI] [Google Scholar]
- 74.Okoro N, Onyeagba RA, Anyim C, Eda OE, Okoli CS, Orji I, et al. Prevalence of Paragonimus Infection. American Journal of Infectious Diseases. 2013; 9(1):17–23. 10.3844/ajidsp.2013.17.23 [DOI] [Google Scholar]
- 75.Abraham JT, Akpan PA. Current status of paragonimiasis in Old Netim 1 Akamkpa Local Government Area of Cross River State-Nigeria. Global Journal of Medical Sciences. 2009; 8(1, 2):17–22. [Google Scholar]
- 76.Asor JE, Ibanga ES, Arene FOI. Paragonimus uterobilateralis: peak period of egg output in sputum of infected subjects in Cross River Basin, Nigeria. Mary Slessor Journal of Medicine. 2003b; 3(1):24–27. 10.4314/msjm.v3i1.10991 [DOI] [Google Scholar]
- 77.Arene FO, Ibanga E, Asor JE. Epidemiology of paragonimiasis in Cross River basin, Nigeria: prevalence and intensity of infection due to Paragonimus uterobilateralis in Yakurr local government area. Public Health. 1998; 112(2):119–122. 10.1038/sj.ph.1900382 . [DOI] [PubMed] [Google Scholar]
- 78.Sachs R, Albiez EJ, Voelker J. Prevalence of Paragonimus uterobilateralis infection in children in a Liberian village. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1986; 80:800–801. 10.1016/0035-9203(86)90387-1 . [DOI] [PubMed] [Google Scholar]
- 79.Udonsi JK. Clinical field trials of praziquantel in pulmonary paragonimiasis due to Paragonimus uterobilateralis in endemic populations of the Igwun Basin, Nigeria. Tropical Medicine and Parasitology. 1989; 40(1):65–68. . [PubMed] [Google Scholar]
- 80.Ibanga ES, Eyo VM. Pulmonary paragonimiasis in Oban community in Akamkpa Local Government area, Cross River State, Nigeria: prevalence and intensity of infection. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001; 95(2):159–160. 10.1016/s0035-9203(01)90144-0 . [DOI] [PubMed] [Google Scholar]
- 81.Permin A, Yelifari L, Bloch P, Steenhard N, Hansen NP, Nansen P. Parasites in cross-bred pigs in the Upper East Region of Ghana. Veterinary Parasitology. 1999; 87(1):63–71. 10.1016/s0304-4017(99)00159-4 . [DOI] [PubMed] [Google Scholar]
- 82.Sachs R, Voelker J. The African civet as a natural host of the lung flukes observed in Liberia. Annual Report, Liberia Research Unit of the Tropical Institute Hamburg. 1985d:p48.
- 83.Uttah EC. Prevalence of human edible crabs infected with Paragonimus Uterobilateralis Metacercariae in southeastern Nigeria. Pacific Journal of Medical Sciences. 2013; 11(1):12–20. [Google Scholar]
- 84.Wokem G. Preliminary Survey of Epidermiological and Socio-Economic Studies on pulmonary paragonimiasis in part of Imo Rivers basin, Imo state, Nigeria on Pulmonary Paragonimiasis in part of Imo Rivers Basin, Imo State, Nigeria. Niger Delta Biologica. 2004; 4(2):82–87. [Google Scholar]
- 85.Ogakwu M, Nwokolo C. Radiological findings in pulmonary paragonimiasis as seen in Nigeria: a review based on one hundred cases. The British Journal of Radiology. 1973; 46(549):699–705. 10.1259/0007-1285-46-549-699 [DOI] [PubMed] [Google Scholar]
- 86.Oloyede IP, Inah GB, Bassey DE, Ekanem EE. Comparative study of radiological findings in pulmonary tuberculosis and paragonimiasis in children in a Southern Nigeria fishing community. West African Journal of Radiology. 2014;21(1):17 10.4103/1115-1474.128075 [DOI] [Google Scholar]
- 87.Umoh NO, Useh MF. Epidemiology of paragonimiasis in Oban community of Cross River State, Nigeria. Mary Slessor Journal of Medicine. 2009; 9(1):1–10 10.4314/msjm.v9i1.46637 [DOI] [Google Scholar]
- 88.Moyou-Somo R, Tagni-Zukam D. Paragonimiasis in Cameroon: clinicoradiologic features and treatment outcome. Médecine tropicale: revue du Corps de santé colonial. 2003; 63(2):163–7. . [PubMed] [Google Scholar]
- 89.Moyou-Somo R, Kefie-Arreym C, Dreyfuss G, Dumas M. An epidemiological study of pleuropulmonary paragonimiasis among pupils in the peri-urban zone of Kumba town, Meme Division, Cameroon. BMC Public Health. 2003; 3:40 10.1186/1471-2458-3-40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ollivier G, Boussinesq M, Albare JJ, Cumberlidge N, Farhati K, Chippaux JP, et al. Étude épidémlologique d’une distomatose à Paragonimus sp. au Sud-Cameroun. Bulletin de la Société de Pathologie Exotique et de ses filiales. 1995; 88(4):164–169. . [PubMed] [Google Scholar]
- 91.Kum PN, Nchinda TC. Pulmonary paragonimiasis in Cameroon. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1982;76(6):768–772. 10.1016/0035-9203(82)90102-x . [DOI] [PubMed] [Google Scholar]
- 92.Volkmer KJ. Diagnostic pattern of African paragonimiasis. Annales de la Société belge de médecine tropicale. 1975; 55(5):535–453. [PubMed] [Google Scholar]
- 93.Volkmer KJ, Braband H. Das Röntgenbild der afrikanischen Paragonimiasis. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren. 1975; 122(3);265–267. 10.1055/s-0029-1230069 [DOI] [PubMed] [Google Scholar]
- 94.Nwokolo C. Treatment of African paragonimiasis. Tropenmedizin und Parasitologie. 1976; 27:53–55. [PubMed] [Google Scholar]
- 95.Ripert C, Carrie J, Ambroise-Thomas P, Baecher R, Kum NP, Same-Ekobo A. Étude épidémiologique et clinique de la paragonimose au Cameroun. Résultats du traitement par le niclofolan. Bulletin de la Société de Pathologie Exotique et de ses filiales. 1981; 74(3):319–331. . [PubMed] [Google Scholar]
- 96.Ripert C., Couprie B, Moyou R, Gaillard F, Appriou M, Tribouley-Duret J. Therapeutic effect of triclabendazole in patients with paragonimiasis in Cameroon: a pilot study. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992; 86(4):417–417. 10.1016/0035-9203(92)90247-a . [DOI] [PubMed] [Google Scholar]
- 97.Nwokolo C, Volkmer KJ. Single dose therapy of Paragonimiasis with menichlopholan. American Journal of Tropical Medicine and Hygiene. 1977; 26:4 10.4269/ajtmh.1977.26.688 . [DOI] [PubMed] [Google Scholar]
- 98.Monson MH, Koenig JW, Sachs A. Successful treatment with praziquantel of six patients infected with the African lung fluke, Paragonimus uterobilateralis. American Journal of Tropical Medicine and Hygiene. 1983; 32(2):371–375. 10.4269/ajtmh.1983.32.371 . [DOI] [PubMed] [Google Scholar]
- 99.Moyou-Somo R, Enyong PA, Kouamouo J, Dinga JS, Couprie B and Ripert C. Etude de la paragonimose dans cinq départements de la Mémé (Sudouest du Cameroun). Résultats du traitement par le praziquantel. Revue Science et Technique, Yaoundé. 1983; 6–7:125–129. [Google Scholar]
- 100.Ochigbo SO, Ekanem EE, Udo JJ. Prevalence and intensity of Paragonimus uterobilateralis infection among school children in Oban village, South Eastern, Nigeria. Tropical Doctor. 2007; 37(4):224–226. 10.1258/004947507782332766 . [DOI] [PubMed] [Google Scholar]
- 101.Osoagbaka OU, Njoku-Obi ANU. Bacterial Etiology of Lower Respiratory Tract Infections in parts of Eastern Nigeria. Nigerian Journal of Microbiology. 1982; 2:131–140. [Google Scholar]
- 102.Njunda AL, Nsagha DS, Assob JCN, Abange BW, Tamoh AJ, Kwenti TE. Pulmonary paragonimiasis and aspergillosis in patients suspected of tuberculosis in Yaounde, Cameroon. British Microbiology Research Journal. 2015; 10(4):1–8. 10.9734/BMRJ/2015/20138 [DOI] [Google Scholar]
- 103.Sachs R. Occurence of Human Lung Fluke Infection in an Endemic Area in Liberia In: Geerts S, Kumar V, Brandt J. (eds) Helminth Zoonoses. Current Topics in Veterinary Medicine and Animal Science, Springer, Dordrecht: 1987; 43: 132–136. 10.1007/978-94-009-3341-5_19 [DOI] [Google Scholar]
- 104.Sachs R, Jarrett B, Attia B. Paragonimiasis and tuberculosis in the Bong area. Annual Report 1985, Liberia Research Unit of the Tropical Institute Hamburg 1985a; p43.
- 105.Adedoyin MA, Awogun IA, Juergensen T. Prevalence of intestinal parasitoses in relationship to diarrhoea among children in Ilorin. West African Journal of Medicine. 1990; 9(2):83–88. . [PubMed] [Google Scholar]
- 106.Yamamoto H, Aanu NO, Chukwuma F, Ugwuegblam I, Ahumibe E. Parasitological Surveys in The East Central State Of Nigeria. Japanese Journal of Tropical Medicine and Hygiene. 1979. 7(1):7–21. [Google Scholar]
- 107.Onubogu UV. Intestinal parasites of school children in urban and rural areas of Eastern Nigeria. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Erste Abteilung Originale. Reihe A: Medizinische Mikrobiologie und Parasitologie. 1978; 242(1):121–132. [PubMed] [Google Scholar]
- 108.Voelker J, Nwokolo C. Human paragonimiasis in eastern Nigeria caused by Paragonimus uterobilateralis. Zeitschrift für Tropenmedizin und Parasitologie. 1973; 24(3):323–328. . [PubMed] [Google Scholar]
- 109.Dierckx P, Coigneau H, Debondt R, Leblanc G. Paragonimose pulmonaire. Annales de la Société Belge de Médecine Tropicale. 1980; 60:111. [Google Scholar]
- 110.Petavy AF, Cambon M, Demeocq F, Dechelotte P. Un cas Gabonais de paragonimose chez un enfant. Bulletin de la Société de Pathologie Exotique et de ses filiales. 1981; 74(2):193–197. . [PubMed] [Google Scholar]
- 111.Simarro PP, Alamo A, Sima FO, Roche J, Mir M, Ndong P. Endemic human paragonimiasis in Equatorial Guinea. Detection of the existence of endemic human paragonimiasis in Equatorial Guinea as a result of an integrated sanitary programme. Tropical and Geographical Medicine. 1991; 43(3):326–328. . [PubMed] [Google Scholar]
- 112.Aka NA, Assoumou A, Adoubryn KD, Djino S, Domoua K, Ouhon J, et al. Un nouveau foyer de paragonimose humaine découvert en Côte d’Ivoire (Afrique de l’Ouest): le cas de l’ île de Lauzoua. Médecine tropicale. 2009; 69(3):263–266. . [PubMed] [Google Scholar]
- 113.Aka NA, Assoumou A, Adoubryn KD, Djino S, Domoua K, Ouhon J, et al. Persistance d’un foyer de paragonimose dans le département de Lakota, Côte d’Ivoire (Afrique de l’Ouest). Bulletin de la Société de Pathologie Exotique et de ses filiales. 2008b; 101(5):407–409. . [PubMed] [Google Scholar]
- 114.Eke RA, Enwereji EE. Re-Emergence of Paragonimiasis in Nigeria: A Case Report and Future Challenges. TAF Preventive Medicine Bulletin. 2010; 9(6):703–706. 10.5455/pmb.20100427030344 [DOI] [Google Scholar]
- 115.Ekanem AM, Oloyede IP, Eduwem DU. Paragonimiasis: A knowledge and awareness survey of clinicians in southern Nigeria on a neglected tropical disease. IBOM Medical Journal. 2020; 13(2):110–119. [Google Scholar]
- 116.Dou G, Aka N, Kouadio-Yapo C, Zika K, Loukou K, Ouhon J, Assoumou A, Adoubryn K. Connaissances Des Medecins Des Services De Pneumologie D’Abidjan Sur La Paragonimose. Revue Bio-Africa. 2018; 17:9–13. [Google Scholar]
- 117.Nkouawa A, Sako Y, Itoh S, Kouojip-Mabou A, Nganou CN, Saijo Y, et al. Ito A. Serological studies of neurologic helminthic infections in rural areas of southwest Cameroon: toxocariasis, cysticercosis and paragonimiasis. PLoS Neglected Tropical Diseases. 2010; 4(7):e732 10.1371/journal.pntd.0000732 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nkouawa A, Sako Y, Moyou-Somo R, Ito A. Serological and molecular tools to detect neurologic parasitic zoonoses in rural Cameroon. Southeast Asian Journal of Tropical Medicine and Public Health. 2011; 42(6):1365 . [PubMed] [Google Scholar]
- 119.Bakuza J. Epidemiological Significance of Parasitic Infections in Olive Baboons (Papio anubis) at Gombe National Park, Tanzania. Tanzania Journal of Science. 2020; 46(1):137–150. [Google Scholar]
- 120.Sachs R, Cumberlidge N. The dwarf river crab Liberonautes latidactylus nanoides Cumberlidge & Sachs, 1989, from Liberia- a new second intermediate host of Paragonimus uterobilateralis. Tropenmedizin und Parasitologie. 1990b; 42(1):73–74. . [PubMed] [Google Scholar]
- 121.Arimoro FO, Orogun EO. Notes on the biology and ecology of Sudanonautes floweri (De Man, 1901; Crustacea: Brachyura: Potamoidea: Potamonautidae) in river Ogbomwen, southern Nigeria. Acta Biologica Colombiana. 2008; 13(1):65–78. [Google Scholar]
- 122.Ogban E, Ojukpong GK, Uttah EC. Assessment of metacercariae infection of edible crabs in an area endemic for paragonimiasis in the Cross River Basin. Pacific Journal of Medical Sciences. 2018; 19(1):30–40. [Google Scholar]
- 123.Sachs R, Cumberlidge N. Distribution of metacercariae in freshwater crabs in relation to Paragonimus infection of children in Liberia, West Africa. Annals of Tropical Medicine and Parasitology. 1990c; 84(3):277–80. 10.1080/00034983.1990.11812467 [DOI] [PubMed] [Google Scholar]
- 124.Sachs R, Cumberlidge N. Metacercarial load of fresh-water crabs Liberonautes latidactylus in an endemic paragonimiasis focus in Liberia, West Africa. Zeitschrift für Angewandte Zoologie. 1991b; 78:161–165. [Google Scholar]
- 125.Sachs R, Cumberlidge N. First record of the spiny river crab, Liberonautes chaperi, A. Milne Edwards, 1887, as second intermediate host of Paragonimus uterobilateralis in Liberia. Annals of Tropical Medicine & Parasitology. 1991c; 85(4):471–472. 10.1080/00034983.1991.11812595 . [DOI] [PubMed] [Google Scholar]
- 126.Fa JE, Seymour S, Dupain JE, Amin R, Albrechtsen L, Macdonald D. Getting to grips with the magnitude of exploitation: bushmeat in the Cross–Sanaga rivers region, Nigeria and Cameroon. Biological Conservation. 2006; 129(4):497–510. 10.1016/j.biocon.2005.11.031 [DOI] [Google Scholar]
- 127.Cabaret J, Bayssade-Dufour C, Tami G, Albaret JL. Identification of African Paragonimidae by multivariate analysis of the eggs. Acta Tropica. 1999; 72(1):79–89. 10.1016/s0001-706x(98)00074-6 . [DOI] [PubMed] [Google Scholar]
- 128.Aka NA, Assoumou A, Adoubryn KD, Djino S, Ouhon J, Rondelaud D, Dreyfuss G. Presence of three egg groups of Paragonimidae in South-Western Ivory Coast (West Africa) In: Dupoy-Camet J, Dei-Cas E. (eds.) X European Multicolloquium of Parasitology. EMOP free papers. Paris, August 24–29 2008. Publ. Medimond, Bologna: 2008; 195–198. [Google Scholar]
- 129.Nozais JP, Doucet J, Dunan J, Assale N’dri G. Les paragonimoses en Afrique Noire. Apropos d’un foyer recent de Côte d’Ivoire. Bulletin de la Société de Pathologie Exotique et de ses filiales. 1980; 73(2):155–163. . [PubMed] [Google Scholar]
- 130.Adoubryn KD, Ouhon J, Assoumou A, Kassi EA, Koné M, Therizol-Ferly M. Champignons et parasites isolés à l’examen de 142 liquides d’aspiration bronchique à Abidjan (Côte d’Ivoire). Médecine d’Afrique Noire, 1999; 46(7):362–365. [Google Scholar]
- 131.Traoré SG. Risques de contraction des affections à Vibrio sp. et à Paragonimus sp. liés à la consommation des crabes et des crevettes vendus sur les marchés d’Abidjan et de Dabou. Doctoral dissertation, Universite Nangui-Abrogoua. 2013. pp. 198. https://hdl.handle.net/10568/33714
- 132.Vandepitte J, Job A, Delaisse J, Tabary M. Quatre cas d’abcès rétroauriculaires chez des Congolais, produits par un nouveau distome. Annales de la Société Belge de Médecine Tropicale. 1957; 37(2):309–316. . [PubMed] [Google Scholar]
- 133.CILSS Landscapes of West Africa—a window on a changing world. Ouagadougou, Burkina Faso. U.S. Geological Survey EROS, 47914 252nd St, Garretson, SD 57030, United States. 2016; pp. 219.
- 134.Ikhuoriah SO, Awharitoma AO. Prevalence of parasitic infestation in freshwater crab, Sudanonautes africanus (A. Milne-Edwards, 1869) (Brachyura: Potamonautidae) from selected Rivers in Edo and Delta States of Nigeria. Journal of Applied Sciences and Environmental Management. 2018; 22(11): 1761–1767. 10.4314/jasem.v22i11.9 [DOI] [Google Scholar]
- 135.Kaze PD, Gam KP. Survey of Cysticercus (Bladder Worm) in Meat Sold for Consumption in Bukuru, Plateau State Nigeria. Academic Journal of Interdisciplinary Studies. 2013; 2(12):45–50. 10.1038/s41437-017-0035-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Demenou BB, Doucet JL, Hardy OJ. History of the fragmentation of the African rain forest in the Dahomey Gap: insight from the demographic history of Terminalia superba. Heredity. 2018; 120(6):547–561. 10.1038/s41437-017-0035-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gouws G, Stewart BA. Potamonautid river crabs (Decapoda, Brachyura, Potamonautidae) of KwaZulu-Natal, South Africa. Water SA. 2001; 27(1):85–98. 10.4314/wsa.v27i1.5015 [DOI] [Google Scholar]
- 138.Sachs R, Kern P, Voelker J. Paragonimus uterobilateralis as the cause of 3 cases of human paragonimiasis in Gabon. Tropenmedizin und Parasitologie. 1983; 34(2):105–108. . [PubMed] [Google Scholar]
- 139.Rabone M, Wiethase J, Allan F, Gouvras AN, Pennance T, Hamidou A, et al. Freshwater snails of biomedical importance in the Niger River Valley: evidence of spatial and temporal patterns in abundance, distribution and infection with Schistosoma spp. Parasites & Vectors. 2019; 12(1):498 10.1186/s13071-019-3745-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]